Abstract
Pseudomonas aeruginosa and many other bacteria can utilize biogenic polyamines, including diaminopropane (DAP), putrescine (Put), cadaverine (Cad), and spermidine (Spd), as carbon and/or nitrogen sources. Transcriptome analysis in response to exogenous Put and Spd led to the identification of a list of genes encoding putative enzymes for the catabolism of polyamines. Among them, pauA1 to pauA6, pauB1 to pauB4, pauC, and pauD1 and pauD2 (polyamine utilization) encode enzymes homologous to Escherichia coli PuuABCD of the γ-glutamylation pathway in converting Put into GABA. A series of unmarked pauA mutants was constructed for growth phenotype analysis. The results revealed that it requires specific combinations of pauA knockouts to abolish utilization of different polyamines and support the importance of γ-glutamylation for polyamine catabolism in P. aeruginosa. Another finding was that the list of Spd-inducible genes overlaps almost completely with that of Put-inducible ones except the pauA3B2 operon and the bauABCD operon (β-alanine utilization). Mutation analysis led to the conclusion that pauA3B2 participate in catabolism of DAP, which is related to the aminopropyl moiety of Spd, and that bauABCD are essential for growth on β-alanine derived from DAP (or Spd) catabolism via the γ-glutamylation pathway. Measurements of the pauA3-lacZ and bauA-lacZ expression indicated that these two promoters were differentially induced by Spd, DAP, and β-alanine but showed no apparent response to Put, Cad, and GABA. Induction of the pauA3 and bauA promoters was abolished in the bauR mutant. The recombinant BauR protein was purified to demonstrate its interactions with the pauA3 and bauA regulatory regions in vitro. In summary, the present study support that the γ-glutamylation pathway for polyamine utilization is evolutionarily conserved in E. coli and Pseudomonas spp. and is further expanded in Pseudomonas to accommodate a more diverse metabolic capacity in this group of microorganisms.
INTRODUCTION
Biogenic polyamines are a group of ubiquitous polycations found in all living organisms. They are essential for cell growth and participate in a variety of physiological functions (2, 30, 31). Depending on the specific biosynthetic pathways (12, 22, 26, 29), different bacteria possess a preferential set of polyamines, which include the diamines diaminopropane (DAP), putrescine (Put), and cadaverine (Cad); the triamines spermidine (Spd) and norspermidine; and the tetramine spermine. It is generally believed that polyamines form complexes with nucleic acid-containing macromolecules through charge interactions in vivo (8, 11, 16). In vitro, excess binding of polyamines to DNA was reported to form very condensed complexes (3), which might cause difficulties in DNA unwinding during replication or transcription. Therefore, the intracellular concentrations of polyamines need to be tightly monitored to prevent adverse effects on cell growth.
When released from the cells into environments, polyamines can be recycled by many bacteria or serve as sources of carbon and nitrogen. Studies conducted by Kurihara and coworkers on Put catabolism in E. coli (17, 19) established a novel γ-glutamylation pathway (Fig. 1), which is initiated by γ-glutamylputrescine synthetase PuuA, followed by three more reactions catalyzed by PuuB, PuuC, and PuuD in sequence, to convert Put into γ-aminobutyrate (GABA). These four enzymes, as well as the Put transporter PuuP and a transcriptional regulator PuuR, are encoded in a single puu gene cluster in E. coli (17).
Fig. 1.
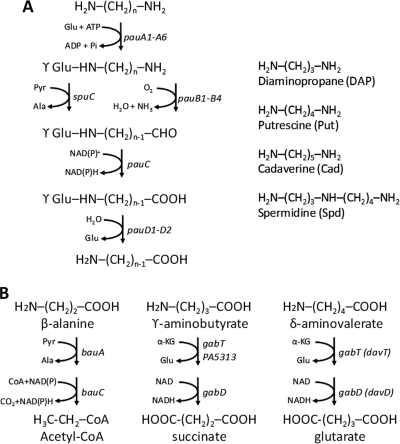
Proposed diamine catabolic pathways of P. aeruginosa. (A) Oxidative deamination of diamines by the γ-glutamylation pathway. The pau genes coding enzymes for polyamine utilization in the pathway were marked. The chemical structures of three common diamines and the triamine spermidine are shown on the right. (B) Divergent catabolic pathways for β-alanine, γ-aminobutyrate, and δ-aminovalerate derived from diaminopropane, putrescine, and cadaverine. Also shown are genes encoding enzymes for the proposed reactions.
Pseudomonas species, including P. aeruginosa, grow on several polyamine compounds as the sole source of carbon and nitrogen. However, the catabolic pathways for polyamine utilization in Pseudomonas were not clear. Characterization of the spu gene cluster identified four putative enzymes encoded by spuIABC for Spd/Put utilization and a Spd/Put uptake system by spuDEFGH (25). The impaired growth of spuA and spuB mutants on Spd and spermine suggests major roles of the SpuA and SpuB enzymes in the catabolism of these polyamines. Although the divergent spuI and spuA promoters were inducible by Put and Spd, most of the spu mutants grew normally on Put. Since the SpuI and SpuB enzymes are E. coli PuuA homologues based on sequence comparison, it is likely that the γ-glutamylation pathway plays a role on polyamine catabolism in P. aeruginosa.
Without γ-glutamylation, spermidine dehydrogenase encoded by the spdH gene was reported to catalyze oxidative cleavage of Spd into DAP and 4-aminobutyraldehyde, as well as spermine, into Spd and 3-aminopropanaldehyde (5). However, synthesis of this enzyme was not inducible by exogenous polyamines, and the spdH knockout mutant grew normally on Spd and spermine. It was concluded that P. aeruginosa PAO1 does not produce sufficient amounts of SpdH to support growth on these two polyamines.
In order to understand how P. aeruginosa responds to the presence of exogenous Put and Spd, DNA microarrays experiments had been conducted by our group to get snapshots of gene expression when the cells were exposed to these compounds in the exponential phase of growth (4, 20, 21). Analysis of these data has led us to report a connection of polyamine and antibiotic resistance through the PhoPQ two-component system, induction of the dadRAX operon for l-alanine catabolism by the putrescine-pyruvate transaminase SpuC, identification of genes that are essential for GABA utilization, and a set of redundant genes that might participate in the γ-glutamylation pathway for polyamine catabolism.
Although E. coli Puu homologues can be identified in the genome of PAO1 by sequence comparison, high redundancy of these homologues posed technical difficulties to conducting genetic analysis. In particular, there are seven puuA homologues in PAO1. In the present study, marker-less gene replacement was applied to construct a series of mutants with different combinations of puuA knockouts, and these mutants were tested for growth on polyamines. Transcriptome analysis indicated that genes inducible by exogenous Spd overlap with those by Put except six genes in two operons—the pauA3B2 operon for γ-glutamylpolyamine synthetase and oxidoreductase and the bauABCD operon for β-alanine catabolism and uptake. Further analysis led to the conclusion that pauA3B2 genes participate in the catabolism of DAP, the aminopropyl moiety of Spd, and that bauABCD genes are essential for growth on β-alanine derived from DAP (or Spd) catabolism via the γ-glutamylation pathway. A transcriptional regulator of the LysR family was identified in control of these two operons in response to β-alanine.
MATERIALS AND METHODS
Strains and growth conditions.
Bacterial strains used in the study include E. coli DH5α and Top10 (Invitrogen) and P. aeruginosa PAO1. Mutants derived from PAO1 were constructed as described below or acquired from the strain stock center at University of Washington. Luria-Bertani (LB) medium was used for strain construction with the following supplements as required: ampicillin at 100 μg/ml for E. coli and carbenicillin at 100 μg/ml, streptomycin at 500 μg/ml, and gentamicin at 100 μg/ml for P. aeruginosa. Minimal medium P was used for the growth of P. aeruginosa supplemented with specific carbon (C) and nitrogen (N) sources, as indicated (9).
Construction of knockout mutants.
For the bauRABCD locus, the flanking regions of the intended knockout gene were amplified by PCR, and restriction enzymes sites were introduced into the primers so that the PCR products possess configurations of 5′-BamHI-[left arm]-EcoRI-3′ and 5′-EcoRI-[right arm]- HindIII-3′. After restriction enzyme digestion, these two DNA fragments were ligated and cloned into the BamHI and HindIII sites of pRTP2 (24). The resulting plasmid was subjected to EcoRI digestion to insert a 1.6-kb EcoRI fragment containing the gentamicin resistance (Gmr) cassette from plasmid pGMΩ1 (28). The final plasmid construct was introduced into E. coli SM10 to serve as the donor in the biparental conjugation (7), with a spontaneous streptomycin-resistant mutant of PAO1 as recipient. After incubation at 37°C for 6 h, the transconjugants were spread and selected on LB plates supplemented with streptomycin and gentamicin. For the construction of a series of pauA mutants, the protocol for gene replacement and in vivo excision by the Flp-FRT recombination system (10) was used to generate unmarked mutants of PAO1 with deletions on multiple genes. Expected deletions in these mutants were confirmed by PCR.
Construction of PbauA::lacZ and PpauA3::lacZ fusions.
A DNA fragment of 429 bp covering the bauR-bauA intergenic region was amplified by PCR from the genomic DNA of PAO1 with the following two primers: 5′-TCTA GAG CGC AGG TTG AGT TCG CTG GAAC-3′ and 5′-TCT AGA CTC GCG GCC CTC GTC GGT CAG-3′. The PCR products were digested with XbaI restriction enzyme and cloned into the XbaI site of pQF50 (6), and the resulting plasmid pBAU1 was confirmed by nucleotide sequencing. For construction of the PpauA3::lacZ fusion plasmid pPAU3, the two primers (5′-CGC GGA TCC GCC GCT TTC CGG GCG TCT CT-3′ and 5′-CCC AAG CTT GGG GCT CTC TTG TCG GTC TTG-3′) were used to amplify a DNA fragment of 467 bp and clone it into the BamHI and HindIII sites of pQF50.
Electrophoretic mobility shift assays.
DNA fragments covering the regulatory region of bauA and pauA3 (Fig. 2) in pBAU1 and pPAU3 as described above were PCR amplified with specific pairs of oligonucleotide primers. For the binding reactions, the DNA probe (1.0 ng) was allowed to interact with different concentrations of purified BauR in a mixture of 20 μl containing 50 mM Tris-Cl (pH 7.5), 50 mM NaCl, 1 mM EDTA, 4 mM dithiothreitol, 5% (vol/vol) glycerol, negative-control DNA (1.0 ng), and 200 μg of acetylated bovine serum albumin/ml. After incubation for 20 min at room temperature, 10 μl of each reaction mixture was loaded onto a polyacrylamide gel (6%) in Tris-borate-EDTA buffer (pH 8.0). The gels were stained with SYBR green I solution (Invitrogen) for 20 min, washed twice with deionized H2O, and scanned with an imaging system (Omega UltraLum) with excitation set at 473 nm and emission set at 520 nm.
Fig. 2.
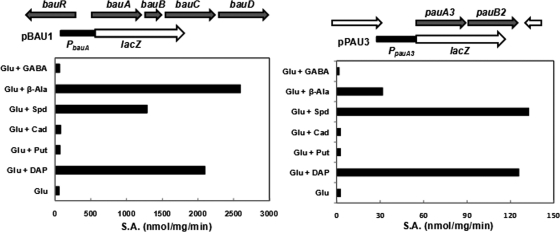
Expression profile of the bauA and pau3A promoters in P. aeruginosa PAO1. The gene organizations of the bauRABCD and pauA3B2 loci were depicted on top of each panel, followed by the schematic presentation of the respective promoter-lacZ fusions, pBAU1 and pPAU3. The expression of these two promoters from the fusion plasmids in strain PAO1 was monitored by measurements of the β-galactosidase activity. The cells were grown in glutamate (Glu) minimal medium in the absence or presence of diaminopropane (DAP), Put (Put), Cad (Cad), spermidine (Spd), β-alanine (β-Ala), and γ-aminobutyrate (GABA).
For assays with crude extracts, the wild-type strain PAO1 and its BauR mutant were grown in glutamate minimal medium till mid-log phase. The cells were broken by Aminco French press followed by centrifugation, and the protein concentrations in the collected supernatants were determined by the Bradford method (1) using bovine serum albumin as standard. The probe covering the bauA regulatory region was amplified by PCR from pBAU1 and labeled with [γ-32P]ATP and T4 DNA kinase according to standard protocols. The binding reactions were conducted under the conditions described above with the radioactive labeled probe and crude extracts.
Expression of BauR in E. coli.
The structural gene of bauR was amplified by PCR from the genomic DNA of PAO1 using the following primer pair: 5′-GCC AAA TCC CGC AAC CCG TC-3′ and 5′-GCC GAA TTC TCA CGA CAC CGC CAC CGC-3′. The resulting PCR product was digested with EcoRI and cloned into the SmaI and EcoRI sites of the expression vector pBAD-HisD (23) so that the N terminus of BauR was fused in-frame with the hexahistidine tag preceded by a ribosomal binding site and an arabinose-inducible promoter in the plasmid. The resulting plasmid, pBAUR, was introduced into E. coli Top10 (Invitrogen). For overexpression of BauR, the recombinant strain of E. coli was grown in LB medium containing ampicillin (100 μg/ml) at 37°C until the optical density at 600 nm reached 0.5, at which point 0.2% (wt/vol [final concentration]) arabinose was added to the culture for induction. Culture growth was continued for another 4 h under the same condition before harvest by centrifugation.
Purification of hexahistidine-tagged BauR.
The cell pellets of bauR overexpression were suspended in phosphate buffer A (20 mM sodium phosphate, 0.5 M NaCl, 20 mM imidazole [pH 7.4]) plus protease inhibitors cocktail (Roche) as a protease inhibitor, and the cells were ruptured by an Aminco French press at 17,000 lb/in2. Cell debris was removed by centrifugation at 20,000 × g for 30 min. The supernatant was applied to a HisTrap HP column (GE Healthcare) equilibrated with the same buffer. After the unbound proteins were washed off with equilibration buffer, His-tagged BauR was eluted at 50% of buffer B (20 mM sodium phosphate, 0.5 M NaCl, 1 M imidazole [pH 5.5]). The target fractions were pooled together and concentrated using an Aminco Ultra-15 centrifugal filter unit (molecular mass cutoff, 50 kDa; Millipore) to change the buffer to 20 mM potassium phosphate (pH 7.6) containing 1 mM EDTA.
Chemical cross-linkage of BauR (30 μg) was conducted in 10 mM HEPES buffer (pH 7.5) in a total volume of 50 μl with 10 μl of 2.3% freshly prepared solution of glutaraldehyde for 2 to 5 min at 37°C. The reaction is terminated by the addition of 5 μl of 1 M Tris-HCl (pH 8.0).
RESULTS
DNA microarrays analysis of polyamine utilization.
The puuABCD genes (putrescine utilization) of E. coli were recently reported (17) to encode four enzymes catalyzing the conversion of Put into GABA via the γ-glutamylation pathway (Fig. 1). In an attempt to explore polyamine metabolism in P. aeruginosa, we conducted DNA microarray experiments in strain PAO1 grown in glutamate minimal medium with or without the supplement Put or Spd (4, 21). Taking E. coli Puu polypeptides as templates, sequence comparison allowed us to identify Puu homologues in PAO1 genome that were found inducible by either Put or Spd from DNA microarrays experiments (Table 1). Synonymous to the nomenclature of E. coli puu genes, we thus designated identified six puuA homologues as pauA1 to pauA6 (polyamine utilization), four puuB homologues as pauB1 to pauB4, one puuC homologue as pauC, and two puuD homologues as pauD1 and pauD2 (Table 1).
Table 1.
Selected genes from DNA microarrays analysis of putrescine and spermidine catabolism
| PA IDa | Gene | Signal valueb |
Annotations | ||
|---|---|---|---|---|---|
| Glu | Glu Put | Glu Spd | |||
| PA0129* | bauD | 166 | 85 | 2,311 | Amino acid permease |
| PA0130* | bauC | 147 | 210 | 4,498 | 3-Oxopropanoate dehydrogenase |
| PA0131* | bauB | 124 | 134 | 3,783 | Cupin-2 domain; unknown function |
| PA0132* | bauA | 58 | 263 | 9,571 | β-Alanine:pyruvate transaminase |
| PA0133 | bauR | 129 | 224 | 163 | Transcriptional regulator; LysR family |
| PA0265 | gabD | 448 | 6,923 | 4,434 | Succinate semialdehyde dehydrogenase |
| PA0266 | gabT | 721 | 9,244 | 6,136 | GABA transaminase |
| PA0296 | pauA1 or spuI | 1,113 | 7,613 | 5,044 | Glutamylpolyamine synthetase |
| PA0297 | pauD1 or spuA | 275 | 1,663 | 1,926 | Peptidase C26 family; PFAM7722 |
| PA0298 | pauA2 or spuB | 582 | 2,625 | 4,244 | Glutamylpolyamine synthetase |
| PA0299 | spuC | 1,203 | 6,179 | 7,115 | Polyamine:pyruvate transaminase |
| PA0534 | pauB1 | 26 | 683 | 34 | FAD-dependent oxidoreductase |
| PA1565* | pauB2 | 58 | 42 | 2,111 | FAD-dependent oxidoreductase |
| PA1566* | pauA3 | 23 | 13 | 2,057 | Glutamylpolyamine synthetase |
| PA1742 | pauD2 | 366 | 2,335 | 2,535 | Glutamine amidotransferase class I |
| PA2040 | pauA4 | NA | NA | NA | Glutamylpolyamine synthetase |
| PA2041 | 107 | 1,245 | 971 | Amino acid permease | |
| PA2776 | pauB3 | 194 | 3,674 | 2,397 | FAD-dependent oxidoreductase |
| PA3356 | pauA5 | 591 | 3,232 | 3,240 | Glutamylpolyamine synthetase |
| PA5301 | pauR | 1,930 | 4,044 | 4,083 | Transcriptional regulator; cupin-2 domain |
| PA5309 | pauB4 | 398 | 1,363 | 1,872 | FAD-dependent oxidoreductase |
| PA5312 | pauC or kauB | 922 | 7,744 | 5,540 | Aldehyde dehydrogenase |
| PA5313 | gabT2 | 144 | 4,226 | 2,095 | Transaminase |
| PA5508 | pauA7 | 118 | 140 | 79 | Glutamylpolyamine synthetase homologue |
| PA5522 | pauA6 | 116 | 591 | 566 | Glutamylpolyamine synthetase |
The PA identification (ID) numbers were taken from the PAO1 genome annotation project (www.pseudomonas.com). Genes that are induced specifically by spermidine are marked with asterisks. These genes are divided by horizontal lines based on possible operon structures.
GeneChip raw data are mean values from two independent sets of cultures. Cells were grown in minimal medium P supplemented with 20 mM concentrations of the following supplements as indicated: Glu, glutamate; Put, putrescine; or Spd, spermidine. NA, not available.
The pauA4 gene (PA2040) was not included in the original design of GeneChip due to >96% nucleotide sequence identity to pauA1; however, induction of its downstream PA2041 gene by Put and Spd (Table 1) led us to propose that pauA4 is subjected to polyamine regulation. Gene PA5508 (pauA7) encodes the seventh and the last PuuA homologue in PAO1, and it is the only one that was not induced by Put or Spd (Table 1).
One interesting observation was that the list of Spd-inducible genes overlaps almost completely with that of Put-inducible ones, except for six genes in two putative operons, as shown in Table 1. The first operon is composed of pauA3 and pauB2 in polyamine catabolism, specifically for DAP as described in later sections. The second operon is composed of four genes, encoding two potential enzymes (PA0132 and PA0130), one small peptide of unknown function in the Cupin-2 super family (PA0131) with the conserved beta barrel domain, and an amino acid permease (PA0129). These four genes participate in utilization of β-alanine (pertinent data will be presented below), which is an intermediate compound of Spd and DAP degradation and hence were designated bauABCD (β-alanine utilization) for PA0132 to PA0129, respectively. In addition, PA0133 divergently transcribed from bauA encodes a possible transcriptional regulator of the LysR family and was designated bauR due to its function in control of the bauA promoter.
Growth phenotype analysis of pauA knockout mutants on polyamine utilization.
In comparison to the single puuA gene in E. coli, there are seven pauA homologues in PAO1. To elucidate the physiological functions of these redundant genes in polyamine catabolism, a series of pauA mutants were constructed by the unmarked gene knockout approach as described in Materials and Methods, and the resulting mutants were tested for growth as the sole source of carbon and nitrogen on three diamines of different methylene chain length (DAP, Put, and Cad) and the triamine Spd.
As shown in Table 2, a single-knockout mutation on the pauA2 gene was sufficient to block completely the growth on Spd, while the utilization of diamines remained normal. Single-knockout mutants of other six pauA genes grew normally on all tested polyamines (except pauA3 on DAP as described below). Among PauA proteins, PauA1 and PauA4 exhibited >44% sequence identity to E. coli PuuA, and indeed growth of the pauA1A4 double mutant on Put was severely retarded but not completely blocked. Combination of pauA1A2A4 in strain M3A-124 and pauA1A4A5 in strain M3A-145 were required to abolish growth on Put and Cad, respectively.
Table 2.
Growth phenotypes of pauA and pauB2 mutants on polyamines, β-alanine, and GABA
| Strain | Genotype | Growth with various supplementsa |
||||||
|---|---|---|---|---|---|---|---|---|
| Glu | Spd | Cad | Put | DAP | β-Ala | GABA | ||
| PAO1 | Wild type | + | + | + | + | + | + | + |
| M7A | ΔpauA1-pauA7 | + | − | − | − | − | + | + |
| M3A-124 | ΔpauA1A2A4 | + | − | + | − | + | + | + |
| M3A-145 | ΔpauA1A4A5 | + | − | − | +/− | + | + | + |
| M2A-14 | ΔpauA1A4 | + | + | + | +/− | + | + | + |
| M1A-1 | ΔpauA1 | + | + | + | + | + | + | + |
| M1A-2 | ΔpauA2 | + | − | + | + | + | + | + |
| M1A-3 | ΔpauA3 | + | + | + | + | +/− | + | + |
| M1A-4 | ΔpauA4 | + | + | + | + | + | + | + |
| M1B-2 | ΔpauB2 | + | + | + | + | − | + | + |
The cells were grown agar plates prepared in minimal medium with the indicated supplements as the sole source of carbon and nitrogen. Glu, glutamate; Spd, spermidine; Cad, cadaverine; Put; putrescine; DAP, diaminopropane; β-Ala, β-alanine; GABA, γ-aminobutyrate. All supplements were added to 10 mM except β-alanine to 2.5 mM. Growth on the plates was recorded in 48 h at 37°C. −, No growth after 48 h; +, growth after 24 h; +/−, growth after 48 h. All unmarked deletion mutants were derived from PAO1.
The pauA3B2 operon was found to be inducible by Spd, as revealed from transcriptome analysis (Table 1). However, deletion of either of these two genes did not exert any apparent effect on Spd utilization. Instead, growth of the pauA3 mutant on DAP was retarded, and the pauB2 mutant cannot grow on DAP completely (Table 2). These results led us to propose that the pauA3B2 operon encodes enzymes catalyzing the first two steps in DAP catabolism (Fig. 1). The presence of redundant PauA glutamylpolyamine synthetases in PAO1 likely accounts for the leaky growth phenotype of the pauA3 mutant on DAP. In comparison, the M7 mutant deleting all seven pauA genes cannot grow on any tested polyamines including DAP; however, M7, as well as other pau mutants tested in the present study, grew normally on l-glutamate, β-alanine, and GABA.
Reminiscent of Put catabolism in E. coli, these results support γ-glutamylation as the first step of polyamine catabolism in P. aeruginosa. Via the γ-glutamylation pathway, the diamines DAP, Put, and Cad are converted into β-alanine, GABA, and δ-aminovalerate, respectively (Fig. 1A). The situation for Spd was not clear since there are two terminal amino groups for γ-glutamylation and two possible internal C-N bonds to split the molecule into separate aminopropyl and aminobutyl moieties. Regardless, the hypothesis was that Spd could be split into β-alanine and GABA after γ-glutamylation.
Differential induction of the bauA and pauA3 promoters by polyamines.
The bauABCD and pauA3B2 operons were found to be inducible by Spd but not by Put based on DNA microarray analysis. To substantiate this finding, the bauA and pauA3 promoters in response to the presence of Spd and Put were monitored in PAO1 harboring pBAU1 (PbauA::lacZ) or pPAU3 (PpauA3::lacZ). As shown in Fig. 2, both promoters were specifically induced by exogenous Spd but not by Put. We also tested the potential effects of DAP and Cad on these promoters. Interestingly, DAP exerted an induction effect stronger than Spd did on the bauA promoter, whereas induction of the pauA3 promoter by DAP was comparable to that by Spd. In comparison, Cad showed no effect on either promoter. Since Spd is composed of two methylene chains of three and four carbons (Fig. 1), these results support the view that the bauABCD and pauA3B2 operons are related to DAP catabolism specifically.
β-Alanine but not GABA induces the bauA promoter.
Polyamine catabolism is proposed to be initiated by γ-glutamylation of one terminal amino group, as first revealed in Put utilization of E. coli. After converting another terminal amino group into carboxyl group, the intermediate compound was catalyzed by hydrolase to release and recycle glutamate. In this scheme, GABA and β-alanine were generated from Put and DAP, respectively. Although the detailed pathway was still not clear, GABA and β-alanine were likely the intermediate compounds of Spd after splitting its aminobutyl and aminopropyl moieties, respectively.
The bauA and bauC genes have been predicted (5) to encode an β-alanine:pyruvate transaminase and a malonate semialdehyde dehydrogenase to convert β-alanine into acetyl coenzyme A (acetyl-CoA) (Fig. 1), and the proposed enzymatic activity of BauA has been reported (13). Along this line, we hypothesized that induction of the bauABCD operon by Spd and DAP was due to the common catabolic intermediate β-alanine and that GABA, the intermediate compound of Put catabolism, should have no effect on this operon. The activities of β-galactosidase expressed from the PbauA::lacZ fusion pBAU1 was measured in PAO1 grown in the presence or absence of GABA or β-alanine, and the results shown in Fig. 2 support this hypothesis of bauA induction by β-alanine.
Mutations in the bauRABCD genes affect β-alanine, DAP, and Spd utilization.
A series of bau::GmΩ knockout mutants was constructed, and the growth phenotypes of these mutants on polyamines, β-alanine, and GABA as the sole source of carbon and nitrogen are shown in Table 3. All bau mutants grew normally on Put, Cad, and GABA, but growth on β-alanine was completely abolished when any of the bauRABC genes were deleted and was only partially retarded in the bauD mutant. These bauRABC mutants also exhibited different extents of growth handicap on Spd and DAP. Similar growth phenotypes were also observed in another set of mutants by transposon insertion from the University of Washington Genome Center (14). These results were in agreement with the proposed physiological functions of BauA and BauC in DAP and β-alanine catabolism and of BauD in β-alanine transport (Fig. 1) and provide a link of BauR and BauB to β-alanine catabolism.
Table 3.
Growth phenotypes of bauRABCD mutants on polyamines, β-alanine, and GABA
| Strain | Genotype | Growth with various supplementsa |
||||||
|---|---|---|---|---|---|---|---|---|
| Glu | Spd | Cad | Put | DAP | β-Ala | GABA | ||
| PAO1 | Wild type | +++ | +++ | +++ | +++ | + | +++ | +++ |
| ΔbauD::GmΩ | +++ | +++ | +++ | +++ | + | ++ | +++ | |
| ΔbauC::GmΩ | +++ | ++ | +++ | +++ | − | − | +++ | |
| ΔbauB::GmΩ | +++ | + | +++ | +++ | − | − | +++ | |
| ΔbauA::GmΩ | +++ | + | +++ | +++ | − | − | +++ | |
| ΔbauR::GmΩ | +++ | + | +++ | +++ | − | − | +++ | |
| MPAO1 | Wild type | +++ | +++ | ND | +++ | + | +++ | +++ |
| bauD::IS | +++ | +++ | ND | +++ | + | ++ | +++ | |
| bauC::IS | +++ | + | ND | +++ | − | − | +++ | |
| bauA::IS | +++ | ++ | ND | +++ | − | − | +++ | |
| bauR::IS | +++ | ++ | ND | +++ | − | − | +++ | |
The cells were grown in minimal medium with the indicated supplements as the sole source of carbon and nitrogen. Glu, glutamate; Spd, spermidine; Cad, cadaverine; Put, putrescine; DAP, 1, 3 diaminopropane; β-Ala, β-alanine; GABA, γ-aminobutyrate. All supplements were added to 10 mM except β-alanine to 2.5 mM. The optical density at 600 nm (OD600) of each culture (2 ml in 15-ml culture tubes) was recorded after growth for 48 h at 37°C. −, OD600 < 0.1; +, 0.1 < OD600 < 0.3; ++, 0.3 < OD600 < 0.8; +++, OD600 > 0.8; ND, not determined. Deletion mutants with a gentamicin-resistant cassette (GmΩ) were derived from PAO1, and transposon insertion (IS) mutants were derived from MPAO1 and obtained from the stock center at University of Washington.
Effects of bauR and bauA mutations on the bauA promoter.
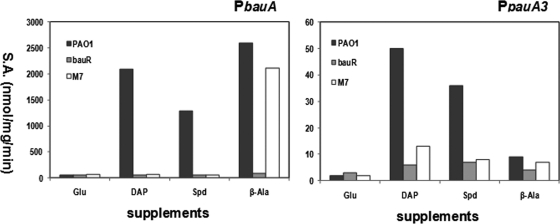
Since the bauR mutant cannot grow on β-alanine, we proposed that the expression of the bauABCD operon is controlled by BauR, a transcriptional regulator of the LysR family. As shown in Fig. 3, induction of the bauA promoter by β-alanine, DAP, and Spd was completely abolished in the bauR mutant, supporting BauR as transcriptional activator of the bauABCD operon.
Fig. 3.
Effects of bauR and γ-glutamylation on expression profiles of the bauA and pauA3 promoters. The specific activities of β-galactosidase expressed from pBAU1 for the bauA promoter or pPAU3 for the pauA3 promoter were measured from the host strains PAO1 (□), the bauR mutant (▩), and the M7 mutant (■) devoid of all seven pauA genes. The cells were grown in glutamate (Glu) minimal medium in the absence of presence of indicated supplements: diaminopropane (DAP), spermidine (Spd), and β-alanine (β-Ala).
The bauA promoter activity in response to exogenous β-alanine was also measured in the bauA mutant harboring pBAU1. Growth handicap of the bauA mutant on β-alanine (Table 3) indicates that BauA is the major, if not the only, source of β-alanine:pyruvate transaminase, and therefore β-alanine was expected to accumulate without degradation in the bauA mutant. The bauA promoter was still inducible by exogenous β-alanine (data not shown), supporting β-alanine as the authentic signal compound for the bauA promoter activation.
γ-Glutamylation is essential for polyamine-dependent induction of the bauA and pauA3 promoters.
To convert into β-alanine and to induce the bauABCD operon, DAP or Spd needs to be glutamylated by γ-glutamylpolyamine synthetase as the first step in the proposed catabolic pathway. Therefore, one would expect abolishment of induction when there is no polyamine glutamylation and hence no β-alanine from catabolism. To test this hypothesis, the expression profile of the PbauA::lacZ fusion was measured in strain M7, which has deletions of seven pauA genes. Consistent with this hypothesis, the bauA promoter was not subjected to induction by exogenous DAP or Spd in M7, while the induction effect of exogenous β-alanine remained (Fig. 3).
Similar experiments were also conducted in M7 harboring the PpauA3::lacZ fusion, and to our surprise polyamine-dependent induction of this promoter was significantly diminished in M7. These results prompted us to test whether BauR also plays a role in regulation of the pauA3 promoter. As shown in Fig. 3, the pauA3 promoter cannot be induced by DAP or Spd in the bauR mutant, supporting the pivotal role of BauR on pauA3B2 expression.
Binding of BauR to the bauA and pauA3 regulatory regions.
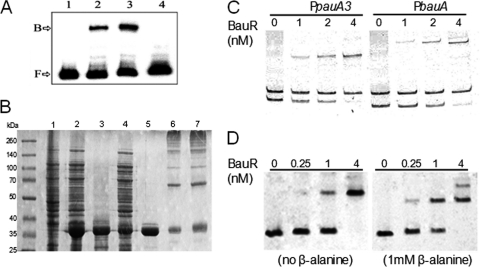
Cell-free crude extracts were prepared from PAO1 and its bauR deletion mutant to conduct electrophoretic mobility shift assays with a 32P-labeled probe covering the bauR-bauA intergenic region. As shown in Fig. 4 A, one distinct nucleoprotein complex with slower mobility can be detected from the crude extract of PAO1 but not the bauR mutant. This result supports possible binding of BauR to the divergent bauR-bauA promoter region.
Fig. 4.
Purification of BauR and demonstration of BauR-DNA interactions. (A) The presence of a nucleoprotein complex with the 32P-labeled bauA promoter region as a probe was demonstrated by the electromobility shift assays. Lane 1, probe only; lane 2, PAO1 crude extract (4 μg); lane 3, PAO1 crude extract (9 μg); lane 4, crude extract of the bauR mutant (9 μg). (B) SDS-PAGE of protein fractions from BauR purification from E. coli Top10 harboring pBAUR. Lane 1, before arabinose induction; lane 2, after arabinose induction; lanes 3 and 4, pellet and supernatant, respectively, of the protein sample in lane 2 after 20,000 × g centrifugation; lane 5, eluted BauR from the nickel column; lanes 6 and 7, the purified BauR subjected to cross-linkage by glutaraldehyde without or with 1 mM β-alanine, respectively. (C) Electrophoretic mobility shift assays with the purified BauR. Two DNA probes covering the bauA or pauA2 regulatory regions were used in the experiments in the presence of a nonspecific DNA fragment as negative control. (D) Binding of BauR to the bauA probe was analyzed in the presence or absence of 1 mM β-alanine in the reaction mixture and the running buffer.
The recombinant BauR protein with a hexahistidine tag attached to its amino terminus (H-BauR) was expressed and purified from E. coli Top10. Although the majority of overexpressed H-BauR formed insoluble inclusion bodies, we have managed to purify this recombinant BauR protein from the remaining soluble fraction by column chromatography as described in Materials and Methods. To understand possible subunit configurations, BauR (0.5 mg/ml) was subjected to chemical cross-linkage with glutaraldehyde and monitored by SDS-PAGE. Additional polypeptides with higher molecular weights were detected (Fig. 4B), corresponding to the estimated sizes of dimers, trimers, and tetramers of BauR, plus other higher-order oligomers that were not able to resolve clearly on the gel. The inclusion of β-alanine (1 mM) in the reaction did not show any apparent effect on the distribution of these cross-linked products.
The purified H-BauR was used to demonstrate its specific interactions with the bauA and pauA3 regulatory regions by electrophoretic mobility shift assays, as shown in Fig. 4C. Consistent with the conclusion from the genetic studies as described above, BauR formed specific nucleoprotein complexes with these DNA fragments, supporting BauR as a transcriptional regulator of the bauA and pauA3 promoters. Although β-alanine was not required to demonstrate the DNA-binding activity of BauR, a marginal 2- to 3-fold increase on the estimated affinity of BauR to the bauA probe was observed by the presence of β-alanine in the reaction (Fig. 4D), as revealed from a plot of the percentage of free probe against BauR concentrations.
DISCUSSION
Redundancy and complexity of polyamine catabolism.
In this study we were able to demonstrate that the M7 mutant strain of P. aeruginosa PAO1 devoid of seven E. coli PuuA homologues cannot grow on any tested polyamines as the sole source of carbon or nitrogen. In addition, it requires specific combinations of pauA knockouts to abolish utilization of different polyamines. These lines of genetic evidence support the functional redundancy of PauA enzymes and the importance of γ-glutamylation for polyamine catabolism in P. aeruginosa.
The complexity of polyamine catabolism is not limited to the presence of multiple pauA genes. After γ-glutamylation as the first step in the proposed pathway, the free amino group distal to the γ-glutamylation site is subjected to deamination, which can be accomplished either by oxidation or transamination (Fig. 1). There are four pauB genes for oxidative deamination (Table 1), which is supposed to operate under aerobic conditions. We have previously reported that mutants of spuC encoding a putrescine:alanine transaminase exhibited growth handicap on Put (25), and hence proposed the existence of a traditional transamination pathway for Put utilization (4). Since operation of the proposed traditional transamination pathway was excluded in the present study, it is likely that SpuC may participate in transamination of γ-glutamylated Put or other diamines regardless of the oxygen status.
After deamination, the aldehyde compounds were oxidized to carboxylates by PauC, an NAD(P)-dependent dehydrogenase of broad substrate specificity. The pauC gene was initially identified as the kauB gene for 4-guanidinobutyraldehyde dehydrogenase in ketoarginine utilization (15). The kauB mutant also cannot grow on Put, DAP, Spd, and spermine (5). Therefore, PauC (KauB) is the only entity for the proposed reaction without other redundant enzymes, even although many PauC homologues with very high sequence similarities exist in P. aeruginosa by BLAST search.
Two putative hydrolases were proposed (PauD1 and PauD2) to release and recycle glutamate in the last step of the γ-glutamylation pathway. The E. coli PuuD protein possesses the γ-glutamyl GABA hydrolase activity that is essential for Put utilization (18). PauD1 is the only E. coli PuuD homologue in P. aeruginosa PAO1 by sequence comparison (44% identity). However, we reported previously that a lesion in pauD1 (spuA) causes a significant growth defect on Spd but not Put. Both PauD1 and PauD2 belong to the glutamine amidotransferase super family even though they bear no significant sequence homology. On the other hand, PauD1 was predicted by PFAM as peptidase C26 (PF07722) that has γ-glutamyl hydrolase activity. Among Put-inducible genes (4), PA2268 also encodes a polypeptide of the same predicted peptidase C26 function. Further studies are in progress to elucidate the biochemical and physiological functions of these putative enzymes.
Cleavage of Spd.
Although the data represented here clearly demonstrate the importance of γ-glutamylation in Spd utilization, how Spd is cleaved into aminopropyl and aminobutyl units remains unknown. Genes that were specifically induced by Spd but not Put in DNA microarrays analysis and characterized in the present study are in fact designated for catabolism of DAP and β-alanine, which are two compounds related to the aminopropyl unit of Spd. It is possible that some PauB enzymes might be able to perform the oxidative cleavage of Spd in question.
Although the exact pathway for Spd degradation through γ-glutamylation was not clear, the fact that the pauA3 and pauB2 mutants exhibited growth defect on DAP but grew normally on Spd would strongly suggest that Spd catabolism does not generate DAP or glutamyl-DAP per se. Instead, we predicted that the cleaved products of Spd might be either glutamyl 3-aminopropan-aldehyde plus GABA or 3-aminopropan-aldehyde plus glutamyl Put.
Amino carboxylates derived from diamines.
DAP, Put, and Cad are three natural diamines found in living organisms. Through the γ-glutamylation pathway, they are converted into β-alanine, GABA, and AMV, respectively. Different routes are taken to further degrade these amino carboxylates into carbon and nitrogen sources in Pseudomonas. BauA and BauC catalyze two contiguous reactions to convert β-alanine into acetyl-CoA, and mutations in either of the cognate coding genes result in complete abolishment of growth on β-alanine. For GABA conversion into succinate, it involves two transaminases (GabT and PA5313) and one dehydrogenase (GabD) that are inducible by Put (4) in P. aeruginosa. Conversion of AMV to glutarate in P. putida requires DavT and DavD (27), which are orthologues of GabT and GabD of P. aeruginosa.
Regulation of polyamine catabolism.
In E. coli, expression of the puu genes is subjected to regulation by the PuuR repressor. Due to the redundancy of pau genes, one would expect a more complicated regulatory system in P. aeruginosa. Based on sequence analysis and the physical proximity to several polyamine-inducible genes, there are at least three transcriptional regulators that likely participate in control of polyamine catabolism—PA0535, PA2267, and PA5301. In particular, PA5301 encodes a transcriptional regulator that exhibits 44% sequence identity to E. coli PuuR (17). Elucidating the potential functions of these regulators in control of pau gene expression is currently in progress.
The BauR protein was identified in the present study as a transcriptional activator for the bauABCD and pauA3B2 operons in β-alanine and DAP catabolism, respectively. Induction of the pauA3 promoter by DAP was abolished in the M7 mutant blocking all seven γ-glutamylpolyamine synthetases, suggesting that the signal molecule is the degradation product of DAP. The bauR gene is divergent from the bauABCD operon, and induction of the bauA promoter by β-alanine requires a functional BauR. Since β-alanine is considered as product of DAP degradation, these results suggest the presence of an unusual regulatory circuit in which an intermediate compound serves as a signal molecule for the induction of upstream and downstream genes of a catabolic pathway.
In summary, the results presented here show that the γ-glutamylation pathway for polyamine utilization is evolutionarily conserved in E. coli and Pseudomonas and is further expanded in Pseudomonas to accommodate the more diverse metabolic capacity in this group of microorganisms.
ACKNOWLEDGMENTS
This study was supported in part by National Science Foundation grant NSF0950217 to C.-D.L. and by a Molecular Basis of Disease Program fellowship from Georgia State University to X.Y.
Footnotes
Published ahead of print on 27 May 2011.
REFERENCES
- 1. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 2. Casero R. A., Pegg A. E. 2009. Polyamine catabolism and disease. Biochem. J. 421:323–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Childs A. C., Mehta D. J., Gerner E. W. 2003. Polyamine-dependent gene expression. Cell. Mol. Life Sci. 60:1394–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chou H. T., Kwon D. H., Hegazy M., Lu C. D. 2008. Transcriptome analysis of agmatine and putrescine catabolism in Pseudomonas aeruginosa PAO1. J. Bacteriol. 190:1966–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dasu V. V., Nakada Y., Ohnishi-Kameyama M., Kimura K., Itoh Y. 2006. Characterization and a role of Pseudomonas aeruginosa spermidine dehydrogenase in polyamine catabolism. Microbiology 152:2265–2272 [DOI] [PubMed] [Google Scholar]
- 6. Farinha M. A., Kropinski A. M. 1990. Construction of broad-host-range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 172:3496–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gambello M. J., Iglewski B. H. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 173:3000–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ha H. C., et al. 1998. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. U. S. A. 95:11140–11145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haas D., Holloway B. W., Schambock A., Leisinger T. 1977. The genetic organization of arginine biosynthesis in Pseudomonas aeruginosa. Mol. Gen. Genet. 154:7–22 [DOI] [PubMed] [Google Scholar]
- 10. Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 11. Huang S. C., Panagiotidis C. A., Canellakis E. S. 1990. Transcriptional effects of polyamines on ribosomal proteins and on polyamine-synthesizing enzymes in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 87:3464–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ikai H., Yamamoto S. 1997. Identification and analysis of a gene encoding l-2,4-diaminobutyrate:2-ketoglutarate 4-aminotransferase involved in the 1,3-diaminopropane production pathway in Acinetobacter baumannii. J. Bacteriol. 179:5118–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ingram C. U., et al. 2007. One-pot synthesis of amino-alcohols using a de-novo transketolase and beta-alanine: pyruvate transaminase pathway in Escherichia coli. Biotechnol. Bioeng. 96:559–569 [DOI] [PubMed] [Google Scholar]
- 14. Jacobs M. A., et al. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 100:14339–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jann A., Matsumoto H., Haas D. 1988. The fourth arginine catabolic pathway of Pseudomonas aeruginosa. J. Gen. Microbiol. 134:1043–1053 [DOI] [PubMed] [Google Scholar]
- 16. Khan A. U., Di Mascio P., Medeiros M. H., Wilson T. 1992. Spermine and spermidine protection of plasmid DNA against single-strand breaks induced by singlet oxygen. Proc. Natl. Acad. Sci. U. S. A. 89:11428–11430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kurihara S., et al. 2005. A novel putrescine utilization pathway involves gamma-glutamylated intermediates of Escherichia coli K-12. J. Biol. Chem. 280:4602–4608 [DOI] [PubMed] [Google Scholar]
- 18. Kurihara S., Oda S., Kumagai H., Suzuki H. 2006. Gamma-glutamyl-gamma-aminobutyrate hydrolase in the putrescine utilization pathway of Escherichia coli K-12. FEMS Microbiol. Lett. 256:318–323 [DOI] [PubMed] [Google Scholar]
- 19. Kurihara S., et al. 2008. γ-Glutamylputrescine synthetase in the putrescine utilization pathway of Escherichia coli K-12. J. Biol. Chem. 283:19981–19990 [DOI] [PubMed] [Google Scholar]
- 20. Kwon D. H., Lu C. D. 2007. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob. Agents Chemother. 51:2070–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwon D. H., Lu C. D. 2006. Polyamines induce resistance to cationic peptide, aminoglycoside, and quinolone antibiotics in Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 50:1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee J., et al. 2009. An alternative polyamine biosynthetic pathway is widespread in bacteria and essential for biofilm formation in Vibrio cholerae. J. Biol. Chem. 284:9899–9907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li C., Lu C. D. 2009. Arginine racemization by coupled catabolic and anabolic dehydrogenases. Proc. Natl. Acad. Sci. U. S. A. 106:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li C., Lu C. D. 2009. Unconventional integration of the bla gene from plasmid pIT2 during ISlacZ/hah transposon mutagenesis in Pseudomonas aeruginosa PAO1. Curr. Microbiol. 58:472–477 [DOI] [PubMed] [Google Scholar]
- 25. Lu C. D., Itoh Y., Nakada Y., Jiang Y. 2002. Functional analysis and regulation of the divergent spuABCDEFGH-spuI operons for polyamine uptake and utilization in Pseudomonas aeruginosa PAO1. J. Bacteriol. 184:3765–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakada Y., Jiang Y., Nishijyo T., Itoh Y., Lu C. D. 2001. Molecular characterization and regulation of the aguBA operon, responsible for agmatine utilization in Pseudomonas aeruginosa PAO1. J. Bacteriol. 183:6517–6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Revelles O., Espinosa-Urgel M., Molin S., Ramos J. L. 2004. The davDT operon of Pseudomonas putida, involved in lysine catabolism, is induced in response to the pathway intermediate delta-aminovaleric acid. J. Bacteriol. 186:3439–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schweizer H. D. 1993. Small broad-host-range gentamicin resistance gene cassettes for site-specific insertion and deletion mutagenesis. Biotechniques 15:831–834 [PubMed] [Google Scholar]
- 29. Tabor C. W., Tabor H. 1985. Polyamines in microorganisms. Microbiol. Rev. 49:81–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walters D. R. 2003. Polyamines and plant disease. Phytochemistry 64:97–107 [DOI] [PubMed] [Google Scholar]
- 31. Wortham B. W., Patel C. N., Oliveira M. A. 2007. Polyamines in bacteria: pleiotropic effects yet specific mechanisms. Adv. Exp. Med. Biol. 603:106–115 [DOI] [PubMed] [Google Scholar]