Abstract
Flavohemoglobins are widely distributed in both prokaryotes and eukaryotes. These proteins are involved in reducing nitric oxide levels. Deletion of the Aspergillus nidulans flavohemoglobin gene fhbA induced sexual development and decreased sterigmatocystin production. Supplementation with a nitric oxide-releasing compound promoted cleistothecial formation and increased nsdD and steA expression, indicating that nitric oxide induces sexual development. This is the first study on the effect of nitric oxide on morphogenesis and secondary metabolism in fungi.
TEXT
Nitric oxide (NO) is a signaling compound of great importance in biological systems (7, 10, 21). This molecule can also cause nitrosative stress which is potentially remediated by the widely distributed flavohemoglobins (FHbs) found in both eukaryotes and prokaryotes (4, 7). Extensive research on FHb proteins from bacteria and yeast revealed their structure, function, and mechanism of action (3, 7, 17). Earlier studies have shown that FHbs in organisms such as Saccharomyces cerevisiae, Alcaligenes eutrophus, and Escherichia coli share similar steady-state NO dioxygenation kinetics (7). Through a dioxygenase-mediated reaction, FHbs, in the presence of molecular O2, converts NO into nontoxic nitrate ions. FHb proteins contain a hemoglobin-like domain with a noncovalently bound heme B protein and a reductase domain with binding sites for FAD and NAD(P)H. It is known that fhb genes are activated by various agents, such as nitrate, nitrite, NO, and NO-releasing agents (5, 7, 8, 11, 20, 24). In aspergilli, the conversion of NO to NO3− by FHbs, Fhb1 and Fhb2 in Aspergillus oryzae (30) and FhbA and FhbB in the filamentous fungus model Aspergillus nidulans, has been demonstrated (24). The present work involves the study of the role of FHbs and NO in fungal development and secondary metabolism.
Fungal strains and growth conditions.
Aspergillus nidulans Cib08 (biA1 yA2), CibA (biA1 yA2 ΔfhbA::argB), CibB (biA1 yA2 ΔfhbB::argB) (described in reference 24), and FGSCA4 were used in this study. The strains were cultured on glucose minimum medium (GMM) plus the appropriate supplements for the corresponding auxotrophic markers (13). Medium was supplemented with 1.5 mM diethylenetriamine-NoNoate (Sigma), a NO-releasing compound, after sterilization when indicated. Solid medium was prepared by adding 10 g/liter agar. Strains were stored as 30% glycerol stocks at −80°C.
Morphological studies.
Plates containing 25 ml of solid GMM plus the appropriate supplements were top agar inoculated with 5 × 106 spores per plate of medium. The cultures were wrapped and incubated at 37°C in the dark. Cores (8-mm diameter) were collected from each spread plate and examined for cleistothecium production. Ethanol (70%) was sprayed on the core surface to facilitate the visualization of cleistothecia under the microscope. The experiments included five replicates and were repeated twice, with similar results.
mRNA analysis.
Total RNA was isolated from mycelia at 48 h and 72 h after inoculation as previously described (23). Five micrograms of total RNA was treated with DNase I RQI (Promega) and reverse transcribed with Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega). Quantitative reverse transcription-PCR (qRT-PCR) was performed with an Mx3000P thermocycler (Stratagene) using SYBR green JumpStart Tag Ready mix (Sigma). The primer pairs used for qRT-PCR are listed in Table 1.
Table 1.
Primers used in this study
| Primer | Sequence |
|---|---|
| actin-F | 5′-ATGGAAGAGGAAGTTGCTGCTCTCGTTATCGACAATGGTTC-3′ |
| actin-R | 5′-CAATGGAGGGGAAGACGGCACGGG-3′ |
| nsdD-F | 5′-CATCTCACCAGCCACAATTACAGGCGGAACCATCAC-3′ |
| nsdD-R | 5′-TTGCGAGCCAGACACAGAGGTCATAACAGTGCTTGC-3′ |
| steA-F | 5′-TCCAGCAAATGGAACCGTGGAATCAGGTGCTC-3′ |
| steA-R | 5′-GAAGGGATGGGGCAAGAATGAGACTTCTGCGGGTAA-3′ |
| aflR-F | 5′-ATGGAGCCCCCAGCGATCAGCCAG-3′ |
| aflR-R | 5′-TTGGTGATGGTGCTGTCTTTGGCTGCTCAAC-3′ |
ST analysis.
Three cores (16-mm diameter) from each replicate of cultures grown on GMM were harvested after 5 days of incubation and placed in a 50-ml Falcon tubes. Sterigmatocystin (ST) was extracted by adding 5 ml of CHCl3. Extracts were fractionated by thin-layer chromatography using toluene-ethyl acetate-formic acid (60:30:10 [vol/vol]) as a solvent system. The thin-layer chromatography (TLC) silica plates were sprayed with aluminum chloride (12.5% in ethanol) to intensify fluorescence upon exposure to long-wave (375-nm) UV light and baked for 10 min at 80°C prior to viewing.
The fhbA and fhbB gene products are highly conserved in aspergilli (see Fig. S1 and S2 in the supplemental material) and are part of a specific phylogenetic group (25). In A. nidulans, fhbA has been shown to be AreA independent and a NirA target, which allows FhbA to avoid nitrogen metabolite repression (24). On the other hand, fhbB was found constitutively expressed (24). A report by te Biesebeke et al. (25) has suggested a role in mycelial branching. However, whether NO and related FHbs have a function in regulating development or secondary metabolism in the fungal kingdom was completely unknown. In this report, we describe for the first time that NO and FHbs are regulators of sexual development and ST toxin production in A. nidulans.
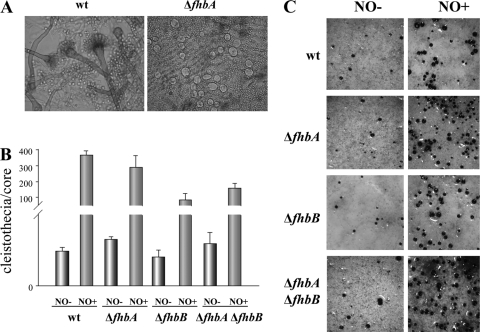
NO has been shown to regulate multiple physiological processes, particularly in mammals (6, 12, 15, 18, 22, 31). These reports reflect that one of the important roles of NO in higher eukaryotes is the regulation of reproduction in males and females. Our study revealed that NO also is involved in the control of sexual development in fungi. We found that the A. nidulans fhbA deletion (ΔfhbA) mutant presented an increase in Hülle cell production with respect to the control strain. Hülle cells are postulated to be nursing cells for the formation of fruiting bodies called cleistothecia (27). This result suggests that NO accumulation in the absence of fhbA might have a positive effect on the induction of sexual development (Fig. 1A). ΔfhbA ΔfhbB presented the same phenotype as ΔfhbA; however, ΔfhbB did not show this effect (data not shown). This is in agreement with the higher NO stress susceptibility observed in the ΔfhbA mutant (24). The increase in Hülle cell numbers observed in the ΔfhbA strain at early time points was found independent of nsdD and steA expression, both genes encoding transcription factors necessary for sexual development (9, 26) (data not shown). In older cultures, ΔfhbA (and ΔfhbA ΔfhbB) resulted in a slight increase in cleistothecia with respect to the level for the wild type (Fig. 1B). Cleistothecia produced by ΔfhbA, ΔfhbB, or ΔfhbA ΔfhbB produced viable ascospores (data not shown). With respect to asexual development, we did not find significant differences under the experimental conditions assayed (data not shown).
Fig. 1.
Deletion in fhbA or supplementation of nitric oxide (NO) promotes A. nidulans sexual development. (A) Micrographs showing Hülle cells produced by ΔfhbA 5 days after inoculation on solid GMM (right), while no Hülle cells were detected in cultures of the isogenic control strain (left). wt, wild type. (B) Quantification of cleistothecia produced by the wild type and the fhb mutants with and without NO supplementation (core = 16-mm diameter). (C) Micrographs of the wild type and the fhb mutants with and without nitric oxide (NO) supplementation (35×). Cultures were sprayed with 70% ethanol to facilitate the visualization of cleistothecia.
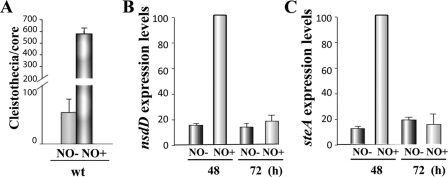
Interestingly, addition of the NO-releasing compound drastically increased the formation of cleistothecia in A. nidulans (Fig. 1B and C). This increase in sexual development was accompanied by a higher accumulation of nsdD and steA transcripts in the NO-treated cultures than in those cultures without the treatment (Fig. 2). Overall, these results support the role of NO as a positive regulator of sexual development in A. nidulans. It is known that the presence of NO at a low concentration acts as a signaling molecule in biological systems (7, 10, 21), while at higher levels this compound causes nitrosative stress (4, 7). It is possible that although either fhbA deletion or NO supplementation is conducive to an increase of fungal sexual development, only higher nitrosative stress levels lead to the increase of nsdD and steA expression, resulting in a drastic increase in cleistothecium formation in NO-supplemented cultures at notably higher levels than those in the wild-type or fhb mutants. Future studies will examine the effect of NO level in these processes. The same induction effect of NO on sexual development was observed in both a strain carrying the veA1 allele (Fig. 1 and 2) and also in a strain carrying the veA+ allele (see Fig. S3 in the supplemental material), indicating that this effect is independent of the veA allele.
Fig. 2.
Addition of nitric oxide increases nsdD and steA expression. (A) Quantification of cleistothecia produced by the wild type with and without NO supplementation (core = 16-mm diameter). (B) qRT-PCR results showing nsdD expression levels in NO-treated versus nontreated cultures 48 h and 72 h after inoculation. (C) steA expression levels in NO-treated versus nontreated cultures 48 h and 72 h after inoculation. The relative expression levels were calculated using the method described by Kenneth and Schmittgen (14), and all values were normalized to the expression of the A. nidulans actin gene.
Recent studies showed that FHbs in aspergilli could also be related to the oxidative stress response (29). It is known that oxidative stress influences sexual development in A. nidulans (16). It is possible that the effect of NO on sexual development could also be in part linked to self-inflicted reactive oxygen species (ROS) production that occurs during the initiation of sexual development (16).
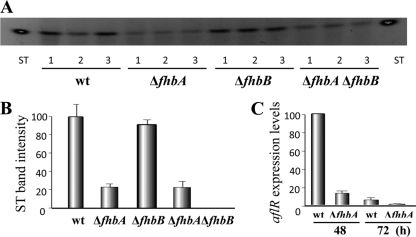
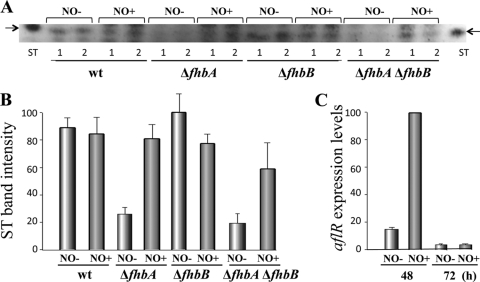
In addition to its effect on fungal morphogenesis, fhbA also influences mycotoxin biosynthesis in A. nidulans (Fig. 3). ΔfhbA (or ΔfhbA ΔfhbB) showed a reduction of ST production. This reduction coincided with a decrease in the expression of aflR, a transcription factor necessary for the activation of the ST gene cluster (28) (Fig. 3). However, ΔfhbB produced the same amount of toxin as the control strain. Surprisingly, supplementation with NO showed a recovery of ST near wild-type level in strains with a ΔfhbA background (Fig. 4A and B). NO supplementation resulted in an increase of aflR expression levels (Fig. 4C). Our experiments suggest that different NO threshold levels could have opposite effects on mycotoxin production. It is also possible that fhbA in A. nidulans could influence other regulatory mechanisms in addition to those related to NO detoxification. Due to the conservation of FHbs in other aspergilli and due to the fact that ST and aflatoxin are synthesized through the same biosynthetic pathway, it is possible that fhbA could also play a role in regulating the biosynthesis of the potent carcinogenic mycotoxin aflatoxin (1, 2, 19).
Fig. 3.
Production of sterigmatocystin (ST) in A. nidulans is regulated by fhbA. (A) ST TLC analysis from wild-type, ΔfhbA, ΔfhbB, and ΔfhbA ΔfhbB cultures grown on GMM for 5 days. (B) Densitometry of the TLC image in panel A. Band intensity quantification was carried out with Scion Image Beta 4.03 software. The normalized ST band intensity values were normalized with respect to the highest level of intensity, considered 100. (C) aflR expression levels in the ΔfhbA strain and its isogenic control strain at 48 h and 72 h after inoculation on solid GMM. The relative expression levels were calculated using the method described by Kenneth and Schmittgen (14), and all values were normalized to the expression of the A. nidulans actin gene.
Fig. 4.
Supplemenation of NO positively affects ST production. (A) ST TLC analysis from wild-type, ΔfhbA, ΔfhbB, and ΔfhbA ΔfhbB cultures with and without NO supplementation grown on GMM for 5 days. (B) Densitometry of the TLC image in panel A. Band intensity quantification was carried out with Scion Image Beta 4.03 software. The normalized ST band intensity values were normalized with respect to the highest level of intensity, considered 100. (C) aflR expression levels in NO-treated versus nontreated cultures 48 h and 72 h after inoculation. The relative expression levels were calculated using the method described by Kenneth and Schmittgen (14), and all values were normalized to the expression of the A. nidulans actin gene.
This is the first study of the effect of NO on sexual development and secondary metabolism in fungi, further revealing NO as a signaling molecule in another kingdom of lower eukaryotes, suggesting an ancient role for this simple molecule in complex regulatory pathways affecting numerous cellular processes, including morphogenesis and metabolism.
Supplementary Material
Acknowledgments
This study was funded by Northern Illinois University and by USDA-SCA 58-6435-9-386.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Bennett J. W., Klich M. 2003. Mycotoxins. Clin. Microbiol. Rev. 16:497–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cary J. W., Calvo A. M. 2008. Regulation of aflatoxin biosynthesis. J. Toxicol. Toxin Rev. 27:347–370 [Google Scholar]
- 3. Cassanova N., O'Brien K. M., Stahl B. T., McClure T., Poyton R. O. 2005. Yeast flavohemoglobin, a nitric oxide oxidoreductase, is located in both the cytosol and the mitochondrial matrix: effects of respiration, anoxia, and the mitochondrial genome on its intracellular level and distribution. J. Biol. Chem. 280:7645–7653 [DOI] [PubMed] [Google Scholar]
- 4. Cooper C. E. 1999. Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411:290–309 [DOI] [PubMed] [Google Scholar]
- 5. Crawford M. J., Goldberg D. E. 1998. Role for the Salmonella flavohemoglobin in protection from nitric oxide. J. Biol. Chem. 273:12543–12547 [DOI] [PubMed] [Google Scholar]
- 6. Edelmann M., Wolfe C., Scordalakes E. M., Rissman E. F., Tobet S. 2007. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev. Neurobiol. 67:1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gardner P. R., et al. 2000. Nitric-oxide dioxygenase activity and function of flavohemoglobins. Sensitivity to nitric oxide and carbon monoxide inhibition. J. Biol. Chem. 275:31581–31587 [DOI] [PubMed] [Google Scholar]
- 8. Gardner P. R., Gardner A. M., Martin L. A., Salzman A. L. 1998. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc. Natl. Acad. Sci. U. S. A. 95:10378–10383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han K. H., et al. 2001. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol. Microbiol. 41:299–309 [DOI] [PubMed] [Google Scholar]
- 10. Hardison R. 1998. Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J. Exp. Biol. 201:1099–1117 [DOI] [PubMed] [Google Scholar]
- 11. Hausladen A., Gow A. J., Stamler J. S. 1998. Nitrosative stress: metabolic pathway involving the flavohemoglobin. Proc. Natl. Acad. Sci. U. S. A. 95:14100–14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ignarro L. J., et al. 1990. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem. Biophys. Res. Commun. 170:843–850 [DOI] [PubMed] [Google Scholar]
- 13. Käfer E. 1977. The anthranilate synthetase enzyme complex and the trifunctional trpC gene of Aspergillus. Can. J. Genet. Cytol. 19:723–738 [DOI] [PubMed] [Google Scholar]
- 14. Kenneth J. L., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT Method. Methods Enzymol. 25:402–408 [DOI] [PubMed] [Google Scholar]
- 15. Kim H. C., et al. 2007. Expression of nitric oxide synthase isoforms in the testes of pigs. Anat. Histol. Embryol. 36:135–138 [DOI] [PubMed] [Google Scholar]
- 16. Lara-Ortiz T., Riveros-Rosas H., Aguirre J. 2003. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol. Microbiol. 50:1241–1255 [DOI] [PubMed] [Google Scholar]
- 17. Lewis M. E. S., Corker H. A., Gollan B., Poole R. K. 2008. A survey of methods for the purification of microbial flavohemoglobins. Methods Enzymol. 436:169–186 [DOI] [PubMed] [Google Scholar]
- 18. Masuda M., Kubota T., Aso T. 2001. Effects of nitric oxide on steroidogenesis in porcine granulosa cells during different stages of follicular development. Eur. J. Endocrinol. 144:303–308 [DOI] [PubMed] [Google Scholar]
- 19. Paterson R. R., Lima N. 2010. Toxicology of mycotoxins. EXS 100:31–63 [DOI] [PubMed] [Google Scholar]
- 20. Poole R. K., et al. 1996. Nitric oxide, nitrite, and Fnr regulation of hmp (flavohemoglobin) gene expression in Escherichia coli K-12. J. Bacteriol. 178:5487–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poole R. K., Hughes M. N. 2000. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 36:775–783 [DOI] [PubMed] [Google Scholar]
- 22. Rosselli M., Keller P. J., Dubey R. K. 1998. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum. Reprod. Update 4:3–24 [DOI] [PubMed] [Google Scholar]
- 23. Sambrook J., Russell D. W. 2006. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 24. Schinko T., et al. 2010. Transcriptome analysis of nitrate assimilation in Aspergillus nidulans reveals connections to nitric oxide metabolism. Mol. Microbiol. 78:720–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. te Biesebeke R., et al. 2010. Phylogeny of fungal hemoglobins and expression analysis of the Aspergillus oryzae flavohemoglobin gene fhbA during hyphal growth. Fungal Biol. 114:135–143 [DOI] [PubMed] [Google Scholar]
- 26. Vallim M. A., Miller K. Y., Miller B. L. 2000. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 36:290–301 [DOI] [PubMed] [Google Scholar]
- 27. Yager L. N. 1992. Early developmental events during asexual and sexual sporulation in Aspergillus nidulans. Biotechnology 23:19–41 [PubMed] [Google Scholar]
- 28. Yu J. H., et al. 1996. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 29:549–555 [DOI] [PubMed] [Google Scholar]
- 29. Zhou S., et al. 2010. Aspergillus oryzae flavohemoglobins promote oxidative damage by hydrogen peroxide. Biochem. Biophys. Res. Commun. 394:558–561 [DOI] [PubMed] [Google Scholar]
- 30. Zhou S., et al. 2009. Cloning and characterization of two flavohemoglobins from Aspergillus oryzae. Biochem. Biophys. Res. Commun. 381:7–11 [DOI] [PubMed] [Google Scholar]
- 31. Zini A., O'Bryan M. K., Magid M. S., Schlegel P. N. 1996. Immunohistochemical localization of endothelial nitric oxide synthase in human testis, epididymis, and vas deferens suggests a possible role for nitric oxide in spermatogenesis, sperm maturation, and programmed cell death. Biol. Reprod. 55:935–941 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.