Abstract
The development of T helper 17 (TH17) cells is a well-established adaptive mechanism for the production of interleukin-17A (IL-17A), a cytokine involved in neutrophil recruitment. However, pathways contributing to mucosal expression of IL-17A during the initial phase of a bacterial infection have received less attention. Here we used the mouse colitis model of Salmonella enterica serotype Typhimurium infection to investigate the contribution of myeloid differentiation primary response protein 88 (MyD88) to inflammation and mucosal IL-17A expression. Expression of IL-23 in the cecal mucosa during S. Typhimurium colitis was dependent on the presence of MyD88. Furthermore, initial expression of IL-17A at 24 h after S. Typhimurium infection was dependent on MyD88 and the receptor for IL-1β. IL-23 and IL-1β synergized in inducing expression of IL-17A in splenic T cells in vitro. In the intestinal mucosa, IL-17A was produced by three distinct T cell populations, including δγ T cells, TH17 cells, and CD4−CD8− T cells. The absence of IL-1β signaling or IL-17 signaling reduced CXC chemokine expression but did not alter the overall severity of pathological lesions in the cecal mucosa. In contrast, cecal pathology and neutrophil recruitment were markedly reduced in Myd88-deficient mice during the initial phases of S. Typhimurium infection. Collectively, these data demonstrate that MyD88-dependent mechanisms, including an initial expression of IL-17A, are important for orchestrating early inflammatory responses during S. Typhimurium colitis.
INTRODUCTION
Salmonella enterica serotype Typhimurium is associated with gastroenteritis in humans, a diarrheal disease characterized by acute neutrophilic intestinal inflammation (29). Analysis of responses in bovine, simian, and murine hosts has shown that IL-17A and IL-22 are among the cytokines whose expression is most prominently induced in the intestinal mucosa at early time points after S. Typhimurium infection (8, 24, 25). In the mouse colitis model of S. Typhimurium infection, IL-17A contributes specifically to neutrophil recruitment into the cecal mucosa (25) but is not required for the development of other pathological changes (33). The cytokine IL-17A has received considerable attention, because one of its cellular sources is a distinct CD4+ helper T cell subset, termed TH17, which is involved in various inflammatory and autoimmune disorders (16). However, antigen-dependent differentiation of naïve T cells into TH17 cells is inadequate to explain the rapid initial release of IL-17A in response to pathogen exposure (6). For example, a marked induction of IL-17A expression is observed within 2 to 5 h after S. Typhimurium infection of bovine or simian ligated ileal loops (24, 25), which does not provide enough time for the development of TH17 cells. The mechanisms that lead to this early, innate release of IL-17A during infection have received less attention than the role of TH17 cell development in states of deregulated inflammation (16).
The role of IL-23 in enhancing or maintaining adaptive responses of TH17 cells has been well described (14). However, recent reports suggest that IL-23 also functions in orchestrating innate inflammatory responses. For example, IL-23 was necessary for inducing innate IL-17A production in a mouse model of Helicobacter hepaticus-induced colitis (5). Similarly, mice deficient for the p19 subunit of IL-23 were unable to express IL-17A during S. Typhimurium-induced colitis (9). A second cytokine implicated in inducing innate production of IL-17A is IL-1β. Ex vivo stimulation with IL-23 or IL-1β elicits an innate release of IL-17A from NKT cells, γδ T cells, or TH17 cells (10, 22, 35). Collectively, these data suggest that IL-23 and/or IL-1β is an important mediator of an initial, innate release of IL-17A during infection.
Myd88 is required for signaling through Toll-like receptors (TLRs) (20) as well as the receptor for IL-1β (21, 41). Salmonella-infected dendritic cells can produce IL-23 in vitro through a MyD88-dependent pathway that involves stimulation of TLR4 (31). However, IL-23 production can also be induced in vitro through alternative, MyD88-independent mechanisms, such as activation of intracellular bacterial sensor nucleotide-binding and oligomerization domain 2 (NOD2) (37). An initial report suggests that pathological changes during the early, innate phase of S. Typhimurium-induced colitis are independent of the presence of Myd88 (12), although cytokine responses were not monitored in that study. As a result, it remains unclear which mechanisms contribute to innate production of IL-17A during the early phase of S. Typhimurium colitis. The goal of this study was to investigate the role of MyD88 in inducing early, mucosal IL-17A expression during S. Typhimurium infection by the use of the mouse colitis model.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. Typhimurium strain IR715, a fully virulent, nalidixic acid-resistant derivative of isolate ATCC 14028, was used as wild-type isolate (34). ZA21 is a noninvasive derivative of S. Typhimurium strain IR715 carrying mutations in all T3SS-1 effector genes required for invasion (sipAsopABDE2) that has been described previously (26, 44). Bacteria were grown aerobically at 37°C in Luria-Bertani (LB) broth supplemented with antibiotics.
Intestinal model epithelia.
CaCo-2 cells (ATCC HTB-37) were grown in minimal essential media (MEM) (Invitrogen) complemented with 10% fetal calf serum. One day before the assay, the medium of the cells was changed to MEM complemented with 2% fetal calf serum. Cells were seeded on the apical compartment of a 12-mm-diameter Transwell plate (Corning Costar, Lowell, MA). The cells were utilized when a transepithelial resistance of 0.6 Ω·cm2 was reached. Polarized CaCo-2 cells were stimulated with recombinant human Il-1β (eBioscience) (20 ng/ml), IL-17 (R&D Systems) (150 ng/ml), and IL-22 (R&D Systems) (100 ng/ml) in the basolateral compartment for 3 h.
Animal experiments.
Streptomycin (Sigma) (20 mg/mouse)-pretreated C57BL/6 mice (Jackson Laboratory), MyD88-deficient mice (1), IL-1 receptor-deficient mice (mutation in the Il1r1 gene; Jackson Laboratory), and IL-17 receptor-deficient mice (mutation in the Il17ra gene; Jay Kolls) were infected orally with 1 × 109 CFU (in 0.1 ml of LB broth) of S. Typhimurium strain IR715 or ZA21 or were mock infected with 0.1 ml of sterile LB broth. At 12 h, 24 h, or 48 h after infection, mice were sacrificed, and samples of the cecum were snap-frozen in liquid nitrogen for isolation of mRNA. For determination of bacterial numbers, cecal contents, Peyer's patches, mesenteric lymph nodes, and liver tissue were homogenized in 1× phosphate-buffered saline (PBS), and serial 10-fold dilutions were plated on LB agar plates containing nalidixic acid.
Histopathology.
Formalin-fixed, hematoxylin and eosin (H&E)-stained cecal tissue sections were evaluated in a blind manner by a veterinary pathologist using the scoring scheme shown in Table 1. Neutrophil counts were determined by high-magnification (×400) microscopy, and numbers were averaged from 10 microscopic fields for each animal. Images were taken using an Olympus BX41 microscope.
Table 1.
Scoring criteria for blinded examination of H&E-stained sections from the cecum
| Score | Neutrophil infiltrationa | Infiltration by mononuclear cellsa | Submucosal edema | Epithelial damage | Exudate |
|---|---|---|---|---|---|
| 0 | No changes (0–5) | No changes (0–5) | No changes | No changes | No changes |
| 1 | 6–20 | 5–10 | Detectable (<10%) | Desquamation | Slight accumulation |
| 2 | 21–60 | 10–20 | Mild (10–20%) | Mild erosion and mild loss of goblet cells/undifferentiated enterocyte hyperplasia | Mild accumulation |
| 3 | 61–100 | 20–40 | Moderate (20–40%) | Marked erosion and moderate loss of goblet cells/undifferentiated enterocyte hyperplasia | Moderate accumulation |
| 4 | >100 | >40 | Marked (>40%) | Ulceration and marked loss of goblet cells/undifferentiated enterocyte hyperplasia | Marked accumulation |
Numbers in parentheses indicate the numbers of cells per high-power field (magnification, ×400).
Isolation and stimulation of splenocytes and T cells.
Single-cell suspensions were prepared by mechanical dissociation of mouse spleens. Prepared single-cell suspensions were treated with ACK buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2-EDTA, pH 7.4) in order to lyse red blood cells. Splenocyte single-cell suspensions were incubated with S. Typhimurium at a multiplicity of infection (MOI) of 1 for 1 h in complete Iscove's modified Dulbecco's medium (IMDM) (Invitrogen). Cells were washed and placed in complete IMDM containing 25 μg/ml of gentamicin for an additional 3 h. Isolation of untouched splenic T cells was performed using a pan-T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) per the manufacturer's instructions. Cells were treated with 10 ng/ml of recombinant mouse IL-23 and/or 10 ng/ml of IL-1β (eBioscience, San Diego, CA), for a total of 5 h. RNA was isolated using a Qiagen Qiashredder/RNEasy kit per the manufacturer's instructions.
Isolation of intestinal lymphocytes.
Three groups of two naive mice each (C57BL/6; Taconic) were euthanized, and organ tissue samples from each group of mice were combined. Intestines were removed beginning at the duodenum and ending at the proximal colon. Intestines were dissected by removing the remaining mesentery and vasculature. Then, each segment was opened longitudinally, the luminal content was gently removed, and the tissue was cut into 5-mm-thick sections with a scalpel. The sections were subsequently washed in cold 1× Hanks balanced salt solution (HBSS) (catalog no. 14185; Gibco) containing 0.015 M HEPES (catalog no. 15630; Gibco) a total of six times to remove mucus and remaining fecal matter. Intestinal tissue was placed in prewarmed (37°C) 1× HBSS containing 10% fetal bovine serum (catalog no. 10082; Gibco), 0.015 M HEPES, and 5 mM EDTA and stirred for 15 min at room temperature and then for 15 min at 37°C, followed by three 15-min washes with buffer adjusted to room temperature. The remaining tissue was stirred in prewarmed (37°C) 1× RPMI medium (catalog no. R1145; Sigma) containing 10% fetal bovine serum, penicillin-streptomycin (catalog no. 15240-062; Gibco), 0.015 M HEPES, and 1.6 mg of collagenase (catalog no. C6885; Sigma-Aldrich)/ml for 45 min at 37°C. The resulting cell suspension was washed twice with 1× HBSS containing 0.015 M HEPES.
Flow cytometric analysis of intestinal lymphocytes.
A total of 4 × 106 intestinal cells were resuspended in 2 ml of IMDM and treated with 25 μg/ml of phorbol-12-myristate 13 acetate (PMA) (catalog no. P8139; Sigma), 1 μM ionomycin (catalog no. I0634; Sigma), and 10 μg/ml of brefeldin A (catalog no. B7651; Sigma) for a total of 4 h at 37°C and 5% CO2. After stimulation, cells were stained with Aqua LIVE/DEAD cell discriminator (catalog no. L34597; Invitrogen) per the manufacturer's protocol. Cells were then stained for 1 h in the dark at 4°C with optimized concentrations of anti-CD3 Alexa 750–allophycocyanin (APC) (eBioscience clone 17A2), anti-CD8 Alexa 700 (eBioscience clone 53-6.7), anti-CD4 Pacific Blue (eBioscience clone RM4-5), and anti-TCR GD R-PE (BD Pharmingen clone GL3). Cells were washed twice with PBS containing 1% bovine serum albumin (fluorescence-activated cell sorter [FACS] buffer). After staining, cells were fixed and permeabilized using BD Cytofix/Cytoperm (catalog no. 51-2090KZ; BD). Permeabilized cells were then stained with anti-IL-17A Alexa Fluor 488 (clone eBio17B7; eBioscience) and anti-gamma interferon (anti-IFN-γ) APC (clone XMG1.2; eBioscience). Cells were washed twice and resuspended in FACS buffer and analyzed using an LSR II flow cytometer (Beckman-Coulter, San Jose, CA). The resulting data were analyzed using Flowjo software (Treestar, Inc., Ashland, OR). Gates were based on Fluorescence-Minus-One controls.
Real-time PCR.
Samples of the cecum were collected and immediately snap-frozen in liquid nitrogen and stored at −80°C. RNA was extracted using TriReagent (Molecular Research) according to the manufacturer's instructions. Isolated RNA was treated with DNase (Applied Biosystems), and reverse transcription (RT) was performed using1 μg of DNase-treated RNA with TaqMan reverse transcription reagent (Applied Biosystems). cDNA (4 μl) was used for each real-time reaction. RT-PCR was performed using Sybr green (Applied Biosystems) and an ABI 7900 RT-PCR machine (Applied Biosystems). The change in mRNA levels was determined using the following formula: fold change = 2[ΔCt(infected) − ΔCt(control)], where ΔCt(control) = threshold cycle (Ct) for target gene mock infection − Ct for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mock infection and ΔCt(infected) = Ct for target gene infection − Ct for GAPDH infection.
Protein expression analysis.
Proteins from the cecum were extracted using TriReagent (Molecular Research) according to the manufacturer's instructions. The protein concentration was determined using a Micro BCA kit (Thermo Scientific) according to the recommendations of the manufacturer. A 0.02-mg volume of total protein was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene fluoride membrane (Millipore) in a semidry transfer process (Bio-Rad). Nonspecific binding was blocked by incubating membranes in PBS containing 3% nonfat dry milk and 0.05% Tween 20. Membranes were incubated with polyclonal primary antibodies raised against tubulin (Cell Signaling Technology) and murine IL-1β (Abcam) overnight at 4°C. Horseradish peroxidase-conjugated anti-rabbit IgG (Promega) was used as the secondary antibody. Horseradish peroxidase activity was visualized by adding Immobilon Western chemiluminescent substrate (Millipore) to the membrane. Images were recorded and processed using a BioSpectrum imaging system (UVP).
Statistical analysis.
To determine the statistical significance of the results obtained with treatment groups in the animal experiments, an unpaired Student t test was used. A paired Student t test was used to confirm statistical significance in the tissue culture experiments. A two-tailed P < 0.05 was taken to represent statistical significance.
RESULTS
Expression of Il23a during S. Typhimurium colitis is dependent on the presence of MyD88.
To initiate our investigation, we determined the contribution of MyD88 to S. Typhimurium-induced IL-23 expression by the use of in vitro infections of splenocytes from wild-type mice (C57BL/6) or congenic Myd88-deficient mice. S. Typhimurium infection of splenocytes from wild-type mice resulted in increased Il23a (the gene encoding the p19 subunit of IL-23) mRNA levels compared to mock-infected control results (P < 0.05) (see Fig. S1 in the supplemental material). In contrast, Il23a expression was not induced during S. Typhimurium infection of splenocytes from MyD88-deficient mice.
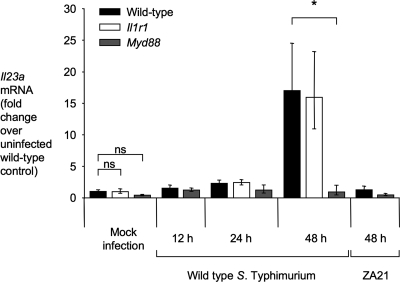
Mice pretreated with streptomycin develop acute inflammation of the cecum in response to S. Typhimurium infection (mouse colitis model). We determined whether MyD88 contributes to IL-23 expression in the mouse colitis model. Wild-type mice (C57BL/6) exhibited increased Il23a transcript levels in the cecal mucosa by 48 h after infection with the S. Typhimurium wild type compared to animals infected with an invasion-deficient mutant (ZA21) or mock-infected control animals (P < 0.05) (Fig. 1). Increased Il23a transcript levels were dependent on the presence of MyD88, because no increase in Il23a mRNA levels was observed in MyD88-deficient mice. MyD88 is required for signaling through TLRs (20) and the IL-1β receptor (21, 41). However, signaling through the IL-1β receptor was not required for Il23a expression, because Il23a transcript levels were not blunted in mice deficient for the IL-1R1 chain of the IL-1β receptor (Fig. 1).
Fig. 1.
Expression of Il23a is dependent on the presence of MyD88. Relative Il23a transcript levels in the cecal mucosa were determined by quantitative real-time PCR using the mouse colitis model. C57BL/6 mice (wild type), MyD88-deficient mice, or IL-1 receptor-deficient mice were inoculated with wild-type S. Typhimurium, a noninvasive S. Typhimurium mutant (ZA21), or sterile medium (Mock infection), and RNA was extracted from the cecal mucosa at the indicated time points. Bars for mock-infected animals represent the combined geometric means ± standard error of data from samples collected at 12, 24, and 48 h after inoculation. All other bars represent geometric means ± standard error of data from at least four different animals. Brackets indicate the statistical significance of differences. *, P < 0.05; ns, not significant.
Early expression of Il17a during S. Typhimurium colitis is dependent on the presence of MyD88 and IL-1R1.
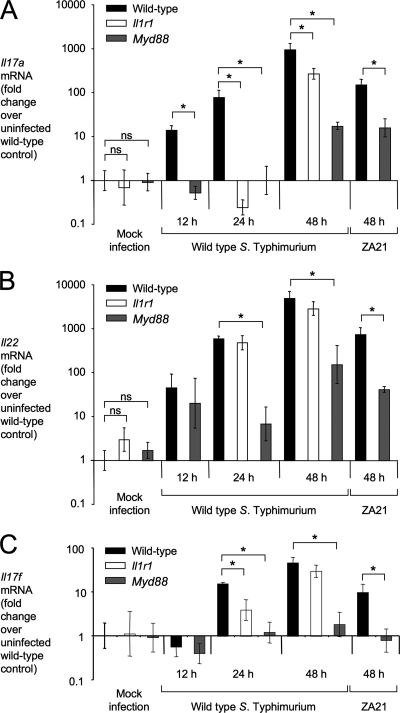
Next, we investigated expression of Il17a, Il17f, Il22, and Il1b during S. Typhimurium infection by the use of the mouse colitis model. Compared to the results seen with mock-infected animals, transcripts for Il17a and Il22 were induced in the cecal mucosa by 12 h after S. Typhimurium infection and mRNA levels continued to increase until 48 h after infection (Fig. 2). No induction of Il17a mRNA was observed by 24 h after infection of MyD88-deficient mice or IL-1R1-deficient mice, suggesting that both MyD88 and IL-1R1 are necessary for the initial production of IL-17A during colitis (Fig. 2A). However, by 48 h after S. Typhimurium infection, Il17a expression was only partially dependent on MyD88 and IL-1R1. Thus, MyD88-independent mechanisms contribute to production of IL-17A at later time points in the mouse colitis model. Expression of Il22 was not dependent on IL-1R1 and was blunted, but not abrogated, in Myd88-deficient mice (Fig. 2B). These data suggested that expression of Il17a and Il22 is differentially regulated during S. Typhimurium colitis. The pattern of Il17f expression was similar to that of Il17a (Fig. 2C). Finally, the expression pattern of Il1b, the gene encoding IL-1β, was similar to that of the Il22 gene (see Fig. S2A in the supplemental material) and IL-1β protein levels were reduced in the cecal mucosa of Myd88-deficient mice compared to wild-type mice during S. Typhimurium infection (see Fig. S2B in the supplemental material).
Fig. 2.
Expression of Il17a, Il17f, and Il22 in the mouse colitis model. Relative Il17a (A), Il22 (B), and Il17f (C) transcript levels in the cecal mucosa were determined by quantitative real-time PCR using the mouse colitis model. C57BL/6 mice (wild type), MyD88-deficient mice, or IL-1 receptor-deficient mice were inoculated with wild-type S. Typhimurium, a noninvasive S. Typhimurium mutant (ZA21), or sterile medium (Mock infection), and RNA was extracted from the cecal mucosa at the indicated time points. Bars for mock-infected animals represent the combined geometric means ± standard error of data from samples collected at 12, 24, and 48 h after inoculation. All other bars represent geometric means ± standard error of data from at least four different animals. Brackets indicate the statistical significance of differences. *, P < 0.05.
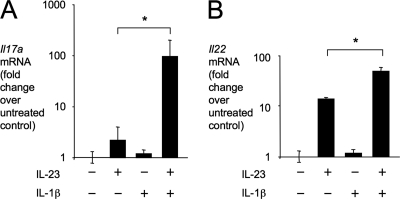
Our finding that IL-1R1 is required for early Il17a expression (Fig. 2A), along with the previous observation that IL-23 is required for Il17a expression in the mouse colitis model (9), suggested that IL-23 and IL-1β might cooperate in inducing IL-17A expression. To test this idea, murine splenic T cells were stimulated with recombinant murine IL-23 and/or Il-1β and expression of Il17a and Il22 was measured by quantitative real-time PCR (Fig. 3). There was a marked synergy between IL-23 and IL-1β in inducing expression of Il17a (Fig. 2A). Expression of Il22 was induced by incubating splenic T cells with IL-23, while a small but significant further increase in Il22 transcript levels was observed after costimulation with IL-23 and IL-1β (Fig. 2B). Our data suggest that IL-23 and IL-1β can synergize in inducing antigen-independent induction of Il17a expression.
Fig. 3.
IL-23 or IL-1β synergizes in inducing Il17a expression. Relative Il17a (A) and Il22 (B) transcript levels determined by quantitative real-time PCR after stimulation of splenic T cells with IL-23 or IL-1β for 5 h. Bars represent geometric means ± standard error of data from three independent experiments. Brackets indicate the statistical significance of differences. *, P < 0.05.
Identification of cellular sources of IL-17A production in the intestinal mucosa.
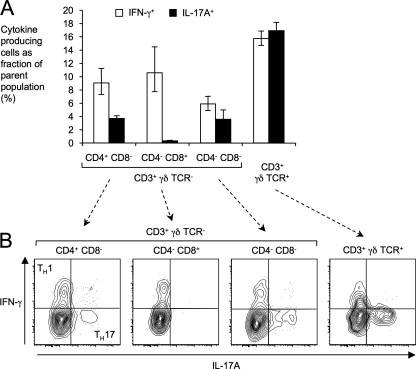
We have recently shown that depletion of T cells results in markedly reduced Il17a expression in the cecal mucosa during S. Typhimurium infection (8). These data suggest that IL-17A is produced by a subset of intestinal T cells. To test this prediction, we isolated intestinal lymphocytes from mice, stimulated them with PMA-ionomycin in the presence of brefeldin A, and analyzed them by flow cytometry for expression of surface markers (CD3, CD4, CD8, and γδ T cell receptor) and intracellular production of IL-17A and IFN-γ (Fig. 4). CD3+ cells (T cells) were divided into cells expressing the γδ T cell receptor (γδ T cells) and γδ T cell receptor-negative cells (representing αβ T cells). The αβ T cell population was further subdivided into CD4+CD8− T cells (T helper cells), CD4−CD8+ T cells (cytotoxic T cells), and CD4−CD8− T cells. The gating strategy is shown in Fig. S3 in the supplemental material.
Fig. 4.
Identification of cellular sources for IL-17A expression in the intestine. CD3+ intestinal lymphocytes (T cells) were differentiated based on expression of surface markers (CD4, CD8, and γδ TCR) and analyzed by flow cytometry for intracellular expression of IL-17A and IFN-γ after stimulation with PMA-ionomycin in the presence of brefeldin A for 4 h. (A) Average number of IFN-γ- or IL-17A-producing intestinal T cells as a fraction of the total number of cells in the indicated subpopulation of T cells. Each bar represents geometric means ± standard error of data from three independent experiments. (B) Representative flow cytometry images for detection of IFN-γ expression (y axis) and IL-17A expression (x axis) within the indicated subsets of intestinal T cells. Gates for detection were set using Fluorescence-Minus-One controls.
Expression of IFN-γ was detected in a subset of T helper cells, a subset of cytotoxic T cells, a subset of CD4−CD8− T cells, and a subset of γδ T cells (Fig. 4A). In contrast, only three T cell populations from the mouse intestine, including a subset of T helper cells, a subset of CD4−CD8− T cells, and a subset of γδ T cells, produced IL-17A. IFN-γ-producing T helper cells and IL-17A-producing T helper cells formed distinct subsets, presumably representing TH1 cells and TH17 cells, respectively (Fig. 3B). Similarly, IFN-γ and IL-17A were produced by distinct subsets of CD4−CD8− T cells and γδ T cells.
IL-22 and IL-1β cooperate in inducing epithelial expression of CXC chemokines in vitro.
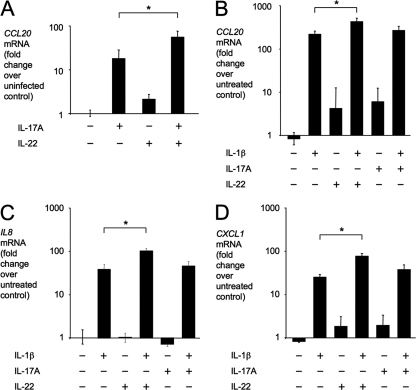
Epithelial cells are an important cellular target of IL-17A and IL-22 (17). For example, IL-17A and IL-22 cooperate in inducing CCL20 expression in polarized human T84 colonic epithelial cells (23). Similarly, we found that CCL20 expression in a model epithelium of polarized CaCo-2 human colonic cells was induced cooperatively by basolateral stimulation with recombinant human IL-17A and IL-22 (Fig. 5 A). However, CCL20 expression was induced at a larger magnitude when model epithelia were stimulated basolaterally with recombinant human IL-1β (Fig. 5B).
Fig. 5.
Cytokine expression by model epithelia after stimulation with IL-17A, IL-22, or IL-1β. Relative CCL20 (A and B), IL-8 (C), or CXCL1 (D) transcript levels were determined by quantitative real-time PCR after basolateral stimulation of polarized CaCo-2 cells with IL-17A, IL-22, or IL-1β. Bars represent geometric means ± standard error of data from three independent experiments. Brackets indicate the statistical significance of differences. *, P < 0.05.
Epithelial cells are the predominant producers of CXC chemokines in the intestinal mucosa during S. Typhimurium infection (28, 43). We therefore investigated expression of the neutrophil chemoattractants CXCL1 and IL-8 (CXCL8) after stimulation of model epithelia with IL-1β, IL-17A, or IL-22. Transcripts of IL-8 were markedly elevated in model epithelia after stimulation with IL-1β but not after stimulation with IL-17A or IL-22 (Fig. 5C). Stimulation with IL-17A or IL-22A resulted in a small increase in CXCL1 expression, while stimulation with IL-1β markedly increased CXCL1 transcript levels (Fig. 5D). A small but significant further increase in IL-8 and CXCL1 mRNA levels was observed when model epithelia were costimulated with IL-1β and IL-22 (Fig. 5C and D).
MyD88 contributes to CXC chemokine expression during S. Typhimurium colitis.
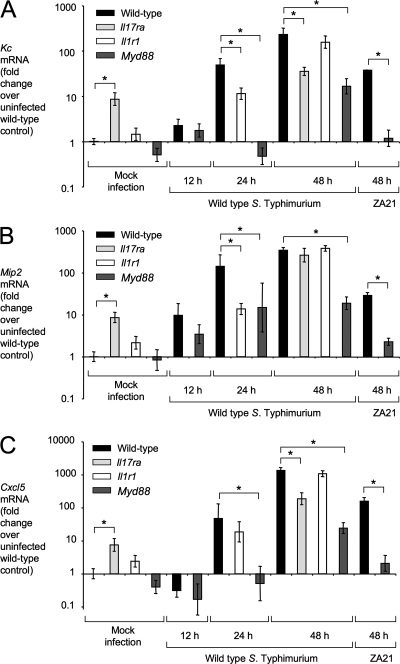
We next investigated the contribution of MyD88-dependent mechanisms to CXC chemokine production in the mouse colitis model (Fig. 6). Expression of Kc, the gene encoding keratinocyte-derived cytokine (KC [CXCL1]), and Cxcl5, encoding epithelial neutrophil-activating protein 78 (ENA-78 [CXCL5]), was induced in the cecal mucosa by 24 h after S. Typhimurium infection in wild-type mice and IL-1R1-deficient mice but not in MyD88-deficient mice (Fig. 6A and C). Expression of Kc and Mip2, the gene encoding macrophage inflammatory protein 2 (MIP2 [CXCL2]), was blunted in the cecal mucosa 24 h after S. Typhimurium infection of IL-1R1-deficient mice (Fig. 6A and B), suggesting that IL-1β signaling contributes to the production of neutrophil chemoattractants during colitis.
Fig. 6.
Expression of Kc, Mip2, and Cxcl5 in the mouse colitis model. Relative Kc (A), Mip2 (B) or Cxcl5 (C) transcript levels in the cecal mucosa were determined by quantitative real-time PCR using the mouse colitis model. C57BL/6 mice (wild type), MyD88-deficient mice, IL-17 receptor-deficient mice, or IL-1 receptor-deficient mice were inoculated with wild-type S. Typhimurium, a noninvasive S. Typhimurium mutant (ZA21), or sterile medium (mock infection), and RNA was extracted from the cecal mucosa at the indicated time points. Bars for mock-infected animals represent the combined geometric means ± standard error of data from samples collected at 12, 24, and 48 h after inoculation. All other bars represent geometric means ± standard error of data from at least four different animals. Brackets indicate the statistical significance of differences. *, P < 0.05.
By 48 h after S. Typhimurium infection, MyD88-dependent and MyD88-independent mechanisms contributed to expression of Kc, Mip2, and Cxcl5 (Fig. 6). The MyD88-independent mechanism contributing to elevated Kc, Mip2, and Cxcl5 expression required S. Typhimurium invasion, because a noninvasive S. Typhimurium mutant (ZA21) was unable to induce CXC chemokine expression in MyD88-deficient mice. Expression of Kc and Cxcl5 was blunted in IL-17 receptor (IL-17RA)-deficient mice 48 h after infection (Fig. 6A and C), suggesting that IL-17 signaling contributes to the production of CXC chemokines during S. Typhimurium colitis.
MyD88 contributes to the development of cecal pathology 48 h after S. Typhimurium infection.
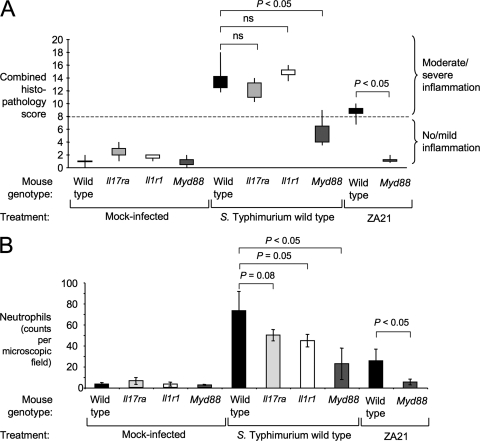
To further explore the consequences of blunted cytokine responses in MyD88-deficient mice during S. Typhimurium infection, we performed an analysis of histopathological changes in the cecum observed at 48 h after infection of wild-type (C57BL/6) mice, MyD88-deficient mice, IL-1R1-deficient mice, and mice deficient for the IL-17 receptor A chain (IL-17RA). Consistent with a recent report (33), IL-17RA deficiency did not markedly reduce the overall severity of intestinal lesions (Fig. 7 A and 8). Furthermore, the levels of severity of intestinal lesions were similar for IL-1R1-deficient mice and wild-type (C57BL/6) mice. However, both IL-1R1-deficient mice and IL-17RA-deficient mice exhibited reduced neutrophil recruitment in the cecal mucosa during S. Typhimurium infection (Fig. 7B), which correlated with the blunted CXC chemokine expression observed in tissue (Fig. 6). These data suggested that IL-1β and IL-17A aid in the recruitment of neutrophils but contribute little to other inflammatory changes observed in tissue. Neutrophil recruitment was reduced at a greater magnitude in MyD88-deficient mice (Fig. 7B), which exhibit a defect in IL-17A production (Fig. 2A) and cannot signal through IL-1R1 (21, 41). These data were consistent with the idea that multiple pathways for neutrophil recruitment were impaired in MyD88-deficient mice, including both IL-1R1-dependent mechanisms and IL-17RA-dependent mechanisms.
Fig. 7.
Histopathological analysis of the cecal mucosa after S. Typhimurium infection. C57BL/6 mice (wild type), MyD88-deficient mice, IL-17 receptor-deficient mice, or IL-1 receptor-deficient mice were inoculated with wild-type S. Typhimurium, a noninvasive S. Typhimurium mutant (ZA21), or sterile medium (Mock infection), and the cecum was collected 48 h after infection for histopathological analysis (Table 1). (A) Boxes in whisker plots represent the second and third quartiles of combined histopathology scores, while lines indicate the first and fourth quartiles. (B) Neutrophil infiltration of the cecal mucosa was quantified. Bars represent averages ± standard error. Brackets indicate the statistical significance of differences. ns, not significant.
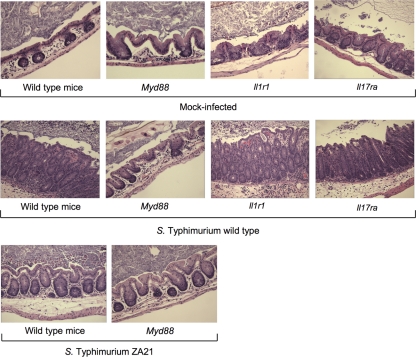
Fig. 8.
Histopathological images of the cecal mucosa after S. Typhimurium infection. Representative images of histopathological lesions in the cecal mucosa (magnification, ×200) of C57BL/6 mice (wild type), MyD88-deficient mice, Il-17 receptor-deficient mice, or Il-1 receptor-deficient mice 48 h after inoculation with wild-type S. Typhimurium, a noninvasive S. Typhimurium mutant (ZA21), or sterile medium (Mock-infected) are shown.
The severity of pathological lesions (Fig. 7A and 8) was markedly reduced during S. Typhimurium infection of MyD88-deficient mice compared to wild-type (C57BL/6) mice (P < 0.05). These data suggested that MyD88-dependent mechanisms contributed significantly to inflammation at 48 h after S. Typhimurium infection, which had been previously noted by gross pathological analysis (39). Since the severity of intestinal lesions was not reduced in IL-1R1-deficient mice, the MyD88-dependent mechanisms contributing to the development of pathological lesions likely represent TLR signaling and/or IL-18-dependent responses. Importantly, mild inflammatory changes still developed in MyD88-deficient mice infected with the S. Typhimurium wild type, which was indicative of the contribution of a MyD88-independent pathway to inflammation at that time point. This MyD88-independent inflammation was no longer observed when MyD88-deficient mice were infected with an invasion-deficient S. Typhimurium mutant (ZA21), which confirms the previous notion that MyD88-independent inflammation is dependent on invasion (12).
DISCUSSION
S. Typhimurium causes a typhoid fever-like disease in mice (mouse typhoid model) in which bacterial loads in the liver and spleen are controlled by TLR4-dependent mechanisms (3, 27, 36, 38, 40). In the mouse typhoid model, inflammatory foci of neutrophils develop in Peyer's patches by day 4 after S. Typhimurium infection in a MyD88-dependent fashion, but neutrophils remain scarce in other parts of the intestinal mucosa (28). This model is therefore not well suited to the study of gastroenteritis, a disease characterized by the rapid development of infiltrates in the ileum and colon that are dominated by neutrophils (29). S. Typhimurium infection of streptomycin-pretreated mice (mouse colitis model) results in a robust and rapidly developing neutrophil influx in the cecum that better models the intestinal pathology characteristic of human gastroenteritis (2). Here we used the mouse colitis model to investigate the contribution of MyD88 to intestinal inflammation during S. Typhimurium infection.
Initial studies using the mouse colitis model suggested that pathological changes in the cecal mucosa are caused by the induction of two distinct pathways. One pathway depends on the action of the invasion-associated type III secretion system (T3SS-1) and is independent of the presence of MyD88, while a second pathway is dependent on the presence of MyD88 but independent of the presence of T3SS-1 (12). Similarly, we found that pathological changes, neutrophil recruitment, and the expression of Kc and Mip2 were induced at 48 h after S. Typhimurium infection by a combination of an invasion-dependent but MyD88-independent mechanism and a MyD88-dependent but invasion-independent mechanism. However, previous studies, which were limited to a histopathological analysis, did not detect a contribution of MyD88 to host responses in the cecal mucosa during the initial 2 days after S. Typhimurium infection (4, 12). Here we show that MyD88 is a major contributor to cytokine production in the cecal mucosa during this early phase of S. Typhimurium infection. For example, expression of Kc and Il17a early (i.e., at 24 h) after S. Typhimurium infection was fully dependent on the presence of MyD88. A role of innate immune recognition through TLR4 in inducing an early expression of IL-17A was first demonstrated in a mouse model of pulmonary Klebsiella pneumoniae infection (13). Subsequent studies implicated MyD88 in induction of IL-17A expression during infections with the pulmonary pathogens Chlamydia muridarum (45) and Streptococcus pneumoniae (18). However, the work presented here represents the first demonstration that MyD88 is required for the initial induction of Il17a expression during an enteric infection, namely, S. Typhimurium-induced colitis.
MyD88 is an adaptor protein required for signaling through receptors for IL-1β (21, 41) and IL-18 (1) and all TLRs recognizing conserved bacterial molecular patterns (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR9) (20). Thus, MyD88 might contribute to an early induction of IL-17A production in the cecal mucosa through at least two distinct pathways. First, a MyD88-dependent mechanism might induce expression of IL-23 (31), a cytokine that mediates innate release of IL-17A in vitro (22, 35). Here we show that expression of IL-23 in the cecal mucosa during S. Typhimurium colitis was dependent on the presence of MyD88. Second, signaling through the MyD88-dependent receptor for IL-1β (21, 41) might stimulate an innate release of IL-17A (10, 35). Our data demonstrate that Il17a expression in the cecal mucosa 24 h after S. Typhimurium infection was dependent on the presence of IL-1R1. Previous studies suggested that early expression of Il17a in the S. Typhimurium mouse colitis model also requires IL-23 (9, 32). Collectively, these data indicate that IL-1β and IL-23 are both necessary for the initial production of IL-17A in the intestinal mucosa during S. Typhimurium colitis. Consistent with this idea, we observed a marked synergy between IL-1β and IL-23 in inducing Il17a expression in vitro.
Depletion of T cells markedly reduces Il17a expression in the mouse colitis model of S. Typhimurium infection, suggesting that T cells are an important cellular source for this cytokine (8). Here we show that three distinct T cell populations, including γδ T cells, TH17 cells, and CD4− CD8− γδTCR− T cells, produced IL-17A in the intestinal mucosa. A contribution of γδ T cells to IL-17A production is suggested by the finding that the absence of this cell type reduces Il17a expression in the cecal mucosa of streptomycin-pretreated mice during S. Typhimurium infection (9). Similarly, TH17 cells have been implicated in early responses to bacterial invasion by the observation that simian immunodeficiency virus-mediated TH17 depletion correlates with reduced mucosal IL-17A and IL-22 expression during S. Typhimurium infection of simian ligated ileal loops (25). CD4−CD8−γδTCR− T cells likely represent NKT cells, a known cellular source of IL-17A (22). However, the relative contribution of NKT cells to S. Typhimurium colitis remains to be investigated.
IL-17A is highly induced at early time points after S. Typhimurium infection of simian and bovine ligated ileal loops (24, 25). A recent study showed that neutralization of IL-17A in the lumen of murine ligated ileal loops exacerbates epithelial damage induced by S. Typhimurium infection (19), which points to a role of IL-17A in the immediate early protection against S. Typhimurium in this model. In the mouse colitis model, IL-17A functions largely in inducing CXC chemokine expression and neutrophil recruitment (25). That is, IL-17RA deficiency modestly reduced neutrophil recruitment to the cecal mucosa. However, IL-17RA deficiency had little effect on the development of submucosal edema, recruitment of mononuclear cells, or the formation of exudates, thus resulting in no significant changes in the overall pathology score, which was consistent with a previous report (33). Similarly, IL-1R1 deficiency modestly reduced neutrophil recruitment without affecting the development of other pathological changes. It appears likely that the more pronounced reduction of neutrophil infiltration observed in MyD88-deficient mice was due to impairment of both IL-17A-dependent and IL-1β-dependent neutrophil recruitment mechanisms. In addition, MyD88-deficient mice were impaired in production of IL-22, a cytokine functioning in defense against luminal bacteria (23) and contributing to protection against systemic Salmonella infection (30). Finally, inactivation of MyD88 prevents signaling through the receptor for IL-18 (1), a cytokine important for IFN-γ production. The impairment of at least four distinct inflammatory pathways (IL-17A, IL-1β, IL-22, and IL-18) might account for the pronounced reduction in cecal pathology observed in MyD88-deficient mice.
Although the severity of pathological lesions was markedly reduced in MyD88-deficient mice, mild inflammatory changes were still observed in these animals 2 days after infection with the S. Typhimurium wild type but not after infection with an invasion-deficient mutant. One MyD88-independent mechanism contributing to intestinal inflammation during S. Typhimurium infection is the activation of nucleotide-binding and oligomerization domain 1 (NOD1) and NOD2, two intracellular sensors of bacterial cell wall fragments (7, 15). A second mechanism that might contribute to invasion-dependent but MyD88-independent mechanisms of inflammation is complement activation by invading bacteria. After T3SS-1-mediated penetration of the epithelial lining, S. Typhimurium becomes exposed to complement, a humoral component of the innate immune system that senses conserved microbial structures, such as lipopolysaccharide (42). The resulting activation of complement through the alternative pathway generates the complement fragments C3a and C5a, which are potent inducers of inflammatory responses (11). However, a possible contribution of complement to S. Typhimurium-induced inflammation has not yet been investigated using the mouse colitis model.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nicole Baumgarth for providing Myd88-deficient mice generated by Shizuo Akira by way of Richard Flavell.
Work in A.J.B.'s laboratory is supported by Public Health Service grants AI040124, AI044170, AI073120, AI076246, AI079173, and AI088122.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Adachi O., et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 2. Barthel M., et al. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernheiden M., et al. 2001. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J. Endotoxin Res. 7:447–450 [PubMed] [Google Scholar]
- 4. Bruno V. M., et al. 2009. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 5:e1000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buonocore S., et al. 2010. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464:1371–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cua D. J., Tato C. M. 2010. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10:479–489 [DOI] [PubMed] [Google Scholar]
- 7. Geddes K., et al. 2010. Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect. Immun. 78:5107–5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godinez I., et al. 2008. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect. Immun. 76:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Godinez I., et al. 2009. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect. Immun. 77:387–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo L., et al. 2009. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. U. S. A. 106:13463–13468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haas P. J., van Strijp J. 2007. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol. Res. 37:161–175 [DOI] [PubMed] [Google Scholar]
- 12. Hapfelmeier S., et al. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675–1685 [DOI] [PubMed] [Google Scholar]
- 13. Happel K. I., et al. 2003. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 170:4432–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korn T., Bettelli E., Oukka M., Kuchroo V. K. 2009. IL-17 and Th17 cells. Annu. Rev. Immunol. 27:485–517 [DOI] [PubMed] [Google Scholar]
- 15. Le Bourhis L., et al. 2009. Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection. Infect. Immun. 77:4480–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Littman D. R., Rudensky A. Y. 2010. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140:845–858 [DOI] [PubMed] [Google Scholar]
- 17. Liu J. Z., Pezeshki M., Raffatellu M. 2009. Th17 cytokines and host-pathogen interactions at the mucosa: dichotomies of help and harm. Cytokine 48:156–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma J., et al. 2010. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect. Immun. 78:830–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayuzumi H., Inagaki-Ohara K., Uyttenhove C., Okamoto Y., Matsuzaki G. 2010. Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology 131:377–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Medzhitov R., et al. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253–258 [DOI] [PubMed] [Google Scholar]
- 21. Muzio M., Ni J., Feng P., Dixit V. M. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 278:1612–1615 [DOI] [PubMed] [Google Scholar]
- 22. Rachitskaya A. V., et al. 2008. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J. Immunol. 180:5167–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raffatellu M., et al. 2009. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raffatellu M., et al. 2007. The capsule-encoding viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 75:4342–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raffatellu M., et al. 2008. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raffatellu M., et al. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 73:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roy M. F., et al. 2006. Incremental expression of Tlr4 correlates with mouse resistance to Salmonella infection and fine regulation of relevant immune genes. Genes Immun. 7:372–383 [DOI] [PubMed] [Google Scholar]
- 28. Rydström A., Wick M. J. 2009. Monocyte and neutrophil recruitment during oral Salmonella infection is driven by MyD88-derived chemokines. Eur. J. Immunol. 39:3019–3030 [DOI] [PubMed] [Google Scholar]
- 29. Santos R. L., et al. 2009. Life in the inflamed intestine, Salmonella style. Trends Microbiol. 17:498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schulz S. M., et al. 2008. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J. Immunol. 181:7891–7901 [DOI] [PubMed] [Google Scholar]
- 31. Siegemund S., et al. 2007. Production of IL-12, IL-23 and IL-27p28 by bone marrow-derived conventional dendritic cells rather than macrophages after LPS/TLR4-dependent induction by Salmonella Enteritidis. Immunobiology 212:739–750 [DOI] [PubMed] [Google Scholar]
- 32. Siegemund S., et al. 2009. Differential IL-23 requirement for IL-22 and IL-17A production during innate immunity against Salmonella enterica serovar Enteritidis. Int. Immunol. 21:555–565 [DOI] [PubMed] [Google Scholar]
- 33. Songhet P., et al. 2010. IL-17A/F-signaling does not contribute to the initial phase of mucosal inflammation triggered by S. Typhimurium. PLoS One 5:e13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stojiljkovic I., Bäumler A. J., Heffron F. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 177:1357–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sutton C. E., et al. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331–341 [DOI] [PubMed] [Google Scholar]
- 36. Talbot S., et al. 2009. Toll-like receptor 4 signalling through MyD88 is essential to control Salmonella enterica serovar typhimurium infection, but not for the initiation of bacterial clearance. Immunology 128:472–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Beelen A. J., et al. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27:660–669 [DOI] [PubMed] [Google Scholar]
- 38. Vazquez-Torres A., et al. 2004. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J. Immunol. 172:6202–6208 [DOI] [PubMed] [Google Scholar]
- 39. Vijay-Kumar M., et al. 2008. Toll-like receptor 5-deficient mice have dysregulated intestinal gene expression and nonspecific resistance to Salmonella-induced typhoid-like disease. Infect. Immun. 76:1276–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiss D. S., Raupach B., Takeda K., Akira S., Zychlinsky A. 2004. Toll-like receptors are temporally involved in host defense. J. Immunol. 172:4463–4469 [DOI] [PubMed] [Google Scholar]
- 41. Wesche H., Henzel W. J., Shillinglaw W., Li S., Cao Z. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 7:837–847 [DOI] [PubMed] [Google Scholar]
- 42. Winter S. E., Keestra A. M., Tsolis R. M., Baumler A. J. 2010. The blessings and curses of intestinal inflammation. Cell Host Microbe 8:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang S., et al. 2003. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 71:4795–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang S., et al. 2002. SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 70:3843–3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X., et al. 2009. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 183:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.