Abstract
Contamination of oysters with human noroviruses (HuNoV) constitutes a human health risk and may lead to severe economic losses in the shellfish industry. There is a need to identify a technology that can inactivate HuNoV in oysters. In this study, we conducted a randomized, double-blinded clinical trial to assess the effect of high hydrostatic pressure processing (HPP) on Norwalk virus (HuNoV genogroup I.1) inactivation in virus-seeded oysters ingested by subjects. Forty-four healthy, positive-secretor adults were divided into three study phases. Subjects in each phase were randomized into control and intervention groups. Subjects received Norwalk virus (8FIIb, 1.0 × 104 genomic equivalent copies) in artificially seeded oysters with or without HPP treatment (400 MPa at 25°C, 600 MPa at 6°C, or 400 MPa at 6°C for 5 min). HPP at 600 MPa, but not 400 MPa (at 6° or 25°C), completely inactivated HuNoV in seeded oysters and resulted in no HuNoV infection among these subjects, as determined by reverse transcription-PCR detection of HuNoV RNA in subjects' stool or vomitus samples. Interestingly, a white blood cell (granulocyte) shift was identified in 92% of the infected subjects and was significantly associated with infection (P = 0.0014). In summary, these data suggest that HPP is effective at inactivating HuNoV in contaminated whole oysters and suggest a potential intervention to inactivate infectious HuNoV in oysters for the commercial shellfish industry.
INTRODUCTION
Human noroviruses (HuNoV) are the most frequent cause of food-borne disease outbreaks in the United States (3, 50), and transmission of HuNoV infection via HuNoV-contaminated shellfish consumption is a worldwide problem (27, 32, 44, 51). HuNoV-contaminated shellfish may result in severe economic losses to the shellfish industry due to product recalls, harvest area closures, and loss of consumer confidence (42, 47). Shellfish are particularly susceptible to contamination, as they can readily bioaccumulate waterborne microbial pathogens from marine and estuarine waters (10). Bioaccumulation is problematic, as HuNoV can persist in seawater for long periods of time (15), may specifically bind and bioconcentrate within shellfish digestive tissues (31, 57), and may remain viable in shellfish tissues for several weeks (21, 58). Unfortunately, methods such as commercial depuration are inadequate for purging HuNoV from shellfish tissues (17, 49, 58) and while thorough cooking inactivates HuNoV in contaminated shellfish, this alters the organoleptic qualities of raw shellfish. At present, there are no commercial postharvest options for bivalve shellfish that inactivate HuNoV and retain an uncooked and commercially attractive appearance.
High hydrostatic pressure processing (HPP) is a commercial intervention currently used for the inactivation of Staphylococcus aureus, Listeria monocytogenes, Vibrio spp., Salmonella spp., and Escherichia coli O157:H7 (reviewed in reference 13) and is proposed for the inactivation of Vibrio parahaemolyticus in oysters (30, 40). In general, HPP has been shown to maintain the appearance, flavor, nutritional quality, and texture of raw shellfish (37, 43). HPP also serves a practical application by facilitating the shellfish shucking process by separating the meat from the shell and has been demonstrated to extend refrigerated shelf life (14, 19, 43). HPP has also been identified recently as a potential means for inactivating parasitic and viral pathogens such as Cryptosporidium parvum oocysts (12), hepatitis A virus, and HuNoV surrogates within raw shellfish (9, 22, 25, 54). The HuNoV surrogate, feline calicivirus (FCV), was inactivated up to 7 log10 in tissue culture medium at a pressure of 275 MPa (11, 18, 26). Murine norovirus (MNV), another HuNoV surrogate, was reduced (4 log10) in live oysters contaminated with MNV in seawater under simulated natural conditions (400 MPa, 5 min, 5°C) (25). The successful inactivation of HuNoV surrogates by HPP suggests that HuNoV may also be inactivated by HPP. Additionally, factors to consider that may influence HPP efficacy against noroviruses include the duration of pressure application, the temperature at which pressure is applied, and food matrix properties such as salinity, pH, and water content (11, 23, 25, 26). If HPP is shown to inactivate HuNoV, the benefits of HPP paired with the potential inactivation of HuNoV in oysters would offer the shellfish industry an effective and multipurpose intervention against HuNoV contamination of oysters.
On the consumer side, there is a need for clinicians to quickly detect HuNoV infection after the ingestion of contaminated oysters or by other transmission routes. Early predictors of HuNoV infection may improve infection control strategies in settings such as hospitals and potentially reduce the economic burden of hospital-acquired norovirus infections (38). White blood cell (WBC) differentials, for example, have been proposed as a surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea (7) and among pediatric patients with viral gastroenteritis (leukocytosis helps distinguish adenovirus from rotavirus) (46). Similarly, early norovirus human challenge studies in the 1970s and 1980s found leukocytosis during acute norovirus infection (2, 16). However, the use of WBC differentials as an adjunctive tool with symptoms for the clinical diagnosis of norovirus remains to be further investigated.
To address these questions, we evaluated several conditions of HPP processing to determine if specific HPP conditions could inactivate 1.0 × 104 genomic equivalent copies (GEC) of Norwalk virus (GI.1), the HuNoV prototype virus, in oysters, rendering the oysters safe for human consumption. We also investigated serum specimens for the presence of WBC differentials as an indicator of HuNoV infection.
MATERIALS AND METHODS
Study participants.
We screened 98 individuals for participation in this study between September 2007 and October 2009, and of those, 44 were selected. All 44 enrolled subjects provided written informed consent and successfully completed a test of understanding. Candidate subjects were excluded if they were H type 1 nonsecretors, food handlers, child or geriatric care givers, health care workers with direct patient contact, persons serologically positive for HIV, individuals positive for bacterial or protozoan enteric infections, persons with abnormal liver or renal functions or blood counts, persons with chronic diseases (i.e., lupus, cancer, renal disease), allergic to shellfish, living with young children or elderly individuals, or pregnant. The secretor status (i.e., genetic susceptibility to GI.1 HuNoV) of each study subject was determined by detection of the H type 1 carbohydrate (35) in saliva, through direct enzyme-linked immunosorbent assays of saliva samples, as described by Azevedo et al. (5), with the following modifications. Polystyrene Costar enzyme immunoassay/radioimmunoassay flat-well medium binding plates (Corning Inc., Corning, NY) were used. Unprocessed saliva was diluted to 0.1% in phosphate-buffered saline (PBS), and 200 μl of diluted saliva was incubated overnight at 4°C in each well. After three washes in PBS, 1 mg/ml horseradish peroxidase-conjugated rabbit anti-UEA-I antibody (EY Laboratories, San Mateo, CA) was diluted to 0.2% in PBS containing 5% defatted milk (BLOTTO) and 100 μl of this mixture was added to each well. After three washes in PBS, reactions were developed with tetramethylbenzidine substrate (BioFX Laboratories Inc., Owings Mills, MD) and quenched with 0.18 M hydrochloric acid (Fisher Scientific). The optical density at 450 nm (OD450) of each well was read using an ELx800 spectrophotometer (BioTek, Winooski, VT) and KCjunior software (BioTek). Positive-secretor samples were defined as having an average triplicate OD450 value equal to or greater than four times the average of a triplicate negative control (a known nonsecretor saliva sample). All subjects enrolled in this study were positive secretors of the H type 1 histo-blood group antigen carbohydrate.
Stopping rules and interim analyses.
Specific study termination criteria were established according to the Data Safety and Monitoring Board (DSMB) adverse-event grading scale. Events warranting study termination included evidence of secondary HuNoV transmission within the General Clinical Research Center (GCRC) requiring immediate infection control measures, serious dehydration requiring intravenous fluids for more than 5 days, and evidence of abnormal blood chemistry, blood cell counts, or liver function tests in the specimens collected at the 35-day postchallenge follow-up visit. Results were reviewed at the close of each study phase to evaluate subject safety and the adverse-event stopping guidelines. Interim analyses consisted of chi-square tests of independence or Fisher's exact test, as applicable. There were no unexpected study-related adverse events during the study.
HuNoV challenges.
This study was conducted in three phases with different HPP test conditions. Subjects within each phase were challenged in the following order: phase 1, 2/7/2008 to 10/23/2008, phase 2, 1/29/2009 to 4/23/2009, phase 3, 6/25/2009 to 9/10/2009. On admission to the Emory University GCRC, subjects provided samples of serum and saliva prior to challenge and daily during days 1 to 5 postchallenge (day 1 represented the challenge day). Postchallenge, subjects were monitored for gastrointestinal symptoms and vital signs two to three times a day by clinical staff over a consecutive 4-night, 5-day period. On days 1, 3, and 4, WBC shift data were collected, defined as an increase in granulocyte production into the upper abnormal range; the normal range for granulocytes is 43 to 72% of the total WBC count. Following discharge, subjects returned for five follow-up visits on approximately days 8, 14, 21, 28, and 35 postchallenge for blood, saliva, and stool sample collection and recording of gastrointestinal symptoms and vital signs. The blood sample collected on the final visit (around day 35) was assayed for ABO status at the Emory University Hospital laboratories. All samples that were not immediately processed were collected and stored at −80°C. Final subject health and safety assessments were performed around 35 days postchallenge.
Norovirus inoculum.
Norwalk virus (genogroup I.1 HuNoV inoculum 8FIIb) was prepared from stool filtrates from a previously HuNoV-infected subject, its titer was determined, and it was safety tested for a range of pathogens and stored at −80°C as previously described (55). The HuNoV stock inoculum 8FIIb was quantified by real-time reverse transcription (RT)-PCR with RNA standards. The geometric mean virus concentration was 7.2 × 108 GEC/ml (36). This stock inoculum was diluted at Emory University using serial 10- and 100-fold dilutions with sterile PBS to a final concentration of 1.0 × 104 GEC/ml. The diluted inocula were aliquoted into 1-ml units, stored at −80°C in CryoTubes, shipped overnight to the ARS-United States Department of Agriculture (USDA) laboratory on dry ice, and stored at −80°C upon receipt until the seeding of oysters. Final diluted inoculum aliquots were not quantified by quantitative RT-PCR (qRT-PCR) because they were too close to the qRT-PCR limit of detection for an accurate measure (36). The study team selected a dose of 1.0 × 104 GEC for seeding of the oysters because published reports on norovirus contamination of oysters reported genogroup I norovirus contamination ranges of 966 to 1,690 GEC/g of oyster digestive tissue (33, 34) and HPP was shown to inactivate 4 logs of murine norovirus (25). The HuNoV inoculum used in this study was tested for infectivity in a pilot study (n = 7) prior to the start of phases 1 to 3.
Oyster screening and preparation.
Commercial oysters (Crassostrea virginica) were obtained from Spatco Inc. (Narragansett, RI). Oysters were of medium market size, with a meat weight of approximately 5 to 10 g per oyster. Throughout this study, oysters were procured from the same vendor. Upon receipt, a representative number of oysters from each lot to be used in the study were tested for total bacteria, fecal coliforms, HuNoV, and hepatitis A virus as described previously (28) and subjected to an initial HPP treatment of 400 MPa for 5 min to (i) ensure inactivation of potentially pathogenic vibrios, (ii) facilitate removal of the oysters from their shells, and (iii) provide an intact oyster for injection of HuNoV inoculum into the digestive tract. This initial treatment of all oysters effectively blinded study participants and Emory investigators to the subsequent HPP treatment status because “untreated” oysters were indistinguishable from subsequently “treated” oysters. However, some whitening of oysters was observed in phase 2, where oysters were given a subsequent treatment of 600 MPa. Three days prior to subject challenge, 333 μl of HuNoV inoculum was injected into the stomach and digestive diverticulum of each of three oysters with a needle and syringe such that the three oysters contained a total of 1.0 × 104 GEC of HuNoV. The three oysters were placed into a plastic bag and heat sealed. Some bags were subjected to HPP treatment as described below, while the remaining bags of oysters were not HPP treated and served as HuNoV-positive controls. Treated and untreated shellfish, in heat-sealed plastic pouches with unique identity (ID) codes, were shipped overnight to Emory investigators at 5°C in cartons containing temperature recorders (ACR Systems, Inc., Surrey, Canada).
High hydrostatic pressure treatment of HuNoV-seeded oysters and randomization.
This study was conducted in three phases with different HPP test conditions. Subjects were divided into study groups, including phase 1, where oysters were subjected to 400 MPa for 5 min at 25°C; phase 2, where oysters were subjected to 600 MPa for 5 min at 6°C; and phase 3, where oysters were subjected to 400 MPa for 5 min at 6°C. The adiabatic temperature rise for HPP treatments at 6°C was 10 to 20°C, and that for HPP treatments at 25°C was 7 to 15°C. These treatments were applied using a Quintus 35-liter food press (QFP 35L-600; Flow International Corporation, Avure Technologies Inc., Kent, WA). Randomized subjects from phases 1 to 3, who ingested non-HPP-treated HuNoV-inoculated oysters, were combined to represent the study's comparison control group. Control subjects were pooled together across phases 1 to 3 because of the identical non-HPP-treated HuNoV-inoculated oysters and challenge conditions.
HuNoV-inoculated oysters were assigned to treatment groups by the USDA and Virginia Polytechnic Institute and State University (Virginia Tech) personnel and assigned unique ID codes known only by USDA and Virginia Tech staff and administered to subjects by randomly pairing each coded bag of oysters with a subject's number by Emory investigators. The method of administering the challenge oysters was consistent between the control and treatment groups. In general, for each enrollment date, at least one treated and one untreated dose was administered. The last six subjects in the study all received HPP-treated oysters and were unblinded to the treatment assignment, upon DSMB recommendation (for additional details, see the supplemental material). For all other subjects, the treatment codes were unblinded at the completion of each phase and reviewed by the DSMB and study investigators.
Norovirus-seeded oyster challenge.
All subjects ingested approximately 2.4 g sodium bicarbonate dissolved in water (approximately 60-ml total volume) 2 min prior to and 5 min after oyster consumption to reduce stomach acidity. Subjects ingested 3 untreated (positive control) or HPP-treated oysters, including oyster juice, with a combined HuNoV dose of 1.0 × 104 GEC. Saltine crackers and/or reduced-fat chocolate milk were offered to subjects to facilitate oyster/oyster juice consumption without affecting stomach acidity.
Detection of norovirus RNA in stool and vomitus samples.
Norovirus infection among the HPP-treated and control groups was the primary endpoint to assess the efficacy of the HPP treatments. Infection was defined as RT-PCR detection of HuNoV RNA in any postchallenge stool or vomitus sample as described in reference 35. For extraction of HuNoV RNA, stool and vomitus samples were suspended in water (20% [vol/vol]), added to an equal volume of Vertrel XF (DuPont, Wilmington, DE), and centrifuged at 13,000 × g for 10 min at room temperature. Viral RNA was extracted from the supernatant using the QIAamp Viral RNA Mini kit vacuum protocol (Qiagen, Valencia, CA), with the following modifications. (i) RNA was eluted with 50 μl of nuclease-free, molecular-grade water (Cellgro, Manassas, VA) after incubation for 5 min, and (ii) vomitus samples were concentrated 5 times through one column membrane. RNA extracts were stored at −20°C until tested. Qiagen columns were used for viral RNA extraction from stool samples because of their documented ability to remove RT-PCR inhibitors from stool samples (1, 8). RT-PCR using the GeneAmp PCR System 9700 thermocycler (PE Applied Biosystems) was performed as described by Moe et al. (41) using HuNoV genogroup I RNA-dependent RNA-polymerase-specific primers NV3 and NV51, with the following modifications. A 20-μl RT mixture was made with 4.5 U avian myeloblastosis virus reverse transcriptase (Promega, Madison WI), 4.0 μl of 5× Green GoTaq reaction buffer (Promega), 20 U RNase inhibitor (Promega), a 0.25 mM deoxynucleoside triphosphate mixture (Qiagen), 5.63 μM primer 51, 1.5 μl of Triton X-100 (Alfa Aesar, Ward Hill, MA), and 5 μl of RNA extracted from a stool or vomitus sample. PCR modifications included an initial denaturation step for 3 min and the addition of a 30-μl PCR mixture to create a final 50-μl reaction volume containing 1.25 U GoTaq DNA polymerase (Promega), 6 μl of 5× Green GoTaq reaction buffer (Promega), and 2.25 μM primer 3. All PCR amplification products were analyzed by gel electrophoresis on ethidium bromide-stained 2% agarose (Promega) gels with the target band at 206 bp (41). Specimens with ambiguous results were retested with the same extract or with a new extract of the sample. Stool specimens from HuNoV-infected subjects were included as positive controls in each extraction and RT-PCR, and water was included as a negative control.
Data collection and statistical methods.
Data compliance was monitored through established sample-tracking sheets and standardized data entry protocols. Standardized error checking was completed through double data entry by separate operators. Database cross-comparisons and any discrepancies were resolved by reviewing the hard-copy files.
All data analyses were performed with SAS 9.2 (SAS Institute Inc., Cary, NC). The primary analysis completed was quasi intention to treat because investigators excluded a pilot study (n = 7) used to confirm inoculum infectivity. Two subjects failed to return for their day 14, 21, 28, and 35 postinoculation follow-up visits in phase 3, but the missing data had a limited effect on our data analyses because our primary outcome of HuNoV infection status was determined prior to the subjects' withdrawal from the study. No outliers were removed during data cleaning or analysis. Due to the small sample size and skewed data within the contingency tables, Fisher's exact two-tailed test and maximum-likelihood Wald chi-square tests were used, where appropriate, to test the significance of the association between the dichotomous variables of infection status and HPP treatment. The backward-elimination technique was applied in the logistic regression modeling using Firth's bias-reducing penalized likelihood method (20), and the final model was selected by the Hosmer and Lemeshow goodness-of-fit test (29) as follows: logit P (infection status = 1∣WBC shift, race, age, gender) = −4.01 + [5.95 × WBC shift] + [2.98 × (race other) + (0.49 × race African-American)] + [1.01 × gender male] − [1.44 × (age 25 to 48 years)]. P < 0.05 was considered significant.
RESULTS
Subject enrollment and follow-up.
All subjects received their oyster challenge with the following proportion of subjects, by study phase, successfully completing the study through the day 35 clinical visit: phase 1, 9/9 (100%); phase 2, 15/15 (100%); phase 3, 18/20 (90%) (see Fig. S1 in the supplemental material). The combined overall rate of completion for the entire study was 95% (n = 44). No significant differences were found between the demographic characteristics of the treatment groups and those of the combined control group of subjects (Table 1), and thus, these groups were appropriate for comparison.
Table 1.
Demographic characteristics of subjects challenged with HPP-treated and untreated oysters
| Characteristic | No. (%) of subjects challenged with: |
χ2P valuec | |
|---|---|---|---|
| HPP-treated oystersa | Untreated oystersb | ||
| Age (yr) at challenge | |||
| 18–24 | 15 (52)e | 9 (60) | |
| 25–48 | 14 (48) | 6 (40) | 0.6013 |
| Ethnicity | |||
| Caucasian | 10 (34) | 5 (33) | |
| African-American | 12 (41) | 7 (47) | |
| Otherd | 7 (24) | 3 (20) | 0.9313 |
| Gender | |||
| Male | 14 (48) | 7 (47) | |
| Female | 15 (52) | 8 (53) | 0.9193 |
Total n = 29.
Total n = 15.
Chi-square goodness-of-fit P values are shown.
The “other” category includes the following ethnicities: Asian, Hispanic, multiracial, and other.
Column percentages may not equal 100 due to rounding.
Efficacy of HPP treatments.
To assess the efficacy of HPP treatments for inactivation of HuNoV and the risk of HuNoV infection among challenged subjects, the proportion of infected subjects was compared to the control group in each phase. The control group represented the combined number of controls over phase 1 through phase 3 (n = 15) because each control received untreated, HuNoV-seeded raw oysters with the same amount of HuNoV inoculum. In phases 1 and 3, there was no significant difference between the HuNoV infection rates of the subjects challenged with HPP-treated oysters and those of the subjects challenged with untreated oysters (Table 2). When the rates of volunteer infection were compared between phases 1 and 3, the rate was lower with HPP at 6°C than with HPP at 25°C; however, these results were not significantly different (P = 0.1285). Conversely, none of the 10 subjects challenged with HuNoV-seeded oysters treated by 600 MPa at 6°C for 5 min (phase 2) became infected with HuNoV. These results suggest that of the three pressure and temperature treatment combinations tested, only the treatment with 600 MPa at 6°C for 5 min successfully inactivated HuNoV within raw oysters and resulted in no infection of any of the subjects.
Table 2.
Distribution of study subject infection status among oyster treatment groups
| Phase | Treatment conditions | No. of subjects infected/total (%) postchallenge with: |
P valueb | |
|---|---|---|---|---|
| HPP-treated oysters | Untreated oystersa | |||
| 1 | 400 MPa, 25°C, 5 min | 3/5 (60) | 7/15 (47) | 1.0000 |
| 2 | 600 MPa, 6°C, 5 min | 0/10 (0) | 7/15 (47) | 0.0202 |
| 3 | 400 MPa, 6°C, 5 min | 3/14 (21) | 7/15 (47) | 0.2451 |
The control group represented the combined number of controls over phase 1 through phase 3 (n = 15) because each control received untreated HuNoV-seeded raw oysters with the same amount of HuNoV inoculum.
Fisher's exact two-sided test compared each treatment group to all of the controls (i.e., the total number of subjects challenged with non-HPP treated oysters).
Clinical disease.
To examine the occurrence of clinical symptoms among HuNoV-infected subjects, we maintained records of self-reported symptoms (nausea, cramping, headache, chills, myalgia, and fatigue) and objective conditions (fever, vomiting, and diarrhea) recorded during days 1 to 5. Subjects were also classified as asymptomatic or overall symptomatic (i.e., the presence of one or more symptoms, with the exception of fever, which had to be associated with at least one other symptom). Among the infected subjects, nine (69%) experienced one or more symptoms and four subjects (31%) had asymptomatic HuNoV infection within the 5 days postchallenge (Table 3). In general, symptomatic subjects exhibited a rapid onset and resolution of symptoms well within the first 5 days postchallenge (Fig. 1). Two subjects experienced diarrhea (≥3 unformed stools in 24 h) but were determined by RT-PCR to be HuNoV RNA negative. The earliest detection of HuNoV RNA in stool and vomitus samples was on day 2 (24 h postchallenge), and the latest detection of HuNoV RNA in stool was on day 34 (33 days postchallenge). There was no significant difference in ABO type between HuNoV-infected and noninfected subjects and between HPP-treated or untreated volunteers (data not shown).
Table 3.
Distribution of symptoms among infected subjects
| Symptom(s) | No. (%) of infected subjects symptomatica |
|---|---|
| Chills | 3 (23) |
| Cramping | 3 (23) |
| Diarrheab | 6 (86) |
| Fatigue | 5 (38) |
| Fever | 12 (92) |
| Headache | 2 (15) |
| Myalgia | 4 (31) |
| Nausea | 8 (62) |
| Overall symptomsc | 9 (69) |
| Emesis | 5 (38) |
| WBC shiftd | 12 (92) |
The total number of infected subjects was 13.
The variable diarrhea had a denominator of 7 due to missing data among infected subjects.
The variable “overall symptoms” was defined as a subject with at least one symptom, not including fever. To be classified as an overall symptom, fever had to be associated with at least one other symptom. For example, a subject with fever and headache was classified as being overall symptomatic; however, a subject with only fever was not considered overall symptomatic.
A WBC shift was defined as an increase in granulocyte production with an abnormal granulocyte count, i.e., >72% of the total WBC count.
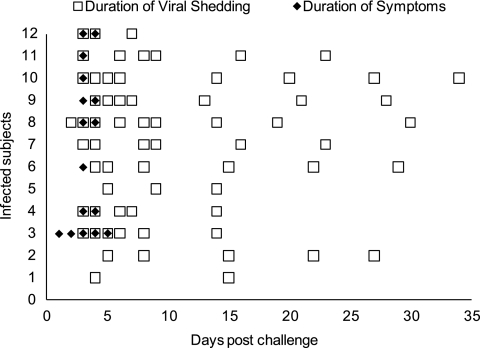
Fig. 1.
Temporal distribution of subject symptoms and HuNoV excretion. Open squares represent RT-PCR-detected HuNoV-positive stool samples collected from infected subjects during days 1 to 5 postchallenge while admitted to the GCRC and during weekly follow-up visits (days 8, 14, 21, 28, and 35 postchallenge). The distribution of squares (e.g., samples/follow-up visits) is the result of the sample collection time line and does not imply intermittent sample positivity or lack of sample positivity. All of the subjects, with the exception of one (11/12), completed all five follow-up visits and provided five stool specimens; one volunteer missed the day 8 follow-up appointment and provided four stool specimens. Filled diamonds represent self-reported and clinically assessed symptoms of infected subjects. The total number of infected subjects was 12 instead of 13 because one infected subject was not available for follow-up after day 8.
WBC shift in HuNoV infection.
Infected subjects exhibited increases in granulocyte blood cell production (WBC shifts; the abnormal range of granulocytes was greater than 72% of the total WBC count occurring after the challenge day) ranging from 77 to 95% of the total WBC count on day 3 or 4 postchallenge. When adjusting for the demographic characteristics of race (African-American, Caucasian, and other), gender, and age group (18 to 24 and 25 to 48 years) in a multivariate logistic model (for model construction and details, see Materials and Methods and the supplemental material), WBC shift was significantly associated with HuNoV infection (odds ratio, 384.90; 95% confidence interval, 9.92 to ∞; Wald P value, 0.0014). The strong correlation between infection status and a WBC shift was supported by the observation that 12 (92%) of 13 infected subjects had a WBC shift and only 1 (3%) of 31 uninfected subjects had a WBC shift (Table 3).
DISCUSSION
The goal of this study was to examine the efficacy of HPP treatments for Norwalk virus inactivation in artificially seeded raw, whole oysters. HPP treatment (600 MPa, 6°C, 5 min) inactivated HuNoV in oysters and prevented infection among challenged volunteers. HuNoV-infected subjects displayed symptoms consistent with the published literature. In addition, 92% of the infected subjects exhibited a unique WBC (granulocyte) shift and this was significantly associated with infection.
To date, this is the first demonstration of HuNoV inactivation by high pressure in a human challenge study. The highest-pressure conditions (600 MPa, 6°C, 5 min), but not the lower-pressure conditions (400 MPa, 6 or 25°C, 5 min), inactivated HuNoV in oysters and prevented HuNoV infection among all of the subjects challenged with HPP-treated oysters. The findings from this study suggest that HuNoV is less sensitive to pressure than animal caliciviruses used in surrogate in vitro PFU reduction studies. Studies of HPP treatments of surrogate HuNoV have reported that 275 MPa for 5 min inactivated >6 log10 PFU of FCV (11, 26) and 400 MPa for 5 min at 5°C inactivated 4 log10 PFU of MNV-1 within oysters (25). Furthermore, research on the mechanism of action of HPP on MNV suggests that HPP may inactivate MNV by disrupting the MNV receptor responsible for MNV binding and cell entry (39, 53). In contrast, 400 MPa was insufficient to prevent HuNoV infections among our human subjects, which suggests that a 4-log10 genome equivalent reduction of HuNoV was not achieved. These results suggest that the mechanism of HPP inactivation may work differently against HuNoV than against animal caliciviruses and may require higher pressures for HuNoV inactivation.
While the magnitude of infectious HuNoV reduction at 400 MPa could not be directly determined in this study because the outcome of this study was dichotomous (the HPP treatment either inactivated or did not inactivate HuNoV in oysters), a proposed model of the expected log10 reduction by the three HPP treatments is included (see Fig. S2 in the supplemental material). Furthermore, it is unclear whether an intermediate pressure (between 400 and 600 MPa) or other pressure-temperature combinations would sufficiently inactivate HuNoV within oysters and prevent HuNoV infection among subjects. The diverse sensitivities seen with these caliciviruses to HPP treatments may be common within families of viruses. Viruses such as foot-and-mouth disease virus, human rhinovirus, and poliovirus within the family Picornaviridae demonstrate a wide range of sensitivities to HPP (24, 45). It is conceivable that different HuNoV genogroups, and perhaps different clusters within a HuNoV genogroup, would exhibit varied sensitivities to HPP (48).
Symptoms associated with HuNoV infection from this study were consistent with the published literature (reviewed in reference 56). The most common symptoms among the infected subjects included nausea, fever, and diarrhea. The duration of symptoms and viral shedding among infected subjects in this study was also consistent with previous studies. Infected subjects experienced symptoms early in the course of infection. These resolved rapidly, followed by an extended period of virus shedding. The longest shedding period observed was through day 34, as detected by RT-PCR in stool samples (Fig. 1). This observation was consistent with a recent clinical trial in which subjects experimentally infected with HuNoV experienced symptomatic illness for 1 to 2 days and shed virus a median of 28 days (range, 13 to 56 days) after challenge (4).
An interesting observation in this study was a transient leukocytosis or “left shift” toward granulocyte production exhibited by volunteers during the early stages of HuNoV infection. This left shift was characterized as a percentage of granulocyte WBCs that was greater than 72% of the total WBC count occurring after the challenge day. In this study, 92% of the infected subjects experienced a left shift toward granulocyte production. Other HuNoV challenge and pediatric viral gastroenteritis studies have reported a similar transient leukocytosis in which a granulocyte shift was identified 48 h postinfection and gradually returned to the baseline by day 5 postinfection (6, 16, 52). These data suggest that left shifts could be used as an adjunctive tool for the clinical diagnosis of norovirus infection in the hospital setting, especially for patients with gastroenteritis.
The practical commercial application of HPP for HuNoV-contaminated shellfish will require treatments to be economical, viable for current commercial units, and acceptable to consumers. The 5-min HPP treatments tested in this study are economical for high-throughput operations. Although the shellfish industry uses HPP at pressures of approximately 300 MPa to facilitate oyster shucking, extend shelf life, and reduce total bacterial counts, including those of Vibrio spp. (19), it is not clear whether the higher pressures required, as suggested by our data, to reduce HuNoV contamination in shellfish will be viable for current commercial units. In addition to evaluation of the upper pressure limits of current commercial units, the consumer acceptability of 600-MPa HPP-treated oysters needs to be investigated. In this study, the high HPP pressure of 600 MPa, which was effective at inactivating HuNoV, induced a mildly cooked whitish appearance. While 400-MPa-treated oysters have been shown to be acceptable to consumers (37), it is uncertain whether an uncooked 600-MPa-treated oyster is also acceptable to consumers. A 600-MPa-treated oyster may be acceptable for consumption if it is subjected to a further processing step such as cooking. Cooking combined with HPP may be a promising strategy because cooked HPP-treated oysters may be organoleptically indistinguishable from cooked non-HPP-treated oysters.
Two limitations of this research were the use of only one HuNoV strain (the prototypical Norwalk virus) and the seeding of oysters with HuNoV via injection instead of natural bioaccumulation. Injection of HuNoV into the oyster digestive tract was the most accurate way to deliver the exact dosage of HuNoV into each oyster, whereas natural bioaccumulation of viruses via natural uptake from HuNoV-seeded seawater would have led to uncertainty regarding the virus levels actually within the shellfish. Two strengths of this research include the randomized, double-blind experimental design and that we directly tested HuNoV instead of using animal caliciviruses as surrogates for HuNoV.
While data from this study suggest a potential intervention to inactivate infectious HuNoV in oysters for the commercial shellfish industry, additional studies are needed to: (i) determine virus reduction levels between 400 and 600 MPa, (ii) examine more closely the effect of temperature on HuNoV inactivation by HPP and consumer acceptability, (iii) evaluate matrix and composition effects such as salinity and pH on virus inactivation, (iv) evaluate the utility of HPP treatment of HuNoV in other foods (e.g., uncooked fruit and vegetable products), (v) determine the mechanism of HPP inactivation for HuNov, and (vi) evaluate the effect of HPP on different strains of HuNoV.
Supplementary Material
ACKNOWLEDGMENTS
All study protocols and methods were approved by an independent DSMB and the Emory University Institutional Review Board. ClinicalTrials.gov identifier: NCT00674336.
This study was supported by the USDA-NIFA (grant 2005-5110-03271) and by the National Institutes of Health Public Health Service (grants UL1 RR025008, KL2 RR025009, TL1 RR025010, and M01 RR0039) from the Clinical and Translational Science Award program. This study was also supported in part by the Virginia Sea Grant Program (grant A/EP-10-01). J.S.L. was supported in part by funds from Emory University Global Health Institute, NIH NIAID (1K01AI087724-01), and USDA NIFA (2010-85212-20608) grants. D.H.K. and G.P.R. were supported by USDA intramural funds (project 1935-42000-059-00D).
We offer special thanks to the GCRC clinical staff at Emory University Hospital, laboratory technicians, collaborators, and subjects for making this project successful.
We have no conflicts of interest to report.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 24 June 2011.
REFERENCES
- 1. Aggarwal R., McCaustland K. A. 1998. Hepatitis E virus RNA detection in serum and feces specimens with the use of microspin columns. J. Virol. Methods 74:209–213 [DOI] [PubMed] [Google Scholar]
- 2. Alexander W. J., Holmes J. R., Shaw J. F., Riley W. E., Roper W. L. 1986. Norwalk virus outbreak at a college campus. South. Med. J. 79:33–36,40 [DOI] [PubMed] [Google Scholar]
- 3. Anonymous 2009. Surveillance for foodborne disease outbreaks—United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 58:609–615 [PubMed] [Google Scholar]
- 4. Atmar R. L., et al. 2008. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14:1553–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Azevedo M., et al. 2008. Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J. Pathol. 215:308–316 [DOI] [PubMed] [Google Scholar]
- 6. Blacklow N. R. 1972. Acute infectious nonbacterial gastroenteritis: etiology and pathogenesis. Ann. Intern. Med. 76:993–1008 [DOI] [PubMed] [Google Scholar]
- 7. Bulusu M., Narayan S., Shetler K., Triadafilopoulos G. 2000. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am. J. Gastroenterol. 95:3137–3141 [DOI] [PubMed] [Google Scholar]
- 8. Burgener M., Candrian U., Gilgen M. 2003. Comparative evaluation of four large-volume RNA extraction kits in the isolation of viral RNA from water samples. J. Virol. Methods 108:165–170 [DOI] [PubMed] [Google Scholar]
- 9. Calci K. R., Meade G. K., Tezloff R. C., Kingsley D. H. 2005. High-pressure inactivation of hepatitis A virus within oysters. Appl. Environ. Microbiol. 71:339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canzonier W. J. 1971. Accumulation and elimination of coliphage S-13 by the hard clam, Mercenaria mercenaria. Appl. Microbiol. 21:1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen H., Hoover D. G., Kingsley D. H. 2005. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J. Food Prot. 68:2389–2394 [DOI] [PubMed] [Google Scholar]
- 12. Collins M. V., et al. 2005. The effect of high-pressure processing on infectivity of Cryptosporidium parvum oocysts recovered from experimentally exposed Eastern oysters (Crassostrea virginica). J. Eukaryot. Microbiol. 52:500–504 [DOI] [PubMed] [Google Scholar]
- 13. Corbo M., et al. 2009. Prolonging microbial shelf life of foods through the use of natural compounds and non-thermal approaches—a review. Int. J. Food Sci. Technol. 44:223–241 [Google Scholar]
- 14. Cruz-Romero M., Kelly A. L., Kerry J. P. 2007. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 8:30–38 [Google Scholar]
- 15. Dancer D., Rangdale R. E., Lowther J. A., Lees D. N. 2010. Human norovirus RNA persists in seawater under simulated winter conditions but does not bioaccumulate efficiently in Pacific oysters (Crassostrea gigas). J. Food Prot. 73:2123–2127 [DOI] [PubMed] [Google Scholar]
- 16. Dolin R., Reichman R. C., Fauci A. S. 1976. Lymphocyte populations in acute viral gastroenteritis. Infect. Immun. 14:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grohmann G. S., Murphy A. M., Christopher P. J., Auty E., Greenberg H. B. 1981. Norwalk virus gastroenteritis in volunteers consuming depurated oysters. Aust. J. Exp. Biol. Med. Sci. 59:219–228 [DOI] [PubMed] [Google Scholar]
- 18. Grove S. F., et al. 2008. Inactivation of hepatitis A virus, poliovirus and a norovirus surrogate by high pressure processing. Innov. Food Sci. Emerg. Technol. 9:206–210 [Google Scholar]
- 19. He H., Adams R. M., Farkas D. F., Morrissey M. T. 2002. Use of high-pressure processing for oyster shucking and shelf-life extension. J. Food Sci. 67:640–645 [Google Scholar]
- 20. Heinze G., Schemper M. 2002. A solution to the problem of separation in logistic regression. Stat. Med. 21:2409–2419 [DOI] [PubMed] [Google Scholar]
- 21. Hewitt J., Greening G. E. 2004. Survival and persistence of norovirus, hepatitis A virus, and feline calicivirus in marinated mussels. J. Food Prot. 67:1743–1750 [DOI] [PubMed] [Google Scholar]
- 22. Kingsley D. H., Calci K., Holliman S., Dancho B., Flick G. J. 2009. High pressure inactivation of HAV within oysters: comparison of shucked oysters with whole-in-shell meats. Food Environ. Virol. 1:137–140 [Google Scholar]
- 23. Kingsley D. H., Chen H. 2008. Aqueous matrix compositions and pH influence feline calicivirus inactivation by high pressure processing. J. Food Prot. 71:1598–1603 [DOI] [PubMed] [Google Scholar]
- 24. Kingsley D. H., Chen H., Hoover D. G. 2004. Inactivation of selected picornaviruses by high hydrostatic pressure. Virus Res. 102:221–224 [DOI] [PubMed] [Google Scholar]
- 25. Kingsley D. H., Holliman D. R., Calci K. R., Chen H., Flick G. J. 2007. Inactivation of a norovirus by high-pressure processing. Appl. Environ. Microbiol. 73:581–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kingsley D. H., Hoover D. G., Papafragkou E., Richards G. P. 2002. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J. Food Prot. 65:1605–1609 [DOI] [PubMed] [Google Scholar]
- 27. Kingsley D. H., Meade G. K., Richards G. P. 2002. Detection of both hepatitis A virus and Norwalk-like virus in imported clams associated with food-borne illness. Appl. Environ. Microbiol. 68:3914–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kingsley D. H., Richards G. P. 2001. Rapid and efficient extraction method for reverse transcription-PCR detection of hepatitis A and Norwalk-like viruses in shellfish. Appl. Environ. Microbiol. 67:4152–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleinbaum D., Klein M. 2010. Logistic regression, 3rd ed. Springer, New York, NY [Google Scholar]
- 30. Kural A. G., Shearer A. E., Kingsley D. H., Chen H. 2008. Conditions for high pressure inactivation of Vibrio parahaemolyticus in oysters. Int. J. Food Microbiol. 127:1–5 [DOI] [PubMed] [Google Scholar]
- 31. Le Guyader F., et al. 2006. Norwalk virus-specific binding to oyster digestive tissues. Emerg. Infect. Dis. 12:931–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Guyader F. S., et al. 2006. Detection of multiple noroviruses associated with an international gastroenteritis outbreak linked to oyster consumption. J. Clin. Microbiol. 44:3878–3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Guyader F. S., et al. 2010. Comprehensive analysis of a norovirus-associated gastroenteritis outbreak, from the environment to the consumer. J. Clin. Microbiol. 48:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Guyader F. S., et al. 2009. Detection and quantification of noroviruses in shellfish. Appl. Environ. Microbiol. 75:618–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindesmith L., et al. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548–553 [DOI] [PubMed] [Google Scholar]
- 36. Liu P., Hsiao H. M., Jaykus L. A., Moe C. 2010. Quantification of Norwalk virus inocula: comparison of endpoint titration and real-time reverse transcription-PCR methods. J. Med. Virol. 82:1612–1616 [DOI] [PubMed] [Google Scholar]
- 37. López-Caballero M. E., Perez-Mateos M., Montero P., Borderias A. J. 2000. Oyster preservation by high-pressure treatment. J. Food Prot. 63:196–201 [DOI] [PubMed] [Google Scholar]
- 38. Lopman B. A., et al. 2004. Epidemiology and cost of nosocomial gastroenteritis, Avon, England, 2002-2003. Emerg. Infect. Dis. 10:1827–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lou F., Neetoo H., Chen H., Li J. 2011. Inactivation of a human norovirus surrogate by high-pressure processing: effectiveness, mechanism, and potential application in the fresh produce industry. Appl. Environ. Microbiol. 77:1862–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma L., Su Y. C. 2011. Validation of high pressure processing for inactivating Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Int. J. Food Microbiol. 144:469–474 [DOI] [PubMed] [Google Scholar]
- 41. Moe C. L., et al. 1994. Application of PCR to detect Norwalk virus in fecal specimens from outbreaks of gastroenteritis. J. Clin. Microbiol. 32:642–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morse D. L., et al. 1986. Widespread outbreaks of clam- and oyster-associated gastroenteritis. Role of Norwalk virus. N. Engl. J. Med. 314:678–681 [DOI] [PubMed] [Google Scholar]
- 43. Murchie L., et al. 2005. High pressure processing of shellfish: a review of microbiological and other quality aspects. Innov. Food Sci. Emerg. Technol. 6:257–270 [Google Scholar]
- 44. Noda M., Fukuda S., Nishio O. 2008. Statistical analysis of attack rate in norovirus foodborne outbreaks. Int. J. Food Microbiol. 122:216–220 [DOI] [PubMed] [Google Scholar]
- 45. Oliveira A. C., et al. 1999. Low temperature and pressure stability of picornaviruses: implications for virus uncoating. Biophys. J. 76:1270–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reina J., Hervas J., Ros M. J. 1994. Differential clinical characteristics among pediatric patients with gastroenteritis caused by rotavirus and adenovirus. Enferm. Infecc. Microbiol. Clin. 12:378–384[In Spanish.] [PubMed] [Google Scholar]
- 47. Richards G. P. 2006. Shellfish-associated viral disease outbreaks, p. 223–238 In Goyal S. M. (ed.), Viruses in foods. Springer, New York, NY [Google Scholar]
- 48. Sánchez G., Aznar R., Martinez A., Rodrigo D. 2011. Inactivation of human and murine norovirus by high-pressure processing. Foodborne Pathog. Dis. 8:249–253 [DOI] [PubMed] [Google Scholar]
- 49. Savini G., Casaccia C., Barile N. B., Paoletti M., Pinoni C. 2009. Norovirus in bivalve molluscs: a study of the efficacy of the depuration system. Vet. Ital. 45:535–539 [PubMed] [Google Scholar]
- 50. Scallan E., et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shieh Y., et al. 2000. Detection of Norwalk-like virus in shellfish implicated in illness. J. Infect. Dis. 181(Suppl. 2):S360–S366 [DOI] [PubMed] [Google Scholar]
- 52. Tajiri H., Kiyohara Y., Tanaka T., Etani Y., Mushiake S. 2008. Abnormal computed tomography findings among children with viral gastroenteritis and symptoms mimicking acute appendicitis. Pediatr. Emerg. Care 24:601–604 [DOI] [PubMed] [Google Scholar]
- 53. Tang Q., et al. 2010. Mechanism of inactivation of murine norovirus-1 by high pressure processing. Int. J. Food Microbiol. 137:186–189 [DOI] [PubMed] [Google Scholar]
- 54. Terio V., et al. 2010. High pressure inactivation of HAV within mussels. Food Environ. Virol. 2:83–88 [Google Scholar]
- 55. Teunis P. F., et al. 2008. Norwalk virus: how infectious is it? J. Med. Virol. 80:1468–1476 [DOI] [PubMed] [Google Scholar]
- 56. Thornton A. C., Jennings-Conklin K. S., McCormick M. I. 2004. Noroviruses: agents in outbreaks of acute gastroenteritis. Disaster Manag. Response 2:4–9 [DOI] [PubMed] [Google Scholar]
- 57. Tian P., Bates A. H., Jensen H. M., Mandrell R. E. 2006. Norovirus binds to blood group A-like antigens in oyster gastrointestinal cells. Lett. Appl. Microbiol. 43:645–651 [DOI] [PubMed] [Google Scholar]
- 58. Ueki Y., et al. 2007. Persistence of caliciviruses in artificially contaminated oysters during depuration. Appl. Environ. Microbiol. 73:5698–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.