Abstract
Bacteria of Bacillus species sporulate upon starvation, and the resultant dormant spores germinate when the environment appears likely to allow the resumption of vegetative growth. Normally, the rates of germination of individual spores in populations are very heterogeneous, and the current work has investigated whether spore-to-spore communication enhances the synchronicity of germination. In order to do this work, time-lapse optical images of thousands of individual spores were captured during germination, and an image analysis algorithm was developed to do the following: (i) measure the positions and germination rates of many thousands of individual spores and (ii) compute pairwise correlations of their germination. This analysis showed that an individual spore's germination rate was dependent on its distance from other spores, especially at short distances. Thus, spores that were within a few micrometers exhibited an increased synchronicity in germination, suggesting that there is a mechanism for short-range communication between such spores during germination. However, two molecules known to be germinants that are released during germination, l-alanine and the 1:1 chelate of Ca2+ and dipicolinic acid, did not mediate spore-to-spore communication during germination.
INTRODUCTION
Bacteria of Bacillus species form spores in order to survive adverse environmental conditions (18, 28). These spores are metabolically dormant and highly resistant to most antibacterial agents. However, the dormant spores continually monitor their environment and can resume vegetative growth once the environment becomes suitable. The first step in a spore's return to growth is germination, which can be initiated by some specific nutrient germinant molecules, including amino acids, nucleosides, and sugars (16, 27). A key early step in germination is the release of the spores' large pool (∼20% of the dry weight of spores' central region or core) of dipicolinic acid (DPA) that is chelated to divalent metal ions, predominantly Ca2+ (Ca-DPA) (12, 25, 27). Recent studies with individual spores have shown that once rapid release of Ca-DPA during germination has begun, it is completed in only a few minutes (4, 13, 22, 27, 35). However, there are generally long lag times (tlag) between the time of addition of a nutrient germinant to the initiation of rapid Ca-DPA release for individual spores, and tlag values vary greatly from spore to spore even in genetically identical populations germinating under the same conditions (4, 35). In contrast, the time needed for release of ∼90% of a spore's Ca-DPA during germination is relatively constant at several minutes.
The precise kinetics of spore germination are of great interest to the food and medical products industries, because once spores have germinated they are much easier to kill than the more resistant dormant spores. Unfortunately, the rates of germination of individual spores are extremely heterogeneous due to the variations in tlag (4, 11, 13, 35). Some of the factors that influence tlag values have recently been elucidated (35), although there could well be additional important factors. The current work has focused specifically on whether spore population density, in particular the distances between individual spores, could impact the germination kinetics by altering tlag values. While bacteria are single-cell organisms, they often release signaling molecules into the environment to coordinate the behavior of individual members of populations in response to environmental cues. For example, the sporulation of many bacteria depends not only on the availability of nutrients within the immediate environment of individual cells but also on the density of the cell population, a mechanism called quorum sensing (1, 15). For bacteria of Bacillus species, the cells carry out quorum sensing for sporulation by both releasing and detecting small peptides derived from the phr genes (23). While cell-to-cell communication in sporulation has been reasonably well studied, less work has been carried out exploring possible spore-to-spore communication in germination. However, results from several studies have suggested that there may be quorum sensing in germination (3, 9, 10, 14, 36). Interestingly, both inhibition and activation of germination have been observed as the result of a higher spore concentration, although the molecular mechanism of these effects is not clear.
In this work, we have evaluated whether the germination of individual Bacillus subtilis and Bacillus megaterium spores is influenced by other spores in the vicinity. This has been done using an imaging-based analysis to directly measure the locations of individual spores in a population as well as their germination time, tlag, and computing pairwise correlation functions for adjacent spores. This analysis has provided evidence that synchronicity of germination is enhanced for spores that are close to each other, and the effects of several potential germinant molecules normally released during germination on this synchronicity have also been examined.
MATERIALS AND METHODS
Strain and spore preparation.
The B. subtilis strains used for this work were derivatives of strain 168—four were isogenic with strain PS832: (i) PS533 (24) (wild type), carrying plasmid pUB110, which encodes resistance to kanamycin (10 μg/ml); (ii) FB20 (21) (gerA), in which the majority of the sequence encoding the GerA nutrient germinant receptor (GR) proteins has been replaced with a spectinomycin resistance (100 μg/ml) cassette; (iii) FB111 (20) (cwlJ), in which the majority of the coding sequence of the gene encoding the cortex-lytic enzyme CwlJ has been replaced with a tetracycline resistance (10 μg/ml) cassette; and (iv) FB112 (20) (sleB), in which the majority of the coding sequence of the gene encoding the cortex-lytic enzyme SleB has been replaced with a spectinomycin resistance cassette. The two other B. subtilis strains used were PY79 derivatives and were PY79 (wild type) and PB705 (32) (prkC) (obtained from J. Dworkin). Spores of B. subtilis strains were prepared on 2× SG agar plates without antibiotics at 37°C, and spores were incubated, harvested, and cleaned as described previously (6, 19). The B. megaterium strain used was QM B1551 (ATCC 12872) (originally obtained from H. S. Levinson). The B. megaterium spores were prepared at 30°C in liquid supplemented nutrient broth without antibiotics, and the spores were harvested and cleaned as described previously (8, 19). All spores used in this work were free (>98%) of growing or sporulating cells, germinated spores, and cell debris, and spores were stored at 4°C and protected from light.
Microscopy and image analysis of spore germination.
Germination of individual spores was analyzed by differential interference contrast (DIC) microscopy, based on a previously described method (35). Prior to germination, spores were heat activated for 30 min at 70°C (B. subtilis) or 15 min at 60°C (B. megaterium) and then cooled on ice for at least 15 min. For microscopy experiments, 0.5 μl of the heat-activated spores was spread on a 3% agarose pad containing the appropriate germinants. Spores were spread at concentrations of 1010 to 4·1010/ml (B. subtilis) and 4·109 to 1010/ml (B. megaterium). The use of various spore concentrations allowed for a broad range of spore-to-spore distances to be achieved in the microscopy samples. Results from different spore concentrations were combined and analyzed together.
Spores of B. subtilis PS832 derivatives were germinated at 30°C in 25 mM HEPES buffer (pH 7.4) plus 10 mM l-valine or at 34°C in AGFK (0.2 mM [each] l-asparagine, d-glucose, and d-fructose and 13 mM phosphate buffer, pH 7.4). Spores of B. subtilis PY79 and its derivative were germinated at 37°C in 25 mM HEPES buffer (pH 7.4) plus 2 mM l-valine. B. megaterium spores were germinated at 25°C in 25 mM phosphate buffer (pH 7.4) with 250 μM d-glucose. Images were taken in time-lapse using an Olympus IX81 microscope equipped with a 60× microscope objective (numerical aperture [NA]=1.45; Olympus), with frame rates of 1 to 2 frames/min.
Image analysis was carried out with the ImageJ and Matlab software programs. To determine the locations and germination times of the spores computationally, we first normalized the intensity of all images. A cross-correlation-based stabilizer filter was then applied to the time-lapse images to compensate for stage drifts and positioning errors during image acquisition. Finally, the center position of each spore was computed based on the DIC contrast using peak detection algorithms. The tlag values of each spore were then obtained by tracing the gray level of the DIC images over time and fitting the intensity transient with a tanh function. For each germination experiment, at least 8,000 individual spores were examined using this procedure.
RESULTS
Analysis of the synchronicity of spore germination.
To analyze the synchronicity of spore germination, we developed an algorithm to computationally determine the tlag values for germination and the positions, r, of individual spores undergoing germination. The basis of the assay is the observation that under DIC imaging, the images of ungerminated spores have a much higher-intensity contrast than those of the spores that are germinated (Fig. 1). The latter reduced contrast is the combined effect of Ca-DPA release from the spore core and water uptake into the core, both to replace the released Ca-DPA and due to the hydrolysis of the peptidoglycan cortex by the cortex-lytic enzymes CwlJ and SleB, which allows the core to swell (27). To verify the validity of the algorithm, positions of spores determined by the computation algorithm were compared with results from manual identification in a few initial experiments. We determined that the algorithm was very accurate in detecting the spores' locations, with essentially no false-negative assignments and only a very small number of incidences of false positives (<0.1%). For each located spore, an intensity transient was obtained from the DIC time-lapse images (Fig. 1). As expected, this intensity changed relatively little over an extended initial period of time, representing tlag, followed by a sudden drop, denoting the release of Ca-DPA and full hydration of the spore core. The tlag values were computationally obtained for more than 90% of spores imaged. The remaining spores were almost exclusively ones that did not germinate during the experiment, possibly because many of these spores were “superdormant” (6, 7, 34), and these spores were not analyzed.
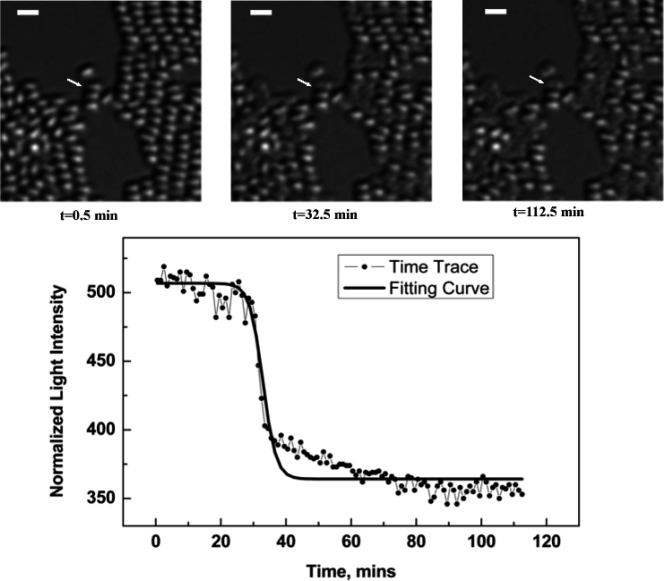
Fig. 1.
Time-lapse images of spores during germination. Bacillus subtilis PS533 (wild-type) spores immobilized on an agarose pad and germinating with 6 mM l-valine were imaged by DIC microscopy every 0.5 min as described in Materials and Methods. Three representative images from the time series are shown (top), in which the contrast changes of one spore have been noted (arrow). The scale bars represent 2 μm. The DIC peak intensities for the designated spore were extracted as described in Materials and Methods and plotted versus time (bottom), showing the characteristic rapid transition in peak intensity following tlag due largely to Ca-DPA release. The solid line represents the best fit of the data.
For each experiment, we calculated the distances between pairs of spores, as well as their differences in the germination lag times:
For a sample of N spores, there are a total of N(N − 1)/2 spore pairs. For each pair of spores, the Δr values measure how close the pair are to each other, while the Δt value reflects the synchronicity of the germination. If germination is a completely single-spore process and spores do not communicate with each other during germination, we should expect Δr and Δt to be completely independent of each other. Otherwise, if spores communicate with each other during germination, we would predict a correlation between Δr and Δt.
Our analysis of the pairwise correlation was based on the probability (Pr) functions. With a large number of measurements of many thousands of spores, we obtained estimates of the cumulative distribution functions for Δr and Δt: Pr(Δr < a) and Pr(Δt < b), as well as the joint cumulative distribution functions, Pr(Δr < a ∩ Δt < b). To determine whether Δr and Δt are correlated with or independent of each other, we can simply calculate the conditional probability:
If Δr and Δt are independent variables, one would predict that F(b;a)=Pr(Δr < a). Thus, the conditional probability, F, should be independent of b. Similarly, we can also compute the conditional probability function
and test if it depends on the value of a.
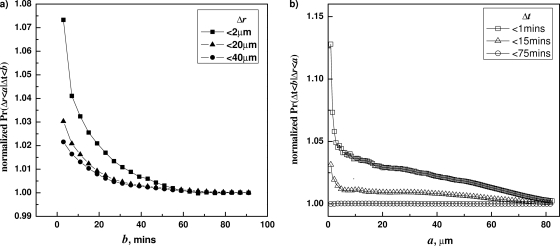
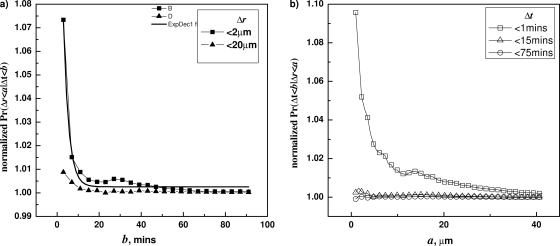
The computed F and G values from wild-type B. subtilis spores germinating with l-valine are plotted in Fig. 2 a and b, respectively. The function F was computed for a=2 μm, 20 μm, and 40 μm. In all cases we found that F is a decaying function of b. The function G was computed for b=1 min, 15 min, and 74 min. Similarily, for smaller b values, G(a;b) is a decaying function of a. These results indicate that Δr and Δt are not independent for B. subtilis spores. Furthermore, the dependence is the strongest for spore pairs that are very close to each other, and the effect gets weaker as the value of a, i.e., the distances between spore pairs, increases. In other words, spores close to each other have an increased synchronicity of germination.
Fig. 2.
Correlation between Δt and Δr in the germination of wild-type B. subtilis (PS533) spores. (a) Conditional probabilities F(b;a) were plotted against b with selected a values (2 μm, 20 μm, and 40 μm), showing decreasing values of F versus b. (b) Conditional probabilities G(a;b) were plotted against a with several different b values (1 min, 15 min, and 75 min), showing decreasing values of G versus a. The spores were germinated at 30°C with 6 mM valine, and germination times and correlations were determined as described in Materials and Methods and the text.
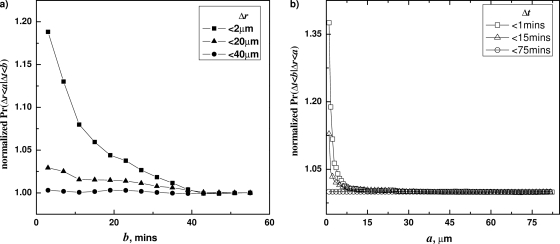
A similar analysis was carried out for B. megaterium spores (Fig. 3). At distance separations (a=2 μm), the computed conditional probability F decreased sharply with increasing b (Fig. 3a). The relative amplitude of the decay at such a short distance is similar to that for B. subtilis spores. However, the decay amplitude decreased rapidly with increasing a and was indistinguishable from the baseline for spores separated by 20 μm (Fig. 3a). The G functions were also consistently a decaying function of a for spore pairs that germinated with higher synchronicity (smaller Δt). However, compared to B. subtilis spores, the G functions for B. megaterium spores exhibited a much faster decay over distance, suggesting that with B. megaterium, spore-to-spore communication is effective only at distances less than 20 μm. This difference between the spores of these two species is probably due to intrinsic differences in the rates of germination, since B. megaterium spores germinate much faster in our conditions, with a most probable tlag of ∼2.5 min, while for B. subtilis spores the most probable tlag was ∼12 min. In other words, the germination of B. megaterium spores was intrinsically more “synchronized.” Therefore, increased germination synchronicity due to spore-to-spore communication is less likely to be significant for spores of B. megaterium than for B. subtilis spores.
Fig. 3.
Correlation between Δt and Δr in the germination of B. megaterium (Bmeg) spores. (a) Conditional probabilities F(b;a) were plotted against b with several different a values (2 μm, 20 μm, and 40 μm), showing decreasing values of F versus b. (b) Conditional probabilities G(a;b) were plotted against a with several different b values (1 min, 15 min, and 75 min), showing decreasing values of G versus a. The spores were germinated at 25°C with glucose, and germination times and correlations were determined as described in Materials and Methods and the text.
Analysis of the mechanism of synchronicity in spore germination.
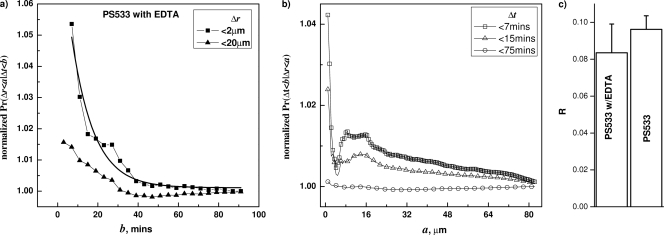
With evidence that germination of one spore can indeed influence the germination of a nearby spore, it was of obvious interest to determine the mechanism of this effect. One molecule released at high levels by a germinating spore is Ca-DPA, and Ca-DPA is a germinant for Bacillus spores, albeit only at concentrations of tens of mM (10, 21, 27). Spore germination by Ca-DPA does not require the GRs but rather is through activation of the cortex lytic enzymes, perhaps directly by Ca-DPA (20, 21, 27). Consequently, the release of Ca-DPA from one germinating spore could potentially stimulate the germination of nearby spores and thus enhance the synchronization of germination of the spore population. To test this hypothesis, we carried out the same analysis with B. subtilis spores germinating with l-valine but with EDTA present. EDTA was used because DPA itself is not especially efficient in triggering spore germination compared to Ca-DPA (20), and a preliminary experiment showed that EDTA in a 10 mM excess over Ca-DPA completely inhibited germination (data not shown). Therefore, we expected that EDTA would block the pairwise correlation if the correlation was mediated by Ca-DPA. However, the results with EDTA present were not significantly different from those of spores germinating with l-valine in the absence of EDTA, since EDTA did not eliminate the correlation between Δr and Δt (Fig. 4 a and b). A quantitative comparison was carried out via fitting the F function (a = 2 μm) with the exponential functions, Ae−λb + B.
Fig. 4.
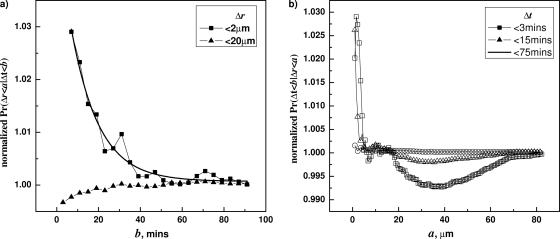
Effect of inhibiting Ca-DPA-mediated germination on correlations between Δt and Δr during germination of wild-type B. subtilis (PS533) spores with l-valine. Correlations between Δt and Δr in wild-type B. subtilis (PS533) spore germinating with l-valine plus EDTA were tested by calculating the conditional probabilities F(b;a) (a) and G(a;b) (b). The spores were germinated at 30°C with 10 mM valine plus 10 mM EDTA, and germination times and correlations were determined as described in Materials and Methods and the text. The comparison between spores under EDTA and without EDTA is shown in panel c.
The relative decay amplitude, R=A/(A + B), from spores germinated in the absence of EDTA was not significantly different from that from spores germinated with EDTA (Fig. 4C). In addition, the l-valine germination of cwlJ B. subtilis spores that cannot undergo Ca-DPA germination exhibited the correlations between Δr and Δt seen with wild-type spores germinating with l-valine (data not shown).
A second type of molecule released from germinating spores that could trigger germination of adjacent spores is l-amino acids. High levels of free l-amino acids are released shortly after Ca-DPA release in germination due to the degradation of 5 to 10% of spore core protein (26). Some l-amino acids, in particular l-alanine and l-valine, alone, are sufficient to trigger B. subtilis spore germination by activating the GerA GR (27). Additionally, in Bacillus cereus, l-alanine released during germination has been suggested to alter the kinetics of inosine-induced germination (5). Thus, we examined spores without the GerA GR to see if the pairwise correlations for these mutant spores were altered. Since spores lacking the GerA GR do not germinate with l-valine or l-alanine alone, these spores, as well as wild-type spores, were germinated with the AGFK mixture that requires both the GerB and GerK GRs but not the GerA GR (27). This analysis showed that gerA and wild-type spores germinating with AGFK behaved similarly (Fig. 5 and 6). Indeed, the decay amplitude of gerA spores was slightly higher. The exact reason for this is unclear. Nevertheless, these results indicated that the correlations we observed were not mediated by l-alanine or l-valine.
Fig. 5.
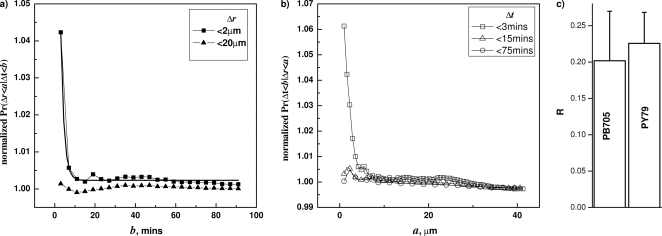
Correlation between Δt and Δr in the germination of wild-type B. subtilis (PS533) spores germinating with AGFK. (a) Conditional probabilities F(b;a) were plotted against b with selected a values (2 μm, 20 μm), showing decreasing values of F versus b. (b) Conditional probabilities G(a;b) were plotted against a with several different b values (3 min, 15 min, and 75 min), showing decreasing values of G versus a. The spores were germinated at 34°C with 0.2 mM AGFK, and germination times and correlations were determined as described in Materials and Methods and the text.
Fig. 6.
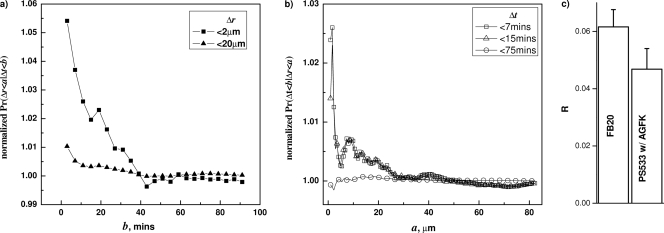
Effect of loss of the GerA GR on correlations between Δt and Δr during B. subtilis spore germination. Correlations between Δt and Δr in gerA B. subtilis (FB20) spores germinating with 0.2 mM AGFK were tested by calculating the conditional probabilities F(b;a) (a) and G(a;b) (b). The comparison of ratio R between the gerA and wild-type spores (data from Fig. 5) is shown in panel c. The spores were germinated at 34°C with 0.2 mM AGFK, and germination times and correlations were determined as described in Materials and Methods and the text.
A third type of molecule that can trigger spore germination is low-molecular-weight fragments of growing cell peptidoglycan (PG). These PG fragments trigger spore germination by activating the protein kinase PrkC (30, 32). While spore PG differs in structure from growing cell PG, and thus cortical PG fragments might not activate PrkC, it still seemed worth examining whether this mechanism was responsible for spore-to-spore communication during spore germination. Consequently, we examined correlations between Δt and Δr during germination of prkC spores (PB705) with l-valine. However, again, these correlations were essentially identical to those observed with the wild-type spores with the same genetic background (PY79) (Fig. 7 and 8). Also note that although the result with PY79 spores is qualitatively consistent with the result with spores of the other wild-type B. subtilis strain, PS533, the decay of the correlation with PY79 spores seems a bit faster, suggesting a slightly less efficient interspore communication in the spores of this strain. However, the exact reason for this difference is not clear.
Fig. 7.
Correlation between Δt and Δr in the germination of wild-type B. subtilis PY79 spores. (a) Conditional probabilities F(b;a) were plotted against b with selected a values (2 μm, 20 μm), showing decreasing values of F versus b. (b) Conditional probabilities G(a;b) were plotted against a with several different b values (1 min, 15 min, and 75 min), showing decreasing values of G versus a. The spores were germinated at 37°C with 2 mM l-valine, and germination times and correlations were determined as described in Materials and Methods and the text.
Fig. 8.
Effect of loss of PrKC on correlations between Δt and Δr during B. subtilis spore germination with l-valine. Correlations between Δt and Δr in B. subtilis PB705 (ΔprkC) spores germinating with l-valine were tested by calculating the conditional probabilities F(b;a) (a) and G(a;b) (b). The comparison of ratio R between mutant and wild-type spores is shown in panel c. The spores were germinated at 37°C with 2 mM valine, and germination times and correlations were determined as described in Materials and Methods and the text.
DISCUSSION
Intercellular communication has been shown to be extremely important for many cellular processes involving growth and survival. In almost all of those cases, signaling pathways based on protein phosphorylation (2) play a significant role. Many quorum-sensing pathways also directly regulate gene expression (1). Spores, on the other hand, do not actively transcribe genes and are metabolically dormant (28, 29). In addition, the germination of spores does not seem to require the synthesis of ATP (27). Therefore, phosphorelays are unlikely to be important for spore germination. Yet we have shown here that communication between spores exists. One of the consequences of such communication was that the spores adjacent to each other tended to have a higher chance that their germination was synchronized.
We note, however, that the synchronization effect was not a significant one under normal lab conditions, since the computed conditional probabilities, F and G, for example, were elevated by only a few percentage points for spore pairs separated by a short distance. Furthermore, the effect decayed as the distances between spores increased. Thus, we expect this synchronization to be difficult to detect using population germination rates under most laboratory conditions, where spores are usually suspended in liquid at moderate concentrations, which may explain why these effects are often not noticed. In nature, tight clusters of spores could potentially exist and thus make the synchronization more significant. Furthermore, at higher spore density, molecules released from multiple spores could exert larger effects on an adjacent spore, therefore enhancing the possibility of detecting the synchronization. This could be a factor in our experiments, which included samples with high spore densities. On the other hand, spore germination is generally very heterogeneous, and individual spores can germinate at very different rates under exactly the same conditions (35). It can be argued that such heterogeneity is a defense mechanism of the bacterium to increase the chance of population survival, because germinated spores and the resultant growing cells are much more vulnerable to environmental hazards. Therefore, germination of all spores at the same time might be disadvantageous if the environment is such that germination is actually a mistake for the spore population (31). Thus, some balance between synchronization and heterogeneity in spore germination is probably the optimal result from evolution, and a very strong effect of synchronization might actually not be expected.
Whatever the value of the synchronization in spore germination observed in this work, an obvious question is what the molecule or molecules are that result in this synchronization. This appears not to be Ca-DPA, even though this molecule can trigger spore germination and large amounts are released when spores germinate (20, 25, 27). Presumably this is because the Ca-DPA released when one spore germinates does not generate a concentration reaching an adjacent spore that exceeds the threshold level of ∼15 mM required to trigger spore germination by exogenous Ca-DPA (20). While Ca-DPA solubility is ∼70 mM, the Ca-DPA concentration in total dormant spore water would be ∼90 mM if all were soluble, assuming the weight of a B. subtilis spore is 6 × 10−13 g, contains 10% dry weight as DPA, and is ∼70% water (17, 28). However, full release of Ca-DPA from the core during germination takes 1 to 3 min for an individual spore (4, 13, 22, 35), indicating that there is ample time for tremendous dilution of this released Ca-DPA well before an adjacent spore is encountered. Consequently, it is perhaps not unexpected that Ca-DPA released from one spore is not especially effective in triggering the germination of an adjacent spore.
The degradation of ∼5% of total spore protein to free amino acids also takes place soon after spore germination, and most of these free amino acids are initially released into the medium and then taken back up and used in metabolism and macromolecular synthesis (27). However, these amino acids would be at least transiently available to trigger germination of other spores, and at least l-alanine is an effective germinant via the GerA GR (27). Indeed, l-alanine concentrations of tens of μM can effectively trigger spore germination. However, l-alanine release by spore protein degradation is well after Ca-DPA release (27), and this degradation presumably takes a significant time to complete, although the kinetics of this process have not been measured for individual spores. Furthermore, the existence of an alanine racemase in the spore coat, which converts l-alanine to d-alanine, could potentially inhibit any interspore communication mediated by l-alanine. In any event, the release of l-alanine by this mechanism also seems not to be important in the synchronization in triggering B. subtilis spore germination via the GerA GR, for the following reasons: (i) this synchronization was seen when germination with high concentrations of l-valine was examined, and these concentrations saturate the GerA GR such that amounts of l-alanine generated by proteolysis during germination would be insignificant compared to the high l-valine concentrations, and (ii) the absence of the GerA GR did not affect the synchronization of germination seen with AGFK.
With l-alanine and Ca-DPA eliminated as major factors in the synchronization of spore germination of adjacent spores, what then are the molecules that cause this effect? At present the answer to this question is not known, although a number of other small molecules are released approximately in parallel with Ca-DPA, including spores' pool of free glutamic acid, which is 5 to 10% the size of the Ca-DPA pool, as well as smaller amounts of other free amino acids (25, 27). While none of these free amino acids alone appear able to trigger B. subtilis spore germination, perhaps multiple small molecules released early in germination act cooperatively to cause the synchronization of spore germination. While at present we cannot identify specific molecules that might be involved in such cooperative action, cooperation between multiple molecules in triggering spore germination certainly takes place in the germination of spores of many Bacillus species (27, 31, 33).
ACKNOWLEDGMENT
This work was supported by a Multi-University Research Initiative (MURI) award from the U.S. Department of Defense (to P.S. and J.Y.).
Footnotes
Published ahead of print on 27 May 2011.
REFERENCES
- 1. Bassler B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582–587 [DOI] [PubMed] [Google Scholar]
- 2. Burbulys D., Trach K. A., Hoch J. A. 1991. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64:545–552 [DOI] [PubMed] [Google Scholar]
- 3. Caipo M. L., Duffy S., Zhao L., Schaffner D. W. 2002. Bacillus megaterium spore germination is influenced by inoculum size. J. Appl. Microbiol. 92:879–884 [DOI] [PubMed] [Google Scholar]
- 4. Chen D., Huang S.-S., Li Y.-Q. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936–6941 [DOI] [PubMed] [Google Scholar]
- 5. Dodatko T., et al. 2009. Bacillus cereus spores release alanine that synergizes with inosine to promote germination. PLoS One 4:e6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghosh S., Setlow P. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghosh S., Setlow P. 2010. The preparation, germination properties and stability of superdormant spores of Bacillus cereus. J. Appl. Microbiol. 108:582–590 [DOI] [PubMed] [Google Scholar]
- 8. Goldrick S., Setlow P. 1983. Expression of a Bacillus megaterium sporulation-specific gene during sporulation of Bacillus subtilis. J. Bacteriol. 155:1459–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gould G. W. 1970. Symposium on bacterial spores. IV. Germination and the problem of dormancy. J. Appl. Bacteriol. 33:34–49 [DOI] [PubMed] [Google Scholar]
- 10. Gould G. W., Hurst A. 1969. Spore germination, p. 397–444. In Gould G. W., Hurst A., (ed.) The bacterial spore. Academic Press Inc., New York, NY [Google Scholar]
- 11. Hashimoto T., Frieben W. R., Conti S. F. 1969. Germination of single bacterial spores. J. Bacteriol. 98:1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang S.-S., et al. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J. Bacteriol. 189:4681–4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kong L., Zhang P., Setlow P., Li Y.-Q. 2010. Characterization of bacterial spore germination using integrated phase contrast microscopy, Raman spectroscopy, and optical tweezers. Anal. Chem. 82:3840–3847 [DOI] [PubMed] [Google Scholar]
- 14. Llaudes M. K., Zhao L., Duffy S., Schaffner D. W. 2001. Simulation and modeling of the effect of small inoculum size on time to spoilage by Bacillus stearothermophilus. Food Microbiol. 18:395–405 [Google Scholar]
- 15. Miller M. B., Bassler B. L. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165–199 [DOI] [PubMed] [Google Scholar]
- 16. Moir A. 2006. How do spores germinate? J. Appl. Microbiol. 101:526–530 [DOI] [PubMed] [Google Scholar]
- 17. Nelson D. L., Kornberg A. 1970. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J. Biol. Chem. 245:1137–1145 [PubMed] [Google Scholar]
- 18. Nicholson W. L., Munakata N., Horneck G., Melosh H. J., Setlow P. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nicholson W. L., Setlow P. 1990. Sporulation, germination and outgrowth, p. 391–450. In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, United Kingdom [Google Scholar]
- 20. Paidhungat M., Ragkousi K., Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paidhungat M., Setlow P. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng L., Chen D., Setlow P., Li Y.-Q. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows the monitoring of spore germination dynamics. Anal. Chem. 81:4035–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pottathil M., Lazazzera B. A. 2003. The extracellular Phr peptide-Rap phosphatase signaling circuit of Bacillus subtilis. Front. Biosci. 8:d32–d45 [DOI] [PubMed] [Google Scholar]
- 24. Setlow B., Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Setlow B., Wahome P. G., Setlow P. 2008. Release of small molecules during germination of spores of Bacillus species. J. Bacteriol. 190:4759–4763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Setlow P. 1988. Small acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu. Rev. Microbiol. 42:319–338 [DOI] [PubMed] [Google Scholar]
- 27. Setlow P. 2003. Spore germination. Curr. Opinion Microbiol. 6:550–556 [DOI] [PubMed] [Google Scholar]
- 28. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525 [DOI] [PubMed] [Google Scholar]
- 29. Setlow P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol. 15:172–180 [DOI] [PubMed] [Google Scholar]
- 30. Setlow P. 2008. Dormant spores receive an unexpected wake-up call. Cell 135:410–412 [DOI] [PubMed] [Google Scholar]
- 31. Setlow P., Liu J., Faeder J. R. Heterogeneity in bacterial spore populations. In Abel-Santos E. (ed.), Bacterial spores: current research and applications, in press. Horizon Scientific Press, Norwich, United Kingdom [Google Scholar]
- 32. Shah I. M., Laaberki M.-H., Popham D. L., Dworkin J. 2008. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell 135:486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wax R., Freese E., Cashel M. 1967. Separation of two functional roles of L-alanine in the initiation of Bacillus subtilis spore germination. J. Bacteriol. 94:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wei J., et al. 2010. Superdormant spores of Bacillus species germinate normally with high pressure, peptidoglycan fragments, and bryostatin. J. Bacteriol. 192:1455–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang P., et al. 2010. Factors affecting variability in time between addition of nutrient germinants and rapid dipicolinic acid release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao L., Montville T. J., Schaffner D. W. 2000. Inoculum size of Clostridium botulinum 56A spores influences time-to-detection and percent growth-positive samples. J. Food Sci. 8:1369–1375 [Google Scholar]