Abstract
We developed a negative counterselection system for Pseudomonas putida based on uracil phosphoribosyltransferase (UPRTase) and sensitivity against the antimetabolite 5-fluorouracil (5-FU). We constructed a P. putida strain that is resistant to 5-FU and constructed vectors for the deletion of the surface adhesion protein gene, the flagellum biosynthesis operon, and two endonuclease genes. The genes were efficiently disrupted and left a markerless chromosomal in-frame deletion.
TEXT
Since the genome of the rod-shaped Gram-negative bacterium Pseudomonas putida KT2440 has been completely sequenced (GenBank accession number AE015451) (10), the study of gene functions is more and more focused. Usually genes are simply replaced by antibiotic resistance markers (15) to see what phenotypic effects result. The generation of a strain with multiple deletions, however, cannot be achieved with this method, because the availability of resistance markers is limited. Therefore, other gene disruption methods based on homologous recombination have been developed. By flanking the resistance cassette with recognition sites for site-specific recombinases, such as Flp/Flp recombination target (FRT) and Cre/loxP (5, 8), the cassette can precisely be removed and used again for the next deletion step. However, scars are left in the chromosome after each deletion step in the form of FRT or loxP sites, respectively. This may cause severe problems because the recognition sites in the chromosome can become recombined, which would then lead to large chromosomal deletions or inversions. Another disadvantage is the elaborate selection for positive clones that have dropped out the resistance cassette. To solve this problem, counterselectable marker systems have been developed. The most powerful are the fusaric acid (tetAR), streptomycin (rpsL), and sucrose (sacB) sensitivity systems (13). Recently, a novel counterselection system has been approved first for Bacillus subtilis (3) and subsequently for several other microorganisms (4, 6, 7). This system is based on upp, encoding the uracil phosphoribosyltransferase (UPRTase). This enzyme (EC 2.4.2.9) belongs to the pyrimidine salvage pathway and creates UMP from uracil and phosphoribosylpyrophosphate (11). The toxic antimetabolite 5-fluorouracil (5-FU) also becomes converted by UPRTase into 5-fluoro-UMP. After metabolization into 5-fluoro-dUMP, it acts as a suicide inhibitor of the thymidylate synthase, subsequently resulting in cell death. Therefore, microorganisms with UPRTase activities are sensitive to 5-FU. In P. putida KT2440, an upp gene (PP_0746) has been identified as well (10).
In this study, we describe a gene deletion procedure based on the upp gene as a new counterselectable marker in P. putida. We deleted the upp gene to get a 5-FU-resistant strain. With that strain, we were able to delete further genes efficiently with regard to creating a strain applicable in industrial production.
Plasmids, bacterial strains, and growth conditions.
The bacterial strains and plasmids used in this study are shown in Table 1. P. putida strains and Escherichia coli JM109 (16) were grown at 30°C in LB medium (2), supplemented with kanamycin (Kan; 50 μg ml−1) (LBKan) or 5-FU (20 μg ml−1). 5-FU was purchased from Sigma-Aldrich Corporation (Taufkrichen, Germany) and prepared as a stock solution of 100 mg ml−1 in dimethyl sulfoxide (DMSO). P. putida strains were also grown in M9 minimal medium (48 mM Na2HPO4·7H2O, 22 mM KH2PO4, 8.6 mM NaCl, 18.7 mM NH4Cl), supplemented with 0.2% glucose, 1 mM MgSO4, 0.1 mM CaCl2, and 6 μM thiamine hydrochloride.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) F′ [traD36 proAB+lacIqlacZΔM15] | 16 |
| P. putida | ||
| KT2440 | Wild type | ATCC 47054 |
| ΔUPP4 | Δupp | This study |
| GN24 | Δupp ΔPP_0168 | This study |
| GN109 | Δupp ΔPP_0168 ΔPP_2451 | This study |
| GN112 | Δupp ΔPP_0168 Δ PP_4333-4396 | This study |
| GN125 | Δupp ΔPP_0168 ΔPP_2451 ΔPP_3375 | This study |
| Plasmids | ||
| pIC20HE | Cloning vector for blue-white screening | 1 |
| pNEO | Cloning vector with kanamycin resistance gene | GenBank accession no. U13862 |
| pJOE6186.1 | pIC20HE with a kanamycin resistance gene cloned into BamHI/ClaI sites | This study |
| pJOE6227.1 | pJOE6186.1 with the up- and downstream regions of upp cloned into BamHI site | This study |
| pJOE6261.2 | pJOE6186.1 with a copy of upp from P. putida KT2440 cloned into NdeI/NheI sites | This study |
| pJOE6348.1 | pJOE6261.2 with the up- and downstream regions of PP_0168 cloned into BamHI site | This study |
| pNG90.7 | pJOE6261.2 with the upstream region of PP_4333 and the downstream region of PP_4396 cloned into BamHI site | This study |
| pNG100.1 | pJOE6261.2 with the up- and downstream regions of PP_3375 cloned into BamHI site | This study |
| pNG105.2 | pJOE6261.2 with the up- and downstream regions of PP_2451 cloned into SalI site | This study |
Plasmid and strain construction for upp-based counterselection.
Standard recombinant DNA techniques and transformation methods were used as described by Sambrook et al. (14). The oligonucleotide primers used in this study are shown in Table S1 in the supplemental material. The usage of the upp counterselection system (Fig. 1) for P. putida requires a strain resistant to 5-FU. The MIC was determined as 10 μg ml−1 5-FU for P. putida KT2440, but 20 μg ml−1 was chosen for selection. That reduced the appearance of false-positive clones without affecting the growth rate. The plasmid used to delete the upp gene in P. putida KT2440 was constructed by starting with plasmid pIC20HE (1). That vector was cut with BamHI/ClaI, and the kanamycin resistance gene (neo), which was cut out from the plasmid pNEO (GenBank accession no. U13862), was inserted by ligation, resulting in pJOE6186.1.
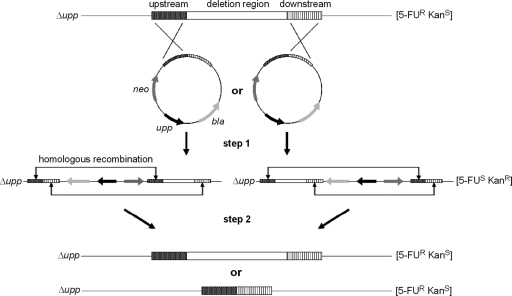
Fig. 1.
Schematic presentation of the upp counterselection system for P. putida. The integration vectors for deletion of a chromosomal region (white box) contain the up- and downstream regions (each about 1 kb; shaded boxes), kanamycin and ampicillin resistance genes (neo and bla), and the upp gene. In the first step, integration occurs via a single-crossover recombination event in either the up- or the downstream region. The resulting strain is sensitive to 5-FU and resistant to kanamycin. In the second step, another recombination event takes place, in which either the original state is restored or the region of interest is deleted. These strains are now resistant to 5-FU and sensitive to kanamycin. When other genes are inserted between the up- and downstream regions of the integration vectors, this method can also be used for gene integration or gene replacement in the P. putida chromosome.
For chromosomal deletion of upp, the up- and downstream regions were PCR amplified, using oligonucleotides s5380/s5381 and s5382/s5383, respectively. Chromosomal DNA from P. putida KT2440 was used as a template. The fragments, which included homologous regions of 792 bp and 883 bp, respectively, were cut with BamHI and HindIII. Integration occurred via 3-fragment ligation into BamHI-cut pJOE6168.1, creating pJOE6227.1.
P. putida KT2440 was transformed with pJOE6227.1 by electroporation. Since the plasmid cannot be autonomously replicated, it has to integrate via single crossover into the chromosome. Selection occurred on LBKan. Positive clones should be Kanr and 5-FUs. Therefore, clones were checked on M9 minimal plates containing 20 μg ml−1 5-FU and 0.2% glucose (M9Glc + 5-FU). One of the Kanr 5-FUs clones was incubated in LB for 24 h at 30°C. During this time the plasmid should have been excised again through homologous recombination and been lost. By this means, either the original genotype would be reestablished or the upp gene would be lost. Afterwards, different dilutions (10−3 and 10−5) were plated on M9Glc + 5-FU. After incubation for 48 h at 30°C, up to 250 CFU was gained. Fifty clones thereof were checked on LBKan and on M9Glc + 5-FU. Of the clones, which were 5-FUr and Kans, 3 were checked by colony PCR: cells were picked from M9 minimal plates and resuspended in 100 μl of double-distilled water. After treatment for 10 min at 99°C, cells were pelletized (5 min, 16.1 g, room temperature). From the supernatant, 10 μl was used as template for the PCR, using s5381/s5383 as primers. The resulting positive strain was designated ΔUPP4. The counterselection system can be used only in P. putida, if 5-FU sensitivity can be restored by reintroducing the upp gene. Therefore, upp was PCR amplified using oligonucleotides s5378/s5379 and chromosomal DNA from P. putida KT2440 as template. The fragment was integrated between the restriction sites NdeI and NheI of vector pJOE6168.1, creating pJOE6261.2. In the BamHI restriction site of that vector, the flanking regions of target genes can now be easily integrated.
Deletion of selected chromosomal genes.
To demonstrate the potential of the new developed upp counterselection system for P. putida (Fig. 1) and with regard to constructing an eligible production strain, different genes were selected and deleted: PP_0168 (lapA), coding for the surface adhesion protein, which is an important stress response element and responsible for the formation of biofilms (12); PP_4333-4396, coding for flagellum biosynthesis; and PP_2451 and PP_3375, coding for endonucleases. Table 2 summarizes the constructions of the different strains. Generally, construction began with PCR amplification of the up- and downstream regions (each about 1 kb), including the start and stop codons of the target gene. The PCR fragments were cut and cloned into pJOE6261.2 according to the data shown in Table 2. Then, P. putida with deleted upp was transformed with the constructed integration vectors by electroporation. Selection steps occurred as described above. The transformation efficiencies were about 25 CFU μg−1 DNA using 5 × 109 electroporated cells. In each case, 10 putative positive clones were checked by colony PCR, whereas the reverse primers were located in the downstream region and the forward primers once in the upstream region (positive control) and once in the region to be deleted (negative control). As further controls, the initial strain and the strain with the integrated vector were used. Theoretically, half of the clones should have lost the gene, while the other half should have restored the original sequence. For reasons already discussed by Fabret et al. (3), the maximum ratio of 50% could not be achieved (Table 2). Deletion of PP_0168 resulted in strains no longer capable of forming biofilms, which was verified by a modified version of a biofilm assay described by Merritt et al. (9) as detailed in the supplemental material. Deletion of the flagellum biosynthesis operon led to strains with a lack of motility, which was observed microscopically and on motility agar plates (see Fig. S2 in the supplemental material). Deletion of the endonucleases, however, had no significant effect on transformation efficiencies.
Table 2.
Overview of the strain constructions
| Parameter | Deleted gene/region |
|||
|---|---|---|---|---|
| PP_0168 (lapA) | PP_4333-4396 (flagellum biosynthesis) | PP_2451 (endA-1) | PP_3375 (endA-2) | |
| Region length (bp) | 26,049 | 64,722 | 693 | 966 |
| Upstream primers (length of homology, bp) | s5278/s5279 (976) | s5840/s5841 (944) | s5943/s5944 (864) | s5947/s5948 (950) |
| Downstream primers (length of homology, bp) | s5280/s5281 (1,031) | s5842/s5843 (1,047) | s5945/s5946 (1,051) | s5949/s5950 (1,052) |
| Restriction sites by which cloned | BamHI/HindIII | BamHI/EcoRI | SalI/HindIII | BamHI/HindIII |
| Integration vector | pJOE6348.1 | pNG90.7 | pNG105.2 | pNG100.1 |
| Target strain | ΔUPP4 | GN24 | GN24 | GN109 |
| Colony PCR primers | s5761/s5762, s5764/s5762 | s6257/s6258, s6368/s6258 | s6261/s6262, s6370/s6262 | s6263/s6264, s6371/s6264 |
| Deletion ratio (%) | 40 | 20 | 10 | 30 |
| Resulting strain | GN24 | GN112 | GN109 | GN125 |
Conclusion.
The upp gene has been approved for B. subtilis, Enterococcus faecalis, Lactobacillus acidophilus, and Desulfovibrio vulgaris already as an efficient negative selection marker for gene deletion, integration, and replacement (3, 4, 6, 7). Here we demonstrate that it can also be an efficient genetic tool for P. putida.
Supplementary Material
Acknowledgments
We thank the Federal Ministry of Science and Education (BMBF), Germany for funding this project (Systembiologie in Pseudomonas, FZK 315406).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 10 June 2011.
REFERENCES
- 1. Altenbuchner J., Viell P., Pelletier I. 1992. Positive selection vectors based on palindromic DNA sequences. Methods Enzymol. 216:457–466 [DOI] [PubMed] [Google Scholar]
- 2. Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fabret C., Ehrlich S. D., Noirot P. 2002. A new mutation delivery system for genome-scale approaches in Bacillus subtilis. Mol. Microbiol. 46:25–36 [DOI] [PubMed] [Google Scholar]
- 4. Goh Y. J., et al. 2009. Development and application of a upp-based counterselective gene replacement system for the study of the S-layer protein SlpX of Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 75:3093–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 6. Keller K. L., Bender K. S., Wall J. D. 2009. Development of a markerless genetic exchange system for Desulfovibrio vulgaris Hildenborough and its use in generating a strain with increased transformation efficiency. Appl. Environ. Microbiol. 75:7682–7691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kristich C. J., Manias D. A., Dunny G. M. 2005. Development of a method for markerless genetic exchange in Enterococcus faecalis and its use in construction of a srtA mutant. Appl. Environ. Microbiol. 71:5837–5849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marx C. J., Lidstrom M. E. 2002. Broad-host-range cre-lox system for antibiotic marker recycling in Gram-negative bacteria. Biotechniques 33:1062–1067 [DOI] [PubMed] [Google Scholar]
- 9. Merritt J. H., Kadouri D. E., O'Toole G. A. 2005. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005:1B.1.1–1.B.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson K. E., et al. 2002. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ. Microbiol. 4:799–808 [DOI] [PubMed] [Google Scholar]
- 11. Neuhard J. 1983. Utilization of preformed pyrimidine bases and nucleosides, p. 95–148 In Munch-Petersen A.(ed.), Metabolism of nucleotides, nucleosides and nucleobases in microorganisms. Academic Press, New York, NY [Google Scholar]
- 12. Reva O. N., et al. 2006. Functional genomics of stress response in Pseudomonas putida KT2440. J. Bacteriol. 188:4079–4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reyrat J. M., Pelicic V., Gicquel B., Rappuoli R. 1998. Counterselectable markers: untapped tools for bacterial genetics and pathogenesis. Infect. Immun. 66:4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 15. Schweizer H. P., Hoang T. T. 1995. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene 158:15–22 [DOI] [PubMed] [Google Scholar]
- 16. Yanisch-Perron C., Vieira J., Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.