Abstract
Little is known about the molecular mechanism for autolysis of Gram-negative bacteria. In the present study, we identified the vvpS gene encoding a serine protease, VvpS, from Vibrio vulnificus, a Gram-negative food-borne pathogen. The amino acid sequence predicted that VvpS consists of two functional domains, an N-terminal protease catalytic domain (PCD) and a C-terminal carbohydrate binding domain (CBD). A null mutation of vvpS significantly enhanced viability during stationary phase, as measured by enumerating CFU and differentially staining viable cells. The vvpS mutant reduced the release of cytoplasmic β-galactosidase and high-molecular-weight extracellular chromosomal DNA into the culture supernatants, indicating that VvpS contributes to the autolysis of V. vulnificus during stationary phase. VvpS is secreted via a type II secretion system (T2SS), and it exerts its effects on autolysis through intracellular accumulation during stationary phase. Consistent with this, a disruption of the T2SS accelerated intracellular accumulation of VvpS and thereby the autolysis of V. vulnificus. VvpS also showed peptidoglycan-hydrolyzing activity, indicating that the autolysis of V. vulnificus is attributed to the self-digestion of the cell wall by VvpS. The functions of the VvpS domains were assessed by C-terminal deletion analysis and demonstrated that the PCD indeed possesses a proteolytic activity and that the CBD is required for hydrolyzing peptidoglycan effectively. Finally, the vvpS mutant exhibited reduced virulence in the infection of mice. In conclusion, VvpS is a serine protease with a modular structure and plays an essential role in the autolysis and pathogenesis of V. vulnificus.
INTRODUCTION
Any form of cell death mediated by an intracellular death program has been referred as programmed cell death (8). Although programmed cell death, classically known as apoptosis, is traditionally associated with eukaryotic cells (25), a substantial body of data suggests that bacteria have also evolved programmed cell death to eliminate cells in the course of development and to eradicate defective or damaged cells (24). Autolysis, perhaps the most commonly observed programmed cell death in bacteria, is a self-lysis of the cell wall by intrinsically produced enzymes called autolysins. Bacterial peptidoglycan (PGN) hydrolases are the best-studied autolysins and can be grouped into glucosidases (N-acetylglucosaminidases and muramidases), amidases, and peptidases according to the cleavage sites of the enzymes (47). PGN peptidases hydrolyze the various ld and dd bonds in the stem peptides and cross bridges of PGN (47).
Autolysis of defective or damaged cells resulting from exposure to antibiotics and other harmful conditions is frequently observed in bacteria (24). It is becoming increasingly apparent that bacteria exhibit social behavior analogous to that found in higher organisms and form complex biofilms, tightly regulated communities of cells (5). From this point of view, autolysis of damaged cells is also altruistic and beneficial to bacterial communities. Autolysis of cells that are seriously damaged could donate their nutrients to kin cells rather than drain limited resources in a hopeless attempt to repair themselves. Maintenance of a low mutation rate, which is important to keep the integrity of a population, is also a benefit obtainable by elimination of cells with damaged DNA. Recent studies have revealed an additional function of autolysis in bacterial communities, as such extracellular chromosomal DNA (eDNA) resulting from autolysis of bacterial cells is a key component of the biofilm matrix and plays an essential role in maintaining intercellular adhesion and biofilm stability (2).
Although biochemical and molecular biological characteristics of the enzymes and their roles in autolysis of many Gram-positive bacteria have been extensively studied (for a recent review, see reference 47), relatively few studies on the enzymes (and any other cellular components) and their functions that contribute to the autolysis of Gram-negative bacteria have been reported (34). A Gram-negative marine bacterium, Vibrio vulnificus, is a causative agent of food-borne diseases, such as life-threatening septicemia and possibly gastroenteritis, in individuals with underlying predisposing conditions (for a recent review, see reference 17). However, no studies have yet been reported on the autolysis of V. vulnificus. Neither autolysins nor other cellular components involved in the autolysis of the bacterium have been identified. Accordingly, V. vulnificus vvpS, encoding a serine protease, VvpS, that appears essential for autolysis of the bacterium was identified and characterized in the present study. It appeared that VvpS, a protein with a highly modular structure, exerts its cell-lytic effects through intracellular accumulation during stationary phase and contains PGN-hydrolyzing activity that is mediated by the C-terminal domain of the protein. Furthermore, the possible role of VvpS in the pathogenesis of V. vulnificus was also explored.
MATERIALS AND METHODS
Strains, plasmids, and culture media.
The strains and plasmids used in this study are listed in Table 1. Unless otherwise noted, the Escherichia coli and V. vulnificus strains were grown in Luria-Bertani (LB) medium at 37°C and LB medium supplemented with 2.0% (wt/vol) NaCl (LBS) at 30°C, respectively.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| Bacterial strains | ||
| V. vulnificus | ||
| MO6-24/O | Clinical isolate; virulent | 47 |
| MO6ΔlacZ | MO6-24/O with ΔlacZ | 1 |
| MS001 | MO6-24/O with vvpS::nptI; Kmr | This study |
| MS003 | MO6-24/O with ΔlacZ vvpS::nptI; Kmr | This study |
| MS023 | MO6-24/O with ΔpilD | This study |
| MS033 | MO6-24/O with ΔpilD vvpS::nptI; Kmr | This study |
| MS042 | MO6-24/O with ΔlacZ ΔpilD | This study |
| MS043 | MO6-24/O with ΔlacZ ΔpilD vvpS::nptI; Kmr | This study |
| E. coli | ||
| DH5α | λ− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−)supE44 thi-1 gyrA relA1 | Laboratory collection |
| SM10 λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir; Kmr; host for π-requiring plasmids; conjugal donor | 27 |
| BL21(DE3) | F−ompT hsdSB(rB− mB−)gal dcm (DE3) | Laboratory collection |
| Plasmids | ||
| pUC18 | Cloning vector; Apr | Laboratory collection |
| pCVD442 | R6K γori sacB; suicide vector; oriT of RP4; Apr | 7 |
| pDS132 | R6K γori sacB; suicide vector; oriT of RP4; Cmr | 31 |
| pJH0311 | 0.3-kb MCS of pUC19 cloned into pCOS5; Apr Cmr | 15 |
| pET28a(+) | His6 tag fusion expression vector; Kmr | Novagen |
| pMBPparallel1 | MBP tag fusion expression vector; Apr | 40 |
| pRKΩlacZ | pRK415 with promoterless lacZ Ω; Smr Tcr | 1 |
| pKC990 | pUC18 with vvpS; Apr | This study |
| pKC9907 | pCVD442 with vvpS::nptI; Apr Kmr | This study |
| pMS0746 | pJH0311 with vvpS; Apr Cmr | This study |
| pMS0806 | pDS132 with ΔpilD; Cmr | This study |
| pMS0734 | pRKΩlacZ; PvvpS-lacZ transcriptional fusion; Smr Tcr | This study |
| pMS0908 | pJH0311 with pilD; Apr Cmr | This study |
| pMS1051 | pMBPparallel1 with vvpS (for wild-type VvpS); Apr | This study |
| pMS1052 | pMBPparallel1 with 3′-truncated vvpS (for VvpS389); Apr | This study |
| pMS1053 | pMBPparallel1 with 3′-truncated vvpS (for VvpS349); Apr | This study |
| pMS1054 | pMBPparallel1 with 3′-truncated vvpS (for VvpS289); Apr | This study |
| pMS1055 | pMBPparallel1 with 3′-truncated vvpS (for VvpS209); Apr | This study |
| pMS1061 | pJH0311 with lacZ; Apr Cmr | This study |
Apr, ampicillin resistant; Cmr, chloramphenicol resistant; Kmr, kanamycin resistant; MCS, multicloning sites.
Cloning of V. vulnificus vvpS and generation of the vvpS and lacZ vvpS mutants.
V. vulnificus genomic DNA was prepared and digested with Sau3AI, ligated with pUC18 (Table 1), and introduced into E. coli DH5α. A positive clone that exhibited proteolytic activity on the LB plate supplemented with skim milk (1.5% [wt/vol]) was obtained. The plasmid, named pKC990, in that clone harbored a 2.4-kb insert with an open reading frame (ORF) of 1,599 nucleotides. Since a database search for homology to the amino acid sequence deduced from the ORF singled out a putative serine protease (NCBI; http://www.ncbi.nlm.nih.gov), the ORF was named vvpS (V. vulnificus serine protease gene).
To inactivate vvpS in pKC990 in vitro, a 1.2-kb nptI DNA conferring resistance to kanamycin (28) was inserted into an EcoRV site present within the coding region of vvpS, and the resulting 3.6-kb vvpS::nptI was subcloned into pCVD442 (7) to generate pKC9907 (Table 1). To generate a vvpS mutant by homologous recombination, E. coli SM10 λpir tra (containing pKC9907) (27) was used as a conjugal donor to V. vulnificus MO6-24/O (47). For the construction of the lacZ vvpS double mutant, MO6ΔlacZ, an isogenic mutant of MO6-24/O that lacks lacZ, was used as the recipient (1) (Table 1). The conjugation and isolation of the transconjugants were conducted using methods previously described (16), and the resulting vvpS and lacZ vvpS mutants chosen for further analysis were named MS001 and MS003, respectively (Table 1). pMS0746 was constructed by subcloning vvpS amplified by PCR using the primers VVPSCF01 and VVPSCR01 into the broad-host-range vector pJH0311 and used for complementation of the vvpS mutation (Tables 1 and 2) (15).
Table 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′→3′)a | Use |
|---|---|---|
| VVPSCF01 | CGAGCTCATAAGCGCCCGATTAAACAA | Complementation of vvpS |
| VVPSCR01 | CCCCGGGAAACGAGGCTTGTGGGTAAG | Complementation of vvpS |
| VVPSPM00 | ATCGGATCCCTGTGAAAGAACCTCTGC | vvpS-lacZ fusion |
| VVPSPM18 | AGGCTGCAGAAACTTTCCGTGATTATGGT | vvpS-lacZ fusion |
| PILDF01 | TGCATGCTGACCACTGGTGTCCCTATT | Construction of pilD mutant |
| PILDF02 | TTTGCGGCCGCAAAAGCAAGGTATTGAGATGG | Construction of pilD mutant |
| PILDR01 | TTTGCGGCCGCAAACAGGGAAGCTAAACCAAT | Construction of pilD mutant |
| PILDR02 | ATCTAGAGTAGGCGCTGTTTCTCAATG | Construction of pilD mutant |
| PILDCF01 | CGAGCTCGATAACCCTCGCAGTTTACC | Complementation of pilD |
| PILDCR01 | CCCCGGGATCCAATCACTAAAGCCATA | Complementation of pilD |
| HISVVPSF01 | CGGATCCACCACCACACCACCAGATGG | Construction of His6 VvpSC |
| HISVVPSR01 | CCTCGAGGTTAAAGTTTTGTCCAAGCT | Construction of His6 VvpSC |
| MBPVVPSF01 | CATGCCATGGCAGAGGTTCTTTCACAGACG | Construction of MBP-VvpS |
| MBPVVPSR01 | AACTGCAGAAACGAGGCTTGTGGGTAAG | Construction of MBP-VvpS |
| MBPVVPSR02 | AACTGCAGTTAATCTTTCGCGCTTGCCGAGAC | Construction of MBP-VvpS389 |
| MBPVVPSR03 | AACTGCAGTTAGGTTTGACCATTAGAAAGCGT | Construction of MBP-VvpS349 |
| MBPVVPSR04 | AACTGCAGTTAAGGCGTATAGCCAACAGAAAC | Construction of MBP-VvpS289 |
| MBPVVPSR05 | AACTGCAGTTATGCTACGTCTCCCACATTGGC | Construction of MBP-VvpS209 |
Regions of oligonucleotides not complementary to the corresponding genes are underlined.
Purification of the truncated VvpSC protein and Western blot analysis.
The DNA encoding the C-terminal 143 amino acids of VvpS, VvpSC, was amplified by PCR using the primers HISVVPSF01 and HISVVPSR01 (Table 2). The PCR product was subcloned into a His6 tag expression vector, pET28a(+) (Novagen, Madison, WI). The resulting His-tagged VvpSC was expressed in E. coli BL21(DE3), and the protein was purified by affinity chromatography according to the manufacturer's procedure (Ni-nitrilotriacetic acid [NTA] agarose; Qiagen, Valencia, CA). The purified VvpSC was used to raise anti-VvpS polyclonal antibodies. Polyclonal antibodies specific for the protein were made by immunizing New Zealand White rabbits on 3 occasions at 3-week intervals with 500 μg of the protein for each immunization (AbFrontier, Seoul, South Korea).
The bacterial cells grown in LBS were harvested, washed, broken by sonication (Ultrasonic processor; Sonics & Materials, Inc., CT), and clarified by centrifugation to generate cell lysates (22). Cellular fractions were prepared by using the PeriPreps Periplasting Kit (Epicentre Biotechnologies, Madison, WI) as previously described (10). The resulting cytoplasm and periplasm fractions, equivalent to 10 μg of total proteins, were subjected to Western blot analyses. Proteins (10 μg) from the cell lysates, cellular fractions, or culture supernatant (10 μl) were resolved by SDS-PAGE (37), and immunoblotting was performed according to the procedure previously described by Lee et al. (22). The protein concentrations were determined by the Bradford method (3), with bovine serum albumin used as the standard.
Construction of MBP-VvpS and MBP-VvpS with C-terminal deletions.
To create a set of mutant VvpS with C-terminal deletions, the primer MBPVVPSF01 (Table 2) was used in conjunction with one of the following primers for PCR amplification of the coding regions of vvpS: MBPVVPSR01 (for amino acids 24 to 532, wild-type VvpS), MBPVVPSR02 (for amino acids 24 to 389, VvpS389), MBPVVPSR03 (for amino acids 24 to 349, VvpS349), MBPVVPSR04 (for amino acids 24 to 289, VvpS289), or MBPVVPSR05 (for amino acids 24 to 209, VvpS209) (Table 2). Since overexpressed VvpS proteins were insoluble (data not shown), the PCR product was used to make an in-frame gene fusion with the 3′ end of the malE gene encoding maltose-binding protein (MBP) in pMBPparallel1 (40) and resulted in pMS1051, pMS1052, pMS1053, pMS1054, and pMS1055, respectively (Table 1). The malE-vvpS (or truncated vvpS) junctions of the plasmids were sequenced to confirm that the two coding regions were in identical reading frames. The resulting MBP-VvpS and MBP-VvpS with C-terminal deletions were expressed and purified by affinity chromatography according to the manufacturer's procedures (Amylose resin; New England BioLabs, Ipswich, MA).
Determination of protease and PGN-hydrolyzing activities.
The protease activity of MBP-VvpS was determined as a casein-hydrolyzing activity, as previously described (45). Briefly, 100 μM MBP-VvpS was added to 500 μl of 0.25% (wt/vol) azocasein (Sigma, St. Louis, MO) solution in 100 mM sodium citrate buffer, pH 6.0. The resulting reaction mixture was incubated for 2 h at 30°C, and the A366 was measured. One unit of enzyme activity is defined as an increase in A366 of 0.01 per hour for protease activity. For PGN-hydrolyzing activity, V. vulnificus PGN was isolated by the method of Glauner (14), and Staphylococcus aureus PGN was purchased (Sigma). The reaction was initiated by addition of 100 μM MBP-VvpS to 500 μl of 0.25 mg/ml PGN solution in 100 mM potassium phosphate buffer, pH 6.0. After incubation for 2 h at 30°C, the A450 was determined (49). One unit of enzyme activity is defined as a decrease in the A450 of 0.01 per hour for PGN-hydrolyzing activity.
Measurement of viability and autolysis.
For determination of viability, cultures in LBS were inoculated with an initial cell density at an A600 of approximately 0.005 and incubated at 30°C with shaking. Samples were removed at the indicated times, and CFU and membrane integrity were measured. The membrane integrity was analyzed using a Live/Dead BacLight viability kit (Molecular Probes, Eugene, OR) as described elsewhere (41). After staining, randomly selected areas were imaged using an epifluorescence microscope (×1,000 magnification; Olympus BX51, Tokyo, Japan) to differentiate viable (with an intact cell membrane; green) and nonviable (lacking an intact cell membrane; red) cells.
To measure autolysis as a function of β-galactosidase release into culture supernatants (42), pMS1061 that constitutively expresses β-galactosidase was constructed by subcloning the lacZ gene from pUC18 into pJH0311 (Table 1) and was introduced into V. vulnificus by conjugation. β-Galactosidase activity in the cell-free supernatants was determined as described previously (42) and reported in Miller units (26). The release of eDNA was measured for determination of autolysis of the cultures as previously reported (46). Briefly, supernatants of the samples were filtered and concentrated 10-fold using a 10-kDa cutoff membrane (Sartorius Stedim Biotech, Gottingen, Germany). The concentrated samples were loaded on a 0.5% agarose gel, and high-molecular-weight eDNAs were visualized by staining with ethidium bromide.
Construction of a PvvpS-lacZ transcriptional fusion.
A PvvpS-lacZ transcriptional fusion reporter was created by subcloning the 477-bp upstream promoter region of vvpS (PvvpS), amplified by PCR using the two primers VVPSPM00 and VVPSPM18, into pRKΩlacZ (Tables 1 and 2). The plasmid carries a promoterless lacZ gene (1). The resulting PvvpS-lacZ reporter, pMS0734, was transferred into MO6ΔlacZ (1) by conjugation, and the β-galactosidase activity was determined by the chloroform-sodium dodecyl sulfate method described previously (26).
Construction of the pilD, pilD vvpS, lacZ pilD, and lacZ pilD vvpS mutants.
The V. vulnificus pilD gene encoding the type IV leader peptidase of the type II secretion system (T2SS) (29) was inactivated in vitro by deletion of about four-fifths (696 bp out of 870 bp) of the pilD ORF using the PCR-mediated linker-scanning mutation method (23). Pairs of primers—PILDF01 and PILDR01 (for amplification of the 5′ amplicon) or PILDF02 and PILDR02 (for amplification of the 3′ amplicon)—were designed and used as listed in Table 2. The 2.1-kb DNA fragment containing ΔpilD was amplified by PCR using the mixture of both amplicons as the template and PILDF01 and PILDR02 as primers and then ligated with SphI-XbaI-digested pDS132 (31), forming pMS0806. E. coli SM10 λpir tra (containing pMS0806) was used as a conjugal donor to MO6-24/O, MS001, MO6ΔlacZ, and MS003 for the construction of the pilD, pilD vvpS double, lacZ pilD double, and lacZ pilD vvpS triple mutants, respectively. The resulting four mutants were named MS023, MS033, MS042, and MS043, respectively (Table 1).
Determination of mouse mortality.
Mouse mortalities from the wild type and vvpS mutant were compared using ICR mice (specific pathogen free; Seoul National University), as described elsewhere (19). The V. vulnificus strains grown in LBS broth at 30°C were harvested and suspended in phosphate-buffered saline (PBS) to the appropriate concentrations. Groups (n = 10) of 7-week-old normal female mice, without an iron-dextran pretreatment, were injected intraperitoneally with 0.1 ml of the bacterial suspensions, and mouse mortalities were recorded for 24 h. All manipulations of mice were approved by the Institute of Laboratory Animal Resources of Seoul National University. To examine the functions of the secreted extracellular VvpS in the pathogenesis of V. vulnificus, the purified VvpS protein (at a concentration of 15 mg kg of body weight−1 per mouse) was injected into the mice, and the mortality of the mice was observed as described above.
Data analyses.
Averages and standard errors of the mean (SEM) were calculated from at least three independent trials. Mouse mortality was evaluated using the log rank test program (http://bioinf.wehi.edu.au/software/russell/logrank/). All other data were analyzed by Student's t test with the SAS program (SAS software; SAS Institute Inc., Cary, NC). The significance of differences between experimental groups was accepted at a P value of <0.05.
RESULTS
Sequence analysis of V. vulnificus VvpS.
The 532-amino-acid sequence of pre-VvpS was deduced from the vvpS nucleotide sequence V. vulnificus MO6-24/O (48) (GenBank, accession number CP002469) and analyzed using the InterProScan program (European Bioinformatics Institute; http://www.ebi.ac.uk/Tools/InterProScan) (Fig. 1). The pre-VvpS protein contains an N-terminal signal peptide with a putative cleavage site located between the 23rd amino acid (Ala) and the 24th (Glu), suggesting that VvpS is a secreted protein. The deduced mature VvpS was comprised of 509 amino acids with a theoretical mass of 52.97 kDa and a pI of 4.69. Further searches for amino acid sequences similar to those of V. vulnificus VvpS identified three coding regions from the V. harveyi, V. parahaemolyticus, and V. alginolyticus genome sequence database (GenBank accession numbers EEZ85352, BAC59905, and EAS77110, respectively), with about 67 to 70% identity in the deduced amino acid sequences (data not shown). However, the functions of the gene products of these three coding regions have not yet been addressed.
Fig. 1.
Sequence analysis of V. vulnificus VvpS. The amino acid sequence of VvpS deduced from the vvpS nucleotide sequence of MO6/24-O (GenBank CP002469) is shown with the PCD (black) and CBD (gray) predicted using the InterProScan program (European Bioinformatics Institute; http://www.ebi.ac.uk/Tools/InterProScan). The putative signal peptide is underlined. The conserved regions of the PCD (TAAHC, DIAL, and GDSGGP) and CBD (stWWst) are indicated by open boxes. The asterisks (*) indicate the conserved catalytic triad (H, D, and S) in the PCD and two solvent-exposed aromatic residues in the CBD, respectively. s, small residue; t, turn-like (4).
The amino acid sequence of VvpS exhibited a modular structure consisting of an N-proximal protease catalytic domain (PCD), a linker domain, and a C-terminal carbohydrate binding domain (CBD), as depicted in Fig. 1. The PCD contains TAAHC, DIAL, and GDSGGP regions that are highly conserved in most serine proteases (35). The three amino acids His85, Asp131, and Ser226 of VvpS, embedded in the TAAHC, DIAL, and GDSGGP regions, respectively, could compose the catalytic triad of VvpS, as observed in most serine proteases (32). The VvpS CBD is grouped into CBD family III based on the sequence homology (data not shown) (4). The primary structure of the VvpS CBD contains the highly conserved stWWst (s, small residue; t, turn-like) motif essential for interaction with carbohydrate substrates (Fig. 1) (4). Common features of bacterial CBDs that include conserved Trp and Gly residues, high contents of hydroxyl amino acids, and low contents of charged amino acids are also found in the VvpS CBD (12). The results indicated that VvpS is a serine protease hydrolyzing specific molecules possessing carbohydrate residues, such as PGN.
Construction of the vvpS mutant and protease activity of VvpS.
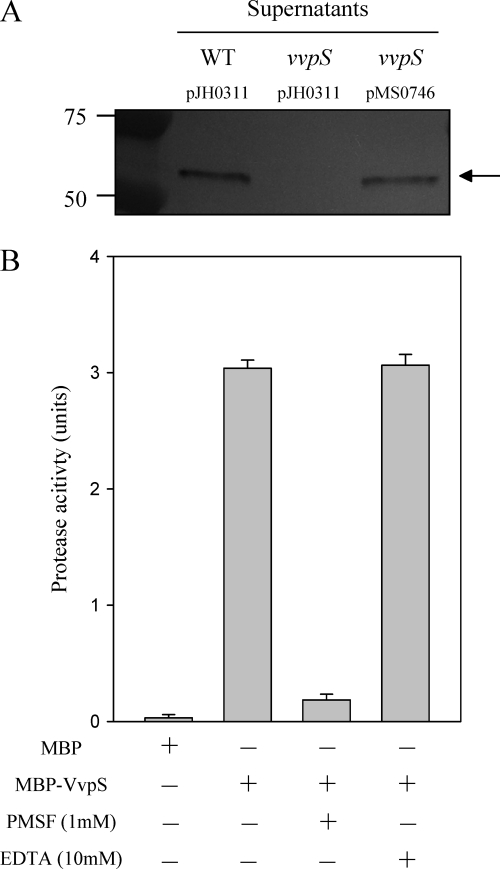
The vvpS isogenic mutant MS001 of V. vulnificus (Table 1) was constructed by an allelic exchange, and the insertional disruption of the vvpS gene in MS001 was confirmed by PCR as previously described (reference 16 and data not shown). By Western blot analysis, a protein of the predicted size of VvpS, about 53 kDa, was detected in the culture supernatant of the wild type harvested at 12 h, supporting the idea that VvpS is secreted to the cell exterior (Fig. 2 A). The VvpS protein in the culture supernatant of the vvpS mutant, however, was not detected by Western blotting, indicating that the disruption of vvpS in the mutant MS001 resulted in complete loss of production of VvpS. We examined whether reintroduction of a recombinant vvpS could complement the loss of VvpS production of MS001. The VvpS production of MS001(pMS0746) was restored to the wild-type level (Fig. 2A).
Fig. 2.
VvpS in the supernatants of V. vulnificus and the effects of the protease inhibitors on VvpS activity. (A) Cultures of the wild type and vvpS mutant harboring each plasmid as indicated were grown in LBS for 12 h at 30°C, and the VvpS proteins in the supernatants were determined by Western blot analysis. Complementation of the mutant with a functional vvpS (pMS0746) is also presented, as indicated. Molecular mass markers (Precision Plus Protein standards; Bio-Rad Laboratories) and VvpS proteins (arrow) are shown in kilodaltons. (B) MBP-VvpS protein (100 μM) was used for the determination of protease activity, defined as an azocasein-hydrolyzing activity in the presence of either 1 mM PMSF or 10 mM EDTA. The same amount of MBP protein was used as a negative control. The error bars represent the SEM.
To characterize the enzymatic properties of VvpS, the protease activity of the purified MBP-VvpS was determined in the presence of different protease inhibitors. The protease activity of MBP-VvpS was apparent and reached 3 units, whereas the protease activity of MBP, as a negative control, was not detectable (Fig. 2B). The addition of 1 mM phenylmethylsulfonyl fluoride (PMSF) into the reaction mixture resulted in a substantial reduction of the protease activity of MBP-VvpS, and the residual level of protease activity corresponded to approximately 1/10 of that observed in the absence of the inhibitor (Fig. 2B). In contrast, the protease activity of MBP-VvpS was not affected significantly in the presence of 10 mM EDTA, indicating that VvpS is a serine protease rather than a metalloprotease (44). Therefore, we have designated the gene vvpS to represent V. vulnificus serine protease to differentiate it from the other genes encoding other potential proteases of V. vulnificus.
Effects of VvpS on the viability of V. vulnificus.
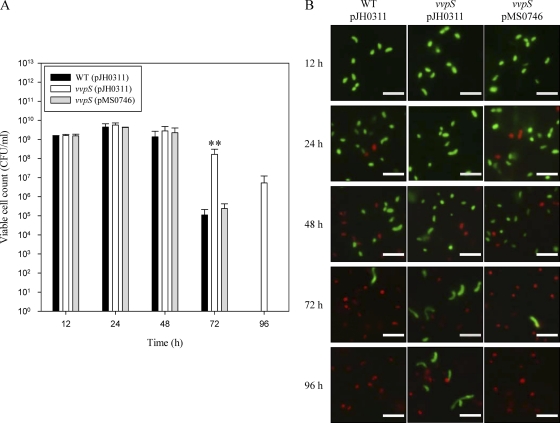
When compared by measuring viable cell numbers as CFU ml−1, the vvpS mutant and parental wild type grew equally well in LBS during exponential phase and reached about 109 to 1010 cells per ml at 24 h (Fig. 3 A). Between 48 and 72 h, the viability of the wild type began to decrease, and no viable cells were detectable after 96 h of culture, whereas the viability of the vvpS mutant was well maintained until 96 h of culture (Fig. 3A). The result indicated that the viability of the vvpS mutant is significantly higher than that of the wild type. Again, the enhanced viability of the vvpS mutant was attenuated to a level comparable to that of the wild type by the reintroduction of vvpS on pMS0746 (Fig. 3A).
Fig. 3.
Effects of the vvpS mutation on the viability of V. vulnificus. Cultures of the wild type and vvpS mutant harboring each plasmid, as indicated, were grown in LBS at 30°C, and samples were removed at the indicated times for enumeration of viable cells as CFU ml−1 (A) and differential staining of viable (green) or dead (red) cells (B). A functional vvpS (pMS0746) was used for complementation of the vvpS mutant. The error bars represent the SEM. **, P < 0.005 relative to the wild type at the indicated time. The V. vulnificus cells were stained with the Live/Dead BacLight viability kit. Bars = 5 μm.
Differential staining of the viable and dead cells using a Live/Dead BacLight viability kit demonstrated that the red dead cells were detected in the wild-type culture at 24 h, whereas none appeared in the vvpS mutant culture until 48 h (Fig. 3B). In addition, the vvpS mutant culture maintained more viable cells than that of the wild type. While a substantial portion of the vvpS mutant cells remained viable, no viable cells were detectable in the wild-type culture at 96 h. Complementation of the vvpS mutation in MS001 with a functional vvpS (pMS0746) reduced the enhanced viability to a level comparable to that of the wild type (Fig. 3B). These results suggested that VvpS could play an essential role in the death of V. vulnificus during stationary phase in batch laboratory culture.
Autolysis and eDNA release.
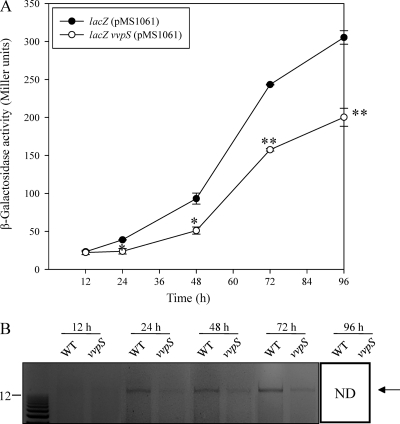
To examine whether VvpS contributes to the death of V. vulnificus by inducing cell lysis, the release of β-galactosidase and eDNA into the culture supernatants was measured at the indicated time points (Fig. 4 A and B). For the parental lacZ strain (MO6ΔlacZ) harboring pMS1061 expressing a lacZ gene constitutively, β-galactosidase release increased during stationary phase and reached a maximum at 96 h (Fig. 4A). The disruption of vvpS in the lacZ vvpS mutant (MO6ΔlacZ vvpS) resulted in a significant reduction of β-galactosidase release into the culture supernatant, suggesting that VvpS contributes to the lysis of V. vulnificus cells during stationary phase.
Fig. 4.
Effects of the vvpS mutation on the autolysis of V. vulnificus. (A) Cultures of the parental lacZ mutant and lacZ vvpS double mutant harboring plasmid pMS1061, which constitutively expresses β-galactosidase, were grown in LBS at 30°C. Samples were removed at the indicated times, and β-galactosidase activities in the supernatant were measured (reported in Miller units). The error bars represent the SEM. *, P < 0.05, and **, P < 0.005 relative to the parental lacZ mutant at the indicated times. (B) Cultures of the wild type and vvpS mutant were grown in LBS at 30°C, and samples were removed at the indicated times for detection of eDNAs released into the supernatant. The eDNAs were stained with ethidium bromide. Molecular size markers (1-kb Plus DNA Ladder; Invitrogen) and eDNAs (arrow) are shown in kilobases. ND, not detected.
When determined by agarose gel electrophoresis, high-molecular-weight eDNAs released from lysed cells were more abundant in the supernatant of the wild type than in the supernatant of the vvpS mutant (Fig. 4B). The eDNAs were detected in the supernatant of the wild type as early as 24 h. For the vvpS mutant, the eDNA initially appeared in the supernatant of the vvpS mutant at 48 h, and its amount was significantly smaller than that of the wild type. When pairs of primers designed to hybridize specifically to the V. vulnificus genomic DNA were used, DNA fragments of identical size were amplified from the eDNAs and V. vulnificus chromosomal DNA by PCR (data not shown). This result indicated that the eDNAs are indeed V. vulnificus chromosomal DNA and confirmed that VvpS contributes to the lysis of the bacteria. Therefore, since VvpS is an endogenously produced enzyme, it is reasonable to assume that VvpS is an autolysin of V. vulnificus and that lysis by VvpS is autolysis.
Intracellular localization of VvpS.
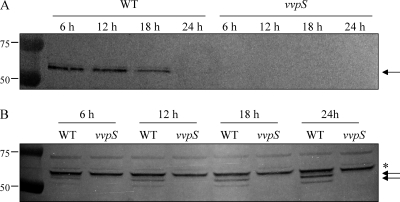
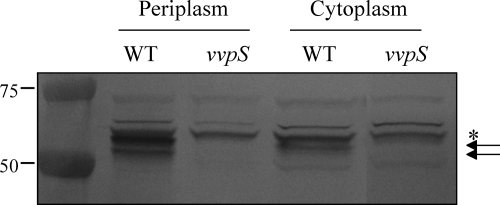
When measured using pMS0734, a PvvpS-lacZ reporter (Table 1), the vvpS expression appeared at the beginning of growth, increased during exponential phase, and then decreased after 24 h (data not shown). Interestingly, when analyzed by Western blotting, the cellular location of the VvpS protein varied at different growth stages. The VvpS protein was found primarily in the culture supernatant harvested at 12 h (Fig. 5 A). However, the extracellular level of VvpS was not detectable after 24 h, and most of the VvpS accumulated in the cells (Fig. 5B). Since autolysis of V. vulnificus became evident after 24 h, this result suggests that autolysis of the bacteria relies, not on the expression level of VvpS, but rather on the accumulation of VvpS in the cells. In addition, when the exact intracellular location of VvpS in cells grown for 24 h was monitored by Western blot analysis, greater amounts of VvpS were detected in the periplasmic space than in the cytoplasmic space (Fig. 6). The periplasmic location of VvpS indicated that the protein might be able to degrade the cell wall as a step in the autolysis of V. vulnificus.
Fig. 5.
Growth phase-dependent localization of VvpS. (A and B) Supernatants (A) and cell lysates (B) of the wild type grown in LBS at 30°C were prepared at the indicated times and examined for the presence of VvpS protein by Western blot analyses. The Western blots are presented as described in the legend to Fig. 2. Molecular mass markers (Precision Plus Protein standards; Bio-Rad Laboratories) for VvpS proteins (arrows) and a cross-reacting protein (asterisk) are shown in kilodaltons.
Fig. 6.
VvpS in the cytoplasmic and periplasmic spaces. Cytoplasm and periplasm fractions of the wild type and vvpS mutant grown in LBS for 24 h were prepared by using the PeriPreps Periplasting Kit (Epicentre Biotechnologies, Madison, WI). The resulting fractions, equivalent to 10 μg of total proteins, were loaded in each lane and examined for the presence of VvpS protein by Western blot analyses. The Western blots are presented as described in the legend to Fig. 2. Molecular mass markers (Precision Plus Protein standards; Bio-Rad Laboratories) for VvpS proteins (arrows) and a cross-reacting protein (asterisk) are shown in kilodaltons.
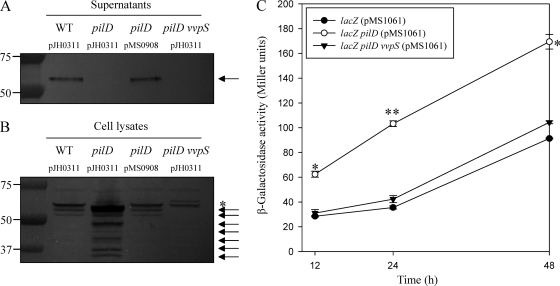
Effect of pilD mutation on the secretion of VvpS and autolysis of V. vulnificus.
V. vulnificus has been shown to possess a T2SS responsible for the extracellular secretion of at least three degradative enzymes (29). In order to determine whether the T2SS is involved in the secretion of VvpS, a mutant that lacks PilD, a component of the T2SS, was constructed and the VvpS secretion of the mutant was compared with that of the parental wild type (Fig. 7 A). A Western blot analysis revealed that the VvpS protein was not detected in the culture supernatant of the pilD mutant at 12 h, indicating that VvpS of V. vulnificus is also secreted via the T2SS. The lack of VvpS secretion in the pilD mutant was restored by the introduction of pMS0908 (Table 1) carrying a recombinant pilD. In contrast, when the intracellular levels of VvpS in the pilD mutant and wild-type cells grown for 12 h were compared, more VvpS (and its degradation products) was accumulated in the pilD mutant cells (Fig. 7B). These results indicated that disruption of the T2SS by the pilD mutation is able to accelerate the accumulation of VvpS in the cells.
Fig. 7.
Secretion and accumulation of VvpS mediated by the T2SS and autolysis of V. vulnificus. (A and B) The VvpS proteins in the supernatants (A) and cell lysates (B) of the wild type, pilD mutant, complemented strain, and pilD vvpS double mutant grown in LBS for 12 h at 30°C were examined by Western blot analyses. The Western blots are presented as described in the legend to Fig. 2. Molecular mass markers (Precision Plus Protein standards; Bio-Rad Laboratories) for VvpS proteins (arrows) and a cross-reacting protein (asterisk) are shown in kilodaltons. (C) Cultures of the parental lacZ and lacZ pilD double and lacZ pilD vvpS triple mutants harboring plasmid pMS1061, which constitutively expresses β-galactosidase, were grown in LBS at 30°C. Samples were removed at the indicated times, and β-galactosidase activities in the supernatant were measured (reported in Miller units). The error bars represent the SEM. *, P < 0.05, and **, P < 0.005 relative to the parental lacZ mutant at the indicated times.
To examine whether this accelerated accumulation of VvpS can affect autolysis, the lysis of V. vulnificus strains was compared by measuring the release of β-galactosidase as shown in Fig. 4A. Based on the β-galactosidase activity in the culture supernatants, the lysis of the pilD mutant was significantly higher and faster than that of the parental wild type (Fig. 7C). This observation indicated that the accelerated accumulation of VvpS in the cells contributed to the enhanced lysis of the pilD mutant. Consistent with this, the increased level of β-galactosidase activity in the supernatant of the pilD mutant was reduced to the level of the parental wild type by additional disruption of vvpS (Fig. 7C).
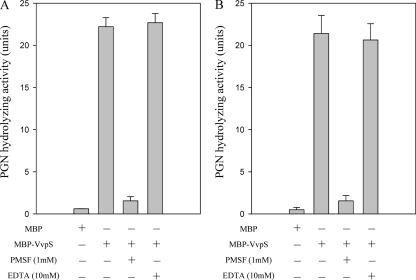
PGN-hydrolyzing activity of VvpS.
All of these observations suggested that VvpS might be able to cleave the cell wall of V. vulnificus. Therefore, PGN was prepared from the V. vulnificus cell wall and used for the determination of the PGN-hydrolyzing activity of MBP-VvpS. As shown in Fig. 8, MBP-VvpS digested the V. vulnificus PGN, indicating that VvpS possesses cell wall-hydrolyzing activity. The presence of 1 mM PMSF, but not 10 mM EDTA, inhibited the PGN-hydrolyzing activity of MBP-VvpS. The patterns of inhibition were similar to the inhibition of protease activities, which were determined by measuring the casein-hydrolyzing activity (Fig. 2B), suggesting that the PGN hydrolysis is attributable to the proteolytic activity of MBP-VvpS (Fig. 8). Since no PGN-hydrolyzing activity of serine proteases with a conserved catalytic His-Asp-Ser triad has been reported, a staphylococcal PGN was purchased and used to reexamine the PGN-hydrolyzing activity of MBP-VvpS. MBP-VvpS hydrolyzed the staphylococcal PGN as well, confirming the PGN-hydrolyzing activity of MBP-VvpS (Fig. 8). Taken together, the data demonstrated that VvpS is responsible for lysis of the cell wall and thus autolysis of V. vulnificus.
Fig. 8.
Peptidoglycan-hydrolyzing activity of MBP-VvpS. MBP-VvpS protein (100 μM) was used for determination of PGN-hydrolyzing activity. PGN-hydrolyzing activity was determined using insoluble PGNs of V. vulnificus (A) and S. aureus (B) as substrates, defined as an activity of hydrolytic solubilization of the PGNs, and measured as a decrease in the A450 in the presence of either 1 mM PMSF or 10 mM EDTA. The same amount of MBP protein was used as a negative control. The error bars represent the SEM.
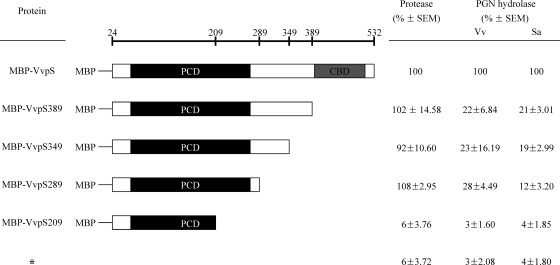
Effects of the CBD on the activity of VvpS.
To examine the role of the CBD in the enzymatic activity of VvpS, we constructed vvpS 3′-deletion mutants and compared the abilities of their products to act as proteases and PGN hydrolases (Fig. 9). MBP-VvpS289 with C-terminal deletions of up to 243 amino acid residues still showed protease activities comparable to those of wild-type MBP-VvpS. The results indicated that the N-terminal amino acid residues prior to position 289 of VvpS constitute an independently folded domain that possesses proteolytic activity. These results were consistent with the prediction that the N-terminal region contains a serine protease catalytic domain based on the VvpS sequence analysis (Fig. 1). Interestingly, the MBP-VvpS with C-terminal deletions of 143 or more amino acid residues revealed significantly reduced PGN-hydrolyzing activity (Fig. 9). These results demonstrated that the C-terminal 143 amino acid residues, predicted to be a CBD, are indeed required for PGN-hydrolyzing activity. As anticipated, MBP-VvpS209 with a deletion of 323 C-terminal amino acid residues, which lacks a part of the PCD, showed no evident protease activity and PGN degradation.
Fig. 9.
C-terminal deletions of VvpS and activities of protease and PGN hydrolase. Shown is a schematic representation of the mutant MBP-VvpS with C-terminal deletions. The amino acid residues up to which each deletion extended are indicated by the numbers at the top. The wild-type MBP-VvpS and its C-terminal deletion mutants are shown below, with the PCD and CBD predicted by the amino acid sequence analysis. The relative activities of the protease and PGN hydrolase are shown on the right. The protease and PGN hydrolase activities were assessed as described in the legends to Fig. 2 and Fig. 8, respectively. Vv, V. vulnificus; Sa, S. aureus; *, MBP protein as a negative control.
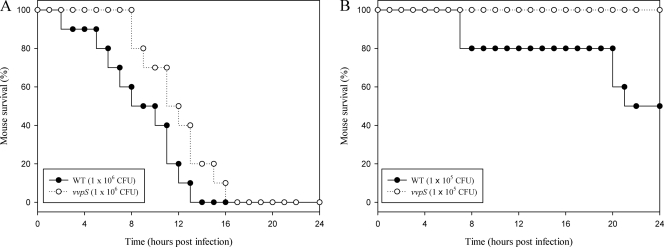
Virulence in mice is dependent on vvpS.
The role of the vvpS gene in virulence was also examined using a mouse model, and survival curves of mice injected intraperitoneally with either the vvpS mutant or the parental wild type at doses of 106 or 105 CFU were monitored for 24 h (Fig. 10 A and B). The results showed that the deaths of mice injected with the vvpS mutant were consistently and significantly delayed (P < 0.05; log rank test) compared to those of mice injected with the parental wild type. Therefore, for the mouse model of intraperitoneal infection, the vvpS mutant appeared to be significantly less virulent than its parental wild type. As such, these results indicated that the V. vulnificus VvpS is apparently important for the pathogenesis of the bacterium.
Fig. 10.
Mortality of the vvpS mutant for mice. (A and B) Seven-week-old specific-pathogen-free female ICR mice were intraperitoneally infected using different concentrations of the wild type or vvpS mutant as indicated. Mouse survival was monitored for 24 h.
The functions of the secreted extracellular VvpS in the pathogenesis of V. vulnificus were also assessed by injecting the MBP or MBP-VvpS protein into mice and by evaluating the mortality of the mice. There was no death in both groups of mice injected with either protein within 24 h (data not shown), indicating that the secreted VvpS is not an important virulence factor. From the result, it seemed unlikely that the increased virulence observed in the wild type resulted from the activity of the extracellular VvpS secreted into the host environment during infection. Thus, when taken together, the results of the present study proposed that the accumulated intracellular VvpS, rather than the secreted extracellular protein, contributes to autolysis and plays an important role in the pathogenesis of V. vulnificus.
DISCUSSION
Over one-third of all known proteases, commonly called peptidases or proteolytic enzymes, are serine proteases. Serine proteases, named after the catalytic serine residue, are usually endopeptidases and catalyze bond hydrolysis in the middle of polypeptide chains (32). Serine proteases are subdivided based on catalytic mechanisms and common ancestry, and trypsin-like proteases are the most abundant among serine proteases (32). VvpS belongs to the trypsin-like proteases and contains the conserved catalytic His-Asp-Ser triad in the PCD (Fig. 1). VvpS is capable of hydrolyzing PGN, as well as casein (Fig. 2B and 8). Although the catalytic mechanism of VvpS is not yet clear, it is reasonable to assume that VvpS is also most likely to cleave a bond (or bonds) in the stem or cross bridge peptides of PGN. Consistent with this, structural analysis reported previously that Ser is implicated in the catalysis of a certain number of PGN peptidases (19, 20, 21, 38, 39). However, these serine PGN peptidases do not contain the catalytic His-Asp-Ser triad and thus do not belong to the trypsin-like proteases. Therefore, VvpS, a trypsin-like protease, seems to be a unique and rare PGN peptidase that is evolutionarily unrelated to previously reported serine PGN peptidases.
Interestingly, VvpS was able to hydrolyze the PGNs of V. vulnificus and S. aureus with comparable efficiencies (Fig. 8). Although information on the molecular features of the V. vulnificus PGN is not yet available, the amino acid constituents of the peptides in the PGNs of the two bacteria would be different from each other. Therefore, it is most likely that VvpS could hydrolyze a bond (or bonds) at residues such as l- and d-Ala that are shared in the stem peptides of both Gram-negative and Gram-positive PGNs (36). Many PGN hydrolases often have one or several domains that bind to PGN (or the cell wall) and greatly affect the specificity and thus activity of the enzymes (47). Consistent with this, VvpS is a modular structure with a C-terminal CBD, and deletion of the CBD significantly impaired PGN-hydrolyzing activity without destroying the casein-hydrolyzing activity (Fig. 9). This indicates that VvpS also needs to bind to the PGN molecules through the CBD to hydrolyze them effectively.
The T2SS allows the secretion of a wide range of proteins from the cytoplasm to the extracellular space via a two-step process (for a recent review, see reference 11). The first step is the transport of unfolded and folded proteins across the cytoplasmic membrane into the periplasmic space by the Sec pathway and the twin-arginine transport (Tat) pathway, respectively (9, 11). Proteins targeted for the Sec or Tat pathway possess an N-terminal signal peptide, and once the preprotein reaches the periplasm, the signal peptide is cleaved off by specific leader peptidases, resulting in a mature protein. The second step mediates the subsequent translocation of the mature protein across the outer membrane and employs a complex machinery consisting of multiple components, including PilD (9). A null mutation in pilD would disconnect this two-step process of V. vulnificus and result in intracellular accumulation of VvpS. Therefore, it is most likely that VvpS is trapped in the periplasmic space, rather than the cytosol, in the pilD mutant, as proposed in Fig. 7. In a similar way, a T2SS that requires ATP for its activity would not be optimally functional during stationary phase, during which the cellular ATP concentration is decreased significantly (9, 30). In addition, the pilD expression was measured using a PpilABCD-lacZ transcriptional fusion reporter and appeared to decrease during stationary phase (data not shown). This growth phase-dependent variation of T2SS activity could explain the stationary-phase-specific accumulation of VvpS in cells and autolysis, as observed in the present study (Fig. 3 and 5).
It is noteworthy that V. vulnificus proliferates for several weeks in sterile seawater (18). Consistent with this, cell death of V. vulnificus was observed in LBS but not in a nutrient-limited minimal medium (data not shown). This indicated that autolysis of V. vulnificus occurs only where nutrients such as amino acids and peptides are present and is more likely to occur in host environments rather than in seawater. If autolysis of V. vulnificus occurs in the host, cell wall- and membrane-associated polysaccharides, such as lipopolysaccharide (LPS), capsular polysaccharide (CPS), and PGN, would be released. The contribution of the cell surface polysaccharides to bacterial pathogenicity has already been extensively studied in terms of the host-pathogen interaction (for a review, see reference 13). It has also been demonstrated that the cell surface polysaccharides protect V. vulnificus by assisting with evasion of phagocytosis by host defense cells and resistance to the bactericidal effects of serum (33, 43). As observed in other pathogens (2), the release of eDNA from lysed V. vulnificus cells could also be used for the development of biofilms, which are known to be resistant to host immune systems (6). Although the vvpS mutant was apparently less virulent in a mouse model than its parental wild type (Fig. 10), it is still unclear whether autolysis of a subpopulation is indeed beneficial for the rest of the bacterial community to survive the host immune system. Therefore, more work is necessary to illuminate the exact function of autolysis in V. vulnificus pathogenesis.
In summary, V. vulnificus vvpS encoding a serine protease with a modular structure was identified in the present study. A null mutation of vvpS significantly reduced autolysis of V. vulnificus, indicating that VvpS is an autolysin responsible for autolysis of the bacteria. VvpS was secreted via the T2SS and accumulated in the periplasmic space in stationary-phase cells to contribute to the autolysis of V. vulnificus. Therefore, a disruption of the T2SS accelerated intracellular accumulation of VvpS and thereby autolysis of V. vulnificus. VvpS also showed PGN-hydrolyzing activity, which is mediated by the presence of a CBD. Finally, the vvpS mutant showed reduced virulence in the infection of mice, indicating that VvpS is essential for pathogenesis, as well as autolysis, of V. vulnificus.
ACKNOWLEDGMENTS
This work was supported by grants to S.H.C from the Agriculture Research Center program, MIFAFF, and the Mid-Career Researcher Program (no. 2009-0092822) and a National Research Laboratory Grant (R0A-2007-000-20039-0) through NRF, MEST, Republic of Korea.
Footnotes
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Baek C. H., Kim K. S. 2003. lacZ- and aph-based reporter vectors for in vivo expression technology. J. Microbiol. Biotechnol. 13:872–880 [Google Scholar]
- 2. Bayles K. W. 2007. The biological role of death and lysis in biofilm development. Nat. Rev. Microbiol. 5:721–726 [DOI] [PubMed] [Google Scholar]
- 3. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 4. Brun E., et al. 1997. Solution structure of the cellulose-binding domain of the endoglucanase Z secreted by Erwinia chrysanthemi. Biochemistry 36:16074–16086 [DOI] [PubMed] [Google Scholar]
- 5. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 6. Donlan R. M., Costerton J. W. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donnenberg M. S., Kaper J. B. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Engelberg-Kulka H., Amitai S., Kolodkin-Gal I., Hazan R. 2006. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Filloux A., Michel G., Bally M. 1998. GSP-dependent protein secretion in Gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol. Rev. 22:177–198 [DOI] [PubMed] [Google Scholar]
- 10. French C., Keshavarz-Moore E., Ward J. M. 1996. Development of a simple method for the recovery of recombinant proteins from the Escherichia coli periplasm. Enzyme Microbial. Technol. 19:332–338 [Google Scholar]
- 11. Gerlach R. G., Hensel M. 2007. Protein secretion systems and adhesins: the molecular armory of Gram-negative pathogens. Int. J. Med. Microbiol. 297:401–415 [DOI] [PubMed] [Google Scholar]
- 12. Gilkes N. R., Henrissat B., Kilburn D. G., Miller R. C., Jr., Warren R. A. 1991. Domains in microbial beta-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol. Rev. 55:303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ginsburg I. 2002. The role of bacteriolysis in the pathophysiology of inflammation, infection and post-infectious sequelae. APMIS 110:753–770 [DOI] [PubMed] [Google Scholar]
- 14. Glauner B. 1988. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal. Biochem. 172:451–464 [DOI] [PubMed] [Google Scholar]
- 15. Goo S. Y., et al. 2006. Identification of OmpU of Vibrio vulnificus as a fibronectin-binding protein and its role in bacterial pathogenesis. Infect. Immun. 74:5586–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeong H. S., Lee M. H., Lee K. H., Park S. J., Choi S. H. 2003. SmcR and cyclic AMP receptor protein coactivate Vibrio vulnificus vvpE encoding elastase through the RpoS-dependent promoter in a synergistic manner. J. Biol. Chem. 278:45072–45081 [DOI] [PubMed] [Google Scholar]
- 17. Jones M. K., Oliver J. D. 2009. Vibrio vulnificus: disease and pathogenesis. Infect. Immun. 77:1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaspar C. W., Tamplin M. L. 1993. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl. Environ. Microbiol. 59:2425–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H. S., Park S. J., Lee K. H. 2009. Role of NtrC-regulated exopolysaccharides in the biofilm formation and pathogenic interaction of Vibrio vulnificus. Mol. Microbiol. 74:436–453 [DOI] [PubMed] [Google Scholar]
- 20. Kishida H., et al. 2006. Crystal structure of penicillin binding protein 4 (dacB) from Escherichia coli, both in the native form and covalently linked to various antibiotics. Biochemistry 45:783–792 [DOI] [PubMed] [Google Scholar]
- 21. Korza H. J., Bochtler M. 2005. Pseudomonas aeruginosa ld-carboxypeptidase, a serine peptidase with a Ser-His-Glu triad and a nucleophilic elbow. J. Biol. Chem. 280:40802–40812 [DOI] [PubMed] [Google Scholar]
- 22. Lee B. C., et al. 2008. Vibrio vulnificus rtxE is important for virulence, and its expression is induced by exposure to host cells. Infect. Immun. 76:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J. H., et al. 2007. Identification and characterization of the Vibrio vulnificus rtxA essential for cytotoxicity in vitro and virulence in mice. J. Microbiol. 45:146–152 [PubMed] [Google Scholar]
- 24. Lewis K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Metzstein M. M., Stanfield G. M., Horvitz H. R. 1998. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 14:410–416 [DOI] [PubMed] [Google Scholar]
- 26. Miller J. H. 1972. Experiments in molecular genetics, p. 352–355 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 27. Miller V. L., Mekalanos J. J. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oka A., Sugisaki H., Takanami M. 1981. Nucleotide sequence of the kanamycin resistance transposon Tn903. J. Mol. Biol. 147:217–226 [DOI] [PubMed] [Google Scholar]
- 29. Paranjpye R. N., Lara J. C., Pepe J. C., Pepe C. M., Strom M. S. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 Cells and virulence in iron-overloaded mice. Infect. Immun. 66:5659–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peterkofsky A., Gazdar C. 1973. Measurements of rates of adenosine 3′:5′-cyclic monophosphate synthesis in intact Escherichia coli B. Proc. Natl. Acad. Sci. U. S. A. 70:2149–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Philippe N., Alcaraz J. P., Coursange E., Geiselmann J., Shneider D. 2004. Improvement of pCVD442, a suicide plasmid for gene allele exchange in bacteria. Plasmid 51:246–255 [DOI] [PubMed] [Google Scholar]
- 32. Polgár L. 2005. The catalytic triad of serine peptidases. Cell. Mol. Life Sci. 62:2161–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powell J. L., Wright A. C., Wasserman S. S., Hone D. M., Morris J. G., Jr 1997. Release of tumor necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in vivo and in vitro models. Infect. Immun. 65:3713–3718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rice K. C., Bayles K. W. 2008. Molecular control of bacterial death and lysis. Microbiol. Mol. Biol. Rev. 72:85–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ross J., Jiang H., Kanost M. R., Wang Y. 2003. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene 304:117–131 [DOI] [PubMed] [Google Scholar]
- 36. Royet J., Dziarski R. 2007. Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences. Nat. Rev. Microbiol. 5:264–277 [DOI] [PubMed] [Google Scholar]
- 37. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 38. Sauvage E., et al. 2007. Crystal structure of the Bacillus subtilis penicillin-binding protein 4a, and its complex with a peptidoglycan mimetic peptide. J. Mol. Biol. 371:528–539 [DOI] [PubMed] [Google Scholar]
- 39. Sauvage E., et al. 2005. Crystal structure of the Actinomadura R39 dd-peptidase reveals new domains in penicillin-binding proteins. J. Biol. Chem. 280:31249–31256 [DOI] [PubMed] [Google Scholar]
- 40. Sheffield P., Garrard S., Derewenda Z. 1999. Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr. Purif. 15:34–39 [DOI] [PubMed] [Google Scholar]
- 41. Smith B., Oliver J. D. 2006. In situ and in vitro gene expression by Vibrio vulnificus during entry into, persistence within, and resuscitation from the viable but nonculturable state. Appl. Environ. Microbiol. 72:1445–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Steinmoen H., Knutsen E., Håvarstein L. S. 2002. Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc. Natl. Acad. Sci. U. S. A. 99:7681–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strom M. S., Paranjpye R. N. 2000. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2:177–188 [DOI] [PubMed] [Google Scholar]
- 44. Supuran C. T., Scozzafava A., Clare B. W. 2002. Bacterial protease inhibitors. Med. Res. Rev. 22:329–372 [DOI] [PubMed] [Google Scholar]
- 45. Swift S., et al. 1999. Quorum sensing-dependent regulation and blockade of exoprotease production in Aeromonas hydrophila. Infect. Immun. 67:5192–5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thomas V. C., Thurlow L. R., Boyle D., Hancock L. E. 2008. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J. Bacteriol. 190:5690–5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vollmer W., Joris B., Charlier P., Foster S. 2008. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol. Rev. 32:259–286 [DOI] [PubMed] [Google Scholar]
- 48. Wright A. C., Simpson L. M., Oliver J. D., Morris J. G., Jr 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zoll S., et al. 2010. Structural basis of cell wall cleavage by a staphylococcal autolysin. PLoS Pathog. 6:e1000807. [DOI] [PMC free article] [PubMed] [Google Scholar]