Abstract
Controlled protein degradation is an important cellular reaction for the fast and efficient adaptation of bacteria to ever-changing environmental conditions. In the low-GC, Gram-positive model organism Bacillus subtilis, the AAA+ protein ClpC requires specific adaptor proteins not only for substrate recognition but also for chaperone activity. The McsB adaptor is activated particularly during heat stress, allowing the controlled degradation of the CtsR repressor by the ClpCP protease. Here we report how the McsB adaptor becomes activated by autophosphorylation on specific arginine residues during heat stress. In nonstressed cells McsB activity is inhibited by ClpC as well as YwlE.
INTRODUCTION
Protein degradation is one major task of cellular protein quality control networks, which ensures the viability and survival of all living organisms. Damaged proteins that may lead to protein aggregation can also occur under normal conditions, but the levels of these protein aggregates are dramatically increased under a variety of stress conditions (17). These protein aggregates not only are unable to fulfill their physiological functions but also can have lethal consequences for the cell. To protect the cell, these irreversibly damaged complexes must be removed through energy-dependent protein degradation when the refolding of misfolded or aggregated proteins by the chaperone networks fails (7, 32, 40).
Energy-dependent protein degradation is achieved by large cylindrical assemblies with a common ring-stacking architecture of diverse complexity in all living organisms. In eubacteria, including the low-GC, Gram-positive model organism Bacillus subtilis, different types of ATP-dependent proteases have evolved (15). For example, the Clp protease complex is formed by a barrel-like structure of two stacked heptameric rings of Clp monomers, which form a catalytic cavity, wherein the 14 active proteolytic sites are separated from the cytosol (38). Hexameric rings of AAA+ ATPases of the Clp/Hsp100 family flank the ends of the ClpP chamber, thereby acting as a gateway to the proteolytic cavity (34).
Hsp100/Clp proteins are responsible not only for ATP-dependent unfolding and translocation of the substrates but also for substrate recognition (39), which prevents the unwanted protein degradation of functional proteins. In many cases, Hsp100/Clp proteins are aided by so-called adaptor proteins, which bind both the AAA+ protein and substrate simultaneously, thereby expanding the substrate spectrum of their cognate AAA+ proteins (20). Nevertheless, most of the Hsp100/Clp proteins are also active independently of an adaptor protein. In contrast, B. subtilis ClpC is unlike most members of this group and requires specific adaptor proteins not only for substrate recognition but also for all its activities (19, 21). Therefore, the regulation of these adaptor proteins is important for controlled degradation by the ClpCP protease.
The McsB adaptor is an arginine kinase (12) that is essential for the regulated proteolysis of the global heat shock regulator CtsR during heat stress (19, 23). The adaptor activity of McsB depends on an active kinase (9, 19), which requires its activator McsA for proper kinase activity. It was shown that ClpC inhibits McsB activity in vitro (23). In addition, McsB is antagonized by the YwlE phosphatase in vitro (23) and in vivo (9). Recently, it was demonstrated that the McsB kinase is inhibited in nonstressed cells and becomes activated upon heat exposure (9).
Here we show how the McsB kinase is activated during heat stress. Upon heat exposure, the McsB/ClpC interaction is dramatically decreased, resulting in an activation of the McsB kinase. Additionally, we demonstrate that the McsB kinase is inhibited by ClpC and YwlE.
MATERIALS AND METHODS
General methods.
Strains and primers used in this study are listed in Tables S1 and S2 in the supplemental material. B. subtilis cells were grown in liquid medium (see below) or on LB agar plates with tetracycline (17 μg/ml), spectinomycin (200 μg/ml), kanamycin (5 μg/ml), or chloramphenicol (5 μg/ml).
DNA manipulation and other molecular biological procedures were carried out according to standard protocols. The transformation of B. subtilis cells was performed by a two-step protocol (18a). Site-directed mutagenesis was conducted by using a plasmid as a template containing the modified clpC operon. PCR was performed by using primers with the desired nucleotide substitutions according to the manufacturer's instructions (Gene Tailor system; Invitrogen).
Culture conditions.
B. subtilis 168 cells and the corresponding mutant cells were inoculated from an exponentially growing culture grown overnight to an optical density at 500 nm (OD500) of 0.08. The cells were routinely grown in synthetic medium (36) at 37°C using 500-ml Erlenmeyer flasks and a shaking-water bath at 180 rpm. When the cells reached the mid-exponential phase (optical density at 500 nm of 0.5), they were shifted to a 50°C prewarmed Erlenmeyer flask for heat stress experiments.
In vivo stability of proteins.
For assaying the stability of CtsR, cells were grown in Belitsky minimal medium without citrate supplemented with 0.01% yeast extract at 37°C. At an optical density at 500 nm of 0.5, cells were labeled with l-35S-labeled methionine (16.7 μCi/ml). After 10 min of labeling, the radioactive methionine was chased by the addition of a 600,000-fold molar excess of cold methionine, and samples of 4 ml were taken. After centrifugation (10,000 × g at 4°C for 10 min), cells were resuspended in 53.3 μl of lysis buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 4 mg/ml [wt/vol] lysozyme, 1.4 mM phenylmethylsulfonyl fluoride [PMSF]) and incubated for 20 min at 37°C. For complete cell lysis, 8 μl of 10% (wt/vol) sodium dodecyl sulfate (SDS) was added, and the samples were incubated for 5 min at 95°C. Next, 720 μl of KI buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 150 mM NaCl, 1% [vol/vol] Triton X-100, 1.4 mM PMSF) was added, and samples were incubated on ice for 15 min. After centrifugation (10,000 × g at 4°C for 45 min), the supernatants were incubated with specific polyclonal antisera of CtsR (diluted 1:30) overnight with slow-tilt rotation at 4°C. A suspension of 40 μl of protein A-coated Dynabeads (Dynal) equilibrated with KI buffer was added to each sample for an additional incubation time of 2 h. The beads were washed three times in 500 μl of KI buffer and finally boiled in 10 μl of SDS sample buffer for 5 min at 95°C. The samples were separated by SDS-PAGE using Mini-Protean cells (Bio-Rad) with an appropriate marker (Page-Ruler prestained protein ladder; Fermentas). After electrophoresis, gels were vacuum dried and exposed to phosphorscreens (Molecular Dynamics) overnight. Autoradiographs were scanned with an SI PhosphorImager (Molecular Dynamics) or a Storm 840 system (Molecular Dynamics).
RNA isolation and Northern blots.
Samples were taken from nonstressed cultures immediately before the shift to 50°C and at different time points during heat exposure. For RNA extraction, cell pellets were resuspended with 0.5 volumes of ice-cold killing buffer (20 mM Tris-HCl, 5 mM MgCl2, 20 mM NaN3). All samples were immediately cooled with liquid nitrogen, spun down at 10.000 × g for 8 min at 4°C, and stored in liquid nitrogen until further preparation. RNA isolation and Northern blot analysis were performed as described previously (31). Each RNA blot was stained with methylene blue prior to hybridization in order to check RNA quality and ensure that equal amounts of RNA were loaded and blotted for each lane. Digoxigenin-labeled antisense RNA probes were used for clpE and clpP mRNAs (13).
Protein purification.
Cells were grown until the mid-exponential phase in LB medium and either heat shocked or not. Prior to cell sampling, proteins were cross-linked according to methods described previously by Herzberg et al. (18) and harvested for 10 min at 15,000 × g. Harvested cells were resuspended in ice-cold buffer W (100 mM Tris-HCl, 150 mM NaCl) and disrupted by using a French press. PMSF was added to a final concentration of 1 mM to prevent protein degradation. Unbroken cells and cell debris were removed by centrifugation at 20,000 × g for 30 min. The proteins were purified by using Ni-nitrilotriacetic acid (NTA) Superflow cartridge H-PR for the purification of overexpressed Escherichia coli proteins and Strep Superflow cartridge H-PR for the purification of proteins from B. subtilis according to the manufacturer's standard procedures (IBA GmbH, Germany).
Phosphorylation assay.
Purified proteins were applied in equal concentrations (1 μM) in phosphorylation assay buffer (25 mM Tris-HCl [pH 8], 300 mM NaCl, 5 mM MgCl2, and 1 mM dithiothreitol [DTT]) at 30°C in the presence of 1 mM ATP in a final volume of 15 μl with the indicated combinations of McsA and McsB.
Coimmunoprecipitation.
Coimmunoprecipitation was performed as described previously (23). Protein A-coated magnetic beads were incubated with 25 μl of antisera directed against McsB at 4°C for 30 min according to the manufacturer's instructions (Dynal). The protein A-antibody complex was incubated with the soluble cell extract, representing 500 μg protein, from B. subtilis 168 and ΔclpC and ΔclpC ΔmcsB(C167S) strains cultivated at 37°C as well as heat-shocked cells. After the formation of the protein A-antibody-antigen complex for 1 h at 4°C, three wash steps using 0.1% Triton X-100 in phosphate-buffered saline (PBS) were performed to prevent the nonspecific binding of abundant proteins. Finally, precipitated proteins were treated with SDS sample buffer at 95°C and then analyzed by SDS-PAGE and subsequent Western blotting.
Protein identification and quantification of the interaction analysis using GeLC-ESI-MS/MS analysis.
In the experimental setup, the control culture was grown in unlabeled 14N medium, while the stressed culture was grown in 15N-enriched medium (Bioexpress 100; CIL US). The cultures were then treated with p-formaldehyde for reversible protein-protein cross-linking (18). Afterwards, either McsB or ClpC was isolated by using streptavidin columns. The purified protein complexes were then analyzed by using GeLC-tandem mass spectrometry (MS) (MS/MS), and their presence was quantified as described previously (2).
Purified protein samples were separated by one-dimensional (1D) SDS-PAGE. After staining with Coomassie silver blue, a complete lane was cut into five equal gel slices. In-gel digestion was performed with trypsin (Promega, Madison, WI) as described previously (11). Peptides obtained from in-gel digestion were separated by liquid chromatography (LC) and measured online by electrospray ionization (ESI)-mass spectrometry. LC-MS/MS analyses were performed by using a nanoACQUITY UPLC system (Waters, Milford, MA) coupled to an LTQ Orbi-trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) as described previously (2), with the exception of the application of a flow rate of 400 nl min−1.
Protein identification and quantification were accomplished as described previously (2). The identification of peptides was carried out by database search using SEQUEST, version 28 (rev. 12) (Thermo Fisher Scientific), against a target-decoy database (8,294 entries). This database was composed of all protein sequences of B. subtilis extracted from UniprotKB (release 12.7) (37a). A set of the reversed sequences created by BioworksBrowser 3.2 EF2 as well as common contaminants such as keratin was appended.
A protein was considered reliably quantified when its ratio was determined in three biological replicates with no fewer than two peptides. Proteins that were identified but failed to give reliable quantitative data were manually inspected for poor quality or presence in only one of the samples, 14N or 15N. If their presence in only one of the samples was based on more than one peptide, they were added to the list of quantified proteins as “on” or “off” proteins. In this case, the corresponding 14N- or 15N-peptide was missing for quantification.
Protein digestion, phosphopeptide enrichment, and identification of phosphate-containing peptides.
Approximately 300 μg (Roti-Nanoquant protein determination; Roth, Karlsruhe, Germany) of purified McsB was digested with activated trypsin (15 min of incubation at 30°C in activation buffer; enzyme-to-trypsin ratio, 1:200) overnight at 37°C. The peptide solution was prepared for phosphopeptide enrichment by using strong-cation-exchange (SCX) chromatography and titanium dioxide beads according methods described previously (30). Peptides were separated and measured according to methods described previously (33).
Proteins were identified by searching all MS/MS spectra in .dta format against a forward-reverse database that was composed of all protein sequences of B. subtilis extracted from UniprotKB, release 12.7, using Sorcerer-SEQUEST (version v.27, rev. 11; ThermoFinnigan, San Jose, CA). The following search criteria were used. Full tryptic specificity was assumed. The maximum mass deviation was set to 10 ppm for the precursor ion and 1 Da for fragment ions. Up to two missed tryptic cleavages were allowed. Methionine oxidation (+15.99492 Da) and phosphorylation of R, S, T, and Y (+79.966331 Da) were set as variable modifications. Proteins were identified by at least two peptides by applying a stringent SEQUEST filter. SEQUEST identifications required at least deltaCn scores of greater than 0.10 and XCorr scores of greater than 1.9, 2.2, 3.3, and 3.8 for singly, doubly, triply, and quadruply charged peptides. Phosphorylated peptides that passed this filter were examined manually and accepted only when b or y ions confirmed the phosphorylation site.
RESULTS
McsB kinase activity is inhibited by ClpC in vivo.
Recently, it was demonstrated that the McsB adaptor is specifically activated upon heat exposure, resulting in the controlled degradation of CtsR (9). We were interested in the molecular details of McsB inhibition in nonstressed cells and the specific activation upon heat exposure. An active McsB kinase leads to a slightly induced transcription of clpE or clpP independent of the intrinsic regulation of CtsR due to the binding of McsB to free CtsR molecules, thereby preventing DNA-repressor interactions (9). This observation gave us the opportunity to monitor the activity of McsB in nonstressed cells, when the transcription of clpE or clpP is strongly repressed by CtsR.
The activation of McsB as an adaptor protein depends on a functional kinase (8, 9, 19), and previous studies revealed that the McsB kinase activity is inhibited by ClpC in vitro (19, 23). When ClpC indeed inhibits McsB adaptor activity in vivo, the McsB adaptor should be activated in a clpC mutant strain due to the lack of ClpC inhibition leading to slightly induced clpE or clpP transcription. In fact, the levels of transcription of clpE as well as clpP were marginally increased in a clpC knockout strain (Fig. 1 A). Nevertheless, CtsR-dependent transcription in a clpC mutant was strongly induced during heat stress caused by the recently described intrinsic, heat-dependent inactivation of CtsR (9) (Fig. 1A). We decided to monitor the impact of McsB on CtsR activity via clpP transcription, because CtsR repression is not as strong as it is for clpE due to the fewer CtsR binding sites in front of clpP (5, 6). Accordingly, clpP transcription is more sensitive to small variances of CtsR activity.
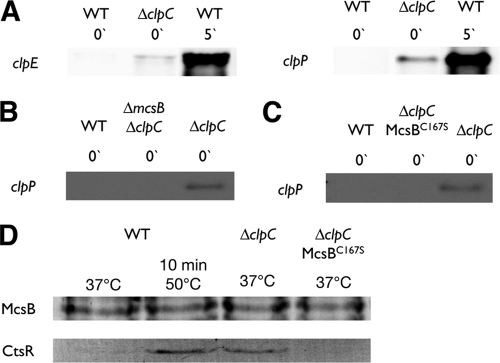
Fig. 1.
ClpC inhibits McsB kinase in vivo. (A to C) Northern blot analysis of clpE or clpP transcription in different mutants. (A) Transcription profiles of the wild type (WT) and a clpC mutant in nonstressed and heat-treated cells. (B and C) Transcription profile of a clpC mcsB or clpC mcsB(C167S) double mutant in comparison to the wild type and a clpC mutant in nonstressed cells. (D) Coimmunoprecipitation of McsB or McsBC167S in the wild type and clpC and clpC mcsB(C167S) mutants under control conditions or 10 min after heat stress, followed by Western blot analysis with McsB and CtsR antibodies.
When slightly enhanced transcription is indeed provoked by an active McsB adaptor, an mcsB knockout should restore the wild-type situation. In fact, an mcsB clpC double mutant displayed a wild-type level of clpP transcription in nonstressed cells (Fig. 1B), demonstrating that McsB is solely responsible for the slightly induced transcription in nonstressed cells observed for a clpC mutant (Fig. 1A). We used our recently developed in vivo expression system (9) to investigate a kinase-inactive McsB point mutant (23) in a ΔclpC background. Also in this mutant, the enhanced transcription disappeared (Fig. 1C), indicating that an active McsB adaptor causes the enhanced transcription of clpP and clpE in a clpC mutant.
Only in its autophosphorylated form is McsB able to bind to the CtsR repressor (9). To further test whether the activation of McsB as an adaptor protein indeed evoked the observed marginal activation of transcription in the clpC mutant, we analyzed the McsB/CtsR interactions in different strains. In nonstressed cells, McsB is not autophosphorylated (9), and McsB did not interact with CtsR (Fig. 1D). However, upon heat exposure McsB displays multiple heat-specific modifications (9), and the ability to bind CtsR is massively enhanced (Fig. 1D). In agreement with the transcriptional data, McsB is able to interact with CtsR in a clpC mutant even in nonstressed cells (Fig. 1D), causing decreased CtsR activity, which finally results in the observed slight increase of clpE and clpP transcription (Fig. 1A). This observation indicates that in a clpC mutant, the McsB adaptor is active as an adaptor protein. In contrast, when the autophosphorylation of McsB is prevented by an inactive McsB kinase (9, 23), McsB is no longer able to bind to CtsR in a clpC knockout strain (Fig. 1D). These results demonstrate that the McsB kinase was activated in the absence of ClpC, suggesting that an inhibition of McsB activity occurs by the direct binding to ClpC in vivo.
Interaction of McsB with ClpC is decreased during heat stress.
McsB kinase is inhibited in the presence of ClpC both in vivo (Fig. 1) and in vitro (23). This leads to an inactive kinase in nonstressed cells. However, upon heat exposure, McsB activity is strongly induced (9). The simplest interpretation of this activation is a diminished inhibition of McsB caused by a decreased interaction with ClpC. To monitor changes in the interaction of McsB with ClpC, we used metabolic labeling with either 14N- or 15N-labeled medium coupled with MS analysis as described previously (8).
We compared the specific McsB/ClpC interaction in nonstressed cells with that of cells that were heat stressed for 5 min. Upon heat exposure, the interaction of McsB with ClpC was dramatically decreased to approximately 30% compared to that of nonstressed cells. Accordingly, almost 70% of the McsB molecules that were associated with ClpC were released upon heat exposure. The percent dissociations determined by a comparison of the McsB/McsA, McsB/ClpC, and ClpC/MecA interactions at 37°C versus 10 min at 50°C (ratios determined by 14N/15N metabolic labeling) were −2.08% ± 0.14%, −68.79% ± 8.73%, and −14.54% ± 1.69%, respectively. This observation shows that McsB is activated when released from ClpC, as was suggested by previous in vitro data (23). In contrast, the binding of McsB with McsA was not altered under these conditions (see above), which is consistent with the fact that the interaction of McsB with McsA is needed for an efficient kinase activity (23).
McsB is probably not displaced by other adaptor proteins on ClpC.
Previous in vitro studies reported that McsB is activated by a titration from ClpC caused by MecA (23). Interestingly, McsB and MecA use the same binding sites on ClpC (19). However, in the above-mentioned quantitative analysis of ClpC interaction partners, we did not detect an increase in levels of known adaptor proteins such as MecA or YpbH during heat stress. In contrast, the interaction of ClpC with MecA was decreased upon heat exposure (see above for dissociation values), and a ClpC-YpbH interaction could not be confirmed by our approach. For further investigation we constructed a strain that ectopically overexpresses clpC (see Fig. S1 in the supplemental material). An increased amount of cellular ClpC molecules should prevent MecA or other adaptor proteins from competitively inhibiting McsB binding to ClpC. If so, the interaction of McsB with ClpC should not be disturbed, and McsB kinase cannot be activated. As a result, CtsR would no longer be degraded during heat stress because McsB kinase activity is required for this process (9, 19).
However, when we investigated CtsR stability in this clpC overexpression strain (see Fig. S1 in the supplemental material), we did not find a stabilization of CtsR indicating an activation of the McsB kinase (Fig. 2 A). These results strongly suggest that McsB is not released from ClpC by a titration but rather by an active stress sensing mechanism. However, the precise molecular details of this process remain unknown, even though we can rule out that other adaptor proteins are involved in vivo, as was suggested by previous in vitro data (23).
Fig. 2.

McsB kinase is activated by a release from ClpC. (A) Stability of CtsR in different mutants. Shown are data from pulse-chase labeling and immunoprecipitation of CtsR after heat stress in the B. subtilis wild type, in a kinase-deficient mcsB mutant (McsBC167S), and in a strain which ectopically overexpresses clpC. (B) Northern blot analysis of clpP in nonstressed wild-type cells, clpC mutant cells, and cells of a clpC knockout strain that ectopically overexpresses clpE.
ClpE does not inhibit the McsB kinase.
The Hsp100/Clp protein ClpE was detected as an interaction partner of McsB but only during heat stress (data not shown). Most probably, this is due to increased amounts of ClpE shortly after heat stress (5). ClpE is known to aid ClpC in CtsR degradation while performing the early proteolysis of CtsR (27). Nevertheless, CtsR degradation always depends on the presence of McsB kinase activity (9, 19). Thus, we were interested in whether ClpE can replace ClpC with regard to the inhibition of the McsB kinase.
In nonstressed cells, ClpE is hardly detectable at the protein level (13). Thus, the overexpression of clpE in trans should decrease the above-mentioned enhanced transcription of clpP to wild-type levels in a clpC mutant strain under standard growth conditions, when ClpE is indeed able to inhibit the McsB kinase. However, this strain displayed a level of clpP transcription similar to that of a clpC knockout strain (Fig. 2B), suggesting that ClpE is not able to inhibit McsB kinase in vivo.
Activated McsB is autophosphorylated on arginine residues.
It has long been known that McsB can be autophosphorylated in vitro (12, 19, 23). Recently, we demonstrated that McsB also underwent autophosphorylation during heat stress in vivo, which is essential for the targeting of CtsR (9). We used phosphopeptide enrichment by titanium dioxide (TiO2) chromatography (30) and online ESI-mass spectrometry with a nanoACQUITY UPLC system coupled to an LTQ Orbi-trap mass spectrometer to determine the specific autophosphorylation sites of McsB in vitro. We detected several phosphate groups on arginine residues at McsB in vitro (Fig. 3), consistent with the previous observation that McsB is an arginine kinase in vitro (12).
Fig. 3.
McsB is autophosphorylated on different arginine residues in vitro. McsB was incubated with its activator McsA for 20 min in the presence of 10 mM ATP, allowing phosphorylation events. The mixture was then trypsin digested, and the phosphopeptides were enriched and analyzed by mass spectrometry (for more details see Materials and Methods). The panels show the spectra of five arginine-phosphorylated peptides of McsB. McsB was phosphorylated on residues R29, R190, R255, R269, and R272. The b and y ions are highlighted, and the peptide sequences are indicated. The amino acids that were identified by mass spectrometric analysis are displayed in colored, bold letters. The upper sequence corresponds to the b ions, and the lower sequence corresponds to the y ions. AMU, atomic mass units.
Interestingly, the detected phospho-sites are located mainly within the C terminus of McsB (Fig. 3). The N terminus of McsB is similar to ATP guanidino-phosphotransferase domains that are critical for kinase activity (23, 25). In contrast, the adjacent C-terminal domain is not homologous to these eukaryotic phosphotransferases and thus may not contain catalytic residues. Because McsB is capable of binding free CtsR only in its autophosphorylated form (9), we hypothesize that these phosphorylations may result in a conformational change that allows McsB to bind to CtsR.
YwlE and ClpC both inhibit the McsB kinase.
Recently, it was reported that the YwlE phosphatase antagonizes McsB in vivo (9, 16, 22). Accordingly, we were interested in whether YwlE contributes to the regulation of McsB activity.
In a clpC mutant, the McsB kinase is activated due to the absence of its inhibitor ClpC, but YwlE could still contribute to McsB inhibition, as was previously shown for the shutdown of the McsB adaptor during heat stress (9). Any increased level of McsB activity in a clpC ywlE double mutant would point to an additional inhibition of McsB by YwlE, even in nonstressed cells. We detected that clpP and clpE transcription levels are once more elevated in nonstressed cells compared to clpC single-knockout mutant cells (Fig. 4 A and B), indicating an enhanced McsB activity in the absence of clpC and ywlE. Nevertheless, transcription is also strongly induced upon heat exposure in this double mutant (Fig. 4A) due to the intrinsic heat sensing of CtsR (9). To confirm that the McsB kinase is not further activated in the absence of YwlE and ClpC, we analyzed CtsRG64P derepression in a clpC ywlE mutant. CtsRG64P is no longer able to respond to heat stress, but it was demonstrated previously that this CtsR variant was still marginally inactivated during heat stress due to the heat-dependent activation of the McsB adaptor (9). CtsRG64P was already partially inactivated in nonstressed cells of a clpC ywlE double mutant comparably to wild-type CtsR in the clpC ywlE mutant, but no further CtsR derepression was found during heat stress, showing that heat-dependent McsB activation does not occur in this mutant (Fig. 4C). Accordingly, McsB was already fully active in nonstressed ywlE clpC mutant cells, demonstrating that ClpC and YwlE are the only inhibitors of McsB.
Fig. 4.
McsB is involved as an adaptor protein in general protein turnover. (A) Northern blot analysis of clpP in cells of the wild type, a ywlE mutant, a clpC mutant, and a clpC ywlE double-knockout strain. (B) clpE-bgaB activity in nonstressed wild-type cells and in cells of a ywlE mutant, a clpC mutant, and a clpC ywlE mutant. (C) Northern blot analysis of clpP in CtsRG64P, CtsRG64P ΔmcsB, and CtsRG64P ΔclpC ΔywlE backgrounds. (D) Western blot analysis of McsB in wild-type and clpC, clpC mcsB(C167S), and ywlE clpC mutant strains after 2D separation. (E) Growth curves of B. subtilis strains, including wild-type strain 168 and ΔclpC, ΔywlE, ΔmcsB, ΔclpC ΔywlE, ΔclpC ΔywlE ΔmcsB, and ΔclpC ΔywlE ΔmcsB(C167S) mutant strains in minimal medium.
We used two-dimensional (2D) PAGE to analyze McsB modifications that may represent autophosphorylated forms of McsB in the different mutant strains. As described above, we found that McsB is present with only one distinct spot in nonstressed cells (Fig. 4D) (9). In contrast, at 10 min upon heat exposure, McsB displayed an elongated spot chain with two additional spots in the acidic area, suggesting autophosphorylated forms of McsB (Fig. 4D) (9). Consistent with the observed activity of the McsB adaptor, McsB exhibits an additional spot in a clpC mutant in nonstressed cells (Fig. 4D). This McsB modification disappeared in the kinase-inactive McsB mutant protein (Fig. 4D), consistent with the above-mentioned transcriptional studies (Fig. 1D). Interestingly, only when both McsB inhibitors, ClpC and YwlE, were mutated did McsB exhibit a heat-shock-like pattern in nonstressed cells (Fig. 4D) (9). These results suggest that both ClpC and YwlE contribute to the inhibition of the McsB adaptor in nonstressed cells.
Active McsB adaptor impairs growth.
The ywlE clpC double mutant displayed a severely impaired growth phenotype in minimal (Fig. 4E) as well as in complex (data not shown) media. The clpC and the ywlE single mutants showed only minor growth defects in minimal medium (Fig. 4E) and exhibited wild-type-like growth in complex medium (data not shown). We propose that the activation of McsB due to the absence of both inhibitors ClpC and YwlE may be responsible for this phenotype. Activated McsB is able to bind substrates such as CtsR (9), thus disturbing protein activity (Fig. 1). Therefore, McsB might bind to other, putative substrates, thereby hampering their physiological function, resulting in a severe disturbance of cellular physiology. If such a theory is true, an mcsB clpC ywlE triple-knockout mutant should suppress the growth phenotype of the double mutant. In fact, the growth of the triple mutant is mostly restored compared with the growth of the ywlE clpC double mutant (Fig. 4E). To test whether this growth defect is provoked by the adaptor activity of McsB, we analyzed a ywlE clpC double mutant, where the McsB kinase was inactivated (9, 23). In this mutant, McsBC167S cannot be autophosphorylated and thus is not hyperactivated as an adaptor protein. As expected, the growth of this mutant was also restored. Both mcsB mutants roughly behave like the single mutants (Fig. 4E). Based on the above-mentioned observation that active McsB binds and inactivates CtsR, one could speculate about additional substrates that are targeted by McsB, whereby McsB interferes with the cell physiology.
DISCUSSION
In this study, detailed molecular insights into how McsB adaptor function is controlled in vivo were gained. Generally, the McsB kinase is inhibited by a direct interaction with ClpC. This interaction is dramatically diminished upon heat exposure, subsequently activating McsB. ClpC not only inhibits the McsB kinase by direct interactions but also degrades activated McsB (9). Consistent with previously described results (9), we confirmed that the YwlE phosphatase is an additional inhibitor of McsB activity. Thus, ClpC and YwlE are both inhibitors of the McsB adaptor in vivo.
Adaptor proteins play an important role in regulated proteolysis in bacteria (1, 20). However, the precise molecular details of how these adaptors are controlled are largely unknown. Only for specific model adaptor proteins, such as MecA in B. subtilis or RssB in Escherichia coli, have regulatory details been reported. MecA is responsible for the degradation of the competence regulator ComK by ClpCP in B. subtilis, thereby regulating natural competence. The activity of MecA is modulated by the antiadaptor protein ComS, which binds with a higher affinity to MecA, subsequently releasing ComK, and activates competence development (37). The phosphorylated adaptor protein RssB targets RpoS in E. coli, the master regulator of the general stress response, for degradation by ClpXP (35, 41). Thus, the phospho-state of RssB, which depends on the activity of the sensor kinase ArcB, is essential for its adaptor function (28). In addition, the activity of RssB is also regulated by different antiadaptor proteins, such as IraP, IaM, or IraD, which all sense and integrate different stress signals (3).
Our results imply a similar multiple regulation of McsB activity by the three regulators of McsB: ClpC, YwlE, and McsA. We suggest that each regulator senses and integrates different stress signals independently regulating McsB activity. The independent regulation of the two inhibitors ClpC and YwlE is suggested by the severe impact of the permanently activated McsB kinase on cell physiology when both inhibitors are mutated (Fig. 4E). This observation indicates that one of the two inhibitors of McsB is always present in its active form, preventing cell damage. Consequently, the activities of both proteins are essential for an appropriate inhibition of McsB. Thus, the inactivation of one of the two inhibitors leads to an activation of McsB. We demonstrated that McsB is activated by a release from ClpC during heat stress but that YwlE still inhibits McsB (9). To date, nothing is known about the regulation of YwlE activity, but most probably, YwlE senses and integrates cellular signals other than ClpC, resulting in a specific YwlE-dependent response. YwlE is highly homologous with low-molecular-weight protein tyrosine phosphatases (LMWPTPs) and contains a conserved cysteine residue in its active center (29). It is known that this cysteine residue possesses regulatory potential in eukaryotes (4) and might also play a role in the regulation of McsB activity in B. subtilis.
ClpC and YwlE do not inhibit McsB with the same intensity. In the absence of ClpC, McsB is strongly activated (Fig. 1A), whereas a ywlE knockout leads to only a minor activation of McsB in nonstressed cells (9). CtsR degradation depends on an active McsB adaptor (9, 19, 23). However, in a ywlE mutant CtsR is only very slowly degraded in nonstressed cells and gets rapidly degraded only upon heat exposure, when the inhibition of McsB by ClpC is attenuated (see Fig. S2 in the supplemental material). Thus, McsB activity is affected primarily by ClpC-dependent inhibition, and we could show that this inhibition is decreased upon heat exposure. Consistent with this finding, the McsB substrate CtsR is degraded only during heat and protein stress (25). Accordingly, this observation also supports the argument that the McsB adaptor exerts its main role in the adaptation of the cell to protein stress.
Nevertheless, McsA is essential for the activation of McsB as an adaptor protein (19, 23). Thus, the interaction of McsA with McsB represents an additional layer of McsB control. Recently, we showed that the strong interaction of McsA with McsB is abolished during thiol-specific stress. This prevents the activation of McsB by either YwlE or ClpC under these conditions. All in all, three different proteins are involved in the control of McsB activity (Fig. 5). Under standard growth conditions, McsB is inhibited by ClpC as well as YwlE, preventing activation by McsA. Probably all three regulators of McsB sense and integrate different stress signals. We were able to demonstrate that the stronger inhibition of ClpC is decreased by protein-folding stress. Under these circumstances, YwlE is active, but the McsB adaptor function is strongly induced.
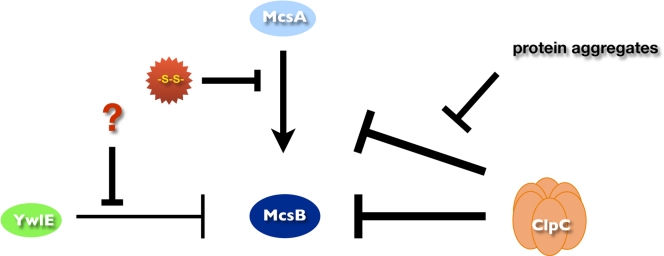
Fig. 5.
Model for the regulation of the McsB adaptor. McsB activity is regulated by its three regulators, ClpC, YwlE, and McsA. McsA is a prerequisite for full McsB activity. McsA permanently interacts with McsB, and this interaction is interrupted only by thiol-specific stresses. ClpC strongly inhibits McsB activation by direct contact with McsB. This inhibition is diminished under different protein stress conditions. In addition, ClpC is able to control McsB activity by regulated proteolysis, when active McsB is degraded by ClpCP. YwlE can also inhibit McsB but with a weaker intensity than that of ClpC.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Tschirner and S. Grund for professional technical assistance, K. Turgay for a stimulating discussion on the phenotype of the ywlE clpC double mutant, and A. Reder and D. Oertel for helpful comments.
This work was supported by grants of the Deutsche Forschungsgemeinschaft (HE1887/7-4 and SFB/TR34) and the European Union Bacell Health program (LSHG-CT-2004-503468) to M.H.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 27 May 2011.
REFERENCES
- 1. Baker T. A., Sauer R. T. 2006. ATP-dependent proteases of bacteria: recognition logic and operating principles. Trends Biochem. Sci. 31:647–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becher D., et al. 2009. A proteomic view of an important human pathogen—towards the quantification of the entire Staphylococcus aureus proteome. PLoS One 4:e8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bougdour A., Cunning C., Baptiste P. J., Elliott T., Gottesman S. 2008. Multiple pathways for regulation of sigmaS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol. Microbiol. 68:298–313 [DOI] [PubMed] [Google Scholar]
- 4. Chiarugi P., Cirri P. 2003. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem. Sci. 28:509–514 [DOI] [PubMed] [Google Scholar]
- 5. Derré I., Rapoport G., Devine K., Rose M., Msadek T. 1999. ClpE, a novel type of HSP100 ATPase, is part of the CtsR heat shock regulon of Bacillus subtilis. Mol. Microbiol. 32:581–593 [DOI] [PubMed] [Google Scholar]
- 6. Derré I., Rapoport G., Msadek T. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31:117–131 [DOI] [PubMed] [Google Scholar]
- 7. Dougan D. A., Mogk A., Bukau B. 2002. Protein folding and degradation in bacteria: to degrade or not to degrade? That is the question. Cell. Mol. Life Sci. 59:1607–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elsholz A. K. W., et al. 2011. CtsR inactivation during thiol-specific stress in low GC, Gram+ bacteria. Mol. Microbiol. 79:772–785 [DOI] [PubMed] [Google Scholar]
- 9. Elsholz A. K. W., Michalik S., Zühlke D., Hecker M., Gerth U. 2010. CtsR, the Gram-positive master regulator of protein quality control, feels the heat. EMBO J. 29:3621–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reference deleted.
- 11. Eymann C., Homuth G., Scharf C., Hecker M. 2002. Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J. Bacteriol. 184:2500–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuhrmann J., et al. 2009. McsB is a protein arginine kinase that phosphorylates and inhibits the heat-shock regulator CtsR. Science 324:1323–1327 [DOI] [PubMed] [Google Scholar]
- 13. Gerth U., et al. 2004. Fine-tuning in regulation of Clp protein content in Bacillus subtilis. J. Bacteriol. 186:179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reference deleted.
- 15. Gottesman S. 2003. Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19:565–587 [DOI] [PubMed] [Google Scholar]
- 16. Hahn J., Kramer N., Briley K., Jr., Dubnau D. 2009. McsA and B mediate the delocalization of competence proteins from the cell poles of Bacillus subtilis. Mol. Microbiol. 72:202–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartl F. U., Hayer-Hartl M. 2009. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16:574–581 [DOI] [PubMed] [Google Scholar]
- 18. Herzberg C., et al. 2007. SPINE: a method for the rapid detection and analysis of protein-protein interactions in vivo. Proteomics 7:4032–4035 [DOI] [PubMed] [Google Scholar]
- 18a. Hoch J. A. 1991. Genetic analysis in Bacillus subtilis. Methods Enzymol. 204:305–320 [DOI] [PubMed] [Google Scholar]
- 19. Kirstein J., Dougan D. A., Gerth U., Hecker M., Turgay K. 2007. The tyrosine kinase McsB is a regulated adaptor protein for ClpCP. EMBO J. 26:2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirstein J., Molière N., Dougan D. A., Turgay K. 2009. Adapting the machine: adaptor proteins for Hsp100/Clp and AAA+ proteases. Nat. Rev. Microbiol. 7:589–599 [DOI] [PubMed] [Google Scholar]
- 21. Kirstein J., et al. 2006. Adaptor protein controlled oligomerization activates the AAA+ protein ClpC. EMBO J. 25:1481–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirstein J., Strahl H., Molière N., Hamoen L. W., Turgay K. 2008. Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol. Microbiol. 70:682–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirstein J., Zühlke D., Gerth U., Turgay K., Hecker M. 2005. A tyrosine kinase and its activator control the activity of the CtsR heat shock repressor in B. subtilis. EMBO J. 24:3435–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reference deleted.
- 25. Krüger E., Zühlke D., Witt E., Ludwig H., Hecker M. 2001. Clp-mediated proteolysis in Gram-positive bacteria is autoregulated by the stability of a repressor. EMBO J. 20:852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reference deleted.
- 27. Miethke M., Hecker M., Gerth U. 2006. Involvement of Bacillus subtilis ClpE in CtsR degradation and protein quality control. J. Bacteriol. 188:4610–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mika F., Hengge R. 2005. A two-component phosphotransfer network involving ArcB, ArcA, and RssB coordinates synthesis and proteolysis of sigmaS (RpoS) in E. coli. Genes Dev. 19:2770–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Musumeci L., et al. 2005. Low-molecular-weight protein tyrosine phosphatases of Bacillus subtilis. J. Bacteriol. 187:4945–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olsen J. V., Macek B. 2009. High accuracy mass spectrometry in large-scale analysis of protein phosphorylation. Methods Mol. Biol. 492:131–142 [DOI] [PubMed] [Google Scholar]
- 31. Reder A., et al. 2008. The Spx paralogue MgsR (YqgZ) controls a subregulon within the general stress response of Bacillus subtilis. Mol. Microbiol. 69:1104–1120 [DOI] [PubMed] [Google Scholar]
- 32. Sauer R. T., et al. 2004. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell 119:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schmidl S. R., et al. 2010. The phosphoproteome of the minimal bacterium Mycoplasma pneumoniae: analysis of the complete known Ser/Thr kinome suggests the existence of novel kinases. Mol. Cell. Proteomics 9:1228–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Striebel F., Kress W., Weber-Ban E. 2009. Controlled destruction: AAA+ ATPases in protein degradation from bacteria to eukaryotes. Curr. Opin. Struct. Biol. 19:209–217 [DOI] [PubMed] [Google Scholar]
- 35. Stüdemann A., et al. 2003. Sequential recognition of two distinct sites in sigma(S) by the proteolytic targeting factor RssB and ClpX. EMBO J. 22:4111–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stülke J., Hanschke R., Hecker M. 1993. Temporal activation of beta-glucanase synthesis in Bacillus subtilis is mediated by the GTP pool. J. Gen. Microbiol. 139:2041–2045 [DOI] [PubMed] [Google Scholar]
- 37. Turgay K., Hahn J., Burghoorn J., Dubnau D. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 17:6730–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a. UniProt Consortium 2007. The Universal Protein Resource (UniProt). Nucleic Acids Res. 35:D193–D197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang J., Hartling J. A., Flanagan J. M. 1997. The structure of ClpP at 2.3 A resolution suggests a model for ATP-dependent proteolysis. Cell 91:447–456 [DOI] [PubMed] [Google Scholar]
- 39. Weber-Ban E. U., Reid B. G., Miranker A. D., Horwich A. L. 1999. Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. Nature 401:90–93 [DOI] [PubMed] [Google Scholar]
- 40. Wickner S., Maurizi M. R., Gottesman S. 1999. Posttranslational quality control: folding, refolding, and degrading proteins. Science 286:1888–1893 [DOI] [PubMed] [Google Scholar]
- 41. Zhou Y., Gottesman S., Hoskins J. R., Maurizi M. R., Wickner S. 2001. The RssB response regulator directly targets sigma(S) for degradation by ClpXP. Genes Dev. 15:627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.