Abstract
Autotransporters are a superfamily of virulence factors produced by Gram-negative bacteria that are comprised of an N-terminal extracellular domain (passenger domain) and a C-terminal β barrel domain (β domain) that resides in the outer membrane (OM). The β domain promotes the translocation of the passenger domain across the OM by an unknown mechanism. Available evidence indicates that an α-helical segment that spans the passenger domain-β domain junction is embedded inside the β domain at an early stage of assembly. Following its secretion, the passenger domain of the serine protease autotransporters of the Enterobacteriaceae (SPATEs) and the pertactin family of Bordetella pertussis autotransporters is released from the β domain through an intrabarrel autoproteolytic cleavage of the α-helical segment. Although the mutation of conserved residues that surround the cleavage site has been reported to impair both the translocation and cleavage of the passenger domain of a SPATE called Tsh, we show here that the mutation of the same residues in another SPATE (EspP) affects only passenger domain cleavage. Our results strongly suggest that the conserved residues are required to position the α-helical segment for the cleavage reaction and are not required to promote passenger domain secretion.
INTRODUCTION
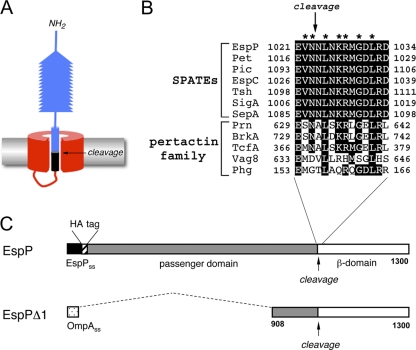
Autotransporters are a large superfamily of virulence factors produced by Gram-negative bacteria. They consist of two domains, an N-terminal extracellular domain (passenger domain) that often exceeds 100 kDa in length, and a C-terminal ∼30-kDa β barrel domain (β domain) that anchors the protein in the outer membrane (OM) (4). Passenger domains are highly divergent in sequence and mediate a wide range of virulence functions (9). Following their transport across the OM, many passenger domains are released from the β domain by a proteolytic cleavage. Despite their sequence diversity, it is likely that the vast majority of passenger domains form an elongated β-helical structure (7, 8, 14, 17, 26). Autotransporter β domains are also highly variable in sequence, but crystallographic analysis indicates that they fold into nearly superimposable 12-stranded β barrel structures (3, 25, 37). The pore of the β barrel is traversed by an α-helical segment that protrudes into the extracellular space and links the β domain to the passenger domain (10, 25, 33, 35). This α-helical segment is incorporated into the pore of the β barrel (which presumably acquires considerable tertiary structure in the periplasm) prior to its integration into the OM and is required for its proper folding and stability (3, 13, 25, 28). Furthermore, deletion of the α-helical segment blocks passenger domain secretion (13, 18, 24, 33, 38).
The mechanism by which the passenger domain is transported across the OM is currently unclear. Based on the observation that the deletion of the β domain abolishes passenger domain secretion, it was originally proposed that the β domain functions as the transporter for the covalently linked passenger domain (29). Because translocation proceeds in a C- to N-terminal direction (13, 16), and most (if not all) β domains are monomeric (11, 20, 22, 31), translocation would almost certainly be initiated by the insertion of a C-terminal segment of the passenger domain into the β domain pore in a hairpin conformation. Subsequently, more N-terminal segments of the passenger domain would slide progressively past a static strand. Several lines of evidence, however, have recently challenged the “autotransporter” hypothesis. First, the pore of the β domain that has been observed in crystal structures is only wide enough to accommodate a hairpin in a fully extended conformation or a single α helix (3, 25, 37), but the aforementioned α-helical segment appears to reside inside the pore throughout the translocation reaction (13, 28). Second, at least some folded polypeptide segments that are too large to fit through the β domain pore (including segments of naturally occurring passenger domains) are secreted efficiently via the autotransporter pathway (15, 31). Third, photo-cross-linking experiments using transiently stalled passenger domain translocation intermediates have strongly suggested that the Bam complex, a hetero-oligomer that facilitates the integration of β barrel proteins into the OM (39, 40), also facilitates passenger domain secretion (12, 28). Finally, the interior of the β domain pore is strikingly diverse in sequence and lacks residues that are conserved throughout the autotransporter superfamily that might play a catalytic role in the translocation reaction. It should be noted, however, that none of these observations exclude the possibility that the passenger domain is secreted through an expanded or incompletely assembled β domain pore.
Regardless of the secretion mechanism, passenger domains are released from the cell surface by a variety of different proteolytic mechanisms (4). The passenger domains of the closely related serine protease autotransporters of Enterobacteriaceae (SPATEs) and many of the members of the pertactin family of Bordetella pertussis autotransporters are cleaved in a unique intrabarrel autocatalytic reaction (3, 6, 35). In this reaction, the cyclization of a conserved asparagine residue on the N-terminal side of the cleavage site (the P1 position) leads to cleavage of the polypeptide chain at a break in the α helix. Following cleavage and release of the passenger domain, only a small fragment of the original α helix remains inside the barrel. Both the activation of the amide group of the catalytic asparagine residue and the nucleophilic attack on the polypeptide backbone appear to require a precise alignment of a number of key residues inside the lumen of the β barrel (3, 35).
In this study, we examined the function of an invariant 14-residue segment of the α helix that spans the passenger domain-β domain junction of the SPATEs using the Escherichia coli O157:H7 autotransporter EspP as a model protein. Given that this segment is required for β domain assembly, and that the β domain of the SPATEs is highly conserved (>60% identity across the entire family), the invariance of this segment might not seem especially noteworthy. This segment is also highly conserved in the pertactin family of autotransporters and other autotransporters, including Hsr from Helicobacter mustelae and AIDA from E. coli, whose β domains are quite divergent (19) (see Fig. 1B). Two leucine residues are invariant within this larger group of proteins, and only very conservative substitutions are observed at several other positions. For this reason, one might imagine that the 14-residue segment has a broad functional role in autotransporter biogenesis. Consistent with this hypothesis, experiments with a SPATE called Tsh have suggested that the mutation of seven of the 14 residues (including the catalytic asparagine that is required for passenger domain cleavage) impairs passenger domain secretion without affecting the insertion of the β domain into the OM (19). In contrast, it was shown that the introduction of a largely nonoverlapping set of mutations in this region in EspP impaired passenger domain cleavage without affecting translocation (6). These experiments, however, utilized a version of EspP that contains a truncated passenger domain whose secretion is less sensitive to mutation than the full-length passenger domain (28, 38). To clarify the role of the invariant 14-residue segment in the biogenesis of SPATEs, we examined the effect of the same 7 mutations on the biogenesis of full-length EspP. We found that none of the mutations significantly affected passenger domain translocation. All of the mutations, however, impaired the cleavage of the EspP passenger domain to the same degree that they impair the processing of Tsh. Our results strongly suggest that the conserved 14-residue segment is required to position the passenger domain for cleavage.
Fig. 1.
The sequence surrounding the passenger domain cleavage site is conserved in SPATEs and pertactin-like proteins. (A) Illustration of the structure of SPATE and pertactin-like proteins prior to passenger domain cleavage (based on references 3 and 35). The passenger domain is separated from the β domain by an intrabarrel proteolytic reaction following its translocation across the OM. (B) Sequence of the α-helical segment surrounding the passenger domain cleavage site in SPATE and pertactin-like proteins. Highly conserved residues are shaded black. Residues that were mutated in EspP in this study (and that were also mutated in Tsh in reference 19) are denoted with an asterisk. (C) Schematic representation of EspP and EspPΔ1. EspP contains a 55-residue signal sequence (SS) and a 968-residue passenger domain. The version of EspP that we studied here also contains an N-terminal HA tag. EspPΔ1 contains the OmpA signal sequence (which is functionally indistinguishable from the native EspP signal peptide [see reference 34]) and only the last 116 residues of the EspP passenger domain.
MATERIALS AND METHODS
Bacterial strains, plasmids, and antisera.
E. coli strain AD202 (MC4100 ompT::kan) (1) was used for all experiments. Plasmid pJH62, which encodes EspPΔ1, plasmid pKMS3, which encodes EspPΔ*1, plasmid pRSL6, which encodes HA-tagged EspP, and plasmid pRSL11, which encodes HA-tagged EspP*, have been described previously (6, 31, 34). Point mutations were introduced into EspP using the PCR overlap extension method (2) with either pJH62 or pRSL6 as a template. The primers that were used to generate all mutations are shown in Table 1. A hexahistidine tag was placed at the N terminus of EspPΔ1 by inserting oligonucleotides that have been previously described (31) into the EagI site of pJH62 to create pND10. Rabbit polyclonal antisera raised against N- and C-terminal peptides of EspP and a C-terminal peptide of YidC, a factor that facilitates the assembly of inner membrane proteins, have been previously described (5, 34). To purify the EspPΔ1 passenger domain, BL21-CodonPlus(DE3)-RIL (Stratagene) transformed with pND10 was grown in 1 liter M9 containing 0.2% glucose and all the l-amino acids except methionine and cysteine (M9-AA). When the culture reached an optical density at 550 nm (OD550) of 0.2, 10 μM IPTG (isopropylthiogalactopyranoside) was added. Following 2.5 h of incubation, the culture was chilled on ice, and cells were removed by centrifugation. The supernatant was applied to 3 ml Ni-nitrilotriacetic acid (NTA) resin (Invitrogen) equilibrated with 50 mM K2HPO4 (pH 7.4). The column was washed once with buffer A (50 mM K2HPO4 [pH 7.4]-200 mM NaCl) containing 20 mM imidazole and once with buffer A containing 50 mM imidazole. The EspPΔ1 passenger domain was then eluted with buffer A containing 200 mM imidazole, precipitated with trichloroacetic acid (TCA), and further purified by SDS-PAGE. The gel-purified protein was then used to generate a polyclonal rabbit antiserum.
Table 1.
Sequences of oligonucleotides used for site-directed mutagenesis
| Primer | Sequence (5′-3′)a |
|---|---|
| Dir-V1022A | TTTCTGAACGAGGCCAACAACCTGA |
| Rev-V1022A | TCAGGTTGTTGGCCTCGTTCAGAAA |
| Dir-V1022R | TTTCTGAACGAGCGCAACAACCTGA |
| Rev-V1022R | TCAGGTTGTTGCGCTCGTTCAGAAA |
| Dir-N1023A | TGAACGAGGTCGCCAACCTGAACAAA |
| Rev-N1023A | TTTGTTCAGGTTGGCGACCTCGTTCA |
| Dir-L1025A | AGTCAACAACGCGAACAAACGTAT |
| Rev-L1025A | ATACGTTTGTTCGCGTTGTTGACT |
| Dir-L1025R | AGTCAACAACCGGAACAAACGTAT |
| Rev-L1025R | ATACGTTTGTTCCGGTTGTTGACT |
| Dir-K1027A | ACAACCTGAACGCACGTATGGGT |
| Rev-K1027A | ACCCATACGTGCGTTCAGGTTGT |
| Dir-R1028A | CAACCTGAACAAAGCTATGGGTGACCT |
| Rev-R1028A | AGGTCACCCATAGCTTTGTTCAGGTTG |
| Dir-R1028K | CAACCTGAACAAAAAAATGGGTGACCT |
| Rev-R1028K | AGGTCACCCATTTTTTTGTTCAGGTTG |
| Dir-G1030A | CAAACGTATGGCTGACCTGCGT |
| Rev-G1030A | ACGCAGGTCAGCCATACGTTTG |
| Dir-G1030R | CAAACGTATGCGTGACCTGCGT |
| Rev-G1030R | ACGCAGGTCACGCATACGTTTG |
| Dir-L1032A | ATGGGTGACGCGCGTGATATC |
| Rev-L1032A | GATATCACGCGCGTCACCCAT |
| Dir-L1032R | GATATCACGCCGGTCACCCAT |
| Rev-L1032R | ATGGGTGACCGGCGTGATATC |
The mutated nucleotides are in boldface.
Kinetic analysis of EspP biogenesis in vivo.
To examine the kinetics of EspP biogenesis, cells were grown in M9-AA. Overnight cultures were washed and diluted into fresh medium at an OD550 of 0.02. When the cultures reached an OD550 of 0.2, synthesis of plasmid-borne genes was induced for 30 min by the addition of 10 μM IPTG. Pulse-chase labeling was then conducted as described previously (35). Aliquots of radiolabeled cells were pipetted over ice and collected by centrifugation. Cell pellets were resuspended in phosphate-buffered saline (PBS) and divided into two equal portions. Cold 10% TCA was added to one half of the cells. The other half was treated with 200 μg/ml proteinase K (PK) for 20 min on ice. The protease reaction was stopped by the addition of 1 mM phenylmethanesulfonyl fluoride (PMSF) before TCA precipitation. TCA precipitates were then solubilized, and immunoprecipitations were performed as described previously (36).
Analysis of EspP translocation at steady state.
To analyze EspP biogenesis under steady-state conditions, cells were grown in LB to an OD550 of 0.2 to 0.3 and EspP synthesis was induced by the addition of 10 μM IPTG. After induction (30 min for EspPΔ1 derivatives or 1 h for hemagglutinin [HA]-tagged full-length EspP derivatives), two 1-ml aliquots were removed from each culture. One aliquot was treated with the indicated amount of PK for 20 min on ice, and the other aliquot was placed on ice and left untreated. Following the addition of 1 mM PMSF to stop proteolysis, all samples were subjected to TCA precipitation. In experiments in which OMs were permeabilized, cells were harvested by centrifugation (20,817 × g, 10 min, 4°C), resuspended in spheroplast buffer (40% sucrose, 33 mM Tris-HCl [pH 8.0]), and incubated with 100 μg/ml lysozyme-2 mM EDTA on ice for 20 min. The permeabilized cell suspension (spheroplast suspension) was then divided in half, and one half was treated with PK as described above.
Gel electrophoresis and Western blotting.
Proteins were resolved by SDS-PAGE using 8 to 16% Tris-glycine minigels (Invitrogen). For Western blotting, horseradish peroxidase (HRP)-linked protein A (Amersham) was used in conjunction with the SuperSignal pico chemiluminescence kit (Pierce) to detect antibody-antigen complexes.
RESULTS
Mutations in a conserved α-helical segment impair the cleavage but not the secretion of a truncated EspP passenger domain.
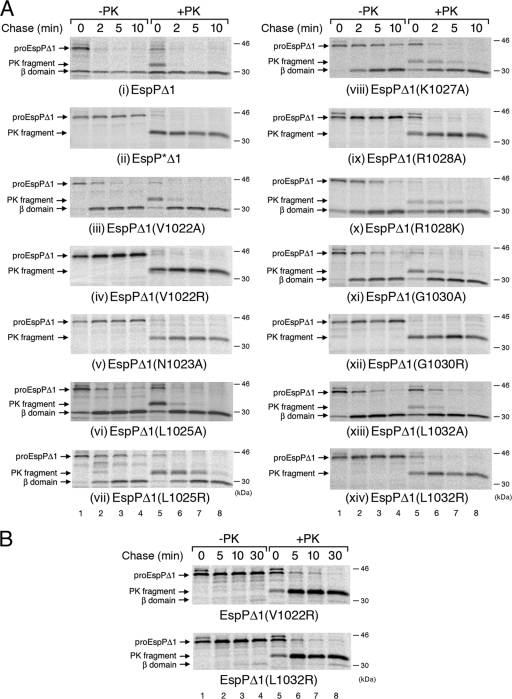
To analyze the function of the invariant motif that spans the SPATE passenger domain-β domain junction (Fig. 1A and B), we first introduced the same point mutations that have been shown to impair the biogenesis of Tsh (19) into EspPΔ1, a truncated version of EspP that contains only the C-terminal 116 residues of the 968-residue passenger domain (Fig. 1C). We used EspPΔ1 in our initial experiments because its passenger domain is secreted and cleaved faster (and more synchronously) than the full-length passenger domain (31, 34), and it shows defects in protein biogenesis (including kinetic defects) more clearly. AD202 was transformed with a plasmid encoding EspPΔ1, EspP*Δ1 (a noncleavable derivative of EspPΔ1 that harbors a N1023S/N1024S double mutation [see reference 31]), or EspPΔ1 containing a mutation at V1022, N1023, L1025, K1027, R1028, G1030, or L1032 under the control of the trc promoter. Cells were grown in minimal medium, and synthesis of the EspP derivatives was induced with IPTG. Following pulse-chase labeling, each sample was divided in half, and one portion was treated with proteinase K (PK) to digest the passenger domain population that was exposed on the cell surface. EspP-containing polypeptides were then immunoprecipitated with an antiserum raised against a C-terminal EspP peptide. Consistent with previous results (31, 34), the passenger domain of wild-type EspPΔ1 was secreted and cleaved rapidly. Nearly half of the pulse-labeled pro-EspPΔ1 (the form of the protein containing covalently linked passenger and β domains) was processed to discrete passenger and β domain fragments (as reflected by immunoprecipitation of the free β domain), and all of the protein was processed within 2 min (Fig. 2A, panel i, lanes 1 to 4). An ∼33-kDa fragment that corresponds to a population of pro-EspPΔ1, whose passenger domain has been exposed on the cell surface but not cleaved (31), was observed only in pulse-labeled cells that were treated with PK (Fig. 2A, panel i, lane 5). In contrast, none of the EspP*Δ1 passenger domain was processed even after 10 min (Fig. 2A, panel ii, lanes 1 to 4). The observation that PK treatment converted most of the pulse-labeled pro form of EspP*Δ1 to an ∼33-kDa fragment shows that the passenger domain was rapidly exposed on the cell surface (Fig. 2A, panel ii, lanes 5 to 8).
Fig. 2.
Effect of cleavage site mutations on the kinetics of EspPΔ1 passenger domain translocation and cleavage. AD202 (MC4100 ompT::kan) producing the indicated mutants was pulse-labeled and subjected to a chase of up to 10 min (A) or 30 min (B) after the addition of IPTG, and PK was added to half of each sample. Immunoprecipitations were then conducted using an antiserum directed against a C-terminal EspP peptide. Proteins were resolved by SDS-PAGE and visualized using a Fuji BAS-2500 phosphorimager.
While most of the mutations that we introduced caused defects in passenger domain cleavage, none of them appeared to significantly affect passenger domain translocation. Consistent with previous results (6), mutation of the catalytic asparagine (N1023) to alanine completely abolished passenger domain cleavage (Fig. 2A, panel v, lanes 1 to 4). The observation that most of the pro form of the protein was accessible to PK digestion even at the earliest time point showed that the mutation had no effect on the cell surface exposure of the passenger domain (Fig. 2A, panel v, lanes 5 to 8). Like the N1023A mutation, four other mutations (V1022R, R1028A, G1030R, and L1032R) caused very strong cleavage defects but did not appear to affect translocation (Fig. 2A, panels iv, ix, xii, and xiv). The V1022R and L1032R mutations did not completely abolish cleavage because small amounts of free β domain could be observed after a very long chase (30 min) (Fig. 2B). The L1025R, K1027A, R1028K, and G1030A mutations did not affect the cell surface exposure of the passenger domain but delayed the appearance of the free β domain and therefore appeared to impair the cleavage reaction (Fig. 2A, panels vii, viii, x, and xi). The V1022A, L1025A, and L1032A mutations were the mildest. While the L1032A mutation appeared to delay the onset of passenger domain translocation slightly, this group of mutations did not otherwise significantly affect protein biogenesis (Fig. 2A, panels iii, vi, and xiii). These results are striking given that mutations corresponding to the V1022R, L1025R, K1027A, R1028A, R1028K, G1030R, and L1032R mutations in EspP have been reported to severely affect the secretion of the closely related Tsh passenger domain (19).
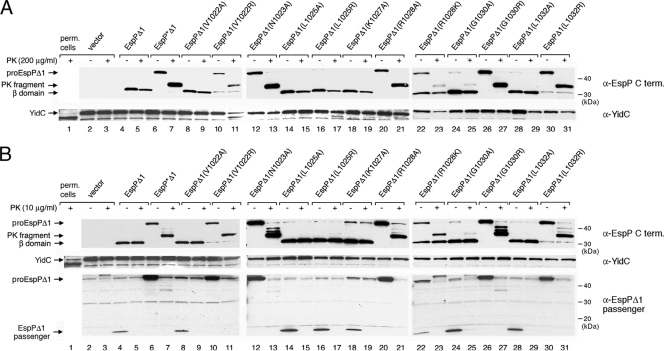
Because growth conditions can influence the efficiency of passenger domain secretion (31), we first considered the possibility that the disparity between our results and previous results might be due to our use of minimal medium instead of LB. To test this possibility, we grew AD202 transformed with a plasmid that bears wild-type espPΔ1 or an espPΔ1 mutant in LB, added IPTG to induce expression of the plasmid-borne gene, and harvested cells 30 min later. Culture samples were then removed and divided in half, and one portion was treated with 200 μg/ml PK. Passenger domain translocation and cleavage were then monitored by Western blotting using the C-terminal anti-EspP antiserum. The results indicated that the mutant phenotypes that we observed when cells were grown in minimal medium were not significantly altered by growing the cells in LB. Mutations that did not significantly affect the cell surface exposure or cleavage of the passenger domain in minimal medium (V1022A, L1025A, and L1032A) did not impair EspPΔ1 biogenesis in rich medium either. The translocation and cleavage of each of the mutant passenger domains appeared to be highly efficient because only an ∼30-kDa band that corresponds to the free β domain could be detected (Fig. 3A, lanes 8 to 9, 14 to 15, and 28 to 29). Four mutations that appeared only to delay passenger domain cleavage in pulse-chase experiments (L1025R, K1027A, R1028K, and G1030A) had either no effect or a very slight effect on EspPΔ1 biogenesis when cells were grown in rich medium (Fig. 3A, lanes 16 to 19 and 22 to 25). Likewise, mutations that blocked the cleavage but not the cell surface exposure of the passenger domain in pulse-chase experiments (N1023A, R1028A, and G1030R) had the same effect when cells were grown in LB. In each case, no free β domain was observed, and PK treatment quantitatively converted the pro-EspPΔ1 to an ∼33-kDa fragment (Fig. 3A, lanes 12 to 13, 20 to 21, and 26 to 27). The V1022R and L1032R mutations, which profoundly delayed the appearance of the β domain in minimal medium, also strongly inhibited passenger domain cleavage in LB (Fig. 3A, lanes 10 to 11 and 30 to 31).
Fig. 3.
Effect of cleavage site mutations on EspPΔ1 passenger domain translocation and cleavage in rich medium. AD202 transformed with plasmids encoding the indicated mutants was grown in LB, and the production of the EspPΔ1 derivatives was induced by the addition of IPTG. Culture samples (containing cells and culture supernatants) were removed, and half of each sample was treated with 200 μg/ml PK (A) or 10 μg/ml PK (B). Western blotting was then conducted using antisera directed against a C-terminal EspP peptide, YidC, and the EspPΔ1 passenger domain.
We next wished to confirm that the ∼33-kDa PK fragment that we observed resulted from cell surface exposure of the passenger domain. It is conceivable that an ∼33-kDa fragment was produced because the OM was leaky and PK had access to a membrane-integrated form of EspPΔ1 whose passenger domain was trapped inside the periplasm. To rule out this possibility, we monitored the status of YidC, an inner membrane protein that is cleaved by exogenous proteases when the OM is permeabilized (30). Consistent with previous results, we found that the ∼60-kDa YidC protein was reduced to an ∼55-kDa fragment when we converted cells to spheroplasts (Fig. 3A, lane 1). YidC generally remained intact in cells that produced the EspPΔ1 mutants, although a fraction of the YidC was cleaved in a few samples (Fig. 3A, lanes 11, 17, and 21). The results suggest that only a minority of the cells were leaky even in the most extreme case. Furthermore, when we repeated the experiment and reduced the PK concentration to 10 μg/ml, the pro-EspPΔ1 was still converted to an ∼33-kDa fragment (albeit slightly less efficiently), but no YidC cleavage was observed (Fig. 3B). Taken together, the results show that the production of the EspPΔ1 mutants does not significantly affect the integrity of the OM.
Finally, to confirm that the mutant passenger domains were cleaved after their transport across the OM, we conducted Western blotting with an antiserum that recognizes the EspPΔ1 passenger domain. As expected, the full-length EspPΔ1 passenger domain (∼13 kDa) was detected whenever efficient passenger domain cleavage was observed (Fig. 3B, bottom panel). Because the passenger domain was completely degraded by PK, it must have been present in the extracellular space and must have been secreted before it was cleaved. These results are consistent with the observation that passenger domain cleavage is dependent on the completion of passenger domain translocation (12, 28, 31) and rule out the possibility that one or more of the mutant passenger domains were retained in the periplasm and cleaved in an aberrant reaction.
Mutations in a conserved α-helical segment impair the cleavage but not the secretion of the full-length EspP passenger domain.
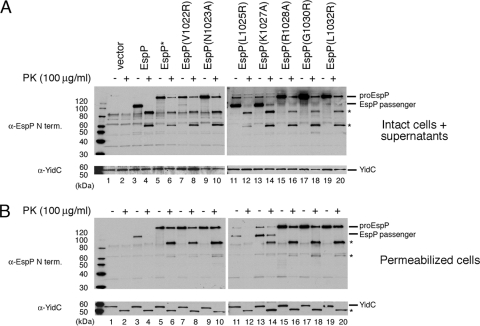
Consistent with previous work on Tsh (19), the results described above indicate that mild mutations in the α-helix that spans the passenger domain-β domain cleavage junction have no effect or a very modest effect on EspP biogenesis, while nonconservative mutations exert a strong effect. Whereas nonconservative mutations in Tsh were reported to impair passenger domain translocation, the equivalent mutations in EspPΔ1 appeared to impair passenger domain cleavage. We could not rule out the possibility, however, that this discrepancy was due to our use of a truncated form of EspP. Indeed, it has been shown that defects in the biogenesis of full-length EspP are not always observed in experiments that involve the use of truncated versions of the protein (38). To address this potential limitation of our experimental design, we next introduced the mutations that produced the most severe defects in EspPΔ1 biogenesis (V1022R, N1023A, L1025R, K1027A, R1028A, G1030R, and L1032R) into the full-length protein. AD202 transformed with a plasmid encoding EspP, EspP* (a derivative that contains an uncleavable passenger domain), or one of the EspP point mutants was grown in LB, and synthesis of the EspP derivatives was induced by the addition of IPTG. Culture samples containing cells and culture supernatants were then removed and divided in half. One half of each sample was treated with 100 μg/ml PK (to eliminate the slight loss of OM integrity observed at a concentration of 200 μg/ml), and Western blotting was conducted using an antiserum directed against an N-terminal EspP peptide. The observation that YidC was completely intact in all the samples showed that the permeability of the OM was not compromised under our experimental conditions (Fig. 4A, bottom panel).
Fig. 4.
Effect of cleavage site mutations on the translocation and cleavage of the full-length EspP passenger domain. (A) AD202 transformed with plasmids encoding the indicated mutants was grown in LB, and the production of the EspP derivatives was induced by the addition of IPTG. Culture samples (containing cells and culture supernatants) were removed, and half of each sample was treated with 100 μg/ml PK. Western blotting was then conducted using antisera directed against an N-terminal EspP peptide and YidC. (B) The experiment described for panel A was repeated, except that cells were permeabilized prior to the addition of PK. Asterisks denote ∼60-kDa and ∼80-kDa N-terminal fragments of EspP and an ∼55-kDa C-terminal fragment of YidC.
Overall, the mutations appeared to affect the biogenesis of the full-length passenger domain to the same degree as they affected the biogenesis of the EspPΔ1 passenger domain. As expected, the wild-type passenger domain was cleaved efficiently, and no unprocessed precursor was observed (Fig. 4A, lane 3). In contrast, several mutations that abolished the cleavage of the truncated passenger domain (V1022R, N1023A, R1028A, G1030R, and L1032R) blocked the production of the cleaved full-length passenger domain and led to the accumulation of pro-EspP (Fig. 4A, lanes 7, 9, 15, 17, and 19). Although the L1025R and K1027A mutations did not strongly affect proteolytic processing, a small amount of the precursor was observed (Fig. 4A, lanes 11 and 13). Because no wild-type pro-EspP could be detected, the results strongly suggest that the L1025R and K1027A mutations delayed the cleavage of the full-length passenger in the same way that they delayed the cleavage of the truncated passenger domain.
The observation that most of the pro-EspP form of the mutant proteins was sensitive to PK digestion showed that the mutations did not exert a significant affect on passenger domain translocation. PK treatment of samples in which pro-EspP was processed efficiently (i.e., samples containing cells that produced wild-type EspP or the L1025R or K1027A mutants) converted about half of the cleaved passenger domain to an ∼80-kDa N-terminal fragment and about half to an ∼60-kDa N-terminal fragment (Fig. 4A, lanes 4, 12, and 14, asterisks). Although the smaller fragment has not been previously observed, the larger fragment presumably corresponds to a PK-resistant fragment that appears in pulse-chase experiments in parallel with the cleaved passenger domain (12, 28). Interestingly, PK treatment also converts Tsh to a ∼90-kDa fragment (19). Thus, it seems likely that the passenger domains of EspP and Tsh adopt similar PK-resistant conformations during their maturation. PK treatment of samples in which large amounts of unprocessed pro-EspP accumulated also converted most of the precursor to a roughly equal mixture of ∼80-kDa and ∼60-kDa fragments (Fig. 4A, lanes 6, 8, 10, 16, 18, and 20). These results not only demonstrate that the passenger domain of the mutant proteins was translocated across the OM effectively (and that the mutations therefore affect the cleavage reaction directly) but also show that the mutations do not significantly affect passenger domain folding.
The finding that PK did not completely digest the precursor form of EspP suggested that the mutations might slightly impair passenger domain translocation. To examine this possibility, we collected cells by centrifugation, generated spheroplasts by permeabilizing the OM, and treated half of each spheroplast suspension with 100 μg/ml PK. The quantitative conversion of YidC to an ∼55-kDa fragment by PK showed that all of the cells were permeabilized (Fig. 4B, bottom panel). As we expected, most of the cleaved passenger domain remained in the supernatant when the cells were isolated and was therefore detected at reduced levels. Nevertheless, the residual passenger domain that was associated with the OM was sensitive to PK digestion (Fig. 4B, lanes 4, 12, and 14). Possibly because the ∼60-kDa PK fragment is somewhat unstable in spheroplast buffer, the ∼80-kDa PK fragment predominated. PK converted the pro-EspP that accumulated in cells that synthesized several of the EspP mutants to the same ∼80-kDa and ∼60-kDa fragments (Fig. 4B, lanes 6, 8, 10, 16, 18, and 20). The observation that these fragments were generated even after the OM was permeabilized rules out the possibility that they correspond to N-terminal segments that were trapped in the periplasm due to the stalling of passenger domain translocation. Interestingly, some of the pro-EspP was completely resistant to PK digestion. These results strongly suggest that the low levels of PK-resistant pro-EspP that we observed are due to the aggregation or aberrant folding of a fraction of the protein rather than to a translocation defect. Because the PK-resistant fraction was observed only when pro-EspP accumulated (for example, it was not observed when wild-type EspP was produced), aggregation likely occurs when the passenger domain is transported across the OM but not cleaved. In any case, taken together, our results show that nonconservative mutations near the passenger domain-β domain junction impair the proteolytic processing but not the translocation of truncated and full-length passenger domains.
DISCUSSION
In this study, we used EspP as a model protein to investigate the function of a sequence motif (1021EVNNLNKRMGDL1032) surrounding the passenger domain cleavage site that is invariant in the SPATEs and highly conserved in the pertactin family of autotransporters. This motif forms a discontinuous α helix that is embedded inside the β domain (3, 35). In both families, the polypeptide chain is cleaved after the first asparagine residue, which is located in a break in the α-helical structure (6, 35). The deletion of residues 1020 to 1032 has previously been shown to destabilize the EspP β domain and prevent its integration into the OM, and the deletion of a similar fragment of the pertactin-like protein BrkA likewise perturbs protein biogenesis (13, 24). We found that conservative mutations in the motif produced no effect or very slight effects on passenger domain translocation and cleavage but that several nonconservative mutations (as well as a mutation that eliminates the catalytic N1023 residue) drastically impaired passenger domain cleavage. The nonconservative mutations, however, had no effect on passenger domain translocation. Very similar defects were observed when the mutations were introduced into either a truncated version of EspP (EspPΔ1) or the full-length protein. In addition, the mutant phenotypes appeared to be independent of growth conditions. Our results are consistent with the results of previous studies showing that the L1025P, K1027I, and D1031P mutations in EspP and a double mutation that alters both asparagine residues flanking the cleavage site of another SPATE (Pet) do not significantly impair passenger domain secretion (23, 38).
Our results are striking because the introduction of the same nonconservative mutations into Tsh (including mutations that are equivalent to V1022R, N1023A, L1025R, K1027A, R1028A, G1030R, and L1032R) has been reported to strongly impair passenger domain translocation (19). The conclusion that the mutations impair the translocation of the Tsh passenger domain was based on the observation that the accumulated pro-Tsh was resistant to PK digestion. Although it is possible that the same mutations would produce very different effects on EspP and Tsh biogenesis, this scenario seems unlikely given the similarity between the sequences of the two proteins and the similarity of the magnitudes of the effects. It is conceivable that the disparity in results is due to the use of different E. coli strains (AD202 versus XL1-Blue). At least in our experience, however, the patterns of autotransporter biogenesis are similar in different strain backgrounds (R. L. Szabady, K. M. Skillman, and H. D. Bernstein, unpublished results). Alternatively, it is possible that the disparity reflects a difference in the level of expression. Although it is unclear whether Tsh was produced at a higher level than EspP, we have observed an increase in the amount of pro-EspP that is resistant to PK digestion when the synthesis of full-length EspP passenger domain cleavage mutants is increased (data not shown). Presumably, the increase in PK resistance is due to enhanced aggregation of the overproduced passenger domain after it is transported across the OM. Finally, the disparity in results may reflect ambiguities in the phenotype of the Tsh mutants that arise from technical considerations. For example, it is unclear why permeabilization of the OM did not sensitize the accumulated pro-Tsh to PK digestion or why the cleaved Tsh passenger domain that was generated during the maturation of the mutant proteins (but not wild-type Tsh) was resistant to PK digestion. The pro-Tsh could be sensitized to PK digestion but only when additional experimental manipulations were conducted to isolate OM vesicles. It is also curious that the introduction of the S259A mutation led to the accumulation of pro-Tsh. While this mutation inactivates the N-terminal serine protease, it has been established that the serine protease of SPATEs is not involved in passenger domain processing (27, 32, 34, 38).
Given the nature of the SPATE (and presumably the pertactin family) passenger domain cleavage mechanism, it is easy to envision how mutations in the passenger domain-β domain junction region might impair proteolysis. As structural analysis has shown, effective autocatalytic cleavage requires both the precise positioning of the catalytic asparagine residue (N1023 in EspP) and an appropriate placement of charged residues within the β barrel (3, 35). The cleavage mechanism has been proposed to involve a deprotonation of the amide group of the catalytic asparagine by a water molecule (35). Mutations such as V1022R, L1025R, and L1032R have the potential to impair cleavage not only by displacing the catalytic residue but also by introducing an uncompensated charge in the vicinity of the cleavage site. Furthermore, previous results strongly suggest that the conserved arginine situated five residues away from the cleavage site (R1028 in EspP) forms a salt bridge with a conserved aspartate (D1120 in EspP) that is essential for proteolysis (3, 6, 35). Consistent with these results, the EspP R1028K mutation (which is predicted to preserve the salt bridge) produces only a small cleavage defect, whereas the R1028A mutation (which generates an uncompensated charge) abolishes cleavage. Finally, G1030 is located at a turn immediately following the α-helical segment (3, 35), and the introduction of a large residue (such as an arginine) at this position could easily produce a conformational change that perturbs the alignment of residues that promote passenger domain cleavage.
In contrast, it is less clear how the sequence of the segment that surrounds the passenger domain cleavage site would exert a direct influence on passenger domain secretion. It was originally proposed that the C terminus of the passenger domain (perhaps together with the N terminus of the β domain) forms a hairpin that inserts into the β domain pore to initiate translocation. This hypothesis implies that the C terminus of the passenger domain is initially in an extended conformation and becomes an α-helix only after completion of the translocation reaction. Recent studies, however, have indicated that the segment that spans the passenger domain cleavage site is embedded inside the β domain in an α-helical conformation prior to the onset of translocation and is maintained in the same conformation throughout the translocation reaction (13, 28). Indeed, it appears that a major function of the α-helical segment is to nucleate early steps in the assembly of the β domain in the periplasmic space. Because assembly presumably involves multiple interactions between the α helix and the β domain (EspP residues 1024 to 1028, 1031, and 1033 all interact with the interior surface of the fully folded β domain [see reference 3]), single point mutations may not have a significant effect on folding. Interestingly, trimeric autotransporters have an α-helical segment that appears to fulfill a similar function. Each of the three subunits of these proteins contributes four β strands to a 12-stranded β barrel as well as an α-helical segment that traverses the β barrel and links the β strands to a passenger domain. Removal of the α helix decreases trimer stability and abolishes passenger domain translocation, but no single residue in the α helix is essential for maintaining the integrity of the β barrel (21). Taken together with previous results, our analysis of the EspP 1021EVNNLNKRMGDL1032 sequence motif supports the view that passenger domain translocation does not proceed simply through the formation of a C-terminal hairpin but instead involves a more complex mechanism that requires other cellular factors.
ACKNOWLEDGMENTS
We thank Travis Barnard for critical reading of the manuscript.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Akiyama Y., Ito K. 1990. SecY protein, a membrane-embedded secretion factor of E. coli, is cleaved by the OmpT protease in vitro. Biochem. Biophys. Res. Commun. 167:711–715 [DOI] [PubMed] [Google Scholar]
- 2. Ansaldi M., Lepelletier M., Mejean V. 1996. Site-specific mutagenesis by using an accurate recombinant PCR method. Anal. Biochem. 234:110–111 [DOI] [PubMed] [Google Scholar]
- 3. Barnard T. J., Dautin N., Lukacik P., Bernstein H. D., Buchanan S. K. 2007. Autotransporter structure reveals intra-barrel cleavage followed by conformational changes. Nat. Struct. Mol. Biol. 14:1214–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernstein H. D. 16 September 2010, posting date Type V secretion: the autotransporter and two-partner secretion pathways. In Böck A., et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC: doi: 10.1128/ecosal.4.3.6 [Google Scholar]
- 5. Choi P. S., Bernstein H. D. 2010. Sequential translocation of an Escherichia coli two-partner secretion pathway exoprotein across the inner and outer membranes. Mol. Microbiol. 75:440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dautin N., Barnard T. J., Anderson D. E., Bernstein H. D. 2007. Cleavage of a bacterial autotransporter by an evolutionarily convergent autocatalytic mechanism. EMBO J. 26:1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emsley P., Charles I. G., Fairweather N. F., Isaacs N. W. 1996. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381:90–92 [DOI] [PubMed] [Google Scholar]
- 8. Gangwer K. A., et al. 2007. Crystal structure of the Helicobacter pylori vacuolating toxin p55 domain. Proc. Natl. Acad. Sci. U. S. A. 104:16293–1629817911250 [Google Scholar]
- 9. Henderson I. R., Navarro-Garcia F., Desvaux M., Fernandez R. C., Ala'Aldeen D. 2004. Type V secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hendrixson D. R., L. de la Morena M., Stathopoulos C., St. Geme J. W., III 1997. Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol. Microbiol. 26:505–518 [DOI] [PubMed] [Google Scholar]
- 11. Hritonenko V., Kostakioti M., Stathopoulos C. 2006. Quaternary structure of a SPATE autotransporter protein. Mol. Membr. Biol. 23:466–474 [DOI] [PubMed] [Google Scholar]
- 12. Ieva R., Bernstein H. D. 2009. Interaction of an autotransporter passenger domain with BamA during its translocation across the bacterial outer membrane. Proc. Natl. Acad. Sci. U. S. A. 106:19120–19125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ieva R., Skillman K. M., Bernstein H. D. 2008. Incorporation of a polypeptide segment into the β-domain pore during the assembly of a bacterial autotransporter. Mol. Microbiol. 67:188–201 [DOI] [PubMed] [Google Scholar]
- 14. Johnson T. A., Qiu J., Plaut A. G., Holyoak T. 2009. Active-site gating regulates substrate selectivity in a chymotrypsin-like serine protease: the structure of Haemophilus influenzae immunoglobulin A1 protease. J. Mol. Biol. 389:559–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jong W. S., et al. 2007. Limited tolerance towards folded elements during secretion of the autotransporter Hbp. Mol. Microbiol. 63:1524–1536 [DOI] [PubMed] [Google Scholar]
- 16. Junker M., Besingi R. N., Clark P. L. 2009. Vectorial transport and folding of an autotransporter virulence protein during outer membrane assembly. Mol. Microbiol. 71:1323–1332 [DOI] [PubMed] [Google Scholar]
- 17. Junker M., et al. 2006. Pertactin β-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. Proc. Natl. Acad. Sci. U. S. A. 103:4918–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klauser T., Krämer J., Otzelberger K., Pohlner J., Meyer T. F. 1993. Characterization of the Neisseria Iga β-core. The essential unit for outer membrane targeting and extracellular protein secretion. J. Mol. Biol. 234:579–593 [DOI] [PubMed] [Google Scholar]
- 19. Kostakioti M., Stathopoulos C. 2006. Role of the α-helical linker of the C-terminal translocator in the biogenesis of the serine protease subfamily of autotransporters. Infect. Immun. 74:4961–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marín E., Bodelón G., Fernández L. Á. 2010. Comparative analysis of the biochemical and functional properties of C-terminal domains of autotransporters. J. Bacteriol. 192:5588–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meng G., Surana N. K., St. Geme III J. W., Waksman G. 2006. Structure of the outer membrane translocator domain of the Haemophilus influenzae Hia trimeric autotransporter. EMBO J. 25:2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Müller D., et al. 2005. Arrangement of the translocator of the autotransporter adhesin involved in diffuse adherence on the bacterial surface. Infect. Immun. 73:3851–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarro-García F., Canizalez-Roman A., Luna J., Sears C., Nataro J. P. 2001. Plasmid-encoded toxin of enteroaggregative Escherichia coli is internalized by epithelial cells. Infect. Immun. 69:1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliver D. C., Huang G., Fernandez R. C. 2003. Identification of secretion determinants of the Bordetella pertussis BrkA autotransporter. J. Bacteriol. 185:489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oomen C. J., et al. 2004. Structure of the translocator domain of a bacterial autotransporter. EMBO J. 23:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Otto B. R., et al. 2005. Crystal structure of hemoglobin protease, a heme binding autotransporter protein from pathogenic Escherichia coli. J. Biol. Chem. 280:17339–17345 [DOI] [PubMed] [Google Scholar]
- 27. Patel S. K., Dotson J., Allen K. P., Fleckenstein J. M. 2004. Identification and molecular characterization of EatA, an autotransporter protein of enterotoxigenic Escherichia coli. Infect. Immun. 72:1786–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peterson J. H., Tian P., Ieva R., Dautin N., Bernstein H. D. 2010. Secretion of a bacterial virulence factor is driven by the folding of a C-terminal segment. Proc. Natl. Acad. Sci. U. S. A. 107:17739–17744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pohlner J., Halter R., Beyreuther K., Meyer T. F. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325:458–462 [DOI] [PubMed] [Google Scholar]
- 30. Sääf A., Monné M., de Gier J. W., von Heijne G. 1998. Membrane topology of the 60-kDa Oxa1p homologue from Escherichia coli. J. Biol. Chem. 273:30415–30418 [DOI] [PubMed] [Google Scholar]
- 31. Skillman K. M., Barnard T. J., Peterson J. H., Ghirlando R., Bernstein H. D. 2005. Efficient secretion of a folded protein domain by a monomeric bacterial autotransporter. Mol. Microbiol. 58:945–958 [DOI] [PubMed] [Google Scholar]
- 32. Stein M., Kenny B., Stein M. A., Finlay B. B. 1996. Characterization of EspC, a 110-kilodalton protein secreted by enteropathogenic Escherichia coli which is homologous to members of the immunoglobulin A protease-like family of secreted proteins. J. Bacteriol. 178:6546–6554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki T., Lett M. C., Sasakawa C. 1995. Extracellular transport of VirG protein in Shigella. J. Biol. Chem. 270:30874–30880 [DOI] [PubMed] [Google Scholar]
- 34. Szabady R. L., Peterson J. H., Skillman K. M., Bernstein H. D. 2005. An unusual signal peptide facilitates the late steps in the biogenesis of a bacterial autotransporter. Proc. Natl. Acad. Sci. U. S. A. 102:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tajima N., Kawai F., Park S.-Y., Tame J. R. 2010. A novel intein-like autoproteolytic mechanism in autotransporter proteins. J. Mol. Biol. 402:645–656 [DOI] [PubMed] [Google Scholar]
- 36. Ulbrandt N. D., Newitt J. A., Bernstein H. D. 1997. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell 88:187–196 [DOI] [PubMed] [Google Scholar]
- 37. van den Berg B. 2010. Crystal structure of a full-length autotransporter. J. Mol. Biol. 396:627–633 [DOI] [PubMed] [Google Scholar]
- 38. Velarde J. J., Nataro J. P. 2004. Hydrophobic residues of the autotransporter EspP linker domain are important for outer membrane translocation of its passenger. J. Biol. Chem. 279:31495–31504 [DOI] [PubMed] [Google Scholar]
- 39. Voulhoux R., Bos M. P., Geurtsen J., Mols M., Tommassen J. 2003. Role of highly conserved bacterial protein in outer membrane protein assembly. Science 299:262–265 [DOI] [PubMed] [Google Scholar]
- 40. Wu T., et al. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235–245 [DOI] [PubMed] [Google Scholar]