Abstract
Here we describe a versatile and sensitive reporter system for actinomycetes that is based on gusA, which encodes the β-glucuronidase enzyme. A series of gusA-containing transcriptional and translational fusion vectors were constructed and utilized to study the regulatory cascade of the phenalinolactone biosynthetic gene cluster. Furthermore, these vectors were used to study the efficiency of translation initiation at the ATG, GTG, TTG, and CTG start codons. Surprisingly, constructs using a TTG start codon showed the best activity, whereas those using ATG or GTG were approximately one-half or one-third as active, respectively. The CTG fusion showed only 5% of the activity of the TTG fusion. A suicide vector, pKGLP2, carrying gusA in its backbone was used to visually detect merodiploid formation and resolution, making gene targeting in actinomycetes much faster and easier. Three regulatory genes, plaR1, plaR2, and plaR3, involved in phenalinolactone biosynthesis were efficiently replaced with an apramycin resistance marker using this system. Finally, we expanded the genetic code of actinomycetes by introducing the nonproteinogenic amino acid N-epsilon-cyclopentyloxycarbonyl-l-lysine with the GusA protein as a reporter.
INTRODUCTION
Actinomycetes have a long-standing history of producing clinically valuable natural products. For this reason, they have been the focus of intensive investigation for many decades. The synthesis of natural products in actinomycetes is tightly controlled by various regulatory proteins (3). Therefore, the regulation of gene expression has been an important topic for many researchers in this field. Deciphering the complex regulatory cascades should create a foundation for the development of antibiotic overproducers and help to clarify the role of the natural product(s) in the bacteria that produce it (2, 10). Understanding regulation is becoming increasingly important, because the majority of the biosynthetic gene clusters potentially responsible for the production of new natural products are not expressed under standard laboratory conditions (9). Further insights into the mechanisms of the regulation of gene expression in actinomycetes will enhance the study of actinomycetes as producers of natural products. Among a variety of genetic and molecular approaches for the study of the regulation of gene expression, the use of reporter genes has become important (8, 18). Reporter genes enable the visualization of various biological processes in living cells. These genes are fused to regulatory sequences, and after being introduced into a biological system, they provide a quantifiable signal. The influence of various physiological conditions, stresses, and trans-acting factors that modulate gene expression at the transcriptional or translational level can be readily detected with the help of reporter genes (45). Recent developments in synthetic biology have expanded the use of reporter genes to monitor the behavior of synthetic genetic circuits and to characterize various synthetic BioBrick parts (e.g., promoters, operators, terminators, and intergenic regions) (21).
Several reporter genes have been used in actinomycete species, including xylE, encoding catechol 2,3-dioxygenase (27), cat, encoding chloramphenicol acetyltransferase (23), neo, encoding neomycin phosphotransferase (7), luxAB and luc, encoding luciferases (13, 34), and gfp, encoding green fluorescent protein (GFP) (19). The most popular reporters available for use in actinomycetes are GFP and luciferase. GFP from jellyfish is one of the most commonly used reporters for studies in many living organisms. Its ability to tolerate N- and C-terminal translational fusions (30) makes GFP a powerful tool to monitor the trafficking and subcellular localization of proteins. For gene expression studies, however, GFP is less sensitive than other systems, due to background fluorescence of all materials used in assays and to its intrinsic limit of one photon of light per GFP molecule (no enzymatic signal amplification) (26). In addition, the assay may suffer from UV-induced toxicity and photobleaching, thus limiting the duration of observation and analysis. In several systems, the formation of GFP aggregates causes cytotoxicity (12, 20). In addition, actinomycetes often have a high level of autofluorescence, which makes the analysis more complicated because of a low signal-to-noise ratio (31).
Luciferase assays can be rapid and sensitive. The rapid turnover of luciferases makes them convenient for monitoring the dynamics of gene transcription (14, 34). The lack of autoluminescence in actinomycetes results in a high signal-to-noise ratio when using luciferases (13). The disadvantage stems from the complexity of enzymatic reactions, which depend on multiple reagents. For example, bacterial luciferase uses FMNH2, O2, and a 10-carbon aldehyde, decanal, whereas eukaryotic luciferases use ATP, Mg2O2, and the substrate luciferin. In addition, the luciferases do not enzymatically amplify the signal, they are labile, and they have a short half-life (53).
In this report, we introduce the protein encoded by gusA, β-glucuronidase (GUS), as a reporter in actinomycetes. GUS hydrolyzes a wide variety of β-glucuronides (29). Several factors make this system superior for the study of gene expression and other applications: GUS offers an unparalleled sensitivity due to the stability and high specific activity of the GUS enzyme, which does not need any cofactors; the enzyme is remarkably tolerant to the most commonly used chemicals and assay conditions (e.g., pH and temperature); most streptomycetes do not carry any endogenous GUS activity; GUS tolerates large N- and C-terminal translational fusions (25, 28, 41). The assay is simple, inexpensive, and sensitive, with a wide range of commercially available substrates that allow different assay types and formats to be used: spectrophotometric (p-nitrophenyl β-d-glucuronide and phenolphthalein-β-d-glucuronide), fluorimetric (4-methylumbelliferyl-β-d-glucuronide and 5-dodecanoylaminofluorescein-di-β-d-glucuronide), chemiluminescent (1,2-dioxetane-β-d-glucuronide), and chromogenic for visual screening (5-bromo-4-chloro-3-indolyl-β-d-glucoronide, other 3-indoxyl derivatives, and naphthol-β-d-glucoronide).
Using the GUS system, we have significantly simplified the method of gene targeting in streptomycetes and showed the utility of the system for gene expression studies at the levels of transcription and translation. We anticipate that the GUS system will provide new possibilities for the study of functional gene expression in actinomycetes and extend the utility of existing reporter systems.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
All strains and plasmids are listed in Table 1. Strains were cultivated on standard media. The following antibiotics were added to media when required: apramycin, kanamycin, hygromycin, thiostrepton, and phosphomycin. For the production of phenalinolactone, NL111 medium was used (4). Phenalinolactone was detected by liquid chromatography-mass spectrometry (LC-MS) as previously described (16). In experiments involving the suppression of a stop codon, N-ε-cyclopentyloxycarbonyl-l-lysine (Cyc) was used.
Table 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| Streptomyces strains | ||
| S. albus J1074 | Wild-type (WT) strain, heterologous host | 35 |
| Streptomyces sp. Tu6071 | WT strain, phenalinolactone producer | 15 |
| Streptomyces sp. Tu6071 ΔR1 | WT with deletion of plaR1 gene | This work |
| Streptomyces sp. Tu6071 ΔR2 | WT with deletion of plaR2 gene | This work |
| Streptomyces sp. Tu6071 ΔR3 | WT with deletion of plaR3 gene | This work |
| E. coli strains | ||
| XL1-Blue | General cloning host | Stratagene |
| ET12567 pUZ8002 | Strain for intergeneric conjugation | 31 |
| Plasmids | ||
| pSET152 | Amr; φC31-based Streptomyces integrative vector | 31 |
| pKCLP2 | Hygr; replicative vector in E. coli; nonreplicative in streptomyces | 36 |
| pKC1139 | Amr; E. coli-Streptomyces shuttle vector | 31 |
| pSOKT | Amr; VWB-based Streptomyces integrative vector | 46 |
| pTESa | Amr; φC31-based Streptomyces integrative vector | 46 |
| pSETGUS | gusA containing 2.0-kb BamHI fragment cloned into BamHI site of pSET152 | This work |
| pKGLP2 | gusA containing 2.0-kb BamHI fragment cloned into BglII site of pKCLP2 | This work |
| pKG1139 | gusA containing 2.0-kb BamHI fragment cloned into BglII site of pKC1139 | This work |
| pKGLPΔR1aac | Vector for deletion of plaR1, based on pKGLP2 | This work |
| pKGLPΔR2aac | Vector for deletion of plaR2, based on pKGLP2 | This work |
| pKGLPΔR3aac | Vector for deletion of plaR3, based on pKGLP2 | This work |
| pGUS | Promoter probe vector; pSETGUS with deleted KpnI fragment containing tipA promoter | This work |
| pGUSR1 | pGUS with plaR1 gene's promoter cloned into XbaI/KpnI sites of pGUS | This work |
| pGUSR2 | pGUS with plaR2 promoter cloned into XbaI/KpnI sites of pGUS | This work |
| pGUSR3 | pGUS with plaR3 promoter cloned into XbaI/KpnI sites of pGUS | This work |
| pGUSM2 | pGUS with plaM2 promoter cloned into XbaI/KpnI sites of pGUS | This work |
| pGUSO2 | pGUS with plaO2 promoter cloned into XbaI/KpnI sites of pGUS | This work |
| pGUSHL4aadA | pTESa-based vector for translational fusion with gusA | This work |
| pGUSHL4plaM2aadA | plaM2 without stop codon cloned into XbaI/EcoRV sites of pGUSHL4aadA | This work |
| pGUSHL4plaM2aadAcontr | Promoterless plaM2 cloned into XbaI/EcoRV sites of pGUSHL4aadA | This work |
| pSETGUSTTG | pSETGUS derivative containing gusA with TTG start codon instead of ATG | This work |
| pSETGUSCTG | pSETGUS derivative containing gusA with CTG start codon instead of ATG | This work |
| pSETGUSGTG | pSETGUS derivative containing gusA with GTG start codon instead of ATG | This work |
| pSETGUSTAG | pSETGUS derivative containing gusA with TAG stop codon directly after ATG start codon | This work |
| pSOKTpyr | DNA fragment containing pylT and pylS cloned into HindIII/SnaBI sites of pSOKT | This work |
| pSOKTpyr-tsr | PCR-amplified tsr marker cloned into SnaBI site of pSOKTpyr | This work |
DNA manipulation.
Isolation of DNA and all subsequent manipulations were performed according to standard protocols (40).
Construction of pKG1139 and pKGLP2 gene inactivation vectors.
A DNA fragment containing the gusA gene under the control of the tipA promoter was synthesized based on the sequence of the Escherichia coli β-glucuronidase protein (NP_416134) and cloned as a BamHI fragment into the BamHI site of pSET152, generating pSETGUS. To construct pKG1139 and pKGLP2, a BamHI fragment containing gusA was subcloned into the BglII sites of pKC1139 and pKCLP2.
Generation of Streptomyces sp. strain Tu6071 ΔR1, ΔR2, and ΔR3 deletion mutants.
Plasmid pKGLPΔR1aac was constructed as follows. Regions flanking the plaR1 gene were amplified by two pairs of primers, R1.1F/R1.1R and R1.2F/R1.2R, yielding two products, R1.1 and R1.2. The primers used in the study are listed in Table 2.
Table 2.
List of the primers used in this work
| Primer | Sequence |
|---|---|
| m2fuscontrxba | CAACTTCTAGACCACTCGAATATGGAAAGGC |
| plarirev2kpn | TATCGGGTACCCTCGTACCTCATCGGGTGCCCT |
| m2gusfus | AAAAGATATCCGCGAGGATCAGCGCCGCGT |
| gusfus1 | AGATATCCTCGCCGAGGCCGCCGCCAAGGAAGCCGCCGCCAAGGAAGCCGCCGCCAAGGAAGCCGCCGCCAAGGCCGCCGCCCTCCGGCCCGTCGAAACCCCGA |
| gusfus2 | ATTTAGGTGACACTATATCTAGAAGATCTGAATTCAAAGATATCCTCGCCGAGGCC |
| gusfus_rev | AAACAATTGTTATCACTGCTTCCCGCCCTGCTG |
| plar2_for | CCTCCCCTCGGTTGAATATG |
| plar2_rev | AAAATCTAGAATCTCAGATGTCGCCCACGC |
| plar2prfrxba | CAACTTCTAGAATGGTCGCAAAACGCGCAGC |
| plar2prrevkpn | TATCGGGTACCCGGCCCGCCCGCGCCCGGTCG |
| plar1prfrxba | CAACTTCTAGACCGTCGACAGGTTGGTGACC |
| plar1prrevkpn | TATCGGGTACCCCGCTGTCGTGGGTATCAGG |
| plar3prfrxba | CAACTTCTAGACGACGGCACCGTTCGTGACC |
| plar3prrevkpn | TATCGGGTACCAGATACCGGTATCGAATCTATACCG |
| plam2prfrxba | CAACTTCTAGAGGCGGCCGTCCATGTTCACC |
| plam2prrevkpn | TATCGGGTACCATTCGAGTGGTCACGATGTCG |
| plao2prfrxba | CAACTTCTAGATTCCACTCCGAGCACCGTGC |
| plao2prrevkpn | TATCGGGTACCTGATCCGCGTGAAAGGGAGC |
| r2.1f psti | CAACTCTGCAGGGAGCTGGCACTCGACGAGC |
| r2.1r | CGACTGGGGTGCACCTTCTCTAGACCGTCCAGCGGCGACCGG |
| r2.2f | CCGGTCGCCGCTGGACGGTCTAGAGAAGGTGCACCCCAGTCG |
| r2.2r nhei | TATCGGCTAGCCGCCTTCCGGCGGCCCATGC |
| tsr for | AATGATCAAGGCGAATACTTCATATGC |
| tsr rev | AAAAAGCTTTCATCACTGACGAATCGAGGTCG |
| gus-tag | AAAACTAGTAGCAACGGAGGTACGGACATGTAGCTCCGGCCCGTCGAAACCC |
| gus-gtg | AAAACTAGTAGCAACGGAGGTACGGACGTGCTCCGGCCCGTCGAAACCC |
| gus-ctg | AAAACTAGTAGCAACGGAGGTACGGACCTGCTCCGGCCCGTCGAAACCC |
| gus-ttg | AAAACTAGTAGCAACGGAGGTACGGACTTGCTCCGGCCCGTCGAAACCC |
| Reverse gus | GATATCTTATCACTGCTTCCCGCCCTG |
| r1.1f | CAACTCTGCAGATCCCTCTCCTGCCGCACTC |
| r1.1r | TATCGTCTAGACCATCCTCTCGGGCGACATG |
| r1.2f | CAACTGGATCCCCCCAGATCTCCCGCATCAG |
| r1.2r | TATCGGATATCTGGCGGCCATCTCCTGTTC |
| r3.1f | CAACTCTGCAGCCAAGAACGTGCCGGAAGG |
| r3.1r | TATCGTCTAGAACGTCCCCGAAATCGAGTC |
| r3.2f | CAACTGGATCCGTACGCCTTTCCCATGACC |
| r3.2r | TATCGGATATCAGAATCCCGTCCCCTCAGC |
R1.1 was digested by PstI and XbaI, whose recognition sites had been introduced into the primers. The resulting fragment was cloned into the corresponding sites of pKGLP2, yielding pKGLPR1.1. R1.2 was digested by BamHI and EcoRV, whose sites had been introduced into the primers, and the resulting fragment was cloned into the corresponding sites of pKGLPR1.1, yielding pKGLPΔR1. The apramycin resistance marker, surrounded by loxP sites, was cut from pLERE with XbaI and SpeI and cloned into the XbaI site of pKGLPΔR1, generating pKGLPΔR1aac.
Plasmid pKGLPΔR2aac was constructed as follows. Regions flanking the plaR2 gene were amplified by two pairs of primers, R2.1F/R2.1R and R2.2F/R2.2R, yielding two products, R2.1 and R2.2. R2.1 was digested by PstI and XbaI, whose sites had been introduced into the primers. The resulting fragment was cloned into the corresponding sites of pKGLP2, yielding pKGLPR2.1. R2.2 was digested by XbaI, and the resulting fragment was cloned into the XbaI and EcoRV sites of pKGLPR2.1, yielding pKGLPΔR2. The apramycin resistance marker, surrounded by loxP sites, was cut from pLERE by XbaI and SpeI and cloned into the XbaI site of pKGLPΔR2, generating pKGLPΔR2aac.
Plasmid pKGLPΔR3aac was constructed as follows. Regions flanking the plaR3 gene were amplified by two pairs of primers, R3.1F/R3.1R and R3.2F/R3.2R, yielding two products, R3.1 and R3.2. R3.1 was digested by PstI and XbaI, whose sites had been introduced into the primers. The resulting fragment was cloned into the corresponding sites of pKGLP2, yielding pKGLPR3.1. R3.2 was digested by XbaI and BamHI, whose sites had been introduced into the primers. The resulting fragment was cloned into the corresponding sites of pKGLPR3.1, yielding pKGLPΔR3.
The gene inactivation plasmids pKGLPΔR1aac, pKGLPΔR2aac, and pKGLPΔR3aac were transferred to Streptomyces sp. Tu6071 by intergeneric E. coli- Streptomyces conjugation (31). The resulting exconjugants were cultivated in liquid Trypticase soy broth (TSB) medium for 10 passages without any antibiotics. After cultivation in liquid medium, each culture was plated on mannitol soy (MS) solid medium (31) supplemented with apramycin at a final concentration of 50 mM. After sporulation, spores were harvested, diluted, and plated to produce single clones on plates of TSB agar. After 5 days of incubation, each plate was overlaid with 1 ml 1 mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-Gluc) solution. White clones were picked from plates of MS agar supplemented with 50 mM apramycin. Gene inactivation was verified by Southern hybridization in the case of the Streptomyces sp. Tu6071 ΔR2aac mutant and by PCR with a pair of primers, either R1.1F/R1.2R or R3.1F/R3.2R, in the case of Streptomyces sp. Tu6071 ΔR1aac or Streptomyces sp. Tu6071 ΔR3aac, respectively. Excision of the apramycin cassette was performed as described elsewhere (17).
Spectrophotometric measurement of glucuronidase activity in cell lysates.
Each strain to be analyzed was inoculated into liquid TSB medium. After the stationary phase had been reached, 1 ml of the culture was used to inoculate 100 ml of liquid medium (TSB, HA, SG, or NL111), which was incubated for 24, 72, or 120 h, depending on the experiment. After cultivation, mycelia were harvested by centrifugation, washed once with distilled water, and resuspended in lysis buffer (50 mM phosphate buffer [pH 7.0], 5 mM dithiothreitol [DTT], 0.1% Triton X-100, 1 mg/ml lysozyme). Lysis was performed at 37°C for 15 min. Lysates were centrifuged at 4,000 rpm for 10 min. Then, 0.5 ml of lysate was mixed with 0.5 ml of dilution buffer (50 mM phosphate buffer [pH 7.0], 5 mM DTT, 0.1% Triton X-100) supplemented with 5 μl 0.2 M p-nitrophenyl-β-d-glucuronide. The optical density at 415 nm (OD415) was measured after 20 min of incubation at 37°C. As a reference, a 1:1 mixture of lysate and dilution buffer was used.
Statistical calculations.
The Microsoft Excel software package was used for statistical calculations. All data presented are means ± standard errors calculated from three independent experiments.
Construction of gusA promoter probe vectors.
To construct a promoter detection vector with a promoterless gusA gene, the KpnI fragment of pSETGUS, containing the tipA promoter, was deleted. To prevent readthrough of the gusA gene from the integrase promoter, a SpeI/XbaI fragment containing the aadA gene surrounded by T4 terminators was cloned into the XbaI site, upstream of the gusA gene. The resulting vector was named pGUS.
The pGUSR1, pGUSR2, pGUSR3, pGUSM2, and pGUSO2 plasmids were constructed as follows. DNA fragments containing the promoters of plaR1, plaR2, plaR3, plaM2, and plaO2 were PCR amplified using the following pairs of primers: plaR1prfrxba/plaR1prrevkpn, plaR2prfrxba/plaR2prrevkpn, plaR3prfrxba/plaR3prrevkpn, plaM2prfrxba/plaM2prrevkpn, and plaO2prfrxba/plaO2prrevkpn, respectively. The resulting PCR products were digested by XbaI and KpnI, and the fragments were cloned into the corresponding sites of the pGUS vector, generating the plasmids pGUSR1, pGUSR2, pGUSR3, pGUSM2, and pGUSO2, respectively.
Construction of plasmids for translational fusion with the gusA gene.
The marker-free integrative plasmid pTESa was used to construct vectors for translational fusion with the gusA gene. The gusA gene, without the start codon, was amplified, and a helical linker was attached to its 5′ end. This was done by two-step PCR, first with the gusfus1/gusrev pair of primers and then with the gusfus2/gusrev pair of primers. The PCR product was digested by MfeI and SspI, and the resulting fragment was cloned into the EcoRI/SspI sites of pTESa. To prevent readthrough of gusA from upstream genes, a SmaI fragment containing the aadA gene surrounded by T4 terminators was cloned upstream of the gusA gene in the SspI site, generating pGUSHL4aadA.
To create a translational fusion of the plaM2 gene with gusA, the plaM2 gene was amplified without the stop codon by using the plaM2prfrxba/M2gusfus pair of primers. The PCR product was digested with XbaI and EcoRV, and the resulting fragment was cloned into the corresponding sites of pGUSHL4aadA, generating pGUSHL4plaM2aadA. As a control we used plasmid pGUSHL4plaM2aadAcontr, which was constructed by cloning the promoterless plaM2 gene into the XbaI/EcoRV sites of pGUSHL4aadA. The promoterless plaM2 gene was generated by PCR using the m2fuscontrxba/M2gusfus pair of primers.
Generation of gusA derivatives with different start codons.
Plasmids pSETGUSTTG, pSETGUSCTG, pSETGUSGTG, and pSETGUSTAG were constructed as follows. The gusA gene with the TTG, CTG, or GTG start codon or with the TAG stop codon as the second codon were PCR amplified with the forward primer GUS-TTG, GUS-CTG, GUS-GTG, or GUS-TAG and the reverse primer reverse-gus. The PCR products were digested by SpeI and XbaI and ligated to the 5.6-kb SpeI/EcoRV fragment of pSETGUS. Derivatives of the gusA gene from pSETGUSTTG, pSETGUSCTG, pSETGUSGTG, and pSETGUSTAG contain the CTC codon in the first position after the initiation codon, compared to the CTG codon in the case of pSETGUS. In addition, gusA gene derivatives from pSETGUSTTG, pSETGUSCTG, pSETGUSGTG, and pSETGUSTAG contain a 2-nucleotide deletion in the region between the tipA promoter and rbs site, in comparison with the initial pSETGUS.
Construction of the pSOKTpyr-tsr plasmid.
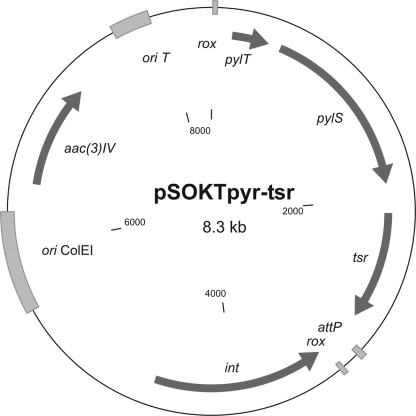
A fragment containing the pylT gene under the control of the tipA promoter and the pylS gene under the control of the ermE promoter was synthesized and cloned into the HindIII/SnaBI sites of a marker-free integration plasmid, generating pSOKTpyr. The thiostrepton resistance gene, tsr, was amplified by PCR using the primers tsrfor and tsrrev and cloned as a blunt fragment into the SnaBI site of pSOKTpyr, generating pSOKTpyr-tsr (see Fig. 10, below).
Fig. 10.
Map of the plasmid pSOKTpyr-tsr. It is based on the pSOKT marker-free integrational plasmid. The pylT and pylS genes encode tRNApyl and pyrrolysyl-tRNA synthetase, respectively. rox, recognition site of Dre recombinase; ori ColEI, origin of replication of E. coli. See also abbreviation definitions in the legend for Fig. 1.
Suppression of nonsense mutations in Streptomyces albus.
Strain S. albus pSOKTpyr-tsrpSETGUSUAG was inoculated into liquid TSB medium supplemented with apramycin and thiostrepton at final concentrations of 50 and 25 μg/ml, respectively. After 48 h of incubation, the mycelium from 1 ml of preculture was used to inoculate TSB medium supplemented with 50 μg/ml apramycin, 25 μg/ml thiostrepton, and 20 mM Cyc. As a control we used the same culture but without adding Cyc. Experimental and control cultures were grown for 24 h before their glucuronidase activities were measured.
RESULTS
Expression of the gusA gene in streptomyces strains.
Currently there are several reporter systems available for actinomycetes, but most of them have limitations. Some are too cumbersome for large-scale screening, while others are not sensitive enough (31). We decided to establish a new reporter system for streptomyces that would be sensitive, simple, and applicable for large-scale screening. We considered several potential candidates for the development of a reporter system, including α-fucosidase, β-fucosidase, β-glucuronidase, N-acetyl-β-galactosamidase, β-ribosidase, and esterase. These enzymes can hydrolyze compounds such as 2-naphthyl α-l-fucopyranoside, p-nitrophenyl-β-l-fucopyranoside, 5-bromo-4-chloro-3-indolyl-β-d-glucuronide, 5-bromo-4-chloro-3-indolyl-β-d-ribofuranoside, 5-bromo-4-chloro-3-indolyl N-acetyl β-d-galactosaminide, and 5-bromo-4-chloro-3-indolyl caprylate, thus providing products for spectrophotometric measurements. We tested Streptomyces coelicolor M600, Streptomyces lividans 1326, Streptomyces sp. Tu6071, Streptomyces bernensis, and S. albus J1074 for the endogenous activity of these enzymes that could potentially mimic the activity of a reporter. All of the strains showed esterase activity, and only S. coelicolor M600 and S. lividans 1326 displayed N-acetyl-β-galactosamidase activity. This indicates that N-acetyl-β-galactosamidase and esterase cannot be used as reporters in streptomyces. Among all the enzymes that did not show any background activity in the streptomycete strains, we chose β-glucuronidase because it is one of the most frequently used reporters (28).
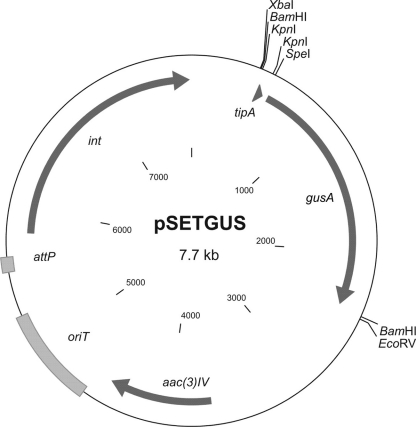
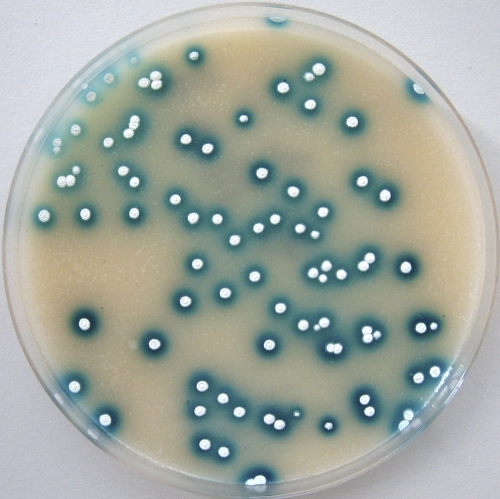
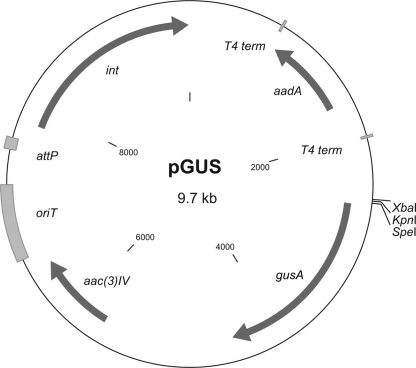
The gene encoding GUS was synthesized according to S. coelicolor codon usage and placed under the control of the thiostrepton-inducible promoter tipA. This synthetic fragment was cloned into the integrative vector pSET152, generating pSETGUS (Fig. 1), which was later transferred to S. lividans 1326, S. coelicolor M600, Streptomyces sp. Tu6071, and S. albus J1074. To detect the GUS activity directly on plates, we used the chromogenic substrate X-Gluc, which forms a blue precipitate, 5,5′-dibromo-4,4′-dichloro-indigo, after hydrolysis by GUS. In all the resulting strains we detected strong GUS activity (Fig. 2) compared to the absence of activity in parental strains. This fact indicates that the synthetic gusA gene can be efficiently expressed in streptomycetes and thus could be used to develop a reporter system.
Fig. 1.
Map of the plasmid pSETGUS. The gusA gene was cloned as a BamHI fragment into the BamHI site of pSET152, generating pSETGUS. tipA, thiostrepton-inducible promoter; gusA, β-glucuronidase gene; aac(3)IV, apramycin resistance gene; oriT, origin of conjugal transfer; attP, attachment site; int, integrase gene.
Fig. 2.
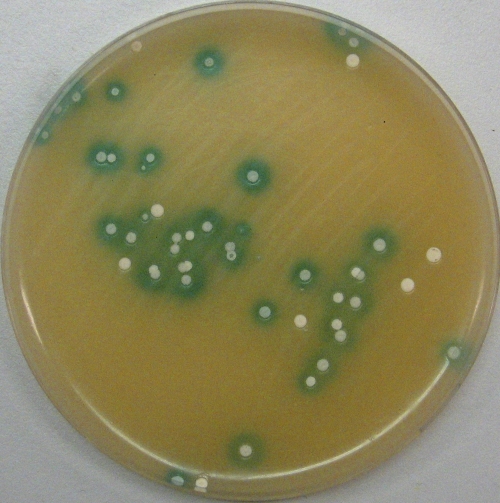
Colonies of Streptomyces sp. Tu6071 pSETGUS overlaid with X-Gluc solution. Blue halos are 5,5′-dibromo-4,4′-dichloro-indigo, formed by the β-glucuronidase activity.
Construction of the gene inactivation vectors containing the gusA gene.
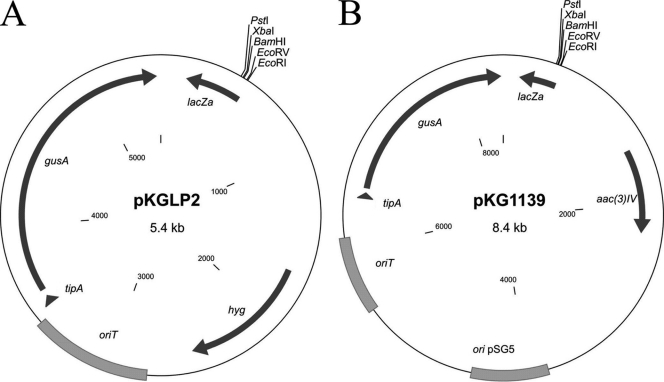
Considering that the activity of GUS can be easily detected visually, it is of great interest to create gene inactivation vectors containing the gusA gene for a simplified method to screen for double crossovers. Such an approach facilitates the selection of clones with double crossovers (those having lost a vector backbone and thus a gusA gene) directly by visual inspection. When overlaid with X-Gluc solution, clones with a single crossover or with the free plasmid will turn blue, while clones with a disrupted gene, which have lost the plasmid's backbone, will stay white. To accomplish this task, we subcloned a gusA gene into the backbone of the suicide vector pKCLP2 and the thermo-sensitive replicative vector pKC1139 (31), yielding pKGLP2 and pKG1139, respectively (Fig. 3). To check the utility of this approach, we inactivated three putative regulatory genes, plaR1, plaR2, and plaR3, of the phenalinolactone biosynthetic gene cluster (16) by using the pKGLP2 vector. The plaR1, plaR2, and plaR3 genes show homology to the streptomyces antibiotic regulatory protein TetR (38) and HxlR transcriptional regulators. Three plasmids, pKGLPΔR1aac, pKGLPΔR2aac, and pKGLPΔR3aac, were constructed to inactivate the corresponding regulatory genes. The inactivation plasmids were transferred into Streptomyces sp. Tu6071 by conjugation. As pKGLP2 is a suicide vector, all the exconjugants contained an inactivation plasmid integrated into their chromosome by homologous recombination. These single-crossover exconjugant strains were passaged 5 to 10 times in liquid medium to accumulate cells with double crossovers. Afterward, cultures were plated on solid medium to obtain spores. Subsequently, the spores were harvested, diluted, and plated to yield single colonies on solid medium. When the colonies reached 3 mm in diameter, plates were covered with X-Gluc solution. White clones (Fig. 4) were picked and analyzed by PCR or Southern hybridization. As expected, all white clones contained deletions of the respective genes. In each of the ΔR1aac, ΔR2aac, and ΔR3aac mutants, the regulatory gene was replaced by an apramycin resistance marker flanked by two loxP sites. Marker-free ΔR1, ΔR2, and ΔR3 deletion mutants of Streptomyces sp. Tu6071 were generated after the expression of the synthetic cre(a) gene (17).
Fig. 3.
Maps of gusA-containing vectors. (A) Suicide vector pKGLP2. (B) Replicative thermo-sensitive vector pKG1139. hyg, hygromycin resistance gene; ori pSG5, thermo-sensitive origin of replication. See also the abbreviations explained in the legend to Fig. 1.
Fig. 4.
Colonies of Streptomyces sp. Tu6071 overlaid with X-Gluc solution. Colonies with blue halos contain the pKGLP2-based vector for gene inactivation integrated into the chromosome by a single crossover. White colonies indicate a deletion of the targeted gene, resulting from double crossover and the loss of the backbone of pKGLP2.
Phenalinolactone production of the Streptomyces sp. Tu6071 ΔR1, ΔR2, and ΔR3 deletion mutants was analyzed by high-performance LC-MS. In the Streptomyces sp. Tu6071 ΔR1 mutant, phenalinolactone production was completely abolished, while in Streptomyces sp. Tu6071 ΔR3 it was the same as in the wild-type strain. Streptomyces sp. Tu6071 ΔR2 produced phenalinolactone in trace amounts.
Utilization of the gusA gene for transcriptional fusion.
Transcriptional fusion with a reporter is the easiest way to determine the transcriptional level of any particular gene. To establish transcriptional fusions with the gusA gene, we constructed a promoter probe vector using the pSETGUS plasmid as a backbone. The tipA promoter of pSETGUS was deleted, and an additional spectinomycin cassette was inserted upstream of the gusA gene, but in the opposite orientation. The spectinomycin cassette we used contained two T4 terminators that should block readthrough of the gusA gene from the integrase promoter. The resulting plasmid was called pGUS (Fig. 5).
Fig. 5.
Map of the promoter probe vector pGUS. It is based on the pSET152 integrative vector and contains the promoterless gusA gene. The spectinomycin resistance cassette aadA, with two T4 terminators, was cloned upstream of the gusA gene to block the transcription of gusA from upstream promoters. See also the abbreviations defined in Fig. 1.
To reveal regulatory targets of the plaR1, plaR2, and plaR3 genes, we measured transcriptional levels of these regulators and of two other structural genes, plaM1 and plaO2, in each deletion mutant and in the wild-type strain. Promoters of these genes were fused to the gusA gene, generating the pGUSR1, pGUSR2, pGUSR3, pGUSM2, and pGUSO2 plasmids. Each transcriptional fusion construct was introduced into each of the Streptomyces sp. Tu6071 ΔR1, ΔR2, and ΔR3 strains and into the wild-type Streptomyces sp. Tu6071. The GUS activities were assayed for these strains grown in liquid TSB medium as described in Materials and Methods, and the quantitative results are shown in Fig. 6.
Fig. 6.
Glucuronidase activity in cell lysates of Streptomyces sp. Tu6071 strains. WT, R1, R2, and R3 correspond to wild-type, ΔplaR1, ΔplaR2, and ΔplaR3 strains, respectively, of Streptomyces sp. Tu6071 expressing gusA from different promoters. PplaR1, PplaR2, PplaR3, PplaM2, and PplaO2 correspond to promoters of plaR1, plaR2, plaR3, plaM2, and plaO2, respectively.
Expression levels of plaR1-, plaR3-, plaM2-, and plaO2-promoter fusions with gusA in the Streptomyces sp. Tu6071 ΔR1 mutant were 168%, 35.5%, 81.5%, and 58% of the wild-type level, respectively. This indicates that the product of the plaR1 gene exhibits positive effects on the transcription of plaR3, plaM2, and plaO2 and negative effects on its own transcription. We did not observe any changes in the transcription from the plaR2 promoter in the Streptomyces sp. Tu6071 ΔR1 background. Expression levels of plaM2-, plaO2-, and plaR3-promoter fusions with gusA in Streptomyces sp. Tu6071 ΔR2 were 46%, 58%, and 19% of the wild-type levels, respectively. From these data it is evident that the product of the plaR2 gene exerts a positive effect on the transcription of plaM2, plaO2, and plaR3. We could not detect any effect of PlaR2 on the transcription of the plaR1 gene or on the transcription of its own gene. The plaR3 gene encodes a homolog of the HxlR regulator of unknown function that belongs to the DUF24 protein family (54). Although it has been shown that the HxlR protein is responsible for formaldehyde-induced expression of the hxlAB operon (54), in our case PlaR3 reduced the transcriptional levels of plaR2, plaM2, and plaO2 and upregulated the transcription of its own gene. The expression levels of plaR2-, plaR3-, plaM2-, and plaO2-promoter fusions with gusA in the Streptomyces sp. Tu6071 ΔR3 mutant were 207%, 63%, 209%, and 139% of the wild-type levels, respectively. The PlaR3 protein had no effect on the transcription of plaR1.
Translational fusion with the gusA gene.
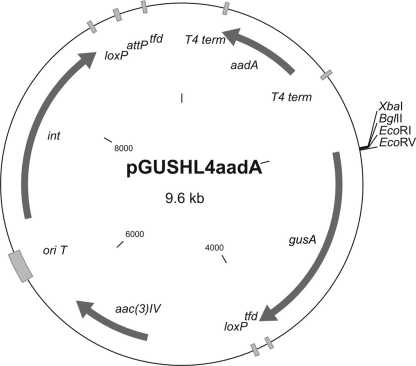
To establish translational fusions with the gusA gene for streptomyces strains, we constructed the vector pGUSHL4aadA (Fig. 7). This vector is based on the pTesa (46) marker-free integrative vector. It contains the gusA gene without a start codon, the helical linker HL4 (1) (which is intended to separate two fused proteins spatially), and a suitable polylinker for cloning genes of interest.
Fig. 7.
Map of the plasmid pGUSHL4aadA. It is based on the pTesa marker-free integrational plasmid. On the 5′ end, gusA is fused to the HL4 helical linker intended to separate two domains of a fused protein. The spectinomycin cassette, with two T4 terminators, is cloned upstream of gusA to block transcription of gusA gene from upstream promoters. tfd, transcriptional terminator of bacteriophage fd; loxP, recognition site of Cre recombinase. See also abbreviations defined in the legends to Fig. 1 and 5.
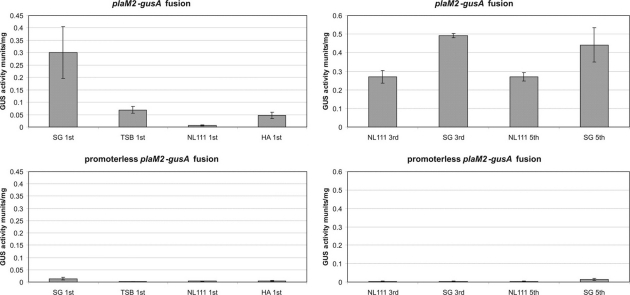
To test this vector, we tried to find an optimal production medium by monitoring the level of the structural enzyme involved in the biosynthesis of a key product. For this purpose we constructed the plasmid pGUSHL4plaM2aadA containing the plaM2 gene fused to gusA. This plasmid was transferred into Streptomyces sp. Tu6071. The activity of the PlaM2-GUS protein was measured in SG, TSB, NL111, and HA media after 1, 3, and 5 days of cultivation. After 24 h of incubation, the average glucuronidase activities in SG, TSB, NL111, and HA media were 0.3, 0.07, 0.0065, and 0.047 mU/mg, respectively (Fig. 8). From our previous studies we know that the highest level of phenalinolactone production is achieved in NL111 medium, in contrast to the low level of plaM2 gene expression in this medium. To study this result further, we decided to compare the activity of the PlaM2-GUS fusant only in SG and NL111 media after 3 and 5 days of incubation. On the third day, GUS activity in SG and NL111 media was 0.49 and 0.27 mU/mg, respectively (Fig. 8). After 5 days of incubation, GUS activity in SG medium decreased slightly, to 0.44 mU/mg, while in NL111 medium it remained at the same level of 0.27 mU/mg (Fig. 8). This result indicates that in SG medium the expression of the plaM2 gene reaches a significant level after 24 h of incubation, whereas in NL111 after 24 h, the level of plaM2 expression reaches only 2.4% of the maximum level achieved after 3 days of incubation. The decrease of PlaM2-GUS fusion activity on day 5 of incubation in SG medium can be probably explained by autolysis of the culture.
Fig. 8.
Glucuronidase activity in cell lysates of Streptomyces sp. Tu6071 pGUSHL4plaM2aadA grown in different media. Plasmid pGUSHL4plaM2aadA contains a fusion of the plaM2 and gusA genes. Glucuronidase activity was measured on days 1, 3, and 5 of incubation. SG, TSB, NL111, and HA are the media used in this experiment.
To ascertain whether the higher level of plaM2 gene expression corresponded to a higher level of phenalinolactone production, we extracted phenalinolactone from SG, HA, TSB, and NL111 media after 6 days of incubation. Extracts were analyzed by LC-MS. We could not detect phenalinolactone in extracts from TSB medium. From HA medium we could extract approximately 4 times more phenalinolactone than from SG. The maximum amount of phenalinolactone was produced in NL111 medium, approximately 5 times more than in SG. In all cases, the amounts of phenalinolactone were recalculated to the same amount of wet biomass. From these data it is evident that the highest productivity was observed in NL111 medium, while the highest level of plaM2 gene expression occurred in SG. These findings indicate that, at least in this case, the highest level of structural gene expression does not reflect the highest productivity, probably because other factors, such as the concentration of precursors and cofactors, play crucial roles in determining the level of antibiotic production.
Creation of gusA gene derivatives with different sensitivities.
When working with reporter genes, it can be important to have variants with different sensitivities. This can be useful for screening mutant colonies in which a particular gene is strongly upregulated. In this case, it is not convenient to use a very sensitive reporter, which even at a low level of transcription translates very efficiently and gives a strong signal, because this makes it difficult to distinguish between clones with the initial level of transcription and those with a strongly increased level. In this case, it would be much better to use a less sensitive reporter that gives a distinct difference in the signal intensity only when the transcription level is increased significantly. The situation can be completely different when the goal is to find mutant clones with an activated gene that is normally silent. In this case, a highly sensitive reporter gene that gives a signal even when the transcription level is very low is necessary.
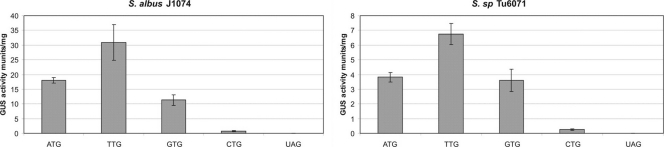
To create gusA derivatives with different sensitivities, we changed their start codons to ascertain whether the efficiency of translation changed. Originally, the gusA gene contained the ATG start codon, so we created three derivatives of gusA, with TTG, GTG, and CTG codons, and cloned them into vector pSET152, generating pSETGUSTTG, pSETGUSCTG, and pSETGUSGTG, respectively. In all of these plasmids, gusA is under the control of the tipA promoter. All these plasmids and the initial pSETGUS were transferred into S. albus J1074 and Streptomyces sp. Tu6071, and the GUS activity was measured for each type of exconjugant. GUS activities in S. albus J1074 exconjugants containing the gusA gene with the ATG, TTG, GTG, and CTG start codons were 18.00, 30.88, 11.31, and 0.76 mU/mg, respectively (Fig. 9). In Streptomyces sp. Tu6071, the GUS activities of exconjugants containing the gusA gene with the ATG, TTG, GTG, and CTG start codons were 3.81, 6.75, 3.59, and 0.25 mU/mg, respectively (Fig. 9). As a control we used the gusA gene with a TAG stop codon as the first codon after the ATG start codon (pSETGUSTAG). The GUS activities of the S. albus J1074 and Streptomyces sp. Tu6071 strains containing this gene were 0.022 and 0.0064 mU/mg (Fig. 9). It is evident from the results that the gusA gene with the TTG codon was the most active in translation initiation; the ATG codon was less active than TTG, while GTG was less active than ATG. The least active codon was CTG. Having gusA genes with different start codons makes it possible to choose one with the necessary sensitivity for any particular application.
Fig. 9.
Glucuronidase activity in cell lysates of different strains containing the gusA gene with different start codons.
A new inducible expression system for streptomycetes using the expanded genetic code.
The availability of an inducible system that is not expressed without induction is of great importance for bacterial genetics. Today there are a few transcriptionally inducible systems for streptomyces, but they share one drawback: they all are leaky. Here we describe the development of a new inducible system that exhibits no leakage under noninductive conditions.
Several natural genetic systems that enable suppression of the in-frame amber UAG (TAG) nonsense codon have been described elsewhere (5, 33). In our system we decided to utilize two genes, pylS and pylT, encoding pyrrolysyl-tRNA synthetase and suppressor tRNAPyl, respectively (33). In the presence of the amino acid pyrrolysine, products of the pylS and pylT genes are able to suppress the amber nonsense codon.
The whole system should function as follows. First, a TAG codon should be inserted in the coding frame of any gene, upstream of the sequence encoding the functional domains. Transcription of this gene should not result in any functional protein, because translation will be interrupted by the amber stop codon. Expression of the pylS and pylT genes will lead to accumulation of pyrrolysyl-tRNA synthetase and suppressor tRNAPyl, but at this stage these products will not be able to suppress the UAG codon because pyrrolysine is missing. Adding pyrrolysine results in induction, suppresses the in-frame stop codon, and enables translation of the complete gene product.
To establish this system, we optimized the sequence of the pylS gene according to the codon usage of S. coelicolor. The pylT gene encodes tRNA, and posttranscriptional processing is critical for the functionality of tRNAs. For processing, flanking regions of tRNA play a crucial role. As the whole system originates from archaebacteria, there is a risk that the tRNAPyl will not be processed properly in streptomyces strains. To overcome this problem, we took the sequence of S. coelicolor tRNAGly (tRNA_100054) with 100-bp flanking regions on each side and substituted the coding region of tRNAGly with the sequence of tRNAPyl. A fragment containing the modified pylT gene under the control of a tipA promoter and the optimized pylS gene under the control of an ermE promoter was synthesized. This fragment was cloned into the VWB-based, marker-free integration vector pSOKT (46), and afterwards a thiostrepton resistance marker was cloned into the resulting plasmid, generating pSOKTpyr-tsr (Fig. 10). Both plasmids, pSETGUSTAG and pSOKTpyr-tsr, were transferred into S. albus J1074. The resulting strain, S. albus pSETGUSTAGpSOKTpyr-tsr, was inoculated into TSB medium supplemented with 50 μg/μl thiostrepton and 20 mM Cyc. Cyc is a commercially available analog of pyrrolysine (37). Under these conditions, both the pylT and pylS genes were expressed well. The gusA gene containing the stop codon was actively transcribed, but only the presence of Cyc in the medium allowed translation to take place. As a control we used the same culture, but without adding the inducer Cyc. GUS activities in cultures of S. albus pSETGUSTAGpSOKTpyr-tsr with and without induction were 0.213 and 0.0002 mU/mg, respectively. The level of GUS activity in the control sample was negligible. This result means that the UAG stop codon is suppressed in a streptomyces background in the presence of pyrrolysine, pyrrolysyl-tRNA synthetase, and suppressor tRNAPyl.
DISCUSSION
Techniques for measuring transcript abundance, including reverse transcription-PCR, microarrays, S1 nuclease protection together with activity measurements, and immunological methods for analyzing proteins encoded by genes of interest, are often not applicable to high-throughput experiments. For such purposes, transcriptional and translational fusions with reporter genes are often used. Unfortunately, the most reliable, convenient, and widely used lacZ reporter gene has strong limitations in streptomyces due to their significant endogenous β-galactosidase activities (32). The current streptomyces reporter toolkit includes neo and cat resistance genes (43, 44, 51), catechol 2,3-dioxygenase (55), green fluorescent protein (49), luciferase (48), and others, but none of them is nearly as good as lacZ. Our aim was to establish a new reporter system for streptomyces with simple and sensitive assays on one hand and without those limitations that current streptomyces reporter systems have on the other. We evaluated a number of Streptomyces spp. strains for endogenous activities of several potent reporter genes, and we found that they lack α-fucosidase, β-fucosidase, β-glucuronidase, and β-ribosidase activities. Among them, we chose β-glucuronidase because it is widely used in plant genetics and has many properties of a nearly perfect reporter (28). Compared to other existing reporters, such as enhanced GFP (EGFP), luciferase, XylE, and Cat/Neo, β-glucuronidase has the advantages of extreme sensitivity, simple assays, enzyme stability, and the possibility of visual, spectrophotometric, and fluorogenic assays. Several of its substrates, including X-Gluc, methylumbelliferyl-β-glucuronide, and p-nitrophenyl-β-d-glucuronide, are commercially available, which is critical for any enzymatic reporter system. To establish the new reporter system, an optimized gusA gene encoding β-glucuronidase was synthesized and expressed in several streptomyces strains. In each strain we could clearly detect strong β-glucuronidase activity, indicating that the gene functions well and can be used as a reporter in streptomyces strains.
One of the applications for which we wanted to use a gusA reporter was the creation of gene inactivation vectors for rapid visual screening of clones with double crossovers. Available systems for gene knockouts in streptomyces include thermo-sensitive or suicide vectors with subsequent laborious screening of clones with the inactivated gene (31). Use of the I-SceI endonuclease (47) and cytosine deaminase (15) for positive selection of knockout clones was reported previously. Both of these systems have some limitations. The I-Sce I endonuclease relies on a high frequency of conjugation, which is often difficult to attain with certain strains, and cytosine deaminase often gives false positives; therefore, a system that speeds up the screening process is of great interest. For this purpose the gusA gene was introduced into the backbone of the common vectors pKCLP2, a suicide vector, and pKC1139, a replicative vector. Such an approach facilitates the selection of clones with double crossovers by visual screening without picking thousands of colonies. Clones containing the vector will turn blue after overlaying substrate solution, and only clones that have lost the plasmid will stay white. The combination of the gusA gene and an antibiotic resistance marker for gene inactivation guarantees that nearly 100% of the white clones contain the inactivated gene. As a proof of this principle, we inactivated three regulatory genes, plaR1, plaR2, and plaR3, in the phenalinolactone cluster of Streptomyces sp. Tu6071. For this purpose we used the suicide vector pKGLP2 containing gusA and the apramycin resistance marker flanked with loxP sites. This allowed us to construct marker-free deletions. In all cases, white clones contained deletions of the genes, and there were no cases of false-positive white clones. Successful utilization of gusA-containing vectors for gene inactivation indicates that this approach can significantly simplify the detection of gene inactivation even in problem strains with low frequencies of conjugation and crossover events.
One of the most frequent uses of reporters is transcriptional fusion. It is probably the easiest way to determine the expression level of any particular gene. Today the most commonly used streptomyces reporters for transcriptional fusion are EGFP and XylE. Utilization of EGFP is limited because a number of streptomyces strains fluoresce in green light and specialized equipment is required to measure fluorescence (52). The main disadvantage of XylE is that the enzymatic assay is lethal to streptomyces cells, so it is impossible to pick viable clones from plates after the assay (27). In this context, the gusA gene has no such limitations: assays are not lethal, a simple spectrophotometer is required for quantitative measurement and, because of the enzymatic amplification of the signal, the assay is very sensitive. To establish transcriptional fusions with the gusA gene, the pGUS promoter probe vector was constructed. To test the vector and the approach in general, transcriptional levels of the regulatory genes plaR1, plaR2, plaR3, and the structural genes plaM2 and plaO2 were measured in a wild-type strain and in ΔR1, ΔR2, and ΔR3 mutants. We found that the putative pathway-specific regulator PlaR1 exerts a positive effect on transcription of plaR3, plaM2, and plaO2 and a negative effect on its own gene. This is consistent with the observation that phenalinolactone production was abolished in the ΔR1 mutant. The downregulation of its own promoter is, however, very surprising. This observation explains why the overexpression of plaR1 in the phenalinolactone producer Streptomyces sp. Tu6071 had no positive effect on antibiotic production (A. Bechthold, personal communication).
The putative TetR repressor PlaR2 upregulated plaR3, plaM, and plaO2, which is consistent with the cessation of phenalinolactone production in the ΔR2 mutant. Because plaR2 encodes a homolog of the TetR family of transcriptional repressors, it seems that it is involved in a complex regulatory network involving a series of regulatory cascades. The product of the plaR3 gene, a homolog of the HxlR regulator, downregulates plaR2, plaM2, and plaO2 and upregulates transcription of its own gene. The Hxl regulator belongs to the DUF24 protein family of unknown function. Inactivation of plaR3 caused no changes in phenalinolactone production. These data indicate that gusA can be successfully exploited to determine transcriptional levels in streptomyces strains. It can be used for large-scale screening of mutants with certain genes upregulated. Such an experiment can be performed in two stages: first, using semiquantitative analysis directly on plates to reduce the number of mutant clones to be analyzed, and second, using quantitative spectrophotometry to find the clones with the desired transcriptional level of the target gene. The simplicity and flexibility of the gusA transcriptional fusion method demonstrate that it is a good alternative to the widely used EGFP and XylE fusions.
Transcriptional fusions place the gusA gene downstream of different promoters. These fusions can be used only to monitor transcription. After this type of fusion, the mRNA of gusA is produced, and all other posttranscriptional events are the same as for gusA mRNA. Thus, any posttranscriptional modifications of the expression of the gene of interest will not be reflected in gusA production. A well-known example of transcription without translation in streptomycetes is bld regulation, during which certain genes are transcribed but translation occurs only if the rare leucine TTA codon-decoding tRNA is present (50). Therefore, the true gene translational fusions that result in the production of a hybrid protein are of great interest, but unfortunately, such methods are poorly represented among streptomyces studies. There is considerable evidence that gusA tolerates large C- and N-terminal fusions without loss of activity (42), so we constructed a vector for N-terminal fusions with gusA. In our system, gusA translational fusions produce a hybrid protein with amino-terminal sequences derived from a protein of interest and a carboxy-terminal fragment of GUS. In this case, any posttranscriptional effects on the expression of a gene of interest will be reflected in GUS production. To evaluate the utility of translational fusions with the gusA gene in streptomyces, we created fusions with the structural gene plaM2 (encoding methyltransferase), which is involved in phenalinolactone production. The level of plaM2 translation was measured in different media: SG, TSB, NL111, and HA. We found that after 24 h of incubation, the translational level of plaM2 was the lowest in NL111 medium, which provided the highest yields of phenalinolactone. The highest level of translation was observed in SG medium. To ascertain whether the situation changed after prolonged cultivation, we measured the level of the PlaM2-GUS fusion in SG and NL111 media after 3 and 5 days. At both times, the activity of the fusant in SG medium was almost twice as high as in NL111. When the levels of phenalinolactone production were measured, the level in NL111 medium was the largest and was approximately five times higher than in SG. This indicates that at least in this case, the highest level of structural gene expression does not correlate with the level of antibiotic production. Obviously, other factors, such as the concentrations of precursors and cofactors, limit phenalinolactone production. The translational fusions with gusA can be easily exploited in streptomyces to directly, quantitatively measure protein yields. Several possible applications of this system include screening for colonies with activated silent genes or pathways, studying the factors affecting translation efficiency, and selecting for the heterologous host yielding the largest amounts of target protein. All of these applications can be realized by semiquantitative visual inspection directly on plates, as well as by quantitative spectrophotometric or fluorogenic measurement.
In mutagenesis experiments dedicated to altering the transcriptional level of any particular gene, the requirement for reporters with different sensitivities is appreciated. For example, when an actively transcribed gene needs to be upregulated, it is preferable to use a less actively translated reporter, which will give a clear difference in signal intensity only if the transcriptional level is increased dramatically. In contrast, when the task is to upregulate a gene that is transcribed at a very low level or is silent, it is better to use a reporter that translates efficiently and gives a signal even if the transcriptional level is very low. To create reporters with different translational efficiencies, we changed the start codons of the gusA gene. Originally, gusA had the ATG codon, and so we created derivatives with TTG, GTG, and CTG start codons. Surprisingly, the highest activity was observed in the gusA gene with the TTG start codon. This construct was almost two times more active than the one with the ATG codon. The gusA gene with the GTG codon turned out to be slightly less active than the one with the ATG codon, while the one with the CTG codon was more than 10 times less active. The fact that the TTG codon in gusA was the most active was unexpected, because streptomycetes have GC-rich DNA, and this codon is employed in the translation initiation of only 4% of the genes in S. coelicolor A3(2). Taking into account E. coli, only 3% of genes in its genome possess a TTG initiation codon (5). Nevertheless, the TTG-gusA reporter gene can be used when a very sensitive reporter is needed. CTG-gusA can be used when mutants with dramatic increases of transcriptional fusion are to be selected. In addition, we have shown that the GUS system may be used in determining the relative translational efficiency of the start codons in streptomycetes.
One of the most essential tasks in current streptomyces genetics is the creation of very tightly regulated systems of gene expression. Existing inducible expression systems in streptomyces are represented by the tipA promoter (24), the nitrilase induction system (22), and the tetracycline repressor (39). All of these systems share one significant limitation: they are leaky. This means that even without induction, they allow transcription of controlled genes, although at low levels. This is the main obstacle to studies of transposon mutagenesis, utilization of site-specific recombinases, lethal phenotypes, and many other investigations, where the target gene expression should be triggered only after induction at a certain time, without any leakage under the noninduced conditions. To establish such an induction system, a very sensitive reporter is required to detect any minor levels of leakage under noninduced conditions.
As a basis for new induction systems, the genetic code expansion cassette from Methanosarcina acetivorans and Methanosarcina barkeri was used (33). This cassette consists of five genes and permits translation of the UAG stop codon as pyrrolysine. The pylT and pylS genes encode Amber-decoding tRNAPyl and pyrrolysyl-tRNA synthetase, respectively. The pylB, pylC, and pylD genes are responsible for the biosynthesis of pyrrolysine. The induction system functions as follows. The first step is introduction of the TAG stop codon into the open reading frame of a controlled gene. This gene should be expressed in the streptomyces host together with pylT and pylS, but translation of the controlled gene is not possible as long as pyrrolysine is absent in the medium. Thus, pyrrolysine acts as the inducer, triggering translation of the controlled gene. To establish this system, a vector containing optimized pylT and pylS was created together with a plasmid carrying the gusA gene with the TAG stop codon as the first codon after the ATG start codon. When these two plasmids were transferred to S. albus J1074, we could not detect any β-glucuronidase activity. This result indicates that translation of gusA is completely aborted. Only when the medium was supplemented with a commercially available pyrrolysine analog, Cyc (37), was gusA successfully translated. In this case, β-glucuronidase activity of 0.213 mU/mg (dry weight) was detected. Thus, we have developed a new induction system and also have added an unnatural amino acid to the streptomyces genetic code. The successful incorporation of a noncanonical amino acid into the proteins produced in actinomycete cells is another tool for studying cell metabolism, physiology, protein processing, and turnover, similarly to the study of other organisms, such as E. coli (6, 11).
In summary, we have constructed a set of reporter genes for numerous types of biological studies in actinomycetes. Several experimental approaches that take advantage of the simplicity and elegance of these reporters have been presented. The studies of gene expression at the transcriptional and translational levels, genetic code expansion, and facilitation of gene targeting procedures comprise only a partial list of experimental approaches that will employ the β-glucuronidase reporter system. The GUS-based system has proved its unparalleled versatility in plant molecular biology and undoubtedly will facilitate a wide variety of studies of actinomycete genetics.
ACKNOWLEDGMENTS
This work was funded by a grant from DFG (Lu1524/2-1) (and BMBF [GenBioCom]) to Andriy Luzhetskyy. Maksym Myronovskyi was supported by a DAAD fellowship.
Footnotes
Published ahead of print on 17 June 2011.
REFERENCES
- 1. Arai R., Ueda H., Kitayama A., Kamiya N., Nagamune T. 2001. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 14:529–532 [DOI] [PubMed] [Google Scholar]
- 2. Baltz R. H. 2001. Genetic methods and strategies for secondary metabolite yield improvement in actinomycetes. Antonie Van Leeuwenhoek 79:251–259 [DOI] [PubMed] [Google Scholar]
- 3. Bibb M. J. 2005. Regulation of secondary metabolism in actinomycetes. Curr. Opin. Microbiol. 8:208–215 [DOI] [PubMed] [Google Scholar]
- 4. Binz T. M., Wenzel S. C., Schnell H. J., Bechthold A., Müller R. 2008. Heterologous expression and genetic engineering of the phenalinolactone biosynthetic gene cluster by using red/ET recombineering. Chembiochem 9:447–454 [DOI] [PubMed] [Google Scholar]
- 5. Blattner F. R., et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462 [DOI] [PubMed] [Google Scholar]
- 6. Böck A., et al. 1991. Selenocysteine: the 21st amino acid. Mol. Microbiol. 5:515–520 [DOI] [PubMed] [Google Scholar]
- 7. Cadenas R. F., Martín J. F., Gil J. A. 1991. Construction and characterization of promoter-probe vectors for Corynebacteria using the kanamycin-resistance reporter gene. Gene 98:117–121 [DOI] [PubMed] [Google Scholar]
- 8. Casadaban M. J., Chou J., Cohen S. N. 1980. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Challis G. 2008. Genome mining for novel natural product discovery. J. Med. Chem. 51:2618–2628 [DOI] [PubMed] [Google Scholar]
- 10. Chater K. F. 2006. Streptomyces inside-out: a new perspective on the bacteria that provide us with antibiotics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1469:761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chin J. W., Martin A. B., King D. S., Wang L., Schultz P. G. 2002. Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:11020–11024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crameri A., Whitehorn E., Tate E., Stemmer W. 1996. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat. Biotechnol. 14:315–319 [DOI] [PubMed] [Google Scholar]
- 13. Craney A., et al. 2007. A synthetic luxCDABE gene cluster optimized for expression in high-GC bacteria. Nucleic Acids Res. 35:e46–e46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Wet J. R., Wood K. V., Helinski D. R., DeLuca M. 1985. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 82:7870–7873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dubeau M. P., et al. 2009. Cytosine deaminase as a negative selection marker for gene disruption and replacement in the genus Streptomyces and other actinobacteria. Appl. Environ. Microbiol. 75:1211–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dürr C., et al. 2006. Biosynthesis of the terpene phenalinolactone in Streptomyces sp. Tü6071: analysis of the gene cluster and generation of derivatives. Chem. Biol. 13:365–377 [DOI] [PubMed] [Google Scholar]
- 17. Fedoryshyn M., Welle E., Bechthold A., Luzhetskyy A. 2008. Functional expression of the Cre recombinase in actinomycetes. Appl. Microbiol. Biotechnol. 78:1065–1070 [DOI] [PubMed] [Google Scholar]
- 18. Ghim C. M., Lee S. K., Takayama S., Mitchell R. J. 2010. The art of reporter proteins in science: past, present and future applications. BMB Rep. 43:451–460 [DOI] [PubMed] [Google Scholar]
- 19. Han L., Khetan A., Hu W. S., Sherman D. H. 1999. Time-lapsed confocal microscopy reveals temporal and spatial expression of the lysine epsilon-aminotransferase gene in Streptomyces clavuligerus. Mol. Microbiol. 34:878–886 [DOI] [PubMed] [Google Scholar]
- 20. Haseloff J., Amos B. 1995. GFP in plants. Trends Genet. 11:328–329 [DOI] [PubMed] [Google Scholar]
- 21. Hasty J., McMillen D., Collins J. J. 2002. Engineered gene circuits. Nature 420:224–230 [DOI] [PubMed] [Google Scholar]
- 22. Herai S., et al. 2004. Hyper-inducible expression system for streptomycetes. Proc. Natl. Acad. Sci. U. S. A. 101:14031–14035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hodge D. R., Thompson D. M., Panayiotakis A., Seth A. 1995. Transcriptional activation analysis by the chloramphenicol acetyl transferase (CAT) enzyme assay. Methods Mol. Biol. 37:409–421 [DOI] [PubMed] [Google Scholar]
- 24. Holmes D. J., Caso J. L., Thompson C. J. 1993. Autogenous transcriptional activation of a thiostrepton-induced gene in Streptomyces lividans. EMBO J. 12:3183–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hull G. A., Devic M. 1995. The beta-glucuronidase (gus) reporter gene system. Gene fusions, spectrophotometric, fluorometric, and histochemical detection. Methods Mol. Biol. 49:125–141 [DOI] [PubMed] [Google Scholar]
- 26. Hutter H. 2006. Fluorescent reporter methods. Methods Mol. Biol. 351:155–173 [DOI] [PubMed] [Google Scholar]
- 27. Ingram C., Brawner M., Youngman P., Westpheling J. 1989. xylE functions as an efficient reporter gene in Streptomyces spp.: use for the study of galP1, a catabolite-controlled promoter. J. Bacteriol. 171:6617–6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jefferson R. A. 1989. The GUS reporter gene system. Nature 342:837–838 [DOI] [PubMed] [Google Scholar]
- 29. Jefferson R. A., Kavanagh T. A., Bevan M. W. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants; EMBO J. 6:3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kain S. R., et al. 1995. Green fluorescent protein as a reporter of gene expression and protein localization. Biotechniques 19:650–655 [PubMed] [Google Scholar]
- 31. Kieser T., Bibb M. J., Buttner M. J., Chater K. F., Hopwood D. A. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom [Google Scholar]
- 32. King A. A., Chater K. F. 1986. The expression of the Escherichia coli lacZ gene in Streptomyces. J. Gen. Microbiol. 132:1739–1752 [DOI] [PubMed] [Google Scholar]
- 33. Longstaff D. G., et al. 2007. A natural genetic code expansion cassette enables transmissible biosynthesis and genetic encoding of pyrrolysine. Proc. Natl. Acad. Sci. U. S. A. 104:1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nordeen S. K. 1988. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques 6:454–458 [PubMed] [Google Scholar]
- 35. Olano C., et al. 2004. Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tü4055: insights into nitrile formation. Mol. Microbiol. 52:1745–1756 [DOI] [PubMed] [Google Scholar]
- 36. Petzke L. 2010. Transgenese in Streptomyceten: transposons, rekombinasen und meganukleasen. Ph.D. thesis. Albert-Ludwigs-Universität Freiburg im Breisgau, Freiburg, Germany [Google Scholar]
- 37. Polycarpo C. R., et al. 2006. Pyrrolysine analogues as substrates for pyrrolysyl-tRNA synthetase. FEBS Lett. 580:6695–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramos J. L., et al. 2005. TheTetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodríguez-García A., Combes P., Pérez-Redondo R., Smith M. C., Smith M. C. 2005. Natural and synthetic tetracycline-inducible promoters for use in the antibiotic-producing bacteria Streptomyces. Nucleic Acids Res. 33:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 41. Schmitz U. K., Lonsdale D. M. 1989. A yeast mitochondrial presequence functions as a signal for targeting to plant mitochondria in vivo. Plant Cell 1:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmitz U. K., Lonsdale D. M., Jefferson R. A. 1990. Application of the beta-glucuronidase gene fusion system to Saccharomyces cerevisiae. Curr. Genet. 17:261–264 [DOI] [PubMed] [Google Scholar]
- 43. Schottel J. L., Bibb M. J., Cohen S. N. 1981. Cloning and expression in Streptomyces lividans of antibiotic resistance genes derived from Escherichia coli. J. Bacteriol. 146:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaw W. V., Hopwood D. A. 1976. Chloramphenicol acetylation in Streptomyces. J. Gen. Microbiol. 94:159–166 [DOI] [PubMed] [Google Scholar]
- 45. Shuman H. A., Silhavy T. J. 2003. The art and design of genetic screen: Escherichia coli. Nat. Rev. Genet. 4:419–430 [DOI] [PubMed] [Google Scholar]
- 46. Siegl T., Herrmann S., Welle E., Bechthold A., Luzhetskyy A. 2010. Abstr. 25th Int. VAAM Workshop 2010, Biol. Bacteria Producing Nat. Products. Universitat Bonn, Bonn, Germany [Google Scholar]
- 47. Siegl T., Petzke L., Welle E., Luzhetskyy A. 2010. I-SceI endonuclease: a new tool for DNA repair studies and genetic manipulations in streptomyces. Appl. Microbiol. Biotechnol. 87:1525–1532 [DOI] [PubMed] [Google Scholar]
- 48. Sohaskey C. D., Im H., Nelson A. D., Schauer A. T. 1992. Tn4556 and luciferase: synergistic tools for visualizing transcription in Streptomyces. Gene 115:67–71 [DOI] [PubMed] [Google Scholar]
- 49. Sun J., Kelemen G. H., Fernández-Abalos J. M., Bibb M. J. 1999. Green fluorescent protein as a reporter for spatial and temporal gene expression in Streptomyces coelicolor A3(2). Microbiology 145:2221–2227 [DOI] [PubMed] [Google Scholar]
- 50. Takano E., et al. 2003. A rare leucine codon in adpA is implicated in the morphological defect of bldA mutants of Streptomyces coelicolor. Mol. Microbiol. 50:475–486 [DOI] [PubMed] [Google Scholar]
- 51. Ward J. M., et al. 1986. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol. Gen. Genet. 203:468–478 [DOI] [PubMed] [Google Scholar]
- 52. Willemse J., van Wezel G. P. 2009. Imaging of Streptomyces coelicolor A3(2) with reduced autofluorescence reveals a novel stage of FtsZ localization. PLoS One 4:e4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wilson T., Hastings J. W. 1998. Bioluminescence. Annu. Rev. Cell Dev. Biol. 14:197–230 [DOI] [PubMed] [Google Scholar]
- 54. Yurimoto H., et al. 2005. HxlR, a member of the DUF24 protein family, is a DNA-binding protein that acts as a positive regulator of the formaldehyde-inducible hxlAB operon in Bacillus subtilis. Mol. Microbiol. 57:511–519 [DOI] [PubMed] [Google Scholar]
- 55. Zukowski M. M., et al. 1983. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc. Natl. Acad. Sci. U. S. A. 80:1101–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]