Abstract
The FlgM secretion checkpoint plays a crucial role in coordinating bacterial flagellar assembly. Here we identify a new role for FlgM and FliA as part of a complex regulatory network which controls flagellum number and is essential for efficient swimming and biofilm formation in the monotrichous bacterium Rhodobacter sphaeroides.
TEXT
Bacterial motility and chemotaxis are implicated in complex prokaryotic behaviors, such as biofilm formation, pathogenesis, and symbiosis (1, 12). The monotrichous alphaproteobacterium Rhodobacter sphaeroides has complex chemotaxis and motility systems (10, 11, 13) and is metabolically diverse, allowing it to exploit many different environmental niches. R. sphaeroides and related organisms are primary colonizers of surfaces in coastal waters (3). Flagellar assembly is an energetically expensive and highly regulated process which in other species has been shown to be intrinsically linked to processes such as biofilm formation (14). This study examined the regulation of synthesis of the single flagellum in R. sphaeroides. Links between flagellum synthesis and biofilm formation were also explored.
R. sphaeroides achieves swimming motility via the unidirectional rotation of a single laterally positioned flagellar filament (2, 5). Assembly of the R. sphaeroides flagellar motor is controlled by a multitiered hierarchy of gene expression which is initiated by the transcriptional activation of the class I master regulator FleQ. FleQ promotes the expression of class II flagellar genes, including FleT, which results in formation of the FleQ/FleT complex. Class III flagellar genes are expressed from FleQ/FleT-dependent σ54 promoters, leading to completion of the flagellar hook basal body (HBB). Class IV flagellar gene expression is then thought to be activated upon the secretion of the anti-sigma factor FlgM through the HBB, resulting in release of the sigma factor FliA (10). Here, we show that FliA/FlgM activity regulates not only flagellar synthesis but also the number of flagella per cell. Not only is the correct assembly of flagella essential for motility, but incorrect assembly also leads to reduced biofilm formation in R. sphaeroides.
Regulation of flagellum number by FliA and FlgM.
To determine the effects of FlgM and FliA activity on the expression of target genes from classes III and IV of the flagellar assembly hierarchy of R. sphaeroides, chromosomal in-frame deletions of flgM and fliA were incorporated into both wild-type strains and a variety of fluorescent-fusion background strains. Protein copy numbers were determined using a fluorescence-based technique similar to that detailed in reference 15; fluorimetry measurements of cellular samples in a FLUOstar Optima plate reader were compared to those of purified yellow fluorescent protein (YFP) of known concentrations. Where antibodies were available, the results were confirmed via quantitative Western blot analysis of the wild-type protein levels.
Fusion proteins were chosen to reflect the different classes of the flagellar assembly hierarchy. The stator protein YFP-MotB, the switch protein YFP-FliM, and the anti-sigma factor FlgM-YFP are all known to be encoded as part of FleQ/FleT-dependent, class III operons (motAB, fliKLMNO, and flhA, respectively) (9). YFP-CheW3 and CheW4-YFP, components of the two pathways essential for chemotaxis in R. sphaeroides (encoded within cheOp2 and cheOp3, respectively), were chosen as class IV gene reporters. Expression of cheOp2 has previously been observed to be regulated by FliA in R. sphaeroides (7). Incorporation of fliA and flgM into the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible expression vector pInd4 (6) allowed the reintroduction and overexpression of these genes within the relevant deletion background strains (Table 1).
Table 1.
Effects of flgM and fliA on target gene expressiona
| Protein | Flagellar gene class(es) | No. of copies of: |
||||
|---|---|---|---|---|---|---|
| Wild type | ΔfliA mutant | ΔflgM mutant | ΔfliA fliA++ strain | ΔflgM flgM++ strain | ||
| YFP-MotB* | III | 610 ± 20 | 1,700 ± 30 | 450 ± 30 | — | 1,500 ± 90 |
| YFP-FliM | III | 1,030 ± 180 | 3,760 ± 60 | 630 ± 20 | — | 3,600 ± 180 |
| FlgM-YFP | III/IV | 8,200 ± 400 | 1,100 ± 200 | N/A | 27,600 ± 750 | NA |
| YFP-CheW3* | IV | 8,700 ± 260 | 6,900 ± 270 | 10,300 ± 210 | 22,400 ± 1,300 | 10,900 ± 300 |
| CheW4-YFP* | IV | 12,700 ± 700 | 5,400 ± 900 | 15,700 ± 400 | 31,300 ± 780 | 15,500 ± 300 |
Copy numbers were measured by fluorimetry and quantified by comparison to numbers in purified YFP samples of known concentration. Numbers represent the means and standard errors of results from at least three independent repeats. *, similar results were also observed via Western blot analysis; —, no expression was detectable; NA, not applicable. fliA++ represents the overexpression of pInd4:fliA at 50 μM IPTG. flgM++ represents the overexpression of pInd4:flgM at 100 μM IPTG.
Table 1 shows that under the tested growth conditions (all cultures were grown in a minimal medium supplemented with 7.5 mM sodium succinate and 0.1% Casamino Acids [sux medium] [7], at 30°C with shaking), both cheOp2 and cheOp3 were regulated by FliA; flgM deletion and fliA overexpression increased chemotaxis gene expression, whereas fliA deletion reduced cellular levels of both class IV YFP-CheW3 and CheW4-YFP. FlgM-YFP expression also showed a high dependence on FliA activity despite being encoded as part of the class III flgA operon (10). This suggests that class III promoter activity may achieve basal levels of expression of FlgM, with larger changes in the cellular concentration of FlgM being controlled by increased FliA activity.
Interestingly, the expression of two chromosomally distant class III flagellar genes, MotB-YFP and YFP-FliM, increased greatly upon both deletion of fliA and overexpression of flgM but decreased upon deletion of flgM and overexpression of fliA. This suggests that FliA may repress flagellar synthesis as part of a feedback loop within the flagellar assembly pathway. Although class III gene expression increased upon overexpression of FlgM, chemotaxis gene expression was the same as that in the parental ΔflgM strain, showing that the mechanisms of regulating class IV and class III genes are different.
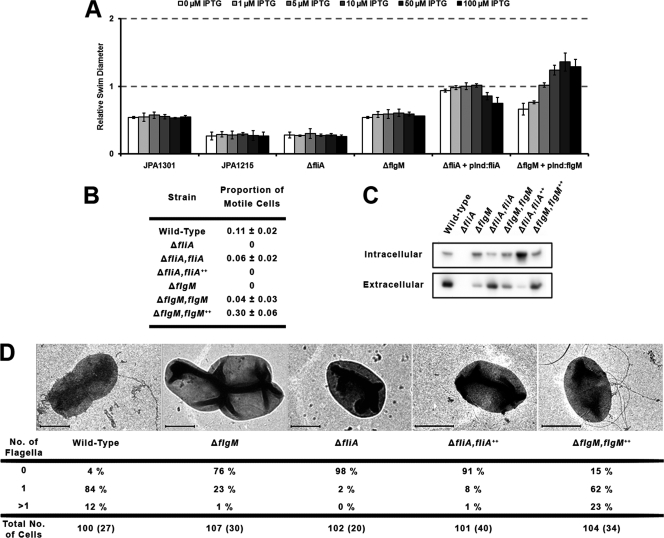
As R. sphaeroides is monotrichous, repression of flagellar synthesis was predicted to affect the number of cells possessing fully formed flagella within a population. Therefore, the proportion of motile cells in mid-log-phase cultures was estimated in phase-contrast microscopy videos (Fig. 1 B), and filaments were observed and their numbers counted for >100 negatively stained cells using a Philips CM120 electron microscope (Fig. 1D). Swimming behavior was also assessed on soft-agar swim plates containing 0.1 mM propionate, with FliA and FlgM levels varied by using the pInd4 expression vector and different concentrations of IPTG (Fig. 1A). Soft-agar swim plates measure swimming in response to chemosensory gradients in a growing population of cells. If only a small subset of the population develop functional flagella, it may be obvious in swim plates but not microscopically. Alternatively, if the chemosensory system is impaired, cells may be seen to swim microscopically but not to move on swim plates.
Fig. 1.
Motility and chemotaxis in ΔfliA and ΔflgM mutant strains. (A) Bacterial swim diameters on soft-agar swim plates. Diameters are measured relative to that of the wild type. fliA and flgM expression was induced in their respective background deletion strains by the addition of different concentrations of IPTG. Data for nonchemotactic (JPA1301) and nonmotile (JPA1215) control strains are also included. Error bars represent the standard deviations of results from three repeats on three separate days. (B) Proportion of motile cells within a population, measured in phase-contrast videos of swimming bacteria. Numbers represent the means and standard deviations of the average proportions of swimming cells over all frames in each ∼30-s video from three repeats on three separate days. The proportions appear low, as nonmotile cells persist from frame to frame, whereas motile cells enter and leave each field of view quickly. (C) Anti-FliC Western blot data for both intracellular and extracellular fractions of the cell. (D) EM flagellum tally. The number of visible flagella on individual cells of a negatively stained preparation of mid-log-phase cells. Sample images are displayed for each condition; scale bars represent 1 μm. Numbers in parentheses show the number of cells with clearly visible division sites within each sample. Throughout, fliA++ represents the overexpression of pInd4:fliA at 50 μM IPTG. flgM++ represents the overexpression of pInd4:flgM at 100 μM IPTG.
As had previously been observed (7), deletion of fliA resulted in nonmotile cells with no flagellar filaments. When intracellular and extracellular flagellin (FliC) was separated by filament shearing and centrifugation, none was detectable in Western blot analyses of either fraction (Fig. 1C). Introduction of pInd4:fliA in the absence of IPTG was sufficient to restore both wild-type proportions of swimming cells and wild-type swimming behavior to the deletion background, as well as wild-type levels of intracellular and extracellular FliC. However, unexpectedly, overexpression of fliA in liquid cultures in the presence of 50 μM IPTG resulted in the majority of cells being nonmotile, with no visible filaments observed by electron microscopy (EM). This observation was also supported by soft-agar swim plate data, where a reduction in swim diameter was seen at higher levels of FliA induction. Western blot analysis showed that intracellular FliC expression increased and extracellular FliC decreased relative to that of the wild type when FliA was overexpressed, suggesting that flagellin secretion was reduced under these conditions.
Deletion of flgM also greatly reduced R. sphaeroides motility, with the proportion of swimming cells and the number of visible filaments within a population being significantly lowered. Intracellular FliC expression was seen to increase slightly upon deletion of flgM, whereas extracellular FliC decreased. Introduction of pInd4:flgM recovered a proportion of swimming cells, and swim diameters were seen to increase above those of the ΔflgM strain. When flgM was overexpressed, the amounts of both intracellular and extracellular FliC were the same as those of the wild type; however, there was a significant increase in swim diameters on swim plates and in the proportion of cells that could swim (observed microscopically). There was also an increase in the number of cells that possessed either zero or multiple flagella when observed by EM. These data suggest that FlgM activity is essential in regulating flagellar number and the rate at which flagella assemble. Relieving FliA's repression of class III gene expression by overexpressing FlgM may increase the rate at which HBBs can form, resulting in increased type III secretion and explaining the appearance of multiple flagella. In wild-type cells, the secretion of FlgM would increase FliA's inhibition of class III gene expression, inhibiting formation of a second flagellum.
The mechanism of class III gene repression by FliA remains unknown, and this inhibition may also affect the earlier, class I and class II flagellar genes. Feedback inhibition of flagellar assembly by FliA demonstrates a new role for the FlgM secretion checkpoint in a monotrichous bacterium, repressing the synthesis of a second flagellum upon completion of the first. The single flagellum of R. sphaeroides is randomly positioned rather than polar. This finding is supported by the lack of FlhF and FlhG, shown to position polar flagella (5). The system of regulating flagellar position in peritrichous systems is unknown, and it is interesting that filament positions appeared random in FlgM-overexpressing R. sphaeroides cells that had an increased number of filaments.
Motility defects in FliA and FlgM mutants impair biofilm formation.
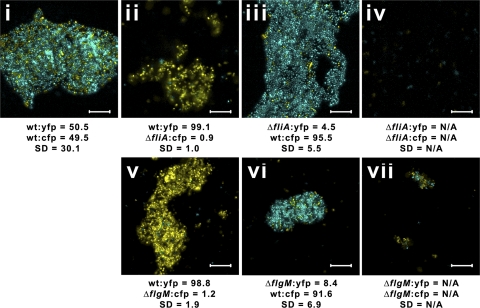
Interestingly, an obvious phenotypic difference in the mutants tested above was their ability to stick to surfaces and form biofilms. Motility is known to be essential for biofilm formation in many different bacterial species (1, 8, 14), although little is known about R. sphaeroides biofilms. In static cultures, the fliA and flgM mutants showed surface growth properties different from those of wild-type cells when CFP or YFP was expressed from pInd4 and when the mutants were visualized on a Nikon A1R confocal microscope after 48 to 72 h (Fig. 2).
Fig. 2.
ΔfliA and ΔflgM mutants show impaired biofilm formation. Sample two-color confocal images of biofilms grown from mixed cultures. Scale bars represent 10 μm. (i) WT:yfp strain/WT:cfp strain; (ii) WT:yfp strain/ΔfliA:cfp strain; (iii) ΔfliA:yfp strain/WT:cfp strain; (iv) ΔfliA:yfp strain/ΔfliA:cfp strain; (v) WT:yfp strain/ΔflgM:cfp strain; (vi) ΔflgM:yfp strain/WT:cfp strain; (vii) ΔflgM:yfp strain/ΔflgM:cfp strain. The means and standard deviations for percentages of bacteria that contained YFP and CFP in at least 15 images from biofilms grown on three separate days were calculated using custom MATLAB software and are displayed beneath each image. Image analysis was impossible via this method for populations where very few cells were seen to form microcolonies/biofilms (not applicable [N/A]). Individual color contrasts have been adjusted using Adobe Photoshop for printing purposes.
Wild-type cells formed classical biofilms, with pillars up to 20 μm high. FliA and FlgM activity had no effect on exopolysaccharide (EPS) production when measured using 10 μM calcofluor in-plate staining (data not shown). However, the ΔflgM strain produced only small microcolonies on surfaces and the ΔfliA strain produced neither biofilms nor microcolonies, suggesting that motility is required for surface adhesion and biofilm nucleation under static conditions. Interestingly, when grown as cocultures with wild-type cells, neither ΔfliA nor ΔflgM cells grew within developing wild-type biofilms but remained planktonic, suggesting that either motility or genes regulated by FliA and FlgM are required for development within growing biofilms.
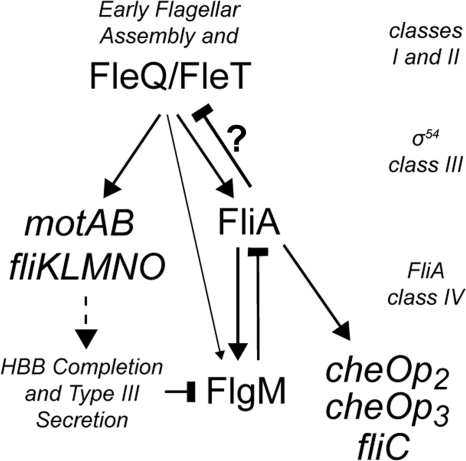
Here, FliA and FlgM have been shown to be part of a complex regulatory network controlling expression of late flagellar genes and chemotaxis genes within R. sphaeroides (Fig. 3). Both fliA and flgM are essential for the coordinated synthesis of a single flagellum. Disruption of either gene results in a dramatic decrease in population motility and the ability to form biofilms. As well as promoting the completion of flagellar assembly by upregulating the expression of class IV flagellar genes (including flagellin), the increase in FliA activity that occurs upon FlgM secretion regulates flagellar number in R. sphaeroides by repressing further expression of early flagellar genes on completion of the first HBB. Although the mechanism for FliA's repression of early flagellar gene expression remains unknown, the all-or-none nature of FlgM secretion through a single flagellum in R. sphaeroides makes this a true checkpoint in the flagellar assembly hierarchy. Interestingly, FlgM mutations in some other species also affect motility (4), suggesting that this mechanism may also be used by other monotrichous bacteria to regulate flagellum number.
Fig. 3.
Schematic representation of FliA and FlgM within the flagellar assembly pathway of R. sphaeroides. In this model, expression from FleQ/FleT-dependent class III promoters of motAB, fliKLMNO, flgM, and fliA is activated by expression of the early flagellar genes and fleQ. FliA activity represses the expression of early flagellar genes (by an unknown mechanism) and promotes expression of the class IV genes, including fliC, two chemotaxis operons, and flgM. This allows completion of the first flagellum and inhibits production of a second. FlgM inhibits FliA activity but is itself secreted from the cell temporally upon completion of the HBB complex, controlling class III and IV gene expression and maintaining the monotrichous nature of the cell. Arrows represent interactions that promote expression. Bars represent inhibitory interactions or processes. The dotted arrow denotes the facilitation of HBB assembly by expression of its components. ? indicates that the mechanism of inhibition of the class III genes is unknown and that it may or may not be achieved via interaction with the class III enhancer proteins FleQ and FleT.
Acknowledgments
This work was supported by the BBSRC and the Oxford Centre for Integrative Systems Biology.
Footnotes
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Anderson J. K., Smith T. G., Hoover T. R. 2010. Sense and sensibility: flagellum-mediated gene regulation. Trends Microbiol. 18:30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armitage J. P., Macnab R. M. 1987. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J. Bacteriol. 169:514–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dang H. Y., Lovell C. R. 2002. Numerical dominance and phylotype diversity of marine Rhodobacter species during early colonization of submerged surfaces in coastal marine waters as determined by 16S ribosomal DNA sequence analysis and fluorescence in situ hybridization. Appl. Environ. Microbiol. 68:496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding L., et al. 2009. Functional characterization of FlgM in the regulation of flagellar synthesis and motility in Yersinia pseudotuberculosis. Microbiology 155:1890–1900 [DOI] [PubMed] [Google Scholar]
- 5. Haya S., Tokumaru Y., Abe N., Kaneko J., Aizawa S. 2011. Characterization of lateral flagella of Selenomonas ruminantium. Appl. Environ. Microbiol. 77:2799–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ind A. C., et al. 2009. Inducible-expression plasmid for Rhodobacter sphaeroides and Paracoccus denitrificans. Appl. Environ. Microbiol. 75:6613–6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin A. C., Gould M., Byles E., Roberts M. A., Armitage J. P. 2006. Two chemosensory operons of Rhodobacter sphaeroides are regulated independently by sigma 28 and sigma 54. J. Bacteriol. 188:7932–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merritt P. M., Danhorn T., Fuqua C. 2007. Motility and chemotaxis in Agrobacterium tumefaciens surface attachment and biofilm formation. J. Bacteriol. 189:8005–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pena-Sanchez J., et al. 2009. Identification of the binding site of the sigma 54 hetero-oligomeric FleQ/FleT activator in the flagellar promoters of Rhodobacter sphaeroides. Microbiology 155:1669–1679 [DOI] [PubMed] [Google Scholar]
- 10. Poggio S., Osorio A., Dreyfus G., Camarena L. 2005. The flagellar hierarchy of Rhodobacter sphaeroides is controlled by the concerted action of two enhancer-binding proteins. Mol. Microbiol. 58:969–983 [DOI] [PubMed] [Google Scholar]
- 11. Porter S. L., Wadhams G. H., Armitage J. P. 2011. Signal processing in complex chemotaxis pathways. Nat. Rev. Microbiol. 9:153–165 [DOI] [PubMed] [Google Scholar]
- 12. Wadhams G. H., Armitage J. P. 2004. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5:1024–1037 [DOI] [PubMed] [Google Scholar]
- 13. Wadhams G. H., Martin A. C., Warren A. V., Armitage J. P. 2005. Requirements for chemotaxis protein localization in Rhodobacter sphaeroides. Mol. Microbiol. 58:895–902 [DOI] [PubMed] [Google Scholar]
- 14. Wang Y., et al. 2007. The flhDC gene affects motility and biofilm formation in Yersinia pseudotuberculosis. Sci. China C Life Sci. 50:814–821 [DOI] [PubMed] [Google Scholar]
- 15. Winkler J., et al. 2010. Quantitative and spatio-temporal features of protein aggregation in Escherichia coli and consequences on protein quality control and cellular ageing. EMBO J. 29:910–923 [DOI] [PMC free article] [PubMed] [Google Scholar]