Abstract
The molecular interactions between staphylococcal phages and host cell surfaces are poorly understood. Employing Staphylococcus aureus teichoic acid mutants, we demonstrate that wall teichoic acid (WTA), but not lipoteichoic acid, serves as a receptor for staphylococcal siphovirus and myovirus, while only the siphovirus requires glycosylated WTA.
TEXT
The horizontal transfer of virulence and resistance genes by bacteriophages has a profound impact on the pathogenicity and environmental adaptation of Staphylococcus aureus and other major human pathogens. The host range of a specific phage is largely determined by its capacity to adsorb to cognate receptor structures on the bacterial cell surface. Understanding the molecular determinants of host specificity is also critical for the design of phage therapies, which are increasingly regarded as an alternative strategy to combat antibiotic-resistant bacteria (15). However, while the receptors for many coliphages have been investigated in detail, the nature of host receptors has remained unknown for most phages infecting Gram-positive pathogens.
The vast majority of known bacteriophages belong to the order Caudovirales or tailed phages, which are composed of an icosahedral head filled with double-stranded DNA and a thin tail. The tailed phages can be further classified into three major families based on tail morphology: Podoviridae with a very short tail, Siphoviridae with a long, noncontractile tail, and Myoviridae with a long, contractile, double-sheathed tail (1). Staphylococcal phages can be assigned to the major serogroups A, B, D, and F. Serogroups A, B, and F are siphoviruses, which differ in tail length, head size, and head shape. Serogroup D phages, on the other hand, belong to the family Myoviridae with double-sheathed, contractile tails (3, 14).
Phage tail tip proteins and/or phage tail fiber proteins are most often involved in recognition of and adsorption to specific components at the host cell surface (11). Many Gram-positive cell envelopes are modified with a unique anionic glycopolymer, the peptidoglycan-anchored wall teichoic acid (WTA), which is one of the most abundant molecules at the bacterial surface (16). Most S. aureus strains express polyribitol phosphate WTA substituted with N-acetylglucosamine (GlcNAc) and d-alanine (20). We have recently identified the S. aureus WTA glycosyltransferase TarM and demonstrated that depletion of TarM leads to a phage-resistant phenotype (21). We concluded that α-GlcNAc glycoepitopes expressed on S. aureus WTA serve as an adsorption receptor for serogroup B phages such as φ11. This study inspired us to further explore the adsorption receptors of staphylococcal phages belonging to other serogroups or morphogroups.
To study the role of wall teichoic acids in staphylococcal phage adsorption, we first created the mutant RN4220ΔtagO (ΔtagO) (Table 1), which is deficient in WTA. ΔtagO was constructed by replacing the tagO gene, which is required for the first step of WTA biosynthesis, with an erythromycin resistance cassette as described previously (18). The mutant was complemented with the plasmid pRB474-tagO, which was constructed by subcloning the tagO gene into the Escherichia coli-S. aureus shuttle expression vector pRB474 (4). The loss of WTA in ΔtagO was verified by no detectable phosphate contents in WTA preparations. Of note, the WTA mutants ΔtagO and K6 were constructed in the genetic background of S. aureus strain RN4220, which is free of capsule (17), prophages, and restriction mechanisms (10). Since this strain is devoid of all of these pre- and postadsorption factors and mechanisms that might lead to phage resistance (12), impaired plaque formation on the mutant lawn indicates impaired adsorption and plaque formation suggests successful adsorption and infection.
Table 1.
Bacterial strains and phages used in this study
| Strain or phage | Description | Source or reference(s) |
|---|---|---|
| S. aureus strains | ||
| RN4220 | Restriction deficient, no capsule, no prophage, transformable strain | 10, 17 |
| 4S5 | RN4220 Δspa ΔltaS | 6 |
| RN4220ΔtagO | RN4220 ΔtagO | This study |
| K6 | RN4220, transposon insertion in tarM | 21 |
| cK6 | K6 complemented with pRB474-tarM | 21 |
| ctagO | ΔtagO complemented with pRB474-tagO | This study |
| SA113 | Derivative of NCTC 8325 harboring prophages φ11, φ12, and φ13 | 8 |
| SA113ΔtagO | SA113 ΔtagO; no wall teichoic acids | 18 |
| Phages | ||
| φ47 | Siphoviridae, serogroup A | NCTCa |
| φSa2mw | Siphoviridae, serogroup A | C. Goerke |
| φ13 | Siphoviridae, serogroup F | G. Bierbaum |
| φ77 | Siphoviridae, serogroup F | NCTC |
| φK | Myoviridae, serogroup D | G. Bierbaum |
| φ812 | Myoviridae, serogroup D | S. Moineau |
NCTC, National Collection of Type Cultures.
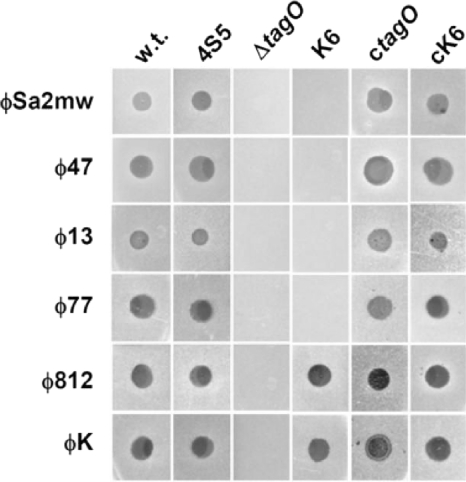
We then challenged wild-type RN4220 and the RN4220-derived mutants with staphylococcal phages of serogroup A (φ47 and φSa2mw), serogroup F (φ13 and φ77), and serogroup D (φK and φ812). Briefly, 10 μl of phage lysate containing approximately 107 PFU was spotted onto soft agar containing test bacteria as described previously (21). All of the phages tested formed plaques on the bacterial lawn of wild-type RN4220 but failed to form plaques on the WTA-deficient ΔtagO mutant (Fig. 1), indicating that the infection is dependent on WTA. This observation was further verified by the fact that the complemented tagO mutant (ctagO) again becomes susceptible to all phages.
Fig. 1.
WTA-dependent phage infection of S. aureus. Phage lysates from serogroup A (φ47, φSa2mw), serogroup F (φ13, φ77), and serogroup D phages (φK and φ812) were spotted onto lawns of wild-type (w.t.) or cell wall mutant S. aureus RN4220. Macroplaque formation indicates successful adsorption and infection by phages. The bacterial strains used include a ΔtagO mutant (deficient in wall teichoic acid), mutant strains K6 (no GlcNAc modification of wall teichoic acid) and 4S5 (no lipoteichoic acids), and ctagO and cK6, which are tagO- and tarM-complemented strains, respectively, and produce wild-type WTA.
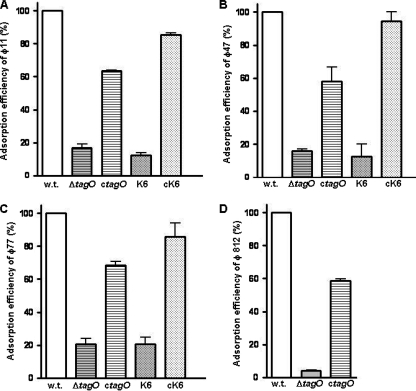
To demonstrate that the impaired phage infection of the S. aureus WTA mutant was indeed caused by impaired adsorption, the adsorption rate was determined for φ11 (serogroup B), φ47 (serogroup A), φ77 (serogroup F), and φ812 (serogroup D). As shown in Fig. 2, all of the phages showed severely impaired adsorption to the tagO mutant, which lacks WTA. Moreover, the adsorption rates of phages infecting the tagO-complemented strain (ctagO) were restored to around 60% of the adsorption rates of phages infecting the wild-type cells, indicating that successful phage infection and adsorption are dependent on WTA.
Fig. 2.
Phage adsorption to S. aureus mutants with altered WTAs in comparison to the wild type (w.t.). S. aureus cells (8 × 107 CFU in 200 μl) were incubated with phage φ11 (A), φ47 (B), or φ77 (C) (5 ×107 PFU in 100 μl) at 37°C for 15 min. The bound phage were separated from unbound free phage by centrifugation at 13,000 × g for 3 min. Similar adsorption experiments were carried out with myovirus φ812 (D), except that 6 × 104 PFU in 100 μl were incubated with 4 × 108 CFU (in 200 μl). Adsorption was calculated by determining the number of PFU of the unbound phage in the supernatant and subtracting it from the total number of input PFU. Adsorption efficiency relative to the adsorption to wild-type strain RN4220, which was set as 100%, is indicated. The data shown are the mean values of three independent measurements. The error bars represent standard deviations.
To investigate the requirement of the GlcNAc glycoepitope on WTA for phage infection, the tarM mutant K6, which lacks GlcNAc on WTA, was infected with various phages. Interestingly, while serogroup A and F phages were not able to form plaques on K6 lawns, the serogroup D phages were still virulent toward this mutant. In addition, K6 complemented with plasmid-encoded tarM was again susceptible to serogroup A and F phages (Fig. 1). This finding leads to the conclusion that the GlcNAc glycoepitope on WTA, introduced by the glycosyltransferase TarM, is required for successful infection by serogroup A and F phages. Further adsorption rate analysis of φ11, φ47, and φ77 (Fig. 2A, B, and C) revealed severely impaired phage adsorption to mutant K6 cells, and the phage adsorption rate was restored to over 80% when the tarM-complemented strain (cK6) was infected. This suggested that α-GlcNAc carried by WTA served as the phage adsorption receptor for serogroup B, A, and F phages of S. aureus. This is also in good agreement with previous observations that phages infecting other Gram-positive bacteria, such as Listeria and Bacillus phages, used the WTA glycoepitope as an adsorption receptor (2, 5, 19, 22). Of note, although adsorption of serogroup D phages is dependent on WTA, neither the glycoepitope nor the alanyl modification of WTA seems to be essential for adsorption since these phages infected both the K6 mutant with tarM disrupted (Fig. 1) and a ΔdltA mutant deficient in alanylation of teichoic acids (data not shown). Thus, the serogroup D phages seem to adsorb to the anionic backbone of WTA.
In a recent study (9), the tail protein ORF636 of φSLT was characterized as an adhesion protein for lipoteichoic acid (LTA) of S. aureus. By in silico analysis, tail proteins that are 99% identical to ORF636 could be identified in the genome of serogroup A phages such as φ47 and φSa2mw, which require, as shown above, the glycoepitopes of WTA for adsorption. To characterize whether LTA is involved in phage infection, especially phage adsorption, a simple, direct, and convincing method would be a spot assay using an LTA-negative S. aureus strain. LTA is synthesized by ltaS, an enzyme that was previously shown to be essential for normal cell division and growth (7). However, it has recently been shown that an ltaS mutant is viable under osmotically stabilizing conditions (13). By adopting a similar strategy, an ltaS knockout mutant was constructed by allelic exchange under conditions that are permissive for growth (broth containing 7.5% NaCl) and the lack of LTA was confirmed by Western blot analysis using a polyglycerolphosphate LTA-specific monoclonal antibody (6; R. M. Corrigan et al., unpublished data). Upon several passages in standard medium without 7.5% NaCl, ΔltaS mutant strain 4S5 regained the ability to grow and divide similar to a wild-type strain. Of note, while LTA was absent from strain 4S5, WTA was still produced by this mutant. We then spotted the phage lysate on the 4S5 lawn and found that all of the phages tested, including serogroup A phages φ47and φSa2mw, were able to form plaques (Fig. 1), indicating that successful phage infection is independent of LTA.
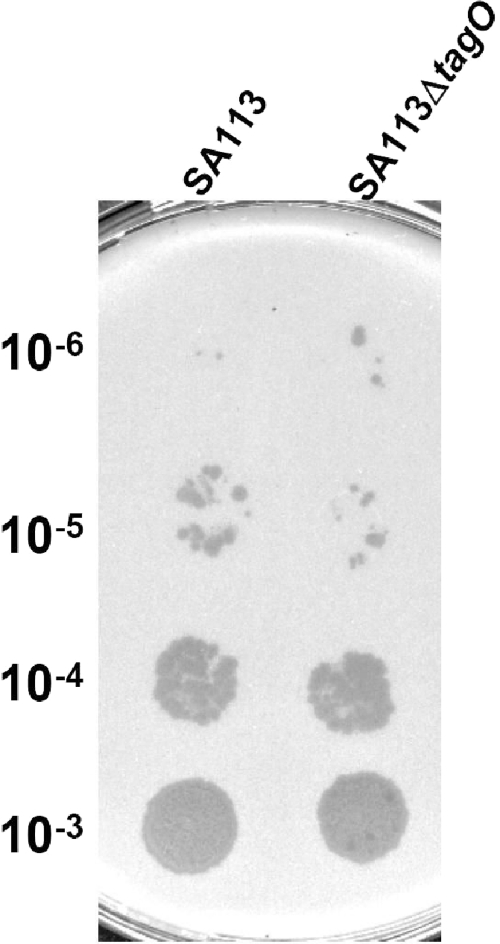
WTA is extremely abundant on the bacterial surface and might also play a role in phage release. To study whether WTA affects the efficiency of phage release, we did a prophage induction experiment by adding mitomycin C to the culture of S. aureus strain SA113, which harbors three prophages in its genome. As shown in Fig. 3, upon mitomycin C induction, the phage titer released by WTA-deficient mutant SA113ΔtagO is comparable to that released by wild-type SA113, indicating that depletion of WTA does dot affect phage release.
Fig. 3.
WTA deficiency does not affect prophage induction and release. Wild-type S. aureus strain SA113 and WTA-deficient mutant SA113ΔtagO were incubated with mitomycin C at a final concentration of 0.5 μg/ml to induce prophage release. After 6 h of induction, the cultures were centrifuged at 5,000 × g for 10 min and then the culture supernatants were serially diluted to 10−6-fold. A 10-μl volume of each dilution (10−3 to 10−6) was then spotted onto a lawn of bacterial indicator strain RN4220 and incubated at 37°C overnight.
In summary, our data clearly demonstrate that WTA, but not LTA, is required for siphovirus and myovirus infection of S. aureus. While siphoviruses need the GlcNAc on WTA for adsorption, myoviruses seem to adsorb to the backbone of WTA. Further studies are necessary to elucidate how WTA is recognized by staphylococcal phage receptor binding proteins and contributes to the strain and species specificity of staphylococcal phages.
Acknowledgments
We thank Cordula Gekeler for technical assistance; Sylvain Moineau, Felix d'Hérelle Reference Center for Bacterial Viruses, Quebec, for providing φ812; and Gabriele Bierbaum, Institute of Medical Microbiology, Immunology and Parasitology, University of Bonn, for providing φK and φ13.
This work was supported by German Research Foundation grants SFB766 to G.X., C.G., and A.P. and TR-SFB34 to A.P. and by Wellcome Trust grant WT084483 to A.G.
Footnotes
Published ahead of print on 3 June 2011.
REFERENCES
- 1. Ackermann H. W. 2006. Classification of bacteriophages, p. 8–16In Calendar R. (ed.), The bacteriophages, 2nd ed. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 2. Baptista C., Santos M. A., Sao-Jose C. 2008. Phage SPP1 reversible adsorption to Bacillus subtilis cell wall teichoic acids accelerates virus recognition of membrane receptor YueB. J. Bacteriol. 190:4989–4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandis H., Lenz W. 1984. Staphylokokken-Bakteriophagen, p. 186–214In Meyer W. (ed.), Staphylokokken und Staphylokokken-Erkrankungen. VEB Gustav Fischer Verlag, Jena, Germany [Google Scholar]
- 4. Brückner R. 1992. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene 122:187–192 [DOI] [PubMed] [Google Scholar]
- 5. Cheng Y., Promadej N., Kim J. W., Kathariou S. 2008. Teichoic acid glycosylation mediated by gtcA is required for phage adsorption and susceptibility of Listeria monocytogenes serotype 4b. Appl. Environ. Microbiol. 74:1653–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corrigan R. M., Gründling A. 2010. Never say never, growth of a lipoteichoic acid negative strain of Staphylococcus aureus, abstr. 191, p. 201 14th International Symposium on Staphylococci and Staphylococcal Infections (ISSSI), Bath, United Kingdom [Google Scholar]
- 7. Gründling A., Schneewind O. 2007. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 104:8478–8483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iordanescu S., Surdeanu M. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96:277–281 [DOI] [PubMed] [Google Scholar]
- 9. Kaneko J., Narita-Yamada S., Wakabayashi Y., Kamio Y. 2009. Identification of ORF636 in phage phiSLT carrying Panton-Valentine leukocidin genes, acting as an adhesion protein for a poly(glycerophosphate) chain of lipoteichoic acid on the cell surface of Staphylococcus aureus. J. Bacteriol. 191:4674–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreiswirth B. N., et al. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712 [DOI] [PubMed] [Google Scholar]
- 11. Kutter E., Raya R., Carlson K. 2004. Molecular mechanisms of phage infection, p. 165–222In Kutter E., Sulakvelidze A. (ed.), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL [Google Scholar]
- 12. Labrie S. J., Samson J. E., Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327 [DOI] [PubMed] [Google Scholar]
- 13. Oku Y., et al. 2009. Pleiotropic roles of polyglycerolphosphate synthase of lipoteichoic acid in growth of Staphylococcus aureus cells. J. Bacteriol. 191:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pantůcek R., et al. 2004. Identification of bacteriophage types and their carriage in Staphylococcus aureus. Arch. Virol. 149:1689–1703 [DOI] [PubMed] [Google Scholar]
- 15. Schoolnik G. K., Summers W. C., Watson J. D. 2004. Phage offer a real alternative. Nat. Biotechnol. 22:505–506 [DOI] [PubMed] [Google Scholar]
- 16. Swoboda J. G., Campbell J., Meredith T. C., Walker S. 2010. Wall teichoic acid function, biosynthesis, and inhibition. ChemBioChem 11:35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wann E. R., Dassy B., Fournier J. M., Foster T. J. 1999. Genetic analysis of the cap5 locus of Staphylococcus aureus. FEMS Microbiol. Lett. 170:97–103 [DOI] [PubMed] [Google Scholar]
- 18. Weidenmaier C., et al. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10:243–245 [DOI] [PubMed] [Google Scholar]
- 19. Wendlinger G., Loessner M. J., Scherer S. 1996. Bacteriophage receptors on Listeria monocytogenes cells are the N-acetylglucosamine and rhamnose substituents of teichoic acids or the peptidoglycan itself. Microbiology 142(Pt. 4):985–992 [DOI] [PubMed] [Google Scholar]
- 20. Xia G., Kohler T., Peschel A. 2010. The wall teichoic acid and lipoteichoic acid polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 300:148–154 [DOI] [PubMed] [Google Scholar]
- 21. Xia G., et al. 2010. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J. Biol. Chem. 285:13405–13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Young F. E. 1967. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc. Natl. Acad. Sci. U. S. A. 58:2377–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]