Abstract
The well-known large catabolic potential of rhodococci is greatly facilitated by an impressive gene multiplicity. This study reports on the multiplicity of kshA, encoding the oxygenase component of 3-ketosteroid 9α-hydroxylase, a key enzyme in steroid catabolism. Five kshA homologues (kshA1 to kshA5) were previously identified in Rhodococcus rhodochrous DSM43269. These KshADSM43269 homologues are distributed over several phylogenetic groups. The involvement of these KshA homologues in the catabolism of different classes of steroids, i.e., sterols, pregnanes, androstenes, and bile acids, was investigated. Enzyme activity assays showed that all KSH enzymes with KshADSM43269 homologues are C-9 α-hydroxylases acting on a wide range of 3-ketosteroids, but not on 3-hydroxysteroids. KshA5 appeared to be the most versatile enzyme, with the broadest substrate range but without a clear substrate preference. In contrast, KshA1 was found to be dedicated to cholic acid catabolism. Transcriptional analysis and functional complementation studies revealed that kshA5 supported growth on any of the different classes of steroids tested, consistent with its broad expression induction pattern. The presence of multiple kshA genes in the R. rhodochrous DSM43269 genome, each displaying unique steroid induction patterns and substrate ranges, appears to facilitate a dynamic and fine-tuned steroid catabolism, with C-9 α-hydroxylation occurring at different levels during microbial steroid degradation.
INTRODUCTION
Rhodoccoci are capable of degrading a wide range of organic compounds (15, 32). This strong catabolic potential is encoded by an extremely large genome, of >9.7 Mb in the case of Rhodococcus jostii RHA1, which also carries numerous gene homologues for various enzyme classes (20). Multiple steroid catabolic gene clusters, for example, have been identified in R. jostii RHA1 (19, 20, 33). In particular, several homologous genes encoding key enzymes involved in steroid ring opening have been identified, i.e., 3-ketosteroid 9α-hydroxylase (KSH), encoded by kshA and kshB, and 3-ketosteroid Δ1-dehydrogenase (KSTD), encoded by kstD (13, 33). Hydroxylation of steroid substrates at the C-9 position, together with dehydrogenation of the A-ring performed by KSTD, leads to opening of the steroid polycyclic ring structure and the formation of 3-hydroxy-9,10-secoandrost-1,3,5(10)-triene-9,17-dione (3-HSA) (7, 31). Knowledge of steroid catabolic enzymes is limited, despite the fact that sterol-degrading rhodococci and mycobacteria are of great industrial and pharmaceutical interest (6, 9, 12, 17, 25, 32). In recent years, interest in steroid catabolic enzymes has gained momentum, following the discovery of cholesterol catabolic gene clusters in R. jostii RHA1 and in the human pathogen Mycobacterium tuberculosis H37Rv (33). Interestingly, kshA and kshB have been identified as essential factors in the pathogenesis of M. tuberculosis H37Rv (10).
KSH is a two-component enzyme system, consisting of a terminal oxygenase, KshA, and a ferredoxin reductase, KshB. The kshA and kshB genes, encoding KshA and KshB, respectively, were first identified in Rhodococcus erythropolis SQ1, a strain possessing at least 3 kshA homologues (31, 34). A crystal structure of KshA of M. tuberculosis H37Rv was recently elucidated and revealed that KshA of H37Rv likely functions as a trimer (α3) (4). KshB from Rhodococcus rhodochrous DSM43269 was shown to contain FAD as flavin cofactor and a plant-type Fe2S2 cluster (4, 24).
We previously reported the cloning, heterologous expression, and characterization of KshA and KshB from R. rhodochrous DSM43269 (IFO3338) (1) and showed that this KSH enzyme displayed activity toward a subtle range of saturated and unsaturated 3-ketosteroid substrates (24). We have also performed cloning of an additional four kshA homologous genes from R. rhodochrous DSM43269 and the construction of a 5-fold kshA null mutant strain, R. rhodochrous RG32, blocked in steroid B-ring opening (35a). To investigate whether each of these five homologous KshA proteins is capable of catalyzing steroid 9α-hydroxylation and to analyze their physiological roles in steroid catabolism, we performed functional complementation experiments with a kshA null mutant strain, RG32 of R. rhodochrous DSM43269, which is blocked in 4-androstene-3,17-dione (AD) degradation. Moreover, kshA transcriptional analyses of wild-type DSM43269 cells induced with a range of steroid substrates were performed. Finally, a biochemical characterization of heterologously expressed and purified KSH enzymes was undertaken to determine the substrate range of the different KshA proteins involved.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli cloning strain DH5α (Stratagene) and expression strains E. coli BL21(DE3) (Invitrogen) and E. coli C41(DE3) (21) were grown in Luria-Bertani (LB) at 37°C, 200 rpm. Wild-type R. rhodochrous DSM43269 was obtained from the DSMZ culture collection. The construction of the 5-fold kshA mutant strain R. rhodochrous RG32 is described elsewhere (35a). R. rhodochrous strains were grown in LB broth or mineral medium at 30°C and 200 rpm. Mineral medium contained K2HPO4 (4.65 g liter−1), NaH2PO4·H2O (1.5 g liter−1), NH4Cl (3 g liter−1), MgSO4·7H2O (1 g liter−1), and Vishniac stock solution (1 ml liter−1). Vishniac stock solution was prepared as follows (modified from the methods described in reference 35): EDTA (10 g liter−1) and ZnSO4·7H2O (4.4 g liter−1) were dissolved in distilled water (pH 8.0, adjusted by using 2 M KOH). Then, CaCl2·2H2O (1.47 g liter−1), MnCl2·7H2O (1 g liter−1), FeSO·7H2O (1 g liter−1), (NH4)6 Mo7O24·4H2O (0.22 g liter−1), CuSO4·5H2O (0.315 g liter−1), and CoCl2·6H2O (0.32 g liter−1) were added in that order at pH 6 and finally stored at pH 4.0. When appropriate, ampicillin, apramycin, or kanamycin was added to a final concentration of 100, 50, or 25 μg ml−1, respectively.
Steroids.
AD, 1,4-androstadiene-3,17-dione (ADD), 19-nor-4-androstene-3,17-dione (nordion), 3α-hydroxy-5α-pregnane-20-one, 5α-androstan-17β-ol- 3-one (stanolon), 3β-hydroxy-5α-androstane-17-one, and 9α-hydroxy-4-androstene-3,17-dione were obtained from Schering-Plough (Oss, Netherlands). 17β-Hydroxy-4-androstene-3-one (testosterone), 11β-hydrocortisone, 3α-,7α-, 12α-trihydroxy-5β-cholan-24-ioc acid (cholic acid), 4-cholestene-3-one (cholestenone), and 5-cholestene-3β-ol (cholesterol) were obtained from Sigma-Aldrich, and 4-pregnene-3,20-dione (progesterone) was obtained from ICN Biomedicals Inc. 1-(5α)-Androstene-3,17-dione, 5α-androstane-3,17-dione, 5β-androstane-3,17-dione, 5α-androstane-17-one, and 23,24-bis-nor-cholesta-4-ene-22-oic acid (4-BNC) were obtained from Steraloids. 23,24-bis-nor-cholesta-1,4-diene-22-oic acid (1,4-BNC) was obtained at near-100% yield using 4-BNC as a substrate for the KSTD1 enzyme of R. erythropolis SQ1 (13, 30). The steroids were extracted from the assay mixture with ethyl acetate and dried by evaporation with N2.
Phylogenetic tree construction.
MEGA version 4.0.2 was used for the construction of a phylogenetic tree (14). The amino acid sequences used to construct the phylogenetic tree were obtained from GenBank databases (accession numbers are shown in parentheses) as follows: HMPREF0063_12465 (ZP_07718021) of Aeromicrobium marinum DSM 15272, BamIOP4010DRAFT_2656 (ZP_02890593) of Burkholderia ambifaria IOP40-10, BCAM1620 (YP_002234232) of Burkholderia cenocepacia J2315, Bcep18194_B1448 (YP_372206) of Burkholderia sp. 383, Gbro_0919 (YP_003272124) of Gordonia bronchialis DSM 43247, H16_B0672 (YP_728834) of Ralstonia eutropha H16, MAB_0080c (YP_001700834), MAB_3627c (YP_001704355), and MAB_4173 (YP_001704900) of Mycobacterium abscessus ATCC 19977, MAV_0633 (YP_879913) and MAV_3037 (YP_882223) of Mycobacterium avium 104, Mb3556 (NP_857195) of Mycobacterium bovis AF2122/97, Mflv_1533 (YP_001132803), Mflv_1546 (YP_001132816), and Mflv_4566 (YP_001135822) of Mycobacterium gilvum PYR-GCK, MintA_010100002014 (ZP_05223666) and MintA_010100021954 (ZP_05227611) of Mycobacterium intracellulare ATCC 13950, Mjls_0287 (YP_001068591) of Mycobacterium sp. JLS, MkanA1_010100023803 (ZP_04751020) of Mycobacterium kansasii ATCC 12478, MMAR_5015 (YP_001853274) of Mycobacterium marinum M, Mmcs_0296 (YP_637473) and Mmcs_4640 (YP_641800) of Mycobacterium sp. MCS, MSMEG_2870 (YP_887190) and MSMEG_5925 (YP_890151) of Mycobacterium smegmatis strain MC2 155, KshA RV3526 (NP_218043) of Mycobacterium tuberculosis H37Rv, MUL_4089 (YP_907606) of Mycobacterium ulcerans Agy99, Mvan_2869 (YP_953681), Mvan_5211 (YP_955988), and Mvan_5225 (YP_956002) of Mycobacterium vanbaalenii PYR-1, Nfa33560 (YP_119567), Nfa4750 (YP_116681), Nfa22480 (YP_118459), and Nfa33740 (YP_119585) of Nocardia farcinica IFM 10152, Noca_2518 (YP_923709) of Nocardioides sp. JS614, RALTA_B0514 (YP_001796945) of Cupriavidus taiwanensis, RER_07540 (YP_002764201), RER_09150 (YP_002764362), RER_13800 (YP_002764827), and RER_51130 (YP_002768560) of Rhodococcus erythropolis PR4, Reut_A1576 (YP_295786) of Ralstonia eutropha JMP134, REQ_06790 (YP_004005481), REQ_08980 (YP_004005694), REQ_15470 (YP_004006310), REQ_40110 (YP_004008669), REQ_42740 (YP_004008918), REQ_43730 (YP_004009014), and REQ_45190 (YP_004009156) of R. equi 103S, RHOER0001-0749 (ZP_04388293), RHOER0001_0834 (ZP_04388135), RHOER0001_2900 (ZP_04382763), and RHOER0001_4808 (ZP_04386887) of Rhodococcus erythropolis SK121, KshA1 (AAL96829) and KshA2 (ACD11366) of Rhodococcus erythropolis SQ1, KshA Ro04538 (YP_704482), KshA2 (Ro02490; YP_702453), KshA3 (ro05811; YP_705746), and KshA4 (ro09003; YP_708205) of Rhodococcus jostii RHA1, ROP_22170 (YP_002779409), ROP_44530 (YP_002781645), and ROP_58730 (YP_002783065) of Rhodococcus opacus B4, KshA1 (ADY18310), KshA2 (ADY18316), KshA3 (ADY18318), KshA4 (ADY18323), and KshA5 (ADY18328) of Rhodococcus rhodochrous DSM43269, Sare_2880 (YP_001537697) of Salinispora arenicola CNS-205, SSMG_02043 (ZP_07278003), and SSMG_04623 (ZP_07280583) of Streptomyces sp. AA4, Strop_2672 (YP_001159494) of Salinispora tropica CNB-440, Tcur_3509 (YP_003301083) of Thermomonospora curvata DSM 43183, and Tpau_3849 (YP_003648763) of Tsukamurella paurometabola DSM 20162.
Electrotransformation of R. rhodochrous strain RG32 with pRRE1-derived plasmids for functional complementation with kshA genes.
Competent cells of strain RG32 were prepared by growing cell cultures in 250 ml LB broth to an optical density at 600 nm (OD600) of 0.2 to 1.0. The cultures were incubated on ice for 1.5 h. All further steps were done at 4°C unless stated otherwise. Cells were pelleted by centrifugation for 10 min at 5,000 × g. The cells were washed twice with MilliQ (125 ml), centrifuged (10 min, 5,000 × g), washed in 10% glycerol (25 ml), and centrifuged for 10 min at 2,000 × g. Cells were resuspended in 10% glycerol (1 ml) and stored in 100-μl aliquots at −80°C until use. For electrotransformation, cells were thawed on ice, and 1 μl (0.1 to 0.4 μg) of plasmid DNA (pA1rho9, pA2rho5, pA3rho5, or pA4rho15 [see Table S1 in the supplemental material]) was added to 100 μl of competent RG32 cells, mixed, and incubated for 1 min on ice. The cells were pulsed at 2.5 kV, 25 μF, and 1,000 Ω (field strength, 12.5 kV cm−1). LB broth (1 ml) was added, and the cells were incubated for 4.5 h at 30°C and 200 rpm prior to plating on selective LB agar supplemented with apramycin.
Growth on different steroids as a sole carbon and energy source.
LB-grown precultures of R. rhodochrous strains were used to inoculate (1:100) 25 ml of mineral liquid medium supplemented with representatives of different classes of steroids, i.e., androstenes, sterols, pregnanes, and bile acids, using 0.5 g liter−1 steroid (AD, cholesterol, progesterone, and cholic acid). Cholesterol was added as a solid to the medium, autoclaved, and dispersed by sonication. The other steroids were dissolved in dimethyl sulfoxide as 25-mg ml−1 stock solutions and then added to the medium. Growth was followed for several days based on OD600 measurements of cell cultures supplemented with AD or cholic acid. Due to the low solubilities of progesterone and cholesterol, total protein content was used to quantify turbidity in cell cultures supplemented with these steroids. Cell cultures (500 μl) were pelleted by centrifugation and resuspended in 100 μl bacterial protein extraction reagent (B-PER; Thermo Scientific). After 5 min, 400 μl MilliQ was added and the mixture was vigorously vortexed and incubated for 10 min. An aliquot of 160 μl was mixed with 640 μl MilliQ and 200 μl protein assay reagent (Bio-Rad). Bovine serum albumin (BSA) was used as a standard to determine the protein content of the sample.
Cholesterol bioconversions.
Cultures of R. rhodochrous strains (25 ml) were grown to an OD600 of approximately 4 in LB broth. Cholesterol (1 g liter−1) was added, and samples (0.5 ml/culture) were taken at different time points for analysis of formed products by high-performance liquid chromatography (HPLC) as described below.
Transcriptomic analysis of R. rhodochrous kshA homologues in steroid-induced cell cultures.
Cultures of R. rhodochrous DSM 43269 were grown in mineral medium (100 ml) supplemented with sodium acetate (2 g liter−1) to an OD600 of approximately 2. The cultures were then induced with 1 g liter−1 steroid (AD, cholesterol, progesterone, or cholic acid). Noninduced cultures were used as negative controls. The cultures were induced for 4 h in the cases of AD, progesterone, and cholic acid and for 24 h in the case of cholesterol. After centrifugation for 30 min at 10,000 × g, pelleted cells were frozen in liquid nitrogen and crushed in an ethanol (70%)-cleaned mortar. Crushed cells were stored at −80°C. RNA was isolated from the crushed cells using the RNeasy kit (Qiagen). An on-column DNase treatment was performed by adding DNase solution to a column that consisted of 40 μl yellow core buffer (Promega), 5 μl 0.09 mM MnCl2, and 5 μl recombinant DNase I (Roche). After incubation at room temperature for 15 min, 200 μl of stop solution (Promega) was added. RNA was eluted in 40 μl MilliQ, subsequently treated with Turbo DNase (Ambion), and stored at −80°C. PCR was performed on RNA to verify that all DNA had been removed. If necessary, DNase treatment was repeated until all DNA was removed from the RNA samples. The PCR mixture (25 μl) consisted of 50 ng RNA, 1× polymerase buffer, 4.0 mM MgCl2, deooxynucleoside triphosphates (0.2 mM), primers (0.2 μM; Table 1), Taq polymerase (1 U; Roche), and MilliQ under the following conditions: 5 min at 95°C; 30 cycles of 30 s at 95°C, 30 s at 68°C, and 1 min at 72°C; and 7 min at 72°C. Reverse transcription-PCR (RT-PCR) was performed using a SuperScript III Platinum one-step qRT-PCR kit (Invitrogen). The mixture (25 μl) contained RNA (50 ng), 12.5 μl 2× reaction buffer, a 0.2 μM concentration of both the forward and the reverse primer (Table 1), MgSO4 to a final concentration of 5 mM, 0.5 μl Platinum Taq mix/SuperScript III and MilliQ. The RNA was incubated at 70°C and kept on ice before being added to the PCR mixture. The PCR conditions were as described for the DNA control, preceded by 15 min at 50°C.
Table 1.
Primers used in this study
| Target | Primer name | Product size (bp) | Sequence (5′–3′) |
|---|---|---|---|
| kshA1 | KshARho-1 | 1,191 | F, CATATGAGCCTCGGCACTTCCGAA |
| R, GCGCTAGCCCGCGGTGGTGGACT | |||
| kshA2 | KshaRho-2 | 1,214 | F2, CATATGGCACCGTGGGTTCCAC |
| R, GCGTCAGGATCCCGATTCGGCGG | |||
| kshA3 | KshaRho-3 | 1,164 | F, GCGCATATGGACATGGCACAGATT |
| R, TCGTCACACCTCCGCTTCCTGCTT | |||
| kshA5 | KshaRho-5 | 1,179 | F, CATATGTCCATCGACACCGCACG |
| R, GCTCTAGGGGGTCGCGGTGGAGC | |||
| kshA1 | KshA1Rho-RT | 374 | F, AACGGCCGCTGCAAGAACATC |
| R1, TTGACCGTTCGCGTGCGGAC | |||
| kshA2 | KshA2Rho-RT | 338 | F2, CCGTTCCGGAGGTTCCCACC |
| R2, TCGGCGTCGACTCGGGATCA | |||
| kshA3 | KshA3Rho-RT | 371 | F, AACGGCAAGTGCACGGACATC |
| R1, CTGCGTCGCCATGCCCTTGT | |||
| kshA4 | KshA4Rho-RT | 333 | F1, CGAAGCTCGGCCGTACCAAG |
| R1, ATCTGCACACCGGTCTTGAT | |||
| kshA5 | KshA5Rho-RT | 407 | F, GCCTGGACGACCCTCGAACGC |
| R1, GTCTGGCCGTTGGCATCGGA | |||
| 16S RNA | 16SF167Ameth | 350 | GTGGCCTACCAAGGCGACGA |
| 16SR483Ameth | AGACGCGACAAGCCGCCTAC |
Construction of kshA and kshB (co)expression plasmids for E. coli Bl21(DE3) and E. coli C41(DE3).
All kshA and kshB genes were amplified via PCR using gene-specific primers from total DNA isolated from R. rhodochrous DSM43269 (Table 1). Gene cloning in expression vector pET15b and construction of the kshA-kshB coexpression plasmids pA1rho3 (kshA1), pA2rho2 (kshA2), pA3rho2 (kshA3), and pA5rho2 (kshA5) were performed as described previously (24) (see Table S1 in the supplemental material). The plasmid used for heterologous coexpression of kshA4 and kshB has been described elsewhere (24).
Heterologous expression of KSH enzymes in E. coli, protein purification, and KSH enzyme activity assay.
Coexpression and copurification of KshA and KshB (together constituting the KSH enzyme), as well as the KSH enzyme activity assay were essentially performed as described by Petrusma et al. (24). KSH activity was assayed by measuring the steroid-dependent NADH consumption over time. The assay mixture (total volume, 500 μl) consisted of 50 mM Tris-HCl buffer (pH 7.0), 25 to 40 μg of KSH enzyme, NADH (105 μM), and 200 μM steroid. NADH consumption was recorded with the Soft-max PRO4 (Life Science edition) program. KSH enzyme stocks were prepared and stored for a maximum of 5 days at a concentration of 1 to 2 mg ml−1 in 20% glycerol. The ratios of coexpressed and copurified KshADSM43269 homologues and KshB were determined using Tricine–SDS-PAGE (28) to separate the KshA and KshB components, followed by densitometry analysis. Uncoupling of the oxygenase enzyme reaction, which would result in NADH oxidation but not product formation, was checked by HPLC-UV (24) or gas chromatography (GC) analysis to confirm product formation. The formation of 9OHAD as a product of the reactions catalyzed by the KshA homologues incubated with AD was analyzed by electrospray ionization–LC-mass spectrometry (MS). Authentic 9OHAD (Schering-Plough, Oss, Netherlands) was used as a standard. Confirmation of 9OHAD formation was also performed using Δ1-KSTD, leading to the formation of 3-HSA as previously described (24). Authentic 3-HSA (Schering-Plough, Oss, Netherlands) was used as a standard.
Steroid analysis.
Steroids were analyzed by HPLC-UV254 on an Alltima C18 column (250 by 4.6 mm, 5 μm) at 35°C using methanol-water (80:20) with 1% formic acid as a mobile phase at a flow rate of 1 ml/min. Samples (0.5 ml) were mixed with 2 ml mobile phase and filtered (0.2 μm) prior to analysis. Samples (0.5 ml) for GC analysis were mixed with 10% H2SO4 (10 μl) and ethyl acetate (2 ml), and the upper organic layer was subjected to GC. GC was performed on a 5% phenyl–5% methoxypoly-siloxane Heliflex AT-5 MS column (30 m by 0.25 mm, inner diameter, 0.25 μm; Alltech, Deerfield, IL) with FID-40 detection at 300°C.
RESULTS
Molecular characterization of five kshA homologues from Rhodococcus rhodochrous DSM43269.
The five kshA genes, described elsewhere for R. rhodochrous DSM43269 (35a), are similar in length (1,137 to 1,200 nucleotides), share 65 to 70% identity at the nucleotide level, and encode proteins with 55 to 62% identity at the amino acid level. Bioinformatics analysis of the KshA homologues showed that all have the typical Rieske Fe2S2 binding domain (C-X-H-X16,17-C-X2-H) and the nonheme Fe2+ motif (D-X3-D-X2-H-X4-H) (31). The eight amino acid residues predicted to be involved in binding of the steroid substrate in KshA of M. tuberculosis H37Rv, i.e., Val176, Gln204, Tyr232, Met238, Asn240, Asn257, Phe301, and Trp308 (H37Rv residue numbering per reference 4), were found to be fully conserved in the KshADSM43269 homologues, with the exception of Val176, which is an Ile in KshA1, and Asn240, which is an Asp in all KshADSM43269 homologues except KshA3.
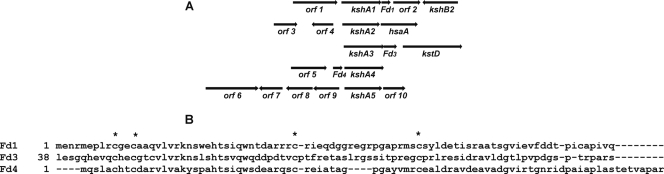
Analysis of the genetic organization of the kshA homologues in DSM43269 revealed that kshA1, kshA2, and kshA3 are located near genes annotated as steroid catabolic genes (Fig. 1A). The kshA genes of R. rhodochrous DSM43269 and the proximal genes all have orthologues in R. jostii RHA1 situated in one of the four previously described steroid catabolic gene clusters, although their genetic organization varied (Table 2) (19, 33). Interestingly, a kshB homologue, kshB2, was identified downstream of kshA1. KshB2 shares 66% amino acid identity with the KshB described previously (24). Adjacent to kshA1, kshA3, and kshA4, small putative genes encoding homologous proteins were found. An alignment of these protein sequences revealed the presence of four conserved cysteines, suggesting these genes may encode ferredoxin-type proteins (Fig. 1B) (18).
Fig. 1.
(A) Schematic of the genomic organization of the kshA homologues of R. rhodochrous DSM43269. Annotation of the identified genes is provided in Table 2. (B) Alignment of the putative ferredoxin-type proteins (Fd) situated adjacent to kshA1, kshA3, and kshA4. *, conserved cysteine.
Table 2.
Comparison of the genetic organization of kshA homologues of DSM43269 and neighboring genes with their orthologous genes in R. jostii RHA1
| R. rhodochrous DSM43269 gene designationa | R. jostii RHA1 orthologue | RHA1 steroid catabolic gene cluster no. | Annotation in R. jostii RHA1 | Amino acid identity (%) |
|---|---|---|---|---|
| orf 1* | ro05822 | 3 | Acyl-CoA synthetase | 54 |
| kshA1 | ro05811 | 3 | KshA3 | 72 |
| Fd1 | ro09013 | 4 | Hypothetical protein | 30 |
| orf 2 | ro05810 | 3 | Dehydrogenase | 74 |
| kshB2 | ro05833 | 3 | KshB3 | 74 |
| orf 3 | ro09032 | 4 | Short-chain dehydrogenase | 82 |
| orf 4 | ro09034 | 4 | IclR family transcriptional regulator | 66 |
| kshA2 | ro09003 | 4 | KshA4 | 72 |
| hsaA | ro09004 | 4 | HsaA4 | 79 |
| kshA3 | ro04538 | 1 | KshA | 75 |
| Fd3 | ro04537 | 1 | Hypothetical protein | 55 |
| kstD | ro04532 | 1 | KstD | 76 |
| orf 5* | ro04742 | 1 | Sensor kinase | 61 |
| Fd4 | ro02482 | 2 | Hypothetical protein | 35 |
| kshA4 | ro09003 | 4 | KshA4 | 57 |
| orf 6 | ro02492 | 2 | Cyclohexanone monooxygenase | 64 |
| orf 7 | ro04889 | Short-chain dehydrogenase | 42 | |
| orf 8 | ro02480 | 2 | Carveol dehydrogenase | 64 |
| orf 9 | ro02481 | 2 | Carveol dehydrogenase | 66 |
| kshA5 | ro02490 | 2 | KshA2 | 72 |
| orf 10* | ro02498 | 2 | Aldehyde dehydrogenase | 65 |
Four steroid catabolic gene clusters have been identified in strain RHA1 (indicated by numbers 1 to 4). *, partially known gene sequence.
Next, phylogenetic analysis was performed on a total of 71 proteins sharing high similarity (>40%) with the KshA homologues of R. rhodochrous DSM43269 (Fig. 2). The phylogenetic tree showed clustering of these proteins into several groups. KshA2, KshA4, and KshA5 of strain DSM43269 appeared to be phylogenetically closely related proteins, belonging to the same group (Fig. 2). The other two KshADSM43269 homologues each belong to different groups.
Fig. 2.
Phylogenetic tree of bacterial KshADSM43269 enzymes and their orthologues in other actinobacteria and Burkholderia species.
Physiological roles of kshA homologues in catabolism of different classes of steroids.
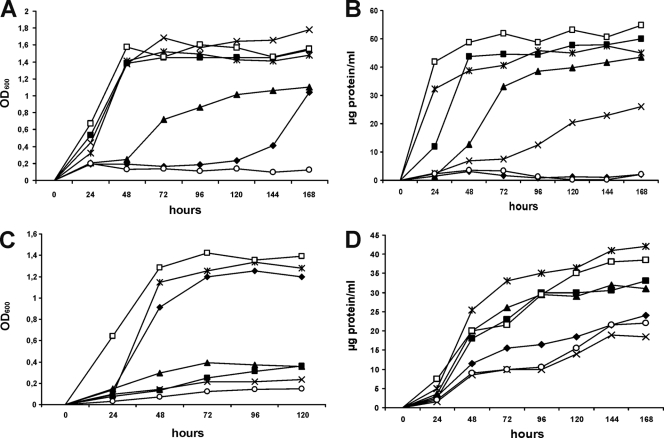
To gain insight into the possible physiological roles of each of the KshADSM43269 homologues in steroid catabolism, growth experiments were performed with representatives of different classes of steroids, i.e., androstenes, sterols, pregnanes, and bile acids, by using AD, cholesterol, progesterone, or cholic acid, respectively, as a sole carbon and energy source. For this purpose, a 5-fold kshA null mutant of strain DSM43269, designated R. rhodochrous strain RG32, was constructed and it lacked kshA1, kshA2, kshA3, kshA4, and kshA5 (35a). Contrary to wild-type DSM43269, the kshA null mutant strain RG32 showed no growth on the steroids tested, except for cholesterol (Fig. 3). Growth of strain RG32 on cholesterol was severely impaired, but not completely blocked. The slow growth of strain RG32 on cholesterol appeared to be supported by side chain degradation, as evident from the accumulation of ADD and 1,4-BNC, which is responsible for the release of propionate and acetate, which could be used as growth substrates (data not shown).
Fig. 3.
Growth of R. rhodochrous DSM43269 wild-type strain (□), the kshA null mutant strain RG32 (○), recombinant strains RG32 transformed with kshA1 (♦), kshA2 (▪), kshA3 (▴), or kshA4 (×), and the kshA1 kshA2 kshA3 kshA4 deletion mutant strain RG31, still harboring kshA5 (*) on 4-androstene-3,17-dione (A), progesterone (B), cholic acid (C), and cholesterol (D). Growth curves were performed in triplicate. See Table S2 of the supplemental material for standard errors of the means.
Next, the kshA1, kshA2, kshA3, and kshA4 genes were individually reintroduced into strain RG32 under the control of their native promoter by using plasmid pRRE1 as a shuttle vector (26) (see Table S1 in the supplemental material). Mutant strain R. rhodochrous RG31, a 4-gene deletion mutant lacking kshA1 to kshA4, was used to study the physiological role of kshA5. The growth experiments indicated that kshA2, kshA3, and kshA5, but not kshA1 and kshA4, were able to restore the growth of mutant strain RG32 on cholesterol to a similar level as observed for wild-type strain DSM43269 (Fig. 3). The kshA2 and kshA5 genes also restored the growth of strain RG32 on both AD and progesterone. Again, kshA1 did not restore growth of strain RG32 on these substrates, whereas the kshA3 gene only partially complemented the growth on AD, progesterone, and cholic acid. Strain RG32 harboring kshA4 was severely delayed in growth on progesterone, whereas full complementation was observed on AD. Interestingly, the growth of strain RG32 on cholic acid was restored only by kshA1 and kshA5, strongly suggesting that kshA1 is specifically involved in cholic acid catabolism. Growth on cholic acid was not restored by kshA2, kshA3, or kshA4.
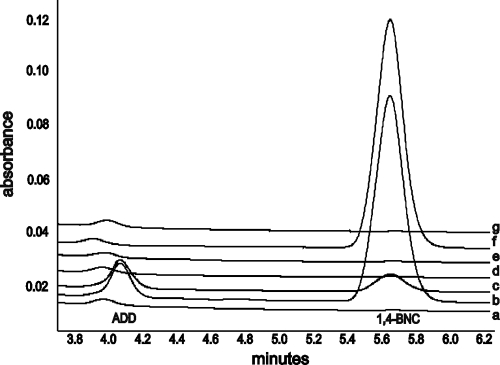
In agreement with our observations in other studies (26, 35a), kshA null mutant strain RG32 was blocked in steroid ring degradation and accumulated the steroid pathway intermediates ADD and 1,4-BNC in cholesterol whole-cell bioconversions (Fig. 4). Subsequently, the role of each of the kshA homologues in cholesterol catabolism was studied in bioconversion experiments with cholesterol and the RG32 strains harboring the individual kshA genes (Fig. 4). The results showed that RG32 cell cultures expressing the kshA2, kshA3, or kshA5 genes were able to degrade 1,4-BNC and ADD when incubated with cholesterol (Fig. 4). This is fully consistent with the results of the growth experiments using cholesterol as a substrate (Fig. 3). Strain RG32 harboring kshA4, however, still accumulated 1,4-BNC, similar to strain RG32 itself, but ADD was not detected. These results are in agreement with the inability of kshA4 to complement growth on cholesterol as a sole carbon and energy source. In contrast, introduction of kshA1 in strain RG32 enabled the partial degradation of 1,4-BNC, whereas ADD was not converted, consistent with the slowed growth observed on cholesterol by this recombinant strain.
Fig. 4.
HPLC elution profiles of cholesterol bioconversions by R. rhodochrous DSM43269 wild type (a), kshA null mutant strain RG32 (b), and recombinant RG32 strains transformed with either kshA1 (c), kshA2 (d), kshA3 (e), kshA4 (f), or mutant strain RG31 harboring kshA5 (g).
None of the strain RG32 cell cultures with the various kshA genes accumulated novel pathway intermediates from cholesterol, which might have been indicative for a KshA enzyme with selectivity other than steroid 9α-hydroxylation.
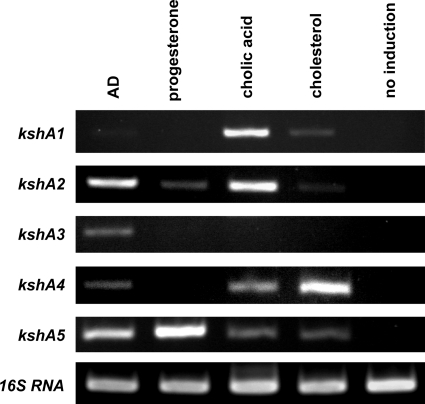
Transcriptional analysis of the kshA homologues.
To further substantiate the involvement of the 5 kshA homologues of DSM43269 in degradation of various classes of steroids, i.e., sterols, pregnanes, androstenes, and bile acids, we performed transcriptional analysis of cell cultures induced with cholesterol, progesterone, AD, or cholic acid, respectively. Wild-type R. rhodochrous strain DSM43269 cells were grown on acetate mineral medium and induced with cholesterol, progesterone, AD, or cholic acid. RT-PCR performed on isolated RNA from noninduced cultures showed that none of the kshA homologues was transcribed, indicating that none of the kshA homologues was constitutively expressed (Fig. 5). The transcriptional profiles of induced cells generally confirmed the results of our growth experiments: expression of kshA1 is induced by cholic acid, and kshA5 is induced by all steroids tested. Intriguingly, transcription of kshA2 and kshA4 was induced by cholic acid in wild-type R. rhodochrous DSM43269, although these KshA homologues did not appear to support growth on this bile acid when introduced in strain RG32. Transcriptional analysis of strain RG32 harboring either kshA2 or kshA4 induced with cholic acid indicated that both enzymes indeed are expressed in this kshA null mutant strain of R. rhodochrous DSM43269.
Fig. 5.
Transcriptional analyses of wild-type R. rhodochrous DSM43269 cell cultures induced with AD, progesterone, cholic acid, or cholesterol. RT-PCRs were performed with gene-specific primers. 16S RNA served as a control.
In wild-type R. rhodochrous DSM43269, kshA3 was specifically induced by AD, whereas only partial complementation of growth of strain RG32 harboring kshA3 on AD was observed. Surprisingly, kshA3 transcripts were not observed when wild-type DSM43269 cells were induced with cholesterol or progesterone, although kshA3 is able to restore growth of strain RG32 on these steroids.
Heterologous expression and purification of KshA homologues of R. rhodochrous DSM43269.
Based on the results of the growth experiments and the transcriptional profiling, differences in the substrate specificities of KSH enzymes with any of these 5 KshA homologues were expected. It also remained to be confirmed whether all 5 kshA homologues actually encode enzymes with 3-ketosteroid 9α-hydroxylase activity or steroid hydroxylases with a different substrate selectivity, e.g., as part of an alternative steroid catabolic pathway. The KshADSM43269 homologues therefore were individually expressed in E. coli together with the KshBDSM43269 enzyme to determine their substrate preference and selectivity.
Cell extracts containing heterologously expressed KshA1, KshA3, or KshA5 all showed enzyme activities with KshB in a standard KSH activity assay using AD as steroid substrate. Consistent with our previous observations, the newly identified KshADSM43269 homologues could not be His tag purified in an active form (24). Using coexpression of KshA1 or KshA3 with KshB of strain DSM43269 in E. coli, and subsequent copurification, we succeeded in obtaining pure and active KSH enzymes. The molar ratios of KshA1:KshB and KshA3:KshB in the purified KSH fractions were 1:1 ± 0.16 and 1:0.72 ± 0.03 (means ± standard errors of the means), respectively. KshA5 could not be obtained in an active form when we used a single expression plasmid with both kshA5 and kshB. When expressing KshA5 [E. coli BL21(DE3)] and KshB [E. coli C41 (DE3)] separately, followed by mixing of the two cell extracts, copurification resulted in an active KSH protein. The molar ratio of KshA5:KshB in the purified KSH fractions was 1:0.59 ± 0.04.
Unfortunately, no expression was observed with KshA2 in E. coli BL21(DE3) or E. coli C41(DE3) despite testing different expression (induction) times, growth temperatures, and isopropyl-β-d-thiogalactopyranoside concentrations for induction. Coexpression of KshA2 with KshB in E. coli BL21(DE3) or E. coli C41(DE3) also did not result in detectable amounts of KshA2 protein nor KSH activity when we used various steroid substrates.
Substrate preference and selectivity of KshA enzymes.
The substrate preferences of the KSH enzymes comprised of KshA1, KshA3, or KshA5 copurified with KshB were determined in a KSH enzyme assay based on their initial enzyme activities on a range of different steroids (Table 3).
Table 3.
Steroid substrate ranges of four KshA homologues of R. rhodochrous DSM43269a
| Steroid substrate | KshA1 |
KshA3 |
KshA4c |
KshA5 |
||||
|---|---|---|---|---|---|---|---|---|
| Enzyme activity | Rel. activity (%) | Enzyme activity | Rel. activity (%) | Enzyme activity | Rel. activity (%) | Enzyme activity | Rel. activity (%) | |
| 4-Androstene-3,17-dione | 2.6 × 102 ± 10 | 100 | 1.7 × 102 ± 22 | 100 | 2.8 × 102 ± 20 | 100 | 1.5 × 102 ± 30 | 100 |
| 1,4-Androstadiene-3,17-dione | 7.5 × 102 ± 90 | 288 | 3.3 × 102 ± 49 | 194 | 2.5 × 102 ± 20 | 89 | 73 ± 11 | 49 |
| 4-Androstene-17β-ol-3-one | 3.3 × 103 ± 70 | 127 | 1.6 × 102 ± 40 | 94 | 2.8 × 102 ± 29 | 101 | 1.7 × 102 ± 24 | 113 |
| 4-Pregnene-3,20-dione | 1.1 × 103 ± 94 | 423 | 1.9 × 102 ± 33 | 112 | 2.7 × 102 ± 38 | 99 | 1.0 × 102 ± 12 | 67 |
| 19-Nor-4-androstene-3,17-dione | 63 ± 7 | 24 | 55 ± 10 | 32 | 2.2 × 102 ± 25 | 78 | 1.6 × 102 ± 25 | 107 |
| 1-(5α)-Androstene-3,17-dione | 59 ± 5 | 23 | 48 ± 5 | 28 | 19 × 102 ± 20 | 68 | 14 × 102 ± 32 | 93 |
| 5α-Androstane-3,17-dione | 23 ± 13 | 9 | ND | ND | 1.8 × 102 ± 19 | 64 | 12 × 102 ± 26 | 80 |
| 5β-Androstane-3,17-dione | 50 ± 7 | 19 | ND | ND | 1.6 × 102 ± 22 | 58 | 1.4 × 102 ± 27 | 93 |
| 5α-Androstane-17β-ol-3-one (stanolon) | ND | ND | ND | ND | ND | ND | 1.4 × 102 ± 27 | 93 |
| 11β-Hydrocortisone | ND | ND | ND | ND | ND | ND | 1.4 × 102 ± 29 | 93 |
| 4-Cholestene-3-oneb | 34 ± 5 | 13 | 39 ± 5 | 23 | ND | ND | 39 ± 5 | 26 |
| 23,24-Bis-nor cholesta-4-ene-22-oic acid | 1.3 × 103 ± 1.2 × 102 | 500 | 2.1 × 102 ± 33 | 124 | —d | 23 | 1.2 × 102 ± 17 | 80 |
| 23,24-Bis-nor-cholesta-1,4-diene-22-oic acid | 1.1 × 103 ± 1.3 × 102 | 423 | 3.0 × 102 ± 44 | 176 | —d | 22 | 64 ± 6 | 43 |
Initial enzyme activities with a 200 μM steroid substrate concentration were calculated in nmol min−1 mg−1 of purified KSH enzyme. The relative activities are expressed as percentages compared to activities with 4-androstane-3,17-dione, which was set at 100%. Standard errors of the means (n = 3) are also reported. ND, no detectable initial activity. No KSH activity was observed with the following compounds: 5-cholestene-3β-ol (cholesterol), 5α-androstane-17-one, 3α-hydroxy-5α-pregnane-20-one, 3β-hydroxy-5α-androstane-17-one, 9α-hydroxy-4-androstene-3,17-dione (negative control).
Due to its limited solubility, this steroid was used at 25 μM. (The lower concentration was also used for 5-cholestene-3β-ol and 3α-hydroxy-5α-pregnane-20-one [data are not shown].)
Data were obtained from reference 24.
Measurements were performed with enzyme batches with higher activities (initial activity on 4-androstane-3,17-dione of 512 ± 62 nmol min−1 mg−1 of purified KSH enzyme) than those described in reference 24 due to the slightly optimized expression and purification methods used in our study. However, the relative activities remained constant. For chemical structures of the tested steroids, see Fig. S1 in the supplemental material.
All KshADSM43269 homologues were able to use AD and ADD as substrates. However, substantial differences were observed in their substrate preferences. KshA1 and KshA3 showed higher activity on ADD than on AD, while AD was the preferred substrate for KshA5. Intriguing was the high preference of KshA1 for 4-BNC and 1,4-BNC. On the other hand, KshA5 had the broadest substrate range, with no apparent preference for any of the tested substrates, but it is unique in its ability to convert 5α-androstan-17β-ol-3-one (stanolon) and 11β-hydrocortisone. KSH activity was only observed with 3-ketosteroid substrates and not with 3-hydroxysteroid substrates (Table 3). The selectivity for C-9 α-hydroxylation of the AD substrate of all the KshADSM43269 homologues was confirmed by LC-MS analyses. Extracted steroid products from the reaction mixtures revealed the formation of 9OHAD from AD for all KSH enzymes. The extracted steroid products from each of the reaction mixtures were also incubated with purified KSTD1 enzyme of R. erythropolis SQ1 (13, 30). HPLC with diode array detection analyses confirmed the disappearance of 9OHAD and the appearance of 3-HSA (data not shown), the expected product of 9OHAD Δ1-dehydrogenation. These experimental data thus conclusively show that KshA1, KshA3, and KshA5 are 3-ketosteroid 9α-hydroxylase isoenzymes.
DISCUSSION
The well-known catabolic potential of rhodococci is greatly facilitated by the impressive gene multiplicity (15, 20, 32). Isoenzymes are involved in degradation pathways of several aromatic compounds, such as biphenyl, phenylacetate, benzoate, and phthalate in rhodococci (5, 8, 11, 22, 23, 27, 36). This study reports on multiple kshA genes, encoding the oxygenase component of KSH, found in R. rhodochrous DSM43269. A total of five kshA genes (kshA1 to kshA5) have been identified (35a). They were shown to encode homologous KshADSM43269 enzymes with C-9 α-hydroxylation selectivity. Consistently, no activity was found with any of the KshADSM43269 homologues on 9OHAD. The latter would have been indicative of a KshA homologue with enzyme selectivity other than C-9 α-hydroxylation. This is further supported by the finding that novel hydroxylated steroid pathway intermediates from cholesterol were not detected in any strain of RG32 complemented with a kshA homologue of DSM43269.
Phylogenetic analysis of the five KshADSM43269 homologues revealed that these are distributed across the different phylogenetic groups that form the phylogenetic tree, although KshA2, KshA4, and KshA5 of strain DSM43269 appear to be phylogenetically closely related proteins (Fig. 2). The kshA genes of DSM43269 and their neighboring genes all have orthologues in R. jostii RHA1, each associated with one of the four steroid catabolic gene clusters (Table 2). Accordingly, we assigned KshA3DSM43269 as a steroid cluster 1 type KshA, KshA5DSM43269 as a cluster 2 type KshA, KshA1DSM43269 as a cluster 3 type, and both KshA2DSM43269 and KshA4DSM43269 as cluster 4 type KshA enzymes. Two groups in the phylogenetic tree appeared to cluster at the genus level, with one group representing homologous proteins of Rhodococcus species and another group representing homologous proteins of Mycobacterium species (Fig. 2). Speculatively, these groups may represent specialized proteins providing catabolic potential to survive in a specific environmental niche. The Rhodococcus cluster, for example, may represent strains capable of growth on cholic acid, since it includes KshA1DSM43269, a KshA specifically linked to cholic acid catabolism. The Mycobacterium cluster includes Rv3526 of M. tuberculosis H37Rv involved in cholesterol degradation (10, 33). Interestingly, KshA1 (Ro04538) of RHA1 is the orthologue of Rv3526 (33) but appears phylogenetically distinct from Rv3526. This may imply that cholesterol catabolism proceeds differently in Mycobacterium than in Rhodococcus, necessitating distinct KshA enzymes.
Bioinformatic analysis of the genetic organization in strain DSM43269 further revealed the presence of a small open reading frame (ORF), encoding a putative ferredoxin associated with three out of the five kshA genes of DSM43269 (Fig. 1B). Although KSH has been defined as a two-component enzyme by the classification system described by Batie et al. (2), these small ORFs may encode a third component of KSH. In the present study KSH activity was obtained when the KshA and KshB components were jointly expressed in E. coli, in the absence of these small proteins. However, a ferredoxin component could enhance efficient electron transfer between KshA and KshB. A small putative protein with four conserved cysteines is also found in close proximity to kshA (ro04538), kshA2 (ro02490), and kshA4 (ro09003) of R. jostii RHA1: ro04537, ro02482, and ro09013, respectively. Further studies are needed to verify a possible role for the putative ferredoxin proteins in KSH activity.
To provide insight into the functionality of the enzymatic multiplicity of the oxygenase component of KSH, we tried to unravel the physiological roles of each of the five KshA homologues in DSM43269. Enzyme activity assays showed that all KSH enzymes with KshADSM43269 homologues studied displayed KSH activity toward 3-ketosteroids (Table 3). KSH activity on 3-hydroxysteroid substrates was not observed with any of the KSH enzymes with KshADSM43269 homologues. KshA5 appeared the most versatile of the KshADSM43269 homologues. KshA5 had the broadest substrate range without a clear substrate preference and was active with Δ4, Δ1,4, 5α-H, and 5β-H steroids, as well as with steroids having bulky aliphatic side chains. Moreover, expression of kshA5 was induced by all steroids tested, and the 4-fold kshA mutant, carrying only kshA5, was capable of growth comparable to wild type on any of the steroid substrates tested (Fig. 3). KshA5 was also the sole KshA enzyme of strain DSM43269 capable of using 5α-androstane-17β-ol-3-one and 11β-hydrocortisone as substrates. Thus, it appears that the other four KshADSM43269 homologues are redundant for growth on steroids. The additional presence of the other KshA homologues, however, may aid in the efficiency of steroid degradation, allowing the bacterium to survive in a competitive environment, like soil. Indeed, it has been shown that the homologous enzymes of the class IIA Rieske oxygenase carbazole 1,9α-dioxygenase of Sphingomonas sp. strain KA1 do not broaden the substrate range, but rather facilitate more efficient growth (29). It has also been suggested that the extensive gene redundancy in rhodococci makes the bacteria better equipped to adapt to new carbon sources (23).
The versatility of KshA5 is in strong contrast with KshA1, which appears to be dedicated to cholic acid degradation. The kshA1 gene, but not kshA2, kshA3, or kshA4, complemented the growth of RG32 on cholic acid to the wild-type level (Fig. 3). In addition, kshA1 was induced only during growth on cholic acid in DSM43269 (Fig. 5). KshA1 is a steroid cluster 3 type KshA, and we thus hypothesize that steroid cluster 3 in R. jostii RHA1 is involved in cholic acid. KshA1 is highly active on 4-BNC and 1,4-BNC compared to the other KshADSM43269 homologues (Table 3). Indeed, compounds such as 7α,12α-dihydroxy-23,24-bis-nor-cholesta-4-ene-22-oic acid and 23,24-bisnorcholesta-1,4-diene-22-oic acid have been found as intermediates in cholic acid degradation (3, 16). Expression of kshA2 and kshA4, both encoding steroid cluster 4 type KshAs, was also induced by cholic acid in wild-type strain DSM43269 (Fig. 5). These genes, however, do not complement the RG32 phenotype in growth on this steroid (Fig. 3). Since the enzymes were expressed in the R. rhodochrous mutant strain RG32 background, it seems that the cholic acid catabolic pathway intermediates are not substrates for the KshA2 and KshA4 isoenzymes.
Gene expression of kshA1 was also induced by cholesterol, albeit at a very low level. The kshA1 gene appeared of no physiological relevance for growth on cholesterol: kshA1 was unable to restore growth of strain RG32 on cholesterol, and accumulation of ADD and 1,4-BNC from cholesterol by a kshA1-complemented RG32 strain was still observed (Fig. 3), while both ADD and 1,4-BNC were preferred substrates of KshA1 (Table 3).
KshA3 is a steroid cluster 1 type kshA (Fig. 2). Steroid cluster 1 is named after the cholesterol catabolic gene cluster found in R. jostii RHA1 (33), thus suggesting that KshA3 is involved in cholesterol degradation. Indeed, kshA3 restores growth of strain RG32 on cholesterol to the wild-type level, confirming a role for kshA3 in cholesterol catabolism (Fig. 3). However, transcriptional analysis with the wild-type strain DSM43269 showed that kshA3 is induced in the presence of AD, but not with cholesterol or any of the other steroids tested. We hypothesize that the formation of AD(D) in strain RG32 carrying kshA3 induces kshA3 expression, after which the substrate range of KshA3 allows full complementation of cholesterol degradation in strain RG32. This strongly implies that cholesterol degradation by wild-type DSM43269 is not accompanied by the formation of AD(D) able to induce kshA3 expression. Several kshA homologues are induced by cholesterol in strain DSM43269 (Fig. 5), likely preventing the accumulation of AD(D) by premature ring opening and thereby kshA3 expression during cholesterol catabolism. Intriguingly, kshA4 is strongly induced by cholesterol but unable to restore growth on cholesterol (Fig. 5). The substrate profile for KshA4 indicated that this homologue has a subtle substrate range (24) (Table 3). Indeed, KshA4 is the only KshA with no detectable activity on cholestenone. In addition, both 4-BNC and 1,4-BNC are poor substrates (Table 3). Consistent with those findings, cholesterol bioconversions with a kshA4-complemented RG32 recombinant strain showed complementation in ADD degradation, but 1,4-BNC still accumulated (Fig. 4). The results taken together suggest that kshA4 is induced by cholesterol but only acts in the degradation pathway after the side chain has been cleaved.
In conclusion, this study provides insight into the KshA multiplicity found in many Rhodococcus species. The KshA homologues of R. rhodochrous DSM43269 show high sequence similarity. They have the same enzymatic selectivity (C-9 α-hydroxylation) and also show overlap in their substrate range. However, the isoenzymes also show interesting differences, each displaying a unique induction pattern and substrate range. Furthermore, both the in vitro and in vivo studies indicated that the KshA homologues are involved in the degradation of specific steroids, acting at different levels in the steroid degradation pathway. The data suggest that the presence of multiple kshA genes in the R. rhodochrous DSM43269 genome facilitates a dynamic and fine-tuned response to the environmental changes encountered by the bacterium.
Supplementary Material
ACKNOWLEDGMENTS
This project was financially supported by the Netherlands Ministry of Economic Affairs and the B-Basic partner organizations (http://www.b-basic.nl) through B-Basic, a public-private Netherlands Organization for Scientific Research-Advanced Chemical Technologies for Sustainability program.
We gratefully acknowledge Theodora Tiemersma-Wegman and Adri Minnaard of the synthetic organic chemistry group of the University of Groningen for LC-MS analyses and Schering-Plough (Oss, Netherlands) for supporting this project. We thank Lindsay Eltis of the Life Sciences Institute, University of British Columbia (Vancouver), for critical reading of the manuscript and helpful suggestions.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Arima K., Nakamatsu W., Beppu T. 1978. Microbial production of 3-oxobisnorchola-1,4-dien-22-oic acid. Agric. Biol. Chem. 42:411–416 [Google Scholar]
- 2. Batie C. J., Ballou D. P., Correll C. J. 1991. Phthalate dioxygenase reductase and related flavin-iron-sulfur containing electron transferases. p. 543–556 In Müller F. (ed.), Chemistry and biochemistry of flavoenzymes. CRC Press, Boca Raton, FL [Google Scholar]
- 3. Birkenmaier A., et al. 2007. Biochemical and genetic investigation of initial reactions in aerobic degradation of the bile acid cholate in Pseudomonas sp. strain Chol1. J. Bacteriol. 189:7165–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Capyk J. K., D'Angelo I., Strynadka N. C., Eltis L. D. 2009. Characterization of 3 ketosteroid 9α-hydroxylase, a Rieske oxygenase in the cholsterol degradation pathway of Mycobacterium tuberculosis. J. Biol. Chem. 284:9937–9946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi K. Y., Kim D., Chae J. C., Zylstra G. J., Kim E. 2007. Requirement of duplicated operons for maximal metabolism of phthalate by Rhodococcus sp. strain DK17. Biochem. Biophys. Res. Commun. 357:766–771 [DOI] [PubMed] [Google Scholar]
- 6. Fernandes P., Cruz A., Angelova B., Pinheiro H. M., Cabral J. M. S. 2003. Microbial conversion of steroid compounds: recent developments. Enzyme Microb. Technol. 32:688–705 [Google Scholar]
- 7. Gibson D. T., Wang K. C., Sih C. J., Whitlock H., Jr 1966. Mechanisms of steroid oxidation by microorganisms. IX. On the mechanism of ring A cleavage in the degradation of 9,10-seco steroids by microorganisms. J. Biol. Chem. 241:551–559 [PubMed] [Google Scholar]
- 8. Gonçalves E. R., et al. 2006. Transcriptomic assessment of isozymes in the biphenyl pathway of Rhodococcus sp. strain RHA1. Appl. Environ. Microbiol. 72:6183–6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holland H. L. 1999. Recent advances in applied and mechanistic aspects of the enzymatic hydroxylation of steroids by whole-cell biocatalysts. Steroids 64:178–186 [DOI] [PubMed] [Google Scholar]
- 10. Hu Y., et al. 2010. 3-Ketosteroid 9α-hydroxylase is an essential factor in the pathogenesis of Mycobacterium tuberculosis. Mol. Microbiol. 75:107–121 [DOI] [PubMed] [Google Scholar]
- 11. Iwasaki T., Miyauchi K., Masai E., Fukuda M. 2006. Multiple-subunit genes of the aromatic-ring-hydroxylating dioxygenase play an active role in biphenyl and polychlorinated biphenyl degradation in Rhodococcus sp. strain RHA1. Appl. Environ. Microbiol. 72:5396–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kieslich K. 1980. Industrial aspects of biotechnological production of steroids. Biotechnol. Lett. 2:211–217 [Google Scholar]
- 13. Knol J., Bodewits K., Hessels G. I., Dijkhuizen L., van der Geize R. 2008. 3-Keto-5α-steroid Δ(1)-dehydrogenase from Rhodococcus erythropolis SQ1 and its orthologue in Mycobacterium tuberculosis H37Rv are highly specific enzymes that function in cholesterol catabolism. Biochem. J. 410:339–346 [DOI] [PubMed] [Google Scholar]
- 14. Kumar S., Nei M., Dudley J., Tamura K. 2008. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 9:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larkin M. J., Kulakov L. A., Allen C. C. 2005. Biodegradation and Rhodococcus: masters of catabolic versatility. Curr. Opin. Biotechnol. 16:282–290 [DOI] [PubMed] [Google Scholar]
- 16. Mahato S. B., Mukherjee E., Banerjee S. 1994. Advances in microbial biotechnology of bile acids. Biotechnol. Adv. 12:357–391 [DOI] [PubMed] [Google Scholar]
- 17. Mahato S. B., Garai S. 1997. Advances in microbial steroid biotransformation. Steroids 62:332–345 [DOI] [PubMed] [Google Scholar]
- 18. Mason J. R., Cammack R. 1992. The electron transport proteins of hydroxylating bacterial dioxygenases. Annu. Rev. Microbiol. 46:277–305 [DOI] [PubMed] [Google Scholar]
- 19. Mathieu J., et al. 2010. 7-Ketocholesterol catabolism by Rhodococcus jostii RHA1. Appl. Environ. Microbiol. 76:352–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McLeod M. P., et al. 2006. The complete genome of Rhodococcus sp. RHA1 provides insights into a catabolic powerhouse. Proc. Natl. Acad. Sci. U. S. A. 103:15582–15587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miroux B., Walker J. E. 1996. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260:289–298 [DOI] [PubMed] [Google Scholar]
- 22. Navarro-Llorens J. M., et al. 2005. Phenylacetate catabolism in Rhodococcus sp. strain RHA1: a central pathway for degradation of aromatic compounds. J. Bacteriol. 187:4497–4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patrauchan M. A., et al. 2005. Catabolism of benzoate and phthalate in Rhodococcus sp. strain RHA1: redundancies and convergence. J. Bacteriol. 187:4050–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petrusma M., Dijkhuizen L., van der Geize R. 2009. Rhodococcus rhodochrous DSM 43269 3-ketosteroid 9α-hydroxylase, a two-component iron-sulfur-containing monooxygenase with subtle steroid substrate specificity. Appl. Environ. Microbiol. 75:5300–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rengarajan J., Bloom B. R., Rubin E. J. 2005. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 102:8327–8332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosłoniec K. Z., et al. 2009. Cytochrome P450 125 (CYP125) catalyses C26-hydroxylation to initiate sterol side-chain degradation in Rhodococcus jostii RHA1. Mol. Microbiol. 74:1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakai M., et al. 2002. Diversity of 2,3-dihydroxybiphenyl dioxygenase genes in a strong PCB degrader, Rhodococcus sp. strain RHA1. J. Biosci. Bioeng. 93:421–427 [DOI] [PubMed] [Google Scholar]
- 28. Schägger H. 2006. Tricine-SDS-PAGE. Nat. Protoc. 1:16. [DOI] [PubMed] [Google Scholar]
- 29. Urata M., et al. 2006. Plasmid pCAR3 contains multiple gene sets involved in the conversion of carbazole to anthranilate. Appl. Environ. Microbiol. 72:3198–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van der Geize R., Hessels G. I., van Gerwen R., van der Meijden P., Dijkhuizen L. 2001. Unmarked gene deletion mutagenesis of kstD, encoding 3-ketosteroid Δ1-dehydrogenase, in Rhodococcus erythropolis SQ1 using sacB as counter-selectable marker. FEMS Microbiol. Lett. 205:197–202 [DOI] [PubMed] [Google Scholar]
- 31. Van der Geize R., Hessels G. I., Van Gerwen R., Van der Meijden P., Dijkhuizen L. 2002. Molecular and functional characterization of kshA and kshB, encoding two components of 3-ketosteroid 9α-hydroxylase, a class IA monooxygenase, in Rhodococcus erythropolis strain SQ1. Mol. Microbiol. 45:1007–1018 [DOI] [PubMed] [Google Scholar]
- 32. Van der Geize R., Dijkhuizen L. 2004. Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr. Opin. Microbiol. 7:255–261 [DOI] [PubMed] [Google Scholar]
- 33. Van der Geize R., et al. 2007. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc. Natl. Acad. Sci. U. S. A. 104:1947–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van der Geize R., Hessels G. I., Nienhuis-Kuiper M., Dijkhuizen L. 2008. Characterization of a second Rhodococcus erythropolis SQ1 3-ketosteroid 9α-hydroxylase activity comprising a terminal oxygenase homologue, KshA2, active with oxygenase-reductase component KshB. Appl. Environ. Microbiol. 74:7197–7203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vishniac W., Santer M. 1957. The thiobacilli. Bacteriol. Rev. 21:195–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a. Wilbrink M. H., Petrusma M., Dijkhuizen L., van der Geize R. 2011. FadD19 of Rhodococcus rhodochrous DSM43269, a steroid-coenzyme A ligase essential for degradation of C-24 branched sterol side chains. Appl. Environ. Microbiol. 77:4455–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamada A., et al. 1998. Two nearly identical aromatic compound hydrolase genes in a strong polychlorinated biphenyl degrader, Rhodococcus sp. strain RHA1. Appl. Environ. Microbiol. 64:2006–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.