Abstract
T cell-mediated immunity is critical for the control of Mycobacterium tuberculosis infection. Identifying the precise immune mechanisms that lead to control of initial M. tuberculosis infection and preventing reactivation of latent infection are crucial for combating tuberculosis. However, a detailed understanding of the role of T cells in the immune response to infection has been hindered. In addition, there are few flow cytometry studies characterizing the Vβ repertoires of T cell receptors (TCRs) at local sites of M. tuberculosis infection in adult tuberculosis. In this study, we used culture filtrate protein 10 (CFP-10) from M. tuberculosis to characterize T cells at local sites of infection. We simultaneously analyzed the correlation of the production of cytokines with TCR Vβ repertoires in CFP-10-specific CD4+ and CD8+ T cell subsets. For the first time, we demonstrate that CFP-10-specific CD4+ or CD8+ T cells from tubercular pleural fluid can produce high levels of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) and upregulate the expression of CD107a/b on the cell surface. The CFP-10-specific cells were effector/memory cells with a CD45RO+ CD62L− CCR7− CD27− expression profile. In addition, we found CFP-10-specific CD4+ and CD8+ T cells in tubercular pleural fluid, with biased usage of TCR Vβ9, Vβ12, or Vβ7.2. Our findings of CFP-10-specific CD4+ and CD8+ T cells in tubercular pleural fluid are critical for understanding the mechanisms of the local cellular immune response and developing more effective therapeutic interventions in cases of M. tuberculosis infection.
INTRODUCTION
Tuberculosis (TB) remains a threat to the health of people worldwide. Unfortunately, vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG) prevents severe disease only for childhood tuberculosis, not for the most common pulmonary infection in adults (7, 38). T cell-mediated immunity is critical for the control of M. tuberculosis infection. Therefore, identifying the precise immune mechanisms that lead to control of initial M. tuberculosis infection and preventing reactivation of latent infection are crucial for combating this disease (25).
CD4+ T cells and Th1 cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), are important in the cell-mediated response to M. tuberculosis infection. IFN-γ is an important cytokine for macrophage activation and contributes to the major effector response to M. tuberculosis. TNF-α is also a key cytokine in host immunity to intracellular bacteria, most notably M. tuberculosis (26). It has repeatedly been demonstrated in murine models that granuloma function, formation, and maintenance are dependent on TNF-α (3). Mice deficient in TNF-α production are highly susceptible to M. tuberculosis infection (16), and depletion of TNF-α results in reactivation of latent disease (1, 5, 6, 32). The incidence of TB is increased in patients given anti-TNF-α treatments for autoimmune diseases (37). CD107a and CD107b are located on the inner membrane of cytotoxic granules and are expressed on the outer cell membrane briefly after degranulation. Therefore, CD107a/b is a good marker for exocytosis of cytotoxic granules in cytotoxic activity.
Region of difference 1 (RD1) encodes the immunogenic mycobacterial proteins, including culture filtrate protein 10 (CFP-10) and early secreted antigenic target 6 (ESAT-6). RD1 is absent from M. bovis BCG. Along with being essential for M. tuberculosis virulence, both CFP-10 and ESAT-6 can stimulate T cells to produce IFN-γ and exhibit cytolytic activity in animals and in humans (21). T cells recognize and respond to antigens via their T cell receptors (TCRs), which are heterodimeric glycoproteins generated by the process of genomic recombination of variable (V), diversity (D), joining (J), and constant (C) regions. TCR Vβ repertoires can indicate which families of T cells are involved in the immune response.
Analysis of the TCR Vβ chain distribution is widely used to characterize alterations in T cell repertoires. Indeed, the diversity of the TCR repertoire has been hypothesized to be associated with effective immune responses against various pathogens, such as in allergies (39), autoimmune disease (8, 14), infection (12, 13, 24), cancer (2), and immunodeficiency (33). However, analysis of TCR V gene usage relies predominantly on PCR-based methods, which do not allow the analysis of precise T cell subsets unless they have been separated previously. Furthermore, antigen-specific TCR Vβ repertoires at local sites of M. tuberculosis infection have rarely been analyzed.
Patients with tuberculous pleurisy have a relatively effective immune response against M. tuberculosis infection (15). Therefore, we took pleural fluid from patients with tuberculous pleurisy to characterize CFP-10-specific T cells at local sites of M. tuberculosis infection. We also analyzed the correlation of TCR Vβ repertoires with the production of cytokines in CFP-10-specific CD4+ and CD8+ T cell subsets. For the first time, our data demonstrate that CFP-10-specific CD4+ and CD8+ T cells exist at local sites of M. tuberculosis infection in patients with tuberculous pleurisy. The CFP-10-specific cells were effector/memory cells with a CD45RO+ CD62L− CCR7− CD27− expression profile. In addition, we found CFP-10-specific CD4+ and CD8+ T cells in tubercular pleural fluid, with biased usage of TCR Vβ9, Vβ12, or Vβ7.2.
MATERIALS AND METHODS
Subjects.
This study was conducted using pleural fluid mononuclear cells (PFMCs) from 14 patients with tuberculous pleurisy (7 females and 7 males, aged 19 to 60 years). All patients with tuberculous pleurisy were from the Chest Hospital of Guangzhou, Guangzhou, China. The diagnosis of tubercular pleural effusion was made by the following criteria: isolation of M. tuberculosis from the pleural fluid or tissue, a positive smear for acid-fast bacilli from the pleural fluid or tissue, and a positive mycobacterium culture. The pleural fluid was obtained during therapeutic thoracentesis. None of the patients were on antituberculosis treatment at the time of enrollment in the study. Exclusion criteria included a positive HIV test or the presence of concurrent infectious diseases. Adequate informed consent was obtained from all individuals involved in this study. The study was approved by the Medical School Review Board at Sun Yat-Sen University, Guangzhou, China.
Cell preparation.
PFMCs from patients with tuberculous pleurisy were isolated by Ficoll-Hypaque density gradient centrifugation. Cells were suspended at 2 × 106 cells/ml in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (Sijiqing, Hangzhou, China), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, and 50 μM 2-mercaptoethanol (all from Gibco).
Antigens and antibodies.
CFP-10 was kindly provided by the Shanghai Haigui Biological Technology Limited Company (Shanghai, China). Anti-CD28 and anti-CD49d (BD Biosciences Pharmingen, San Diego, CA) were used as costimulatory molecules. In all cases, CFP-10 was used at 5 μg/ml, and anti-CD28 and anti-CD49d were used at 1 μg/ml (each). The following antibodies were used for flow cytometry: CD3-phycoerythrin (PE)-Cy7 (clone SK7), CD4-allophycocyanin (APC)-Cy7 (clone RPA-T4), CD8-peridinin chlorophyll protein (CD8-PerCP) (clone SK1), IFN-γ–fluorescein isothiocyanate (FITC) (clone 45.15), IFN-γ–APC (clone B27), TNF-α–PE (clone MAb11), TNF-α–PE-Cy7 (clone MAb11), CD45RO-PE (clone UCHL1), CD62L-APC (clone DREG-56), CCR7-PE-Cy7 (clone 3D12), CD27-APC (clone L128), CD107a-FITC (clone H4A3), CD107b-FITC (clone H4B4) (all from BD Biosciences Pharmingen), and CD3-PE-TR (clone S4.1) (Invitrogen, Carlsbad, CA) antibodies, as well as an IOTest Beta Mark TCR Vβ repertoire kit (Beckman Coulter, Inc., Brea, CA).
Intracellular cytokine staining and flow cytometric analysis.
PFMCs were stimulated with CFP-10 plus anti-CD28 and anti-CD49d. In addition, CD107a-FITC and CD107b-FITC antibodies were added for the detection of degranulation at the beginning of cell culture, for a total of 6 h. Brefeldin A (10 μg/ml; Sigma-Aldrich, St. Louis, MO) and monensin (1 μl/ml; BD Biosciences Pharmingen) were added after 1 h, and plates were incubated at 37°C and 5% CO2. After stimulation, cells were washed with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA) and 0.05% sodium azide. Cells were incubated with antibodies for surface staining at 4°C. Cells were then fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.1% saponin, and stained for intracellular cytokines. Flow cytometry was performed using a BD FACSCalibur flow cytometer, and data were analyzed using FlowJo software (Treestar, San Carlos, CA).
TCR Vβ repertoire analysis.
PFMCs from 10 patients with tuberculous pleurisy were stimulated as described above. After stimulation, cells were stained with CD3-PE-TR, CD4-APC-Cy7, and CD8-PerCP antibodies and an IOTest Beta Mark TCR Vβ repertoire kit, which allowed staining of 24 TCR Vβ chains, at 4°C. Cells were fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.1% saponin, and stained with IFN-γ–APC and TNF-α–PE-Cy7 antibodies at 4°C. Flow cytometry was performed using a BD FACS Aria II flow cytometer. The IOTest Beta Mark TCR Vβ repertoire kit contains 8 vials (A to H) of FITC- and PE-conjugated TCR Vβ antibodies. In each tube (A to H), one TCR Vβ antibody was conjugated to FITC, another to PE, and a third to both FITC and PE. The 24 antibodies were as follows: antibodies to Vβ1 (clone BL37.2), Vβ2 (clone MPB2D5), Vβ3 (clone CH92), Vβ4 (clone WJF24), Vβ5.1 (clone IMMU157), Vβ5.2 (clone 36213), Vβ5.3 (clone 3D11), Vβ7.1 (clone ZOE), Vβ7.2 (clone ZIZOU4), Vβ8 (clone 56C5.2), Vβ9 (clone FIN9), Vβ11 (clone C21), Vβ12 (clone VER2.32), Vβ13.1 (clone IMMU222), Vβ13.2 (clone H132), Vβ13.6 (clone JU74.3), Vβ14 (clone CAS1.1.3), Vβ16 (clone TAMAYA1.2), Vβ17 (clone E17.5F3), Vβ18 (clone BA62.6), Vβ20 (clone ELL1.4), Vβ21.3 (clone IG125), Vβ22 (clone IMMU546), and Vβ23 (clone AF23).
For data analysis, a sequential gating strategy was used to detect cytokine-producing TCR Vβ repertoires among CD3+ CD4+ and CD3+ CD8+ lymphocytes. In our study, we generally acquired 300,000 lymphocytes in total. Lymphocyte gating was set using forward and side light scatter. We further analyzed 70,000 to 210,000 CD3+ CD4+ and 40,000 to 90,000 CD3+ CD8+ cell events. CD3+ CD4+ and CD3+ CD8+ cells were then displayed and further gated to identify IFN-γ- or TNF-α-producing cells.
Statistical analysis.
All statistical tests were performed with GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA). Differences between groups were assessed by the Kruskal-Wallis test with Dunn's multiple comparison test. P values of <0.05 were considered significant (***, P < 0.001; **, P < 0.01; *, P < 0.05).
RESULTS
CFP-10 markedly enhances the production of cytokines by CD4+ and CD8+ T cells from PFMCs.
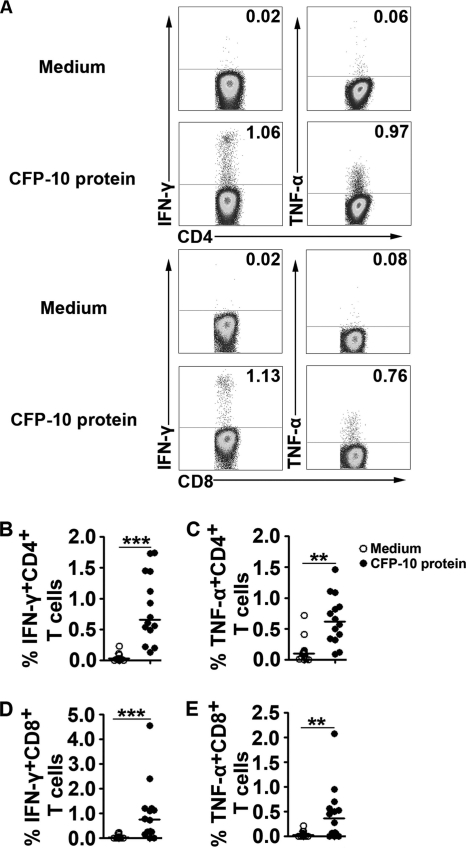
To determine whether CFP-10-specific CD4+ and CD8+ T cells were present at local sites of M. tuberculosis infection, we stimulated PFMCs with CFP-10 for 8 h. The cells were stained with antibodies and detected by fluorescence-activated cell sorting (FACS). The results show that PFMC-derived CD4+ or CD8+ T cells produced very small percentages of IFN-γ and TNF-α in culture medium lacking CFP-10. The addition of CFP-10 to PFMC cultures markedly enhanced the production of IFN-γ and TNF-α (Fig. 1A). The statistical results show that PFMC-derived CD4+ or CD8+ T cells produced significantly higher levels of cytokines following stimulation with CFP-10 (Fig. 1B to E) (P < 0.01). These data show that CFP-10-specific CD4+ T cells and, especially, CD8+ T cells are present at local sites of M. tuberculosis infection in patients with tuberculous pleurisy.
Fig. 1.
CFP-10 markedly enhances the production of cytokines in CD4+ and CD8+ T cells from local sites of M. tuberculosis infection. PFMCs were stimulated with CFP-10 for 8 h. (A) Representative flow cytometry result. Plots shown were gated on CD4+ or CD8+ T lymphocytes, and the numbers represent the percentages of cytokine-producing cells. (B to E) Statistical results for PFMCs (n = 14). Open circles indicate cultures lacking CFP-10, and filled circles indicate cultures with CFP-10 added. Horizontal bars indicate the medians for the different conditions. **, P < 0.01; ***, P < 0.001.
CFP-10-specific CD8+ T cells from PFMCs have more coexpression of CD107a/b with IFN-γ or TNF-α than do CD4+ T cells.
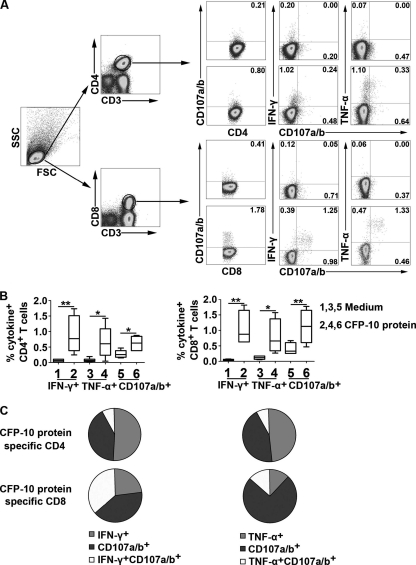
To further measure the biological activity of CFP-10-specific CD4+ and CD8+ T cells at local sites of M. tuberculosis infection, we measured the expression of CD107a/b in PFMCs and the correlation with IFN-γ or TNF-α production. We stimulated PFMCs with CFP-10 for 6 h in the presence of antibodies to CD107a/b. The cells were harvested and detected by FACS. The results show that CFP-10-specific CD4+ and CD8+ T cells from PFMCs produced IFN-γ and TNF-α and upregulated expression of CD107a/b upon activation (Fig. 2A). The statistical results show that both CD4+ and CD8+ T cells from PFMCs produced significantly higher levels of cytokines and CD107a/b following stimulation with CFP-10 than without the protein (Fig. 2B) (n = 6; P < 0.05), and CFP-10-specific CD8+ T cells expressed higher levels of CD107a/b than did CD4+ T cells (medians of 1.29% and 0.62%, respectively). In addition, CFP-10-specific CD4+ and CD8+ T cells from PFMCs coexpressed CD107a/b and either IFN-γ or TNF-α. Importantly, coexpression of CD107a/b and IFN-γ (medians of 37.03% and 7.89% in CD8+ T cells and CD4+ T cells, respectively) or TNF-α (medians of 13.62% and 7.88%, respectively) in CFP-10-specific CD8+ T cells was significantly higher than that in CD4+ T cells (Fig. 2C) (n = 6). These data suggest that CFP-10-specific CD4+ and CD8+ T cells might contribute to important biological activities at local sites of M. tuberculosis infection.
Fig. 2.
CFP-10-specific CD8+ T cells from PFMCs coexpress higher levels of CD107a/b with IFN-γ or TNF-α than do CD4+ T cells. PFMCs were stimulated with CFP-10 for 6 h. (A) One flow cytometry result representative of six independent experiments with similar results. Lymphocyte gating was set using forward and side light scatter. CD4+ and CD8+ T cells were gated according to the expression of CD3, CD4, and CD8. The number in each quadrant represents the percentage of cytokine-producing CD4+ or CD8+ T cells. (B) Statistical analysis of CD107a/b and IFN-γ or TNF-α production by CD4+ or CD8+ T cells following stimulation with CFP-10 (n = 6). For each plot, the median is represented by a horizontal line, the interquartile range by a box, and the range by whiskers. Differences between groups were assessed by the Kruskal-Wallis test. *, P < 0.05; **, P < 0.01. (C) Coexpression of CD107a/b and IFN-γ or TNF-α by CD4+ or CD8+ T cells (n = 6). The pie charts represent median frequencies of CFP-10-specific CD4+ or CD8+ T cells.
CFP-10-specific IFN-γ is produced by effector/memory CD4+ and CD8+ T cells.
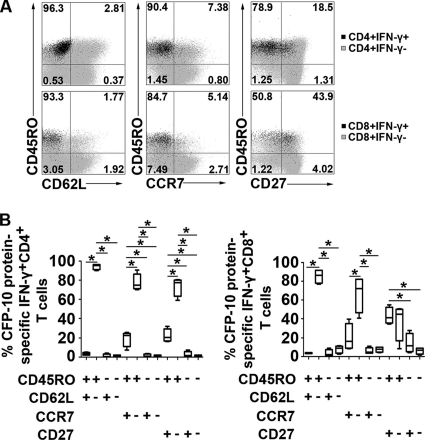
We further measured several cell surface markers of CFP-10-specific CD4+ and CD8+ T cells on the basis of IFN-γ production. The results show that the CFP-10-specific IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells were effector/memory T cells with a CD45RO+ CD62L− CCR7− CD27− expression profile (Fig. 3A). The statistical analysis showed that large proportions of the IFN-γ+ CD4+ and IFN-γ+ CD8+ T cell subsets were CD45RO+ CD62L−, CD45RO+ CCR7−, or CD45RO+ CD27−. Small proportions of the IFN-γ+ CD4+ and IFN-γ+ CD8+ T cell subsets were CD45RO− CD62L+, CD45RO− CCR7−, or CD45RO− CD27− (Fig. 3B). In addition, CFP-10-specific IFN-γ-producing CD8+ T cells expressed slightly higher levels of CD27 than did CD4+ T cells (medians of 41.35% and 20.2%, respectively). Based on these data, effector/memory CFP-10-specific CD4+ and CD8+ T cells might contribute to immediate effector functions in response to M. tuberculosis infection.
Fig. 3.
CFP-10-specific effector/memory CD4+ or CD8+ T cells produce IFN-γ. PFMCs were stimulated with CFP-10 for 8 h. (A) One representative result for IFN-γ-producing CD4+ or CD8+ T cells from four independent experiments with similar results. The gray background of each plot represents the expression of CD45RA, CD62L, CCR7, and CD27 in the IFN-γ− CD4+ or IFN-γ− CD8+ T cell subset. The black dots in each plot represent the expression of relevant phenotypic marker expression in the IFN-γ+ CD4+ or IFN-γ+ CD8+ T cell subset. Figures shown are overlapped images for two CD4+ or CD8+ T cell subsets. The percentages of IFN-γ+ CD4+ or IFN-γ+ CD8+ T cells falling into the respective quadrants are indicated in each plot. (B) Mean distributions of IFN-γ-producing CFP-10-specific CD4+ and CD8+ T cell subsets (n = 4). For each plot, the median is represented by a horizontal line, the interquartile range by a box, and the range by whiskers. Differences between groups were assessed by the Kruskal-Wallis test. *, P < 0.05.
TCR Vβ repertoire analysis of CFP-10-specific CD4+ and CD8+ T cells.
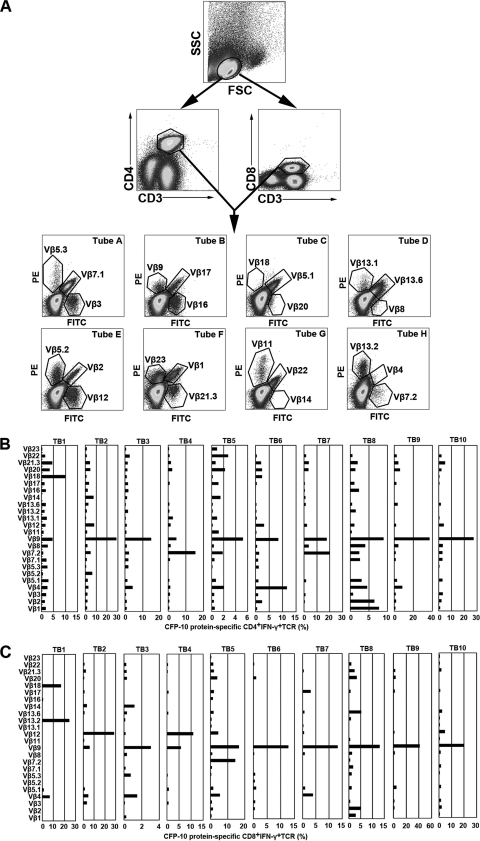
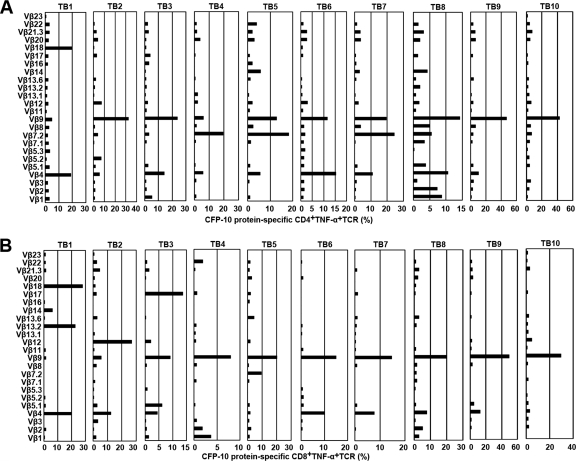
To further characterize the types of CFP-10-specific CD4+ and CD8+ T cells at local sites of M. tuberculosis infection, we determined the TCR Vβ repertoires of CFP-10-specific CD4+ and CD8+ T cells on the basis of IFN-γ and TNF-α production. Figure 4A graphically shows the sequence of flow cytometry analytical steps. For CFP-10-specific CD4+ T cells, six patients with tuberculous pleurisy had the most IFN-γ production by TCR Vβ9 (TB2, TB3, TB5, TB8, TB9, and TB10), two patients had the most by TCR Vβ7.2 (TB4 and TB7), and two patients had the most by TCR Vβ18 (TB1) or Vβ4 (TB6) (Fig. 4B). Meanwhile, for CFP-10-specific CD8+ T cells, two patients had the most IFN-γ production by TCR Vβ12 (TB2 and TB4), seven patients had the most by TCR Vβ9 (TB3, TB5, TB6, TB7, TB8, TB9, and TB10), and one patient had the most by TCR Vβ13.2 (TB1) (Fig. 4C).
Fig. 4.
Analysis of TCR Vβ repertoires in CFP-10-specific IFN-γ-producing CD4+ (B) and CD8+ (C) T cells in tubercular pleural fluid. (A) Representative example of flow cytometry of PFMCs. The lymphocyte gating was set with forward and side light scatter. CD4+ and CD8+ T cells were gated according to the expression of CD3, CD4, and CD8. The expression of 24 TCR Vβ chains by CFP-10-specific CD4+ or CD8+ PFMCs was then assessed. Different TCR Vβ chains were analyzed by use of eight tubes. In each tube, from A to H, one TCR Vβ antibody was conjugated to FITC, another to PE, and a third to both FITC and PE. PFMC TCR Vβ repertoires following stimulation of cells with CFP-10 for 8 h are indicated for 10 patients with tuberculous pleurisy (TB1 to TB10). Percentages of IFN-γ production by CD4+ (B) or CD8+ (C) TCR Vβ chains are indicated for each donor.
The TCR Vβ repertoires of CFP-10-specific TNF-α-producing CD4+ (Fig. 5A) and CD8+ (Fig. 5B) T cells were very similar to those of IFN-γ-producing cells. For CFP-10-specific CD4+ T cells, five patients had the most TNF-α production by TCR Vβ9 (TB2, TB3, TB8, TB9, and TB10), three patients had the most by TCR Vβ7.2 (TB4, TB5, and TB7), and two patients had the most by TCR Vβ18 (TB1) or Vβ4 (TB6) (Fig. 5A). Meanwhile, for CFP-10-specific CD8+ T cells, seven patients had the most TNF-α production by TCR Vβ9 (TB4, TB5, TB6, TB7, TB8, TB9, and TB10), and three patients had the most by TCR Vβ18 (TB1), Vβ17 (TB3), or Vβ12 (TB2) (Fig. 5B).
Fig. 5.
Analysis of TCR Vβ repertoires in CFP-10-specific TNF-α-producing CD4+ (A) and CD8+ T cells (B) in tubercular pleural fluid. PFMCs were stimulated and analyzed as described in the legend to Fig. 4. PFMC TCR Vβ repertoires following stimulation of cells with CFP-10 are indicated for 10 patients with tuberculous pleurisy (TB1 to TB10; n = 10). The percentage of TNF-α production by each CD4+ (A) or CD8+ (B) TCR Vβ chain is indicated for each donor.
Taken together, we found that CFP-10-specific CD4+ and CD8+ T cells in tubercular pleural fluid had biased usage of TCR Vβ9, Vβ12, and Vβ7.2, which indicated potentially important roles for these repertoires in the cellular immune response at local sites of M. tuberculosis infection.
DISCUSSION
In the present study, we used CFP-10 to characterize antigen-specific CD4+ and CD8+ T cells at local sites of M. tuberculosis infection. We found that both CD4+ and CD8+ T cells from tubercular pleural fluid could produce various cytokines following stimulation with CFP-10. In addition to IFN-γ, which had been shown previously to play a key role in the protective function of CD4+ and CD8+ T cells, TNF-α was also produced. These cytokines play important effector roles in activating macrophages, inducing apoptosis of infected cells, and contributing to granuloma formation (4, 6, 16, 22).
It was still unclear whether the accumulation of CFP-10-specific CD4+ and CD8+ T cells resulted from their continuous recruitment to local sites of M. tuberculosis infection or from cell division after these cells entered the tubercular pleural fluid. Recent studies have suggested that small numbers of Th1 cells at the periphery might result from T cells homing to local sites of M. tuberculosis infection (27–29).
In addition to their potential importance as cytokine-producing T cells, CFP-10-specific CD4+ and CD8+ T cells were originally identified based on their cytolytic abilities. CD107a and CD107b are two intracellular proteins that are normally found in lysosomes but that are also structural components of cytotoxic granules. Following exocytosis of cytotoxic granules, CD107a/b is transiently expressed on the surfaces of cytotoxic lymphocytes (40). CD107a/b expression on the surfaces of CFP-10-specific CD4+ and CD8+ T cells supported our hypothesis that these cells might recognize and kill M. tuberculosis-infected cells. In addition, we observed coexpression of cell surface CD107a/b and the intracellular cytokine IFN-γ or TNF-α, which might contribute to the optimal biological activity of CD4+ and CD8+ T cells. T cells can be subdivided into several functional subsets, including naive, effector, and memory T cells (17, 19, 34). In this study, we found that CFP-10-specific IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells in tubercular pleural fluid were effector/memory T cells with a CD45RO+ CD62L− CCR7− CD27− expression profile, consistent with previous studies (23, 35). These CFP-10-specific CD4+ and CD8+ T cells in tubercular pleural fluid might contribute to immediate effector functions in response to M. tuberculosis infection.
Analysis of the TCR Vβ repertoire has been used widely to characterize alterations of T cell repertoires, which range from extensive diversity to expansion of single T cell clones. Some studies have shown that the total available T cell repertoire specific for a given antigen is quite diverse in TCR Vβ usage (9–11, 18, 31, 36). Until now, there have been few studies regarding TCR Vβ repertoires in peripheral blood mononuclear cells (PBMCs) in cases of childhood TB (20). Furthermore, there was little research using flow cytometry to study TCR Vβ repertoires at local sites of M. tuberculosis infection in cases of adult TB.
For the first time, we have combined analysis of the production of cytokines with TCR Vβ repertoire analysis and have described the main functional TCR Vβ chains of CFP-10-specific CD4+ and CD8+ T cells in tubercular pleural fluid. Although there were some distinct TCR Vβ repertoires in different patients with tuberculous pleurisy, we found that CFP-10-specific CD4+ and CD8+ T cells from tubercular pleural fluid had biased usage of TCR Vβ9, Vβ12, or Vβ7.2. One hypothesis states that CFP-10 might be processed into peptides that are differently presented by different HLA molecules. Some variations of CFP-10-specific TCR Vβ repertoires in PFMCs were observed among individuals, which might be due to differences in the HLA background. Additionally, human T cell responses, even those to immunodominant epitopes, may be quite diverse with respect to TCR usage (30), which is a hypothesis consistent with our results. Because we saw such a wide variation in repertoires, we show the data for each individual. We did not use any type of statistical analysis for TCR Vβ repertoires, because it is our belief that any statistical analysis will weaken the measured variations among individuals.
Moreover, we compared CFP-10-specific CD4+ or CD8+ T cells between PBMCs and PFMCs from the same patient with tuberculous pleurisy (data not shown). PBMCs had a very weak response to CFP-10, which made it difficult to further analyze M. tuberculosis antigen-specific TCR Vβ repertoires. At the same time, we found an enrichment of antigen-specific T cells at local sites of M. tuberculosis infection. Our purpose was to characterize the TCR Vβ repertoires of CFP-10-specific CD4+ and CD8+ T cells at local sites of M. tuberculosis infection.
In conclusion, our findings demonstrate for the first time the biased usage of TCR Vβ chains at local sites of M. tuberculosis infection. These findings are critical for understanding the mechanisms of the local cellular immune response and the future design of more effective TCR-based immunotherapies for prevention, treatment, or relief of tuberculosis symptoms. Our ongoing studies aim at characterizing the functions of the main TCR Vβ chains in detail and addressing the question of whether antigen-specific TCR Vβ repertoires are associated with TB severity and susceptibility to recurring disease. Additionally, we urgently need to investigate the exact mechanisms for selection of TCR Vβ repertoires.
ACKNOWLEDGMENTS
This research was supported by grant 115 (no. 2008ZX10003-011) from the Ministry of Health of the People's Republic of China, by the National Nature Science Foundation of China (no. 30872300), and by the National Key Basic Research Program of China (973; no. 2007CB512404).
Footnotes
Published ahead of print on 23 May 2011.
REFERENCES
- 1. Adams L. B., et al. 1995. Exacerbation of acute and chronic murine tuberculosis by administration of a tumor necrosis factor receptor-expressing adenovirus. J. Infect. Dis. 171:400–405 [DOI] [PubMed] [Google Scholar]
- 2. Albers A. E., et al. 2006. Alterations in the T-cell receptor variable beta gene-restricted profile of CD8+ T lymphocytes in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. 12:2394–2403 [DOI] [PubMed] [Google Scholar]
- 3. Algood H. M., Lin P. L., Flynn J. L. 2005. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin. Infect. Dis. 41 (Suppl. 3):S189–S193 [DOI] [PubMed] [Google Scholar]
- 4. Balcewicz-Sablinska M. K., Keane J., Kornfeld H., Remold H. G. 1998. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J. Immunol. 5:2636–2641 [PubMed] [Google Scholar]
- 5. Bean A. G., et al. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504–3511 [PubMed] [Google Scholar]
- 6. Botha T., Ryffel B. 2003. Reactivation of latent tuberculosis infection in TNF-deficient mice. J. Immunol. 171:3110–3118 [DOI] [PubMed] [Google Scholar]
- 7. Brewer T. F. 2000. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin. Infect. Dis. 31:S64–S67 [DOI] [PubMed] [Google Scholar]
- 8. Brogan P. A., Shah V., Bagga A., Klein N., Dillon M. J. 2003. T cell Vbeta repertoires in childhood vasculitides. Clin. Exp. Immunol. 131:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Busch D. H., Pilip I., Pamer E. G. 1998. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J. Exp. Med. 188:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Busch D. H., Pilip I. M., Vijh S., Pamer E. G. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353–362 [DOI] [PubMed] [Google Scholar]
- 11. Casanova J. L., Romero P., Widmann C., Kourilsky P., Maryanski J. L. 1991. T cell receptor genes in a series of class I major histocompatibility complex-restricted cytotoxic T lymphocyte clones specific for a Plasmodium berghei nonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J. Exp. Med. 174:1371–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarencio J., et al. 2006. Characterization of the T-cell receptor Vbeta repertoire in the human immune response against Leishmania parasites. Infect. Immun. 74:4757–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conley D. B., et al. 2006. Superantigens and chronic rhinosinusitis: skewing of T cell receptor V beta-distributions in polyp-derived CD4+ and CD8+ T cells. Am. J. Rhinol. 20:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Englund P., et al. 2007. Restricted T cell receptor BV gene usage in the lungs and muscles of patients with idiopathic inflammatory myopathies. Arthritis Rheum. 56:372–383 [DOI] [PubMed] [Google Scholar]
- 15. Ferrer J. 1997. Pleural tuberculosis. Eur. Respir. J. 10:942–947 [PubMed] [Google Scholar]
- 16. Flynn J. L., et al. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561–572 [DOI] [PubMed] [Google Scholar]
- 17. Geginat J., Lanzavecchia A., Sallusto F. 2003. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 11:4260–4266 [DOI] [PubMed] [Google Scholar]
- 18. Haberman A. M., Moller C., McCreedy D., Gerhard W. U. 1990. A large degree of functional diversity exists among helper T cells specific for the same antigenic site of influenza hemagglutinin. J. Immunol. 145:3087–3094 [PubMed] [Google Scholar]
- 19. Hamann D., et al. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 9:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobsen M., et al. 2007. Clonal expansion of CD8+ effector T cells in childhood tuberculosis. J. Immunol. 2:1331–1339 [DOI] [PubMed] [Google Scholar]
- 21. Kaufmann S. H. E. 2004. New issues in tuberculosis. Ann. Rheum. Dis. 63:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keane J., Shurtleff B., Kornfeld H. 2002. TNF-dependent BALB/c murine macrophage apoptosis following Mycobacterium tuberculosis infection inhibits bacillary growth in an IFN-gamma independent manner. Tuberculosis 82:55–61 [DOI] [PubMed] [Google Scholar]
- 23. Li L., Wu C. Y. 2008. CD4+CD25+ Treg cells inhibit human memory γδ T cells to produce IFN-γ in response to M. tuberculosis antigen ESAT-6. Blood 12:5629–5636 [DOI] [PubMed] [Google Scholar]
- 24. Lima M., et al. 2003. Immunophenotype and TCR-Vbeta repertoire of peripheral blood T-cells in acute infectious mononucleosis. Blood Cells Mol. Dis. 30:1–12 [DOI] [PubMed] [Google Scholar]
- 25. Lin P. L., Flynn J. L. 2010. Understanding latent tuberculosis: a moving target. J. Immunol. 185:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin P. L., Plessner H. L., Voitenok N. N., Flynn J. L. 2007. Tumor necrosis factor and tuberculosis. J. Invest. Dermatol. Symp. Proc. 12:22–25 [DOI] [PubMed] [Google Scholar]
- 27. Lorgat F., Keraan M. M., Lukey P. T., Ress S. R. 1992. Evidence for in vivo generation of cytotoxic T cells. PPD-stimulated lymphocytes from tuberculous pleural effusions demonstrate enhanced cytotoxicity with accelerated kinetics of induction. Am. Rev. Respir. Dis. 145:418–423 [DOI] [PubMed] [Google Scholar]
- 28. Lorgat F., Keraan M. M., Ress S. R. 1992. Cellular immunity in tuberculous pleural effusions: evidence of spontaneous lymphocyte proliferation and antigen specific accelerated responses to purified protein derivative (PPD). Clin. Exp. Immunol. 2:215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manca F., et al. 1991. Limited clonal heterogeneity of antigen specific T cells localizing in the pleural space during mycobacterial infection. Infect. Immun. 2:503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meinl E., et al. 1993. Myelin basic protein-specific T lymphocyte repertoire in multiple sclerosis. Complexity of the response and dominance of nested epitopes due to recruitment of multiple T cell clones. J. Clin. Invest. 92:2633–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mikszta J. A., Jang Y. S., Kim B. S. 1997. Role of a C-terminal residue of an immunodominant epitope in T cell activation and repertoire diversity. J. Immunol. 158:127–135 [PubMed] [Google Scholar]
- 32. Mohan V. P., et al. 2001. Effects of tumor necrosis factor alpha on host immune response in chronic persistent tuberculosis: possible role for limiting pathology. Infect. Immun. 69:1847–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pierdominici M., et al. 2003. Biased T-cell receptor repertoires in patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Clin. Exp. Immunol. 132:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 [DOI] [PubMed] [Google Scholar]
- 35. Scriba T. J., et al. 2008. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J. Immunol. 3:1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sourdive D. J., et al. 1998. Conserved T cell receptor repertoire in primary and memory CD8+ T cell responses to an acute viral infection. J. Exp. Med. 188:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stenger S. 2005. Immunological control of tuberculosis: role of tumor necrosis factor and more. Ann. Rheum. Dis. 64:iv24–iv28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trunz B. B., Fine P., Dye C. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and military tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367:1173–1180 [DOI] [PubMed] [Google Scholar]
- 39. Wahlstrom J., et al. 1997. Lung and blood T-cell receptor repertoire in extrinsic allergic alveolitis. Eur. Respir. J. 10:772–779 [PubMed] [Google Scholar]
- 40. Wolint P., Betts M. R., Koup R. A., Oxenius A. 2004. Immediate cytotoxicity but not degranulation distinguishes effector and memory subsets of CD8+ T cells. J. Exp. Med. 7:925–936 [DOI] [PMC free article] [PubMed] [Google Scholar]