Abstract
Clostridium difficile is an anaerobic, Gram-positive, spore-forming, opportunistic pathogen that is the most common cause of hospital-acquired infectious diarrhea. In numerous pathogens, stress response mechanisms are required for survival within the host. Extracytoplasmic function (ECF) σ factors are a major family of signal transduction systems, which sense and respond to extracellular stresses. We have identified three C. difficile ECF σ factors. These ECF σ factors, CsfT, CsfU, and CsfV, induce their own expressions and are negatively regulated by their cognate anti-σ factors, RsiT, RsiU, and RsiV, respectively. The levels of expression of these ECF σ factors increase following exposure to the antimicrobial peptides bacitracin and/or lysozyme. The expressions of many ECF σ factors are controlled by site 1 and site 2 proteases, which cleave anti-σ factors. Using a retargeted group II intron, we generated a C. difficile mutation in prsW, a putative site 1 protease. The C. difficile prsW mutant exhibited decreased levels of expression of CsfT and CsfU but not of CsfV. When expressed in a heterologous host, C. difficile PrsW was able to induce the degradation of RsiT but not of RsiU. When the prsW mutant was tested in competition assays against its isogenic parent in the hamster model of C. difficile infection, we found that the prsW mutant was 30-fold less virulent than the wild type. The prsW mutant was also significantly more sensitive to bacitracin and lysozyme than the wild type in in vitro competition assays. Taken together, these data suggest that PrsW likely regulates the activation of the ECF σ factor CsfT in C. difficile and controls the resistance of C. difficile to antimicrobial peptides that are important for survival in the host.
INTRODUCTION
Clostridium difficile is an anaerobic, Gram-positive, spore-forming, opportunistic pathogen and is the most common cause of hospital-acquired infectious diarrhea (3, 29, 32). C. difficile infections can range from mild diarrhea to life-threatening pseudomembranous colitis. People who develop C. difficile-associated disease are most commonly patients on antibiotics and/or who reside in hospitals or long-term care facilities. In recent years both the incidence and severity of C. difficile infections have increased in susceptible populations as well groups not traditionally considered to be at risk (33, 38, 39, 43).
In most patients, C. difficile infection is triggered by the disruption of the normal intestinal flora. C. difficile colonizes the intestinal tract of 12 to 20% of the adult population without causing disease (42). The disruption of the normal intestinal flora, most commonly due to treatment with antibiotics such as fluoroquinolones or clindamycin (13, 29), allows C. difficile to grow to high levels. How the normal flora blocks C. difficile growth is unclear. The normal flora may compete with C. difficile for nutrients and for binding sites in the intestinal tract or produce antimicrobial compounds that inhibit C. difficile growth (26, 45).
The major known virulence determinants of C. difficile are TcdA and TcdB, members of the large clostridial glucosylating toxin family (34, 54). Recently, it was shown that both TcdB and, to a lesser degree, TcdA are required for C. difficile pathogenesis but not colonization in a hamster model (31, 34). The toxins are likely responsible for the more severe sequelae of C. difficile-associated disease, and it seems likely that there are other factors required for C. difficile to colonize the host and evade the host immune defenses.
Many bacteria coordinate responses to extracellular stresses, including antimicrobial compounds, by using a class of sigma factors called extracytoplasmic function (ECF) σ factors. ECF σ factors belong to the σ70 family of σ factors, the largest and most diverse subfamily of alternative σ factors (25, 50). The primary amino acid sequences of ECF σ factors are highly divergent. However, ECF σ factors contain two regions with homology to σ70 region 4 and region 2, which are responsible for binding to the −35 and −10 regions, respectively, of their target promoters (25, 50).
The activities of many ECF σ factors are controlled by membrane-bound anti-σ factors, which bind directly to their cognate σ factors and thus inhibit the activity of the ECF σ factors (25). The activation of many of these σ factors is achieved by the proteolytic destruction of the anti-σ factors. In Escherichia coli the ECF σ factor σE is activated in response to outer membrane stress. DegS, a periplasmic serine protease, cleaves the anti-σ factor RseA at site 1 (2, 55, 56). After site 1 cleavage, the site 2 protease (a membrane-embedded metalloprotease), cleaves the ECF anti-σ factor, resulting in increased σ factor activity (1, 2, 27).
The ECF σ factor σW, from Bacillus subtilis, is the most well-characterized Gram-positive ECF σ factor. It induces the expressions of approximately 30 genes in response to cell envelope stresses such as cell wall-acting antibiotics (ampicillin and fosfomycin), detergents, and antimicrobial peptides (6, 15, 23, 44). In B. subtilis, σW activity is inhibited by the membrane-bound anti-σW factor RsiW (46). Although B. subtilis encodes two homologs of E. coli DegS, neither is required for the site 1 cleavage of RsiW (46). We previously identified PrsW as a novel protease that is required for the site 1 cleavage of the RsiW anti-σ factor and thus activates σW activity (15). PrsW is not a serine protease like DegS. PrsW is the founding member of a family of membrane-embedded metalloproteases that share predicted structural similarities to the RCE1 prenyl endopeptidase from Saccharomyces cerevisiae (4).
Studies have shown that ECF σ factors and the proteases that regulate them play an important role in the pathogenesis of numerous bacterial pathogens. Several Gram-negative pathogens, including Salmonella enterica serovar Typhimurium, Vibrio cholerae, and Pseudomonas aeruginosa, use ECF σ factors to coordinate the expression of virulence genes (10, 30, 52). In S. Typhimurium and V. cholerae, the σE system is induced by antimicrobial peptides produced by the host during infection (10, 37). Mycobacterium tuberculosis encodes several ECF σ factors that play important roles in virulence and survival within macrophages (19, 35, 36, 51). Recently, an ECF σ factor from Staphylococcus aureus was suggested to be required for pathogenesis in a mouse model of septic arthritis (48).
In this study we have identified and examined three C. difficile ECF σ factors. We show that the ECF σ factors are required for their own expression and are inhibited by cognate anti-σ factors. The expressions of two of the ECF σ factors, csfT and csfU, are regulated by C. difficile PrsW, a putative site 1 protease of anti-ECF σ factors. The expression of C. difficile PrsW results in the degradation of RsiT but not RsiU in B. subtilis, suggesting that C. difficile PrsW may directly control CsfT activity. We will also provide evidence that suggests that PrsW is required for resistance to bacitracin and lysozyme as well as for the colonization of hamsters during C. difficile infection. PrsW is an important regulator of antimicrobial resistance and may be important for colonization and survival during an infection.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and primers.
The bacterial strains and plasmids used in this study are described in Table 1. The C. difficile strains are isogenic with the erythromycin (Erm)-sensitive strain JIR8094 (41), a derivative of the sequenced clinical isolate 630 (47). C. difficile was grown in or on brain heart infusion medium (Gibco) supplemented with 0.5% yeast extract, 0.4% glucose, and 0.1% l-cysteine (BHIS medium) or in TY medium (0.4% tryptone, 0.5% yeast extract, 0.1% l-cysteine) at 37°C in an atmosphere of 10% hydrogen, 5% CO2, and 8% nitrogen in an anaerobic chamber (Coy Lab Products). C. difficile strains were grown on BHIS agar plates containing thiamphenicol (Thi) (10 μg/ml), Erm (5 μg/ml), kanamycin (Kan) (50 μg/ml), or cefoxitin (Fox) (16 μg/ml), as needed. B. subtilis strains are isogenic derivatives of wild-type B. subtilis strain PY79. B. subtilis and E. coli strains were grown in or on LB medium at 37°C with ampicillin (Amp) (50 μg/ml), spectinomycin (Spt) (100 μg/ml), chloramphenicol (Cam) (10 μg/ml), or erythromycin (50 μg/ml), as needed. The primers used in this work are listed in Table S1 in the supplemental material. All primers were synthesized by IDT DNA Inc. (Coralville, IA).
Table 1.
List of bacterial strains
| Strain | Genotype or description | Reference or source |
|---|---|---|
| Clostridium difficile | ||
| JIR8094 | Spontaneous erythromycin-sensitive derivative of 630 | 41 |
| TCD31 | JIR8094 prsW1::ltrB::ermB | |
| Escherichia coli | ||
| Omnimax-2 T1R | F′ [proAB+lacIqlacZΔM15 Tn10(Tetr) Δ(ccdAB)] mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 Δ(lacZYA-argF)U169endA1recA1supE44 thi-1gyrA96relA1tonA panD | Invitrogen |
| HB101/pRK24 | F−mcrB mrr hsdS20(rB− mB−)recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 | |
| CDE1408 | Omnimax/pCE263 (pDG1663 + PcsfT-lacZ [mls]) | |
| CDE1419 | Omnimax/pCE268 (pDG1663 + PcsfU-lacZ [mls]) | |
| CDE1440 | Omnimax/pCE286 (pDG1663 + PcsfV-lacZ [mls]) | |
| CDE1446 | Omnimax/pCE292 (pDR111 “HindIII” RfA) | |
| CDE1614 | Omnimax/pJK10-1 (pDR111 + Phs-csfT+ Spt) | |
| CDE1615 | Omnimax/pJK12-E5A (pDR111 + Phs-csfT+ rsiT+ Spt) | |
| CDE1616 | Omnimax/pJK8-C42 (pDR111 + Phs-csfU+ Spt) | |
| CDE1617 | Omnimax/pTHE172-82 (pDR111 + Phs-csfU+ rsiU+ Spt) | |
| CDE1618 | Omnimax/pTHE166-V22 (pDR111 + Phs-csfV+ Spt) | |
| CDE1619 | Omnimax/pTHE200-K41 (pDR111 + Phs-csfV+ rsiV+Spt) | |
| CDE1620 | Omnimax/pTHE1031 (pDR111 + Phs-2×Flag-rsiT+ Spt) | |
| CDE1621 | Omnimax/pTHE1038 (pDR111 + Phs-2×Flag-rsiU+ Spt) | |
| CDE1622 | Omnimax/pTHE1046 (pDP150 + Phs-prsWC. difficile+ mls) | |
| Bacillus subtilis | ||
| PY79 | Protrophic derivative of Bacillus subtilis 168 | 58 |
| CDE1410 | PY79 thrC::PcsfT-lacZ (mls) | |
| CDE1601 | PY79 thrC::PcsfT-lacZ (mls) amyE::Phs-csfT+(Spt) | |
| CDE1602 | PY79 thrC::PcsfT-lacZ (mls) amyE::Phs-csfT+ rsiT+ (Spt) | |
| CDE1603 | PY79 thrC::PcsfT-lacZ (mls) amyE::Phs-csfU+(Spt) | |
| CDE1604 | PY79 thrC::PcsfT-lacZ (mls) amyE::Phs-csfV+(Spt) | |
| CDE1420 | PY79 thrC::PcsfU-lacZ (mls) | |
| CDE1605 | PY79 thrC::PcsfU-lacZ (mls) amyE::Phs-csfU+(Spt) | |
| CDE1606 | PY79 thrC::PcsfU-lacZ (mls) amyE::Phs-csfU+ rsiU+ (Spt) | |
| CDE1607 | PY79 thrC::PcsfU-lacZ (mls) amyE::Phs-csfT+(Spt) | |
| CDE1608 | PY79 thrC::PcsfU-lacZ (mls) amyE::Phs-csfV+(Spt) | |
| CDE1609 | PY79 thrC::PcsfV-lacZ (mls) | |
| CDE1610 | PY79 thrC::PcsfV-lacZ (mls) amyE::Phs-csfV+(Spt) | |
| CDE1611 | PY79 thrC::PcsfV-lacZ (mls) amyE::Phs-csfV+ rsiV+ (Spt) | |
| CDE1612 | PY79 thrC::PcsfV-lacZ (mls) amyE::Phs-csfT+(Spt) | |
| CDE1613 | PY79 thrC::PcsfV-lacZ (mls) amyE::Phs-csfU+(Spt) | |
| CDE1623 | PY79 ΔprsW::kan amyE::Phs-2×Flag-rsfT+ (Spt) | |
| CDE1624 | PY79 ΔprsW::kan amyE::Phs-2×Flag-rsfT+(Spt) thrC::Phs-prsW+(mls) | |
| CDE1625 | PY79 ΔprsW::kan amyE::Phs-2×Flag-rsfU+(Spt) | |
| CDE1626 | PY79 ΔprsW::kan amyE::Phs-2×Flag-rsfU+(Spt) thrC::Phs-prsW+(mls) |
Plasmid construction.
We constructed a vector for the generation of targeted mutations in C. difficile by modifying the pJIR750ai plasmid (Sigma) used for the construction of mutants in Clostridium perfringens (9, 18). To generate the targetron vector, we cloned an ermBRAM cassette, which had been synthesized by Geneart Co., into the MluI site of pJIR750ai. The ermBRAM cassette contains a retrotransposition-activated selectable marker (RAM), which consists of an antibiotic resistance gene (ermB) interrupted by a group I self-splicing intron, td, from bacteriophage T4 (60). The presence of the intron blocks the expression of the ermB resistance gene prior to integration into the bacterial chromosome. We generated a C. difficile promoter and a lacZα fragment by using two sequential PCRs. The lacZα fragment from pUC19 was PCR amplified by using primers CDEP605 and CDEP607. The resulting product was then used as the DNA template in a second PCR amplification using primers CDEP606 and CDEP607. The resulting product was digested with SphI and BsrGI and cloned into plasmid pJIR750ai-ermBRAM, which had been digested with the same enzymes. Finally, an RP4 origin of conjugal transfer was added by amplifying oriT from pGP704 (40) using primers CDEP636 and CDEP637. The fragment was digested with NlaIII and cloned into the SphI fragment of pJIR750ai-ermBRAM-lacZα. This vector was renamed pCE240 and was used for subsequent cloning for mutagenesis in C. difficile.
To generate lacZ reporter fusions to the ECF σ factor promoters, we amplified approximately 0.5-kb DNA fragments upstream of the first open reading frame in the putative ECF σ factor gene operon. To generate these DNA fragments, we used C. difficile chromosomal DNA and the primers specified in Table S1 in the supplemental material in a Platinum Pfx protocol (Invitrogen) according to the manufacturer's directions. The resulting PCR products were digested with EcoRI and BamHI (NEB) and ligated into EcoRI- and BamHI-digested pDG1663 (17). The resulting clones placed the C. difficile promoters of interest just upstream of a promoterless lacZ gene. The clones were sequenced and transformed into B. subtilis PY79 cells, where they integrated into the thrC gene of the B. subtilis genome.
The C. difficile ECF σ factor genes were cloned by first PCR amplifying the gene of interest using the primers specified in Table S1 in the supplemental material and Platinum Pfx (Invitrogen). The resulting PCR products were cloned by using the pENTR-D TOPO kit (Invitrogen). The ECF σ factor genes were each moved into plasmid pCE292 (a Gateway destination plasmid constructed by the insertion of an RfA cassette into a blunt-ended SphI site of pDR111) by using LR Clonase II (Invitrogen). The resulting plasmids placed the IPTG-inducible Phyperspank (Phs) promoter (5) upstream of the ECF σ factor gene in each construct. The resulting plasmids were transformed into B. subtilis cells, where they integrated into the amyE gene of the B. subtilis genome.
The plasmid expressing C. difficile 2×Flag-RsiT in B. subtilis was constructed by PCR amplifying rsiT using CDE948 and CDEP187. The resulting PCR product was then PCR amplified by using CDEP950 and CDEP187 and cloned into pENTR-D TOPO (Invitrogen). The Flag-tagged rsiT gene was moved into plasmid pCE292 by using LR Clonase II (Invitrogen), resulting in plasmid pTHE1031. To clone the C. difficile anti-σ factor gene rsiU under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter, the rsiU gene was PCR amplified by using CDE1126 and CDEP775. The resulting PCR product was then PCR amplified by using CDEP1127 and CDEP775 to add the Flag epitope tag. This PCR product was digested with HindIII and SphI and cloned into pDR111 digested with the same enzymes, resulting in pTHE1038. Plasmids pTHE1031 and pTHE1038 placed the IPTG-inducible hyperspace promoter upstream of the Flag-tagged rsiT and risU genes and were transformed into B. subtilis, where they integrated into the amyE gene of the B. subtilis genome.
The C. difficile prsW gene was placed under the control of an IPTG-inducible promoter by PCR amplifying prsW by use of CDEP703 and CDEP704. The resulting PCR product was digested with HindIII and SphI and cloned into pDP150 digested with the same enzymes. The resulting plasmid, pTHE1046, was transformed into B. subtilis cells, where it integrated into the thrC gene of the B. subtilis genome.
Bacterial strain construction.
B. subtilis strains were constructed by transformation using a one-step method described previously (57). The C. difficile prsW mutant was constructed by using a targetron-based system for the mutagenesis of C. difficile. To construct the prsW mutant, we used primers CDEP467, CDEP468, and CDEP469, which were identified by using the targetron algorithm (Sigma), to retarget the intron into prsW. We amplified the retargeted intron according to the manufacturer's instructions (Sigma) and cloned this DNA fragment into pCE240 by using HindIII and BsrGI. The resulting plasmid was transformed into HB101/pRK24 (12). We used this conjugation donor to introduce the retargeted plasmid into C. difficile as previously described, with the exception that we plated the conjugation mixtures onto BHIS plates with Thi, Kan, and Fox (12). Exconjugants that proved to be Thir, Kanr, and Foxr were then streaked onto BHIS plates with Erm, Kan, and Fox. The resulting erythromycin-resistant colonies were screened by PCR using primers CDEP692 and CDEP515 to identify a prsW::ltrB::ermB mutant. The resulting prsW::ltrB::ermB mutant was then tested for the loss of the targetron plasmid (This Cams) (21).
Isolation of C. difficile nucleic acids.
C. difficile chromosomal DNA was purified by resuspending the cells in 0.5 ml lysis buffer (50 mM EDTA, 0.1 M NaCl) supplemented with lysozyme (10 mg/ml). Proteinase K was added, and the mixture was incubated at 65°C for 1 h. DNA was extracted by using phenol-chloroform-isoamyl alcohol (600 μl; Fisher), followed by ethanol precipitation.
C. difficile RNA was isolated from bacteria grown in TY medium. A single colony of C. difficile was inoculated into TY medium and grown for 24 h. The C. difficile strains were subcultured at a 1:30 dilution in TY medium. For time course experiments, cells were resuspended by repeated pipetting at given time points, and the optical density at 600 nm (OD600) was measured (Biowave CO8000). For RNA purification, cells were fixed by the addition of equal volumes of acetone-ethanol (1:1) to cells and incubated at −80°C for 15 min. Fixed cells were pelleted by centrifugation and washed 3 times with 0.75 ml of TE (10 mM Tris [pH 7.0], 1 mM EDTA) and then resuspended in 1 ml of Trizol (Invitrogen). Approximately 0.1 ml of silica beads (diameter, 0.1 mm; RPI Products) was added, and cells were disrupted by vortexing for 3 to 5 min. RNA was isolated according to the manufacturer's protocol (Trizol; Invitrogen). Contaminating DNA was removed according to the protocol of the DNA-free kit (Ambion). Samples were cleaned by use of an RNeasy kit (Qiagen) and tested for DNA contamination by PCR amplification (Thermo Taq polymerase; NEB) using primers TE337 and TE338.
RT-PCR and quantitative reverse transcriptase PCR.
To generate cDNA from RNA samples, Superscript III was used according to the manufacturer's protocol (Invitrogen). For reverse transcriptase PCR (RT-PCR), we used 2 pmol the specified primers (see Table S1 in the supplemental material) in PCR mixtures with 30 cycles of denaturation (94°C for 30 s), annealing (48°C for 30 s), and extension (72°C for 60 s). For quantitative real-time PCR (qRT-PCR) experiments, reverse transcription reaction mixtures were diluted 1:5 in water. For each well, 5 μl of sample was added to 10 μl of Sybr green master mix (Applied Biosystems) and 5 μl gene-specific primers (2.5 μM). Experiments were performed in triplicate on three biologically independent replicates. Data were normalized to RNA levels of the C. difficile housekeeping gene rpoB.
β-Galactosidase activity assays.
The β-galactosidase activities of B. subtilis were determined by using a standard β-galactosidase enzymatic assay modified for 96-well plates. Strains were grown overnight in LB broth at 30°C. Cultures were diluted 1:100 in LB medium plus 1 mM IPTG (RPI Products) and grown for 3 h at 37°C. The cell density was measured at an OD600 by use of a spectrophotometer (EL311sx; Biotek). Cells were lysed by using lysozyme, and the β-galactosidase activity of the cell lysate was determined by the addition of the substrate o-nitrophenyl-β-d-galactopyranoside (ONPG) and measurement of the change in the OD405 over 1 h. The relative β-galactosidase activities were calculated as previously described (49).
Immunoblot analysis.
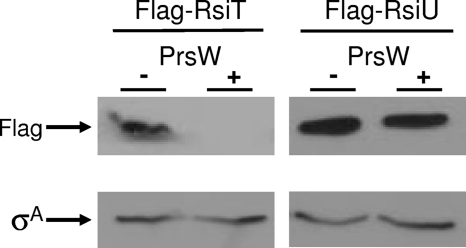
The PrsW-dependent degradation of anti-σ factors was determined by immunoblot analysis. The strains shown in Fig. 6 were grown to an OD600 of 2 in LB medium at room temperature (37°C). The cells were subcultured 1:25 in LB broth supplemented with 1 mM IPTG at 37°C and grown for 2.5 h. The cells were pelleted by centrifugation, resuspended in 100 μl of 2× sample buffer, and lysed by repeated sonication. Samples were electrophoresed on a 15% SDS-polyacrylamide gel (Bio-Rad). The proteins were then blotted onto nitrocellulose, and the proteins were detected by incubation with a 1:10,000 dilution of anti-Flag antibodies (Sigma) or a 1:15,000 dilution of anti-σA antibodies, followed by incubation in a 1:10,000 dilution of goat anti-rabbit IgG(H+L)-horseradish peroxidase (HRP) conjugate (Bio-Rad).
Fig. 6.
PrsW promotes degradation of RsiT but not of RsiU. Shown is the effect of C. difficile PrsW on the stability of the anti-σ factors in B. subtilis. The genotypes of the strains are as follows: Flag-RsiT PrsW−, amyE::Phs-2×Flag-rsiT ΔprsW::kan) (CDE1623); Flag-RsiT PrsWC. difficile+, amyE::Phs-2×Flag-rsiT thrC::Phs-prsWC. difficile+ ΔprsW::kan) (CDE1624); Flag-RsiU PrsW−, amyE::Phs-2×Flag-rsiU ΔprsW::kan) (CDE1625); Flag-RsiU PrsWC. difficile+, amyE::Phs-2×Flag-rsiU thrC:: Phs-prsWC. difficile+ ΔprsW::kan) (CDE1626). Cells were subcultured 1:25 in LB medium with 1 mM IPTG and grown for 2.5 h at 37°C. The cells were harvested and lysed by sonication. Proteins were separated by SDS-PAGE and immunoblotted by using either anti-Flag or anti-σA antibodies as described in Materials and Methods.
C. difficile competition assays.
The C. difficile strains used in competition assays were grown overnight in BHIS broth at 37°C in an anaerobic chamber (Coy Laboratories). The inoculum was prepared by mixing the mutant and wild-type bacteria at a ratio of 1:1 and was then washed and diluted in 0.15 M phosphate-buffered saline plus 0.1% cysteine.
For in vivo competition assays, bacteria were anaerobically loaded into inoculating needles and syringes and sealed in air-tight containers until just prior to inoculation. The actual numbers of wild-type and mutant bacteria present in the inoculum were determined before and after inoculation by serial dilution and plating onto BHIS plates and BHIS plates plus Erm. Female Syrian gold hamsters (Harlan Sprague-Dawley Inc., Indianapolis, IN) were inoculated orally with 0.3 ml clindamycin (10 mg/ml; Sigma) 36 h prior to infection. Hamsters were inoculated with ∼10,000 organisms of an equal mixture of mutant and wild-type strains. After 5 days, hamsters were sacrificed, and their small intestines, ceca, and colons were harvested. All organs were homogenized, diluted, and plated onto BHIS plates with Kan and Fox and on BHIS plates with Kan, Fox, and Erm. The competitive index (CI) was calculated as the ratio of [mutant output (Ermr)/wild-type output]/[mutant input (Ermr)/wild-type input]. The competitive index of each set of assays was analyzed statistically by using a Student's t test.
The in vitro competition assays were performed similarly by the inoculation of BHIS agar with 1,000 organisms from a 1:1 mixture of prsW mutant and wild-type strains as described above. The ratio of mutant to the wild type cells was determined as described above for both the input inoculum and the output.
Similarly, in vitro competition assays were used to test the effect of extracellular stress on the survival of cells of the prsW mutant and wild-type strains. The strains were grown individually overnight in BHIS broth. Each strain was subcultured 1:30 in fresh BHIS broth for 3 h. The mutant and the wild type were mixed at a 1:1 ratio and then incubated with either bacitracin (final concentration, 0.5 mg/ml), lysozyme (final concentration, 1 mg/ml), or water. The cultures were allowed to incubate for 1 h at 37°C. The ratio of mutant to wild-type cells was determined as described above, and the CI was calculated as described above.
RESULTS
Identification and organization of ECF σ factor operons in C. difficile.
In order to survive in the environment and/or the host, bacteria must have methods of detecting and responding to extracellular stresses. One common mechanism for sensing extracellular stresses utilizes ECF σ factors (25). We are interested in understanding how the human pathogen C. difficile uses ECF σ factors to coordinate responses to such assaults. Using bioinformatic analyses (BLAST and MIST), we analyzed the sequenced clinical isolate C. difficile 630 for the presence of putative ECF σ factors (47, 53). We identified three putative C. difficile ECF σ factors, which we named Clostridium sigma factors (CsfT, CsfU, and CsfV) (Fig. 1).
Fig. 1.
Determination of operon structure of C. difficile ECF σ factor genes. At the top is each putative σ factor operon. Black block arrows indicate the putative ECF σ factor genes. Gray block arrows indicate the putative anti-σ factor genes. The capital letters designate pairs of oligomers as follows: A, CDEP741 and CDEP759; B, CDEP833 and CDEP806; C, CDEP767 and CDEP710; D, CDEP530 and CDEP286; E, CDEP823 and CDEP931; F, TE1338 and TE1339; G, CDEP831 and CDEP832; H, CDEP840 and CDEP841; I, CDEP842 and CDEP843; J, CDEP844 and CDEP845; K, CDEP846 and CDEP847; L, CDEP848 and CDEP849; M, CDEP850 and CDEP851. RNA was isolated from wild-type C. difficile JIR8094 and was incubated in a reaction mixture without (−) or with (+) reverse transcriptase. PCR primer pairs were tested by using genomic DNA as a positive control (data not shown). The DNA was then run on a 1.5% agarose gel.
Many ECF σ factor genes are found in an operon with other genes (50). We examined the location of the putative ECF σ factors in the C. difficile chromosome and determined whether they were cotranscribed with other genes. Each pair of primers was tested in a PCR using chromosomal DNA as a template to ensure that the primers were capable of amplifying DNA fragments of the predicted size (data not shown). To control for potential DNA contamination, we included a no-reverse-transcriptase (RT) control for each primer set. We also included a negative control for each putative operon, which spans an intergenic region upstream of the first open reading frame and thus likely would not encode an mRNA. DNA fragments should be generated from the cDNA template only if neighboring genes were cotranscribed on the same contiguous transcript. We were able to PCR amplify a product when we used a primer in the 3′ end of csfT and a primer in the 5′ end of rsiT, suggesting that csfT and cd0676 (rsiT) RNAs are cotranscribed in a single mRNA transcript (Fig. 1). Using RT-PCR, we were able to obtain PCR products by using pairs of oligomers annealing in csfU and cd1888 (rsiU), cd1888 and cd1889, or cd1889 and cd1890 (Fig. 1). This suggests that these genes are cotranscribed with each other. The intergenic regions between the cd1556 and cd1562 genes were also all amplified from cDNA made from mRNA (Fig. 1). This finding suggests that the operon that harbors the csfV gene consists of a seven-gene operon, that csfU is part of a four-gene operon, and that csfT is part of a two-gene operon.
C. difficile ECF σ factors are required for transcription of their own promoters.
Many ECF σ factors facilitate RNA polymerase recognition of their own promoters and thus are required for their own transcription (25, 50). Given the limited genetic tool set available for C. difficile, we used B. subtilis as a heterologous host to test the ability of the C. difficile ECF σ factors to promote their own transcription. The promoter regions of each operon containing an ECF σ factor gene were cloned into a B. subtilis vector that contains a promoterless lacZ gene (17). The resulting lacZ fusion constructs were integrated into the thrC locus of the B. subtilis chromosome to generate Pcsf-lacZ reporter fusion strains.
Constructs expressing each ECF σ factor (csfT, csfU, and csfV) under the control of an IPTG-inducible promoter were introduced into the reporter strains. We assayed the β-galactosidase activities of the resulting strains and found that when the cognate ECF σ factors were expressed, there was an observed 25-fold to 250-fold increase in the expression of the csf genes (Fig. 2). This finding suggests that the C. difficile ECF σ factors are required for their own transcription.
Fig. 2.
C. difficile ECF σ factors induce their own expressions. Shown are the effects of different C. difficile ECF σ factors on the expression of each C. difficile ECF σ factor gene operon in B. subtilis. The relevant genotypes of the strains with respect to the ECF σ factors are noted. (A) The strains indicated as PcsfT harbor thrC::PcsfT-lacZ and have the following genotypes: −, thrC::PcsfT-lacZ (CDE1410); CsfT+ (T), amyE::Phs-csfT+ thrC::PcsfT-lacZ (CDE1601); CsfU+ (U), amyE::Phs-csfU thrC::PcsfT-lacZ (CDE1603); CsfV+ (V), amyE::Phs-csfV thrC::PcsfT-lacZ (CDE1604). The strains indicated as PcsfU harbor thrC::PcsfU-lacZ and have the following genotypes: −, thrC::PcsfU-lacZ (CDE1420); CsfT+, amyE::Phs-csfT+ thrC::PcsfU-lacZ (CDE1607); CsfU+, amyE::Phs-csfU thrC::PcsfU-lacZ (CDE1605); CsfV+, amyE::Phs-csfV thrC::PcsfU-lacZ (CDE1608). (B) The strains indicated as PcsfV harbor thrC::PcsfV-lacZ and have the following genotypes: −, thrC::PcsfV-lacZ (CDE1609); CsfT+, amyE::Phs-csfT+ thrC::PcsfV-lacZ (CDE1612); CsfU+, amyE::Phs-csfU thrC::PcsfV-lacZ (CDE1613); CsfV+, amyE::Phs-csfV thrC::PcsfV-lacZ (CDE1610). Cells were subcultured 1:100 in LB medium with 0.1 mM IPTG and grown for 3 h at 37°C. The cells were harvested, and the β-galactosidase (β-gal) activities were determined as previously described (20). The results shown are the results of three experiments, and the error bars denote standard deviations of the means.
We then tested the ability of each ECF σ factor to promote transcription from the other ECF σ factor promoters. In all but one instance, ECF σ factors were unable to increase the levels of expression of noncognate ECF σ factor promoters, as we observed no change in reporter gene expression (Fig. 2). Only CsfT was able to moderately (3-fold) induce the expression of PcsfV-lacZ (Fig. 2). As a comparison, CsfV induced the expression of PcsfV-lacZ 25-fold (Fig. 2), while CsfT induced the expression of PcsfT-lacZ 250-fold (Fig. 2). This suggests that while the ECF σ factors are required for their own transcription, they do not participate directly in the transcription of other ECF σ factor genes.
Putative anti-ECF σ factors repress expression of ECF σ factor promoters.
The activity of most ECF σ factors is inhibited by membrane-bound anti-σ factors (25, 50). These anti-σ factors are often encoded downstream of the ECF σ factor genes. We used three criteria to predict possible anti-ECF σ factor genes: (i) homology to known anti-ECF σ factors, (ii) proximity to the csf gene, and (iii) the presence of putative transmembrane domains in the predicted protein sequence. Both CsfV and CD1559 (RsiV) have strong homology to B. subtilis σV and anti-σV (RsiV) (Fig. 1). For both csfT and csfU no cognate anti-ECF σ factors could be identified by homology to known anti-σ factors. The genes immediately downstream of the csfT and csfU genes, however, encoded proteins with 1 and 6 predicted transmembrane domains, respectively.
To determine if these genes encoded anti-ECF σ factors, we made constructs expressing both the ECF σ factor and the gene encoding a putative transmembrane protein from IPTG-inducible promoters. These vectors were integrated into the B. subtilis strain containing the Pcsf promoters fused to the lacZ reporter gene. We found that in each case, the coexpression of the ECF σ factor genes and the genes just downstream of the ECF σ factor genes resulted in little or no expression from the lacZ reporter strain (Fig. 3). The expressions of both csfT and cd0676, here referred to as rsiT, resulted in 250-fold less β-galactosidase activity than when csfT alone was expressed. Similar fold decreases in expression were observed for csfU and cd1888 (rsiU) as well as csfV and cd1559 (rsiV) coexpressions (Fig. 3). This finding suggests that these genes encode negative regulators of the ECF σ factors and may function as anti-σ factors.
Fig. 3.
Identification of anti-σ factors for each C. difficile ECF σ factor. Shown are the effects of C. difficile putative anti-σ factors on the expression of each C. difficile ECF σ factor gene operon in B. subtilis. The relevant genotypes of the strains with respect to the ECF σ factors are noted. (A) The strains indicated as PcsfT harbor thrC::PcsfT-lacZ and have the following genotypes: −, thrC::PcsfT-lacZ (CDE1410); csfT, amyE::Phs-csfT+ thrC::PcsfT-lacZ (CDE1601); csfT rsiT, amyE::Phs-csfT+ rsiT+ thrC::PcsfT-lacZ (CDE1602). The strains indicated as PcsfU harbor thrC::PcsfU-lacZ and have the following genotypes: −, thrC::PcsfU-lacZ (CDE1420); csfU, amyE::Phs-csfU+ thrC::PcsfU-lacZ (CDE1605); csfU rsiU, amyE::Phs-csfU+ rsiU+ thrC::PcsfU-lacZ) (CDE1606). (B) The strains indicated as PcsfV harbor thrC::PcsfV-lacZ and have the following genotypes: −, thrC::PcsfV-lacZ (CDE1609); CsfV, amyE::Phs-csfV thrC:: PcsfV-lacZ (CDE1610); csfV rsiV, amyE::Phs-csfV+ rsiV+ thrC::PcsfV-lacZ (CDE1611). Cells were subcultured 1:100 in LB medium with 0.1 mM IPTG and grown for 3 h at 37°C. The cells were harvested, and the β-galactosidase activities were determined as previously described (20). The results shown are the results of three experiments, and the error bars denote standard deviations of the means.
The C. difficile ECF σ factor genes are maximally expressed during stationary-phase growth.
We sought to determine the growth stage at which the ECF σ factors were maximally expressed by using qRT-PCR. We monitored RNA levels of each ECF σ factor during growth in rich medium (TY medium), and RNA was collected at given time points. The RNA levels of the genes encoding the putative ECF σ factors were determined by using qRT-PCR, and differences in total RNA levels were corrected for by use of the housekeeping gene rpoB. We observed that the levels of expression of all three σ factor genes increased over time and were maximal during stationary phase (Fig. 4).
Fig. 4.
Expression of C. difficile ECF σ factors increases during stationary phase. At different time points during growth, the optical density was measured, and RNA was extracted from wild-type strain JIR8094. The relative RNA levels of each ECF σ factor were determined by using qRT-PCR. Listed in Table S1 in the supplemental material are the oligonucleotides used in the qRT-PCR: TEQ003 and TEQ004 for csfT (black bars), TEQ005 and TEQ006 for csfU (hatched bars), TEQ007 and TEQ008 for csfV (gray bars), and TEQ009 and TEQ010 for rpoB (data not shown). RNA levels were normalized to the housekeeping gene rpoB.
Antimicrobial compounds induce expression of C. difficile ECF σ factors.
The activities of ECF σ factors are often induced in response to extracellular signals, including cell envelope stress (25, 50). Since the C. difficile ECF σ factors regulate their own expressions (Fig. 2), increases in gene expression levels are indicative of increases in the activity of the ECF σ factor itself. We tested whether compounds that cause cell envelope stress, including bacitracin and lysozyme, altered ECF gene expression. To monitor gene expression in C. difficile, we performed a qRT-PCR assay. Subinhibitory concentrations of either bacitracin (5 μg/μl) or lysozyme (10 μg/μl) were added to mid-exponential-phase C. difficile cells, and the cultures were incubated for 2 h. RNA was isolated, and the transcript levels were determined by qRT-PCR. RNA levels were normalized to RNA transcript levels of the housekeeping gene rpoB. The RNA levels of csfT and csfU were increased 3- to 4-fold when exposed to either bacitracin or lysozyme (Table 2 ). Interestingly, the csfV RNA level was relatively unchanged in response to bacitracin but was substantially higher following exposure to lysozyme (Table 2). These data suggest that csfT, csfU, and csfV expressions and activities of the ECF σ factors are induced by cell envelope stress.
Table 2.
Relative RNA levels following antibiotic exposure
| Treatment | Mean fold change in RNA level ± SD |
||
|---|---|---|---|
| csfT | csfU | csfV | |
| None | 1.0 ± 0.5 | 1.0 ± 0.4 | 1.0 ± 0.09 |
| Bacitracin (50 μg/ml) | 3.5 ± 0.8 | 3.9 ± 0.07 | 1.3 ± 0.07 |
| Lysozyme (100 μg/ml) | 3.5 ± 0.1 | 2.9 ± 0.8 | 15 ± 1.1 |
Construction of the C. difficile prsW mutant, a homolog of B. subtilis site 1 protease.
In many organisms, ECF σ factor activity is controlled by multiple proteolytic cleavages of the anti-ECF σ factor (2, 15, 25, 46). In B. subtilis PrsW catalyzes the site 1 cleavage of the anti-ECF σ factor RsiW and thereby regulates 1 of 7 ECF σ factors in this bacterium (15, 23). C. difficile encodes a homolog of PrsW that shares 34% identity and 58% similarity to B. subtilis PrsW, which is annotated as SleB.
We constructed a C. difficile prsW mutant using the modified targetron vector (pCE240) described in Materials and Methods. The targetron system is based upon the Lactococcus lactis group II intron Ll.ltrB, which can be “retargeted” to a given gene by mutating the exon-binding sequences of the intron to be complementary to the target gene. The targetron vector was retargeted to PrsW by using an algorithm available from Sigma-Aldrich (18). The retargeted vector was introduced into C. difficile JIR8094 cells via conjugation. We then selected for Ermr colonies, which were screened by PCR to determine if prsW had been disrupted.
We then used primers CDEP515 and CDEP516 for the amplification of the Ermr Kanr strains. We observed a 2.2-kb fragment, compared to the 0.5-kb wild-type prsW fragment (Fig. 5A and B). This increase in size corresponds to the size of the intron. We also used primers specific for the intron (CDEP692) in combination with the prsW-specific primer CDEP515 to show that the Ermr Kanr strain would allow PCR amplification across the insertion points (Fig. 5C). The wild-type parent strain gave no PCR product using the same primers (Fig. 5C). These data confirm the insertion of the intron into the C. difficile prsW gene.
Fig. 5.
Confirmation and gene expression of a C. difficile prsW insertion mutant. (A and B) Schematic representations of the wild-type prsW gene (A) and the prsW gene disrupted by the LtrAL1::ermB intron of C. difficile strain JIR8094 (B). The locations and orientations of the oligomers used for PCR screening (CDEP515, CDEP516, and CDEP692) are represented as arrows. (C) PCR analysis of the JIR8094 parent strain (wild type [wt]) and the prsW::ltrBL1::ermB (prsW mutant) strain. The oligomers CDEP515 and CDEP516 generated a 465-bp product for the wild type and a 2,400-bp product for the prsW mutant. The oligomers CDEP515 and CDEP692 amplified no band for the wild type and a 435-bp product for the prsW mutant. The outside lanes contain a 1-kb DNA ladder from NEB. (D) Relative RNA levels of the different genes in the wild type or the prsW mutant. The oligonucleotides used in the qRT-PCR for csfT (TEQ003 and TEQ004), csfU (TEQ005 and TEQ006), csfV (TEQ007 and TEQ008), tcdA (TEQ017 and TEQ018), and rpoB (TEQ009 and TEQ010) are listed in Table S1 in the supplemental material. RNA levels were normalized to the housekeeping gene rpoB. Experiments were performed in triplicate, and the standard deviations are indicated.
PrsW is required for maximal expression of the ECF σ factor genes csfT and csfU.
In B. subtilis, PrsW is required for the maximal expression of sigW by initiating the degradation of the anti-σ factor RsiW (15, 23). We hypothesized that C. difficile PrsW may be required for the maximal expression of one or more ECF σ factors in C. difficile. Since the C. difficile ECF σ factors regulate their own expressions, the RNA levels of the ECF σ factor genes reflect the activities of the ECF σ factors. To determine if PrsW affected ECF σ factor activity, we monitored the RNA levels of each ECF σ factor in the wild type and the isogenic prsW mutant by using qRT-PCR. We found that the levels of expression of both csfT and csfU were decreased 5-fold in the prsW mutant compared to the wild type (Fig. 5D). However, the prsW mutation had no effect on the expression of csfV or tcdB (Fig. 5D). This finding suggests that in C. difficile, PrsW is required for the maximal activity of the ECF σ factors CsfT and CsfU but not of CsfV or the toxin TcdB.
PrsW can induce degradation of RsiT but not RsiU.
In B. subtilis, PrsW activates σW by initiating the degradation of RsiW (15, 23). Since C. difficile PrsW is required for the maximal expression of two genes encoding ECF sigma factors, csfT and csfU, we sought to determine if PrsW was capable of initiating the degradation of either cognate anti-σ factor. We constructed B. subtilis strains which lack endogenous B. subtilis prsW and then expressed either 2×Flag-RsiT or 2×Flag-RsiU. We then introduced an IPTG-inducible prsW+ promoter from C. difficile. We assayed the levels of the anti-σ factors by immunoblotting using anti-Flag antibodies. In the absence of C. difficile PrsW, we were able to detect both 2×Flag-RsiT and 2×Flag-RsiU (Fig. 6). However, when C. difficile PrsW was coexpressed with 2×Flag-RsiT, we observed a decrease in the level of RsiT (Fig. 6). In contrast, we observed no change in the level of 2×Flag-RsiU (Fig. 6). As a control we also performed an immunoblot analysis for the housekeeping sigma factor σA as a loading control. Levels of σA remained unchanged in all samples (Fig. 6). This finding suggests that C. difficile can induce the degradation of RsiT in a heterologous host and thus likely has a direct role in controlling the activation of CsfT. Interestingly, our data also suggest that PrsW does not degrade RsiU and thus likely controls the expression of csfU indirectly.
PrsW is involved in C. difficile resistance to antimicrobial peptides.
We demonstrated that C. difficile PrsW is required for the expression of csfT and csfU and likely functions by cleaving RsiT directly. In B. subtilis a mutation in prsW is more sensitive to antimicrobial peptides due to an inability to activate σW (6, 15). Thus, we sought to determine if a C. difficile prsW mutant also exhibits increased sensitivity to antimicrobials. We focused on bacitracin and lysozyme, as they were shown to induce both csfT and csfU (Table 2). To determine whether PrsW was required for resistance to these antimicrobials, we compared the survivals of wild-type and prsW mutant C. difficile strains following exposure to bacitracin and lysozyme. Each antimicrobial peptide was added to an aliquot of a 1:1 mixture of cells of the wild-type and prsW mutant strains. Before and after exposure, CFU were determined by diluting and plating bacteria onto BHIS agar and onto BHIS agar plus Erm. We found that the prsW mutant was significantly more sensitive to both bacitracin (median CI, 0.12; P value, 0.003) and lysozyme (median CI, 0.115; P value, 0.031) than were untreated strains (median CI, 1.08; P value, 0.42) (Fig. 7A). This suggests that in C. difficile, PrsW and the signal transduction pathways that PrsW regulates play a role in the resistance of C. difficile to these antimicrobial peptides.
Fig. 7.
The wild-type and prsW C. difficile strains were tested for growth or survival under various in vitro and in vivo conditions. (A) In vitro survival competition assays. Mixtures of wild-type and prsW mutant cells were exposed to water (untreated), bacitracin (0.5 mg/ml), or lysozyme (2.5 mg/ml) for 1 h. Experiments were performed on three independent days. The graph shows the results of one set of experiments (n=5). (B) In vivo competition assays with hamsters. Mixtures of wild-type and prsW mutant cells were orally inoculated into hamsters. At 5 days postinoculation, animals were sacrificed, and relevant organs (small intestine, cecum, and colon) were removed, homogenized, and plated for C. difficile. Experiments were performed on two independent days. The graph shows results from both sets of experiments (n=8). A mixture of wild-type and prsW mutant cells was also used to inoculate fresh BHIS medium and grown overnight at 37°C for the in vitro experiment. The numbers of wild-type and prsW mutant bacteria in all competition assays were determined by dilution plating onto BHIS agar and onto BHIS agar with Erm.
PrsW is required for C. difficile colonization.
Since in B. subtilis, PrsW was shown previously to respond to and protect against antimicrobial peptides, including those of the innate immune system (15, 24, 44), we reasoned that PrsW might be necessary during C. difficile infection of the host. Therefore, we tested the virulence phenotype of the prsW mutant in an animal model of infection by using a competition assay. Hamsters were treated with a single dose of clindamycin (30 mg/kg of body weight) orally 24 h prior to inoculation with C. difficile (34). The hamsters were then infected with an equal mixture of wild-type and prsW mutant cells (∼105 CFU) as previously described (8). The hamsters developed diarrhea at approximately 5 days postinoculation and classical histopathological events, including edema, vascular congestion within the lamina propria, neutrophil infiltration, villous epithelial degeneration, and an increased crypt mitotic rate (data not shown). If allowed to progress, the hamsters became moribund at approximately 7 days postinoculation. The competitive index of the prsW mutant compared to the wild type was determined as described in Materials and Methods.
We found that the prsW mutant caused an approximately 36-fold decrease (P value of <0.00005) in colonization or survival in the cecum of the hamsters (Fig. 7B). The prsW mutation had a very modest but not significant effect on C. difficile colonization in the colon (median CI, 0.4; P value, 0.24) and small intestine (median CI, 0.055; P value, 0.26) (Fig. 7B). Thus, prsW appears to encode a factor required for colonization or survival particularly within the cecum. In contrast, when we performed competition assays in vitro with BHIS medium, we found that the prsW mutant and the wild type competed evenly in vitro (Fig. 7B). This finding suggested that the prsW mutant does not have a general growth defect.
DISCUSSION
Despite the increasing prevalence and severity of C. difficile-associated disease, only a few C. difficile genes have been shown to affect pathogenesis. In particular, toxins, specifically ToxB, are required for C. difficile-induced disease (31, 34). However, mutants in the toxin genes tcdA and tcdB have no defects in the colonization of hamsters (31, 34). In this work, we demonstrate that PrsW is required for the maximum C. difficile colonization of hamsters during an infection (Fig. 7). We compared wild-type and prsW mutant C. difficile strains directly by using in vivo competition assays and found that the prsW mutant was isolated 36-fold less frequently than the wild type in the cecum, the major site of C. difficile 630-induced pathology in the hamster (16, 28). Importantly, since the absence of the toxins does not affect C. difficile colonization (31, 34), it is likely that the effects of PrsW on C. difficile are independent of the known virulence factors tcdA and tcdB. This is also supported by the observation that a prsW mutation does not alter the expression of tcdA in vitro (Fig. 5).
The prsW mutant also showed decreased survival when grown in the presence of compounds that induce cell envelope stress, including the antimicrobial peptide bacitracin, a compound produced by Bacillus species, and lysozyme, a major product of the innate immune response against bacterial infection (7, 11). Given that PrsW is required for resistance to antimicrobials in vitro, it seems likely that the defect that we observed in vivo is due to an increased sensitivity to one of these stresses in the animal. Both the host and the normal flora produce a number of products that may kill or inhibit an incoming pathogen. Since the prsW mutant shows a more pronounced defect in the cecum than in the colon or small intestine, it suggests that the extracellular stresses encountered are different in these tissues. Although we do not yet know the precise in vivo stress(es) for which PrsW is required for defense, we hypothesize that PrsW is required for resistance to an antimicrobial peptide(s) during C. difficile infection (14, 44).
In B. subtilis, PrsW is a site 1 protease that cleaves the anti-σ factor RsiW (15). B. subtilis encodes 7 ECF σ factor systems, only 1 of which is known to be regulated by the site 1 protease PrsW. C. difficile also encodes multiple ECF σ factor systems that are differentially regulated. Our studies show that PrsW affects the expressions of csfT and csfU but not that of csfV in C. difficile. We have found that C. difficile PrsW was able to induce the degradation of RsiT but not that of RsiU by using B. subtilis as a heterologous host (Fig. 6). This finding suggests that PrsW likely regulates CsfT activation in C. difficile directly in a mechanism similar to that of the PrsW-dependent activation of RsiW in B. subtilis (15, 23). In contrast, C. difficile RsiU is not degraded in the presence of PrsW, suggesting that the PrsW regulation of csfU expression is presumably indirect.
It is interesting that when C. difficile PrsW was expressed in B. subtilis, we observed a loss of full-length RsiT, but we did not observe an accumulation of a site 1 product (Fig. 6). This finding is similar to what was observed previously for the PrsW-induced degradation of RsiW in B. subtilis (15, 23). The site 1 product is not observed as the site 2 protease RasP rapidly cleaves RsiW (15, 46). Thus, our inability to detect cleaved RsiT raises the possibility that the B. subtilis site 2 protease RasP is capable of degrading cleaved RsiT in a manner analogous to that of RsiW in B. subtilis.
While B. subtilis PrsW and C. difficile PrsW are 34% identical and 58% similar, the anti-sigma factors RsiT from C. difficile and RsiW from B. subtilis share no detectable homology. However, both RsiT and RsiW contain a single transmembrane domain, suggesting that the overall topologies of these proteins are similar. It is interesting that C. difficile PrsW is unable to complement a B. subtilis prsW mutant (data not shown). This suggests that while the B. subtilis and C. difficile PrsW proteins respond to similar environmental cues, they must also have some substrate specificity.
Although homologs to the three C. difficile ECF σ factors are found only in Gram-positive organisms, their presence in different genera varies greatly. CsfV homologs are well conserved and can be found most broadly in organisms such as B. subtilis and Enterococcus faecalis (22, 59). In contrast, homologs of CsfT are found only in Clostridium and Ruminococcus species, species which can be found free living or in the intestinal tracts of various hosts. While CsfU homologs are present in Gram-positive bacteria associated with the gastrointestinal tract, they are strikingly absent from other more closely related bacteria that do not colonize the gastrointestinal tract. Homologs of PrsW can be found in species of the Firmicutes, the Actinobacteria, and the Cyanobacteria, which encode 1 to 17 putative ECF σ factors. Interestingly, PrsW homologs are also found in a Rickettsia species and Archaea genera, which do not appear to encode any putative ECF σ factors. This finding suggests that PrsW may have another function independent of ECF signal transduction. Indeed, it was shown recently that in Bacillus subtilis, a prsW mutation results in an accumulation of membrane proteins in a manner that is independent of the σW pathway that it controls (61).
The virulence defect of the C. difficile PrsW mutant may be due to one or more different virulence mechanisms. In C. difficile, PrsW directly regulates CsfT activity but indirectly controls CsfU levels. Effectors of either or both CsfT and CsfU regulons may enhance C. difficile survival and colonization in the animal. It is possible that PrsW may regulate the levels of other membrane or secreted proteins in an ECF σ factor-independent manner to aid C. difficile survival in the host. Although we have yet to demonstrate the specific molecular mechanism by which PrsW functions to contribute to the in vivo and in vitro phenotypes, information from these studies provides a starting point to understand factors required for colonization and survival during C. difficile infection.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Sorg (Texas A&M University) and A. L. Sonenshein (Tufts University) for helpful suggestions and Rob Britton (Michigan State University) for the group II intron algorithm.
This work was supported by Public Health Service grant AI-087834 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Akiyama Y., Kanehara K., Ito K. 2004. RseP (YaeL), an Escherichia coli RIP protease, cleaves transmembrane sequences. EMBO J. 23:4434–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alba B. M., Leeds J. A., Onufryk C., Lu C. Z., Gross C. A. 2002. DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Genes Dev. 16:2156–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartlett J. G. 1992. Antibiotic-associated diarrhea. Clin. Infect. Dis. 15:573–581 [DOI] [PubMed] [Google Scholar]
- 4. Boyartchuk V. L., Ashby M. N., Rine J. 1997. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275:1796–1800 [DOI] [PubMed] [Google Scholar]
- 5. Britton R., et al. 2002. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J. Bacteriol. 184:4881–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butcher B. G., Helmann J. D. 2006. Identification of Bacillus subtilis sigma-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by bacilli. Mol. Microbiol. 60:765–782 [DOI] [PubMed] [Google Scholar]
- 7. Callewaert L., Michiels C. 2010. Lysozymes in the animal kingdom. J. Biosci. 35:127–160 [DOI] [PubMed] [Google Scholar]
- 8. Chen X., et al. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992 [DOI] [PubMed] [Google Scholar]
- 9. Chen Y., McClane B. A., Fisher D. J., Rood J. I., Gupta P. 2005. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl. Environ. Microbiol. 71:7542–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crouch M., et al. 2005. The alternative sigma factor sigma is required for resistance of Salmonella enterica serovar Typhimurium to anti-microbial peptides. Mol. Microbiol. 56:789–799 [DOI] [PubMed] [Google Scholar]
- 11. Dann S. M., Eckmann L. 2007. Innate immune defenses in the intestinal tract. Curr. Opin. Gastroenterol. 23:115–120 [DOI] [PubMed] [Google Scholar]
- 12. Dineen S. S., Villapakkam A. C., Nordman J. T., Sonenshein A. L. 2007. Repression of Clostridium difficile toxin gene expression by CodY. Mol. Microbiol. 66:206–219 [DOI] [PubMed] [Google Scholar]
- 13. Dubberke E. R., Wertheimer A. I. 2009. Review of current literature on the economic burden of Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 30:57–66 [DOI] [PubMed] [Google Scholar]
- 14. Ellermeier C. D., Hobbs E. C., Gonzalez-Pastor J. E., Losick R. 2006. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell 124:549–559 [DOI] [PubMed] [Google Scholar]
- 15. Ellermeier C. D., Losick R. 2006. Evidence for a novel protease governing regulated intramembrane proteolysis and resistance to antimicrobial peptides in Bacillus subtilis. Genes Dev. 20:1911–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goulding D., et al. 2009. Distinctive profiles of infection and pathology in hamsters infected with Clostridium difficile strains 630 and B1. Infect. Immun. 77:5478–5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guerout-Fleury A. M., Frandsen N., Stragier P. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57–61 [DOI] [PubMed] [Google Scholar]
- 18. Gupta P., Chen Y. 2008. Chromosomal engineering of Clostridium perfringens using group II introns. Methods Mol. Biol. 435:217–228 [DOI] [PubMed] [Google Scholar]
- 19. Hahn M. Y., Raman S., Anaya M., Husson R. N. 2005. The Mycobacterium tuberculosis extracytoplasmic-function sigma factor SigL regulates polyketide synthases and secreted or membrane proteins and is required for virulence. J. Bacteriol. 187:7062–7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harwood C. 1990. Molecular biological methods for Bacillus. Wiley, Chichester, NY [Google Scholar]
- 21. Heap J. T., Pennington O. J., Cartman S. T., Carter G. P., Minton N. P. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70:452–464 [DOI] [PubMed] [Google Scholar]
- 22. Hebert L., et al. 2007. Enterococcus faecalis constitutes an unusual bacterial model in lysozyme resistance. Infect. Immun. 75:5390–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heinrich J., Wiegert T. 2006. YpdC determines site-1 degradation in regulated intramembrane proteolysis of the RsiW anti-sigma factor of Bacillus subtilis. Mol. Microbiol. 62:566–579 [DOI] [PubMed] [Google Scholar]
- 24. Helmann J. D. 2006. Deciphering a complex genetic regulatory network: the Bacillus subtilis sigmaW protein and intrinsic resistance to antimicrobial compounds. Sci. Prog. 89:243–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helmann J. D. 2002. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46:47–110 [DOI] [PubMed] [Google Scholar]
- 26. Hookman P., Barkin J. S. 2009. Clostridium difficile associated infection, diarrhea and colitis. World J. Gastroenterol. 15:1554–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanehara K., Ito K., Akiyama Y. 2002. YaeL (EcfE) activates the sigma(E) pathway of stress response through a site-2 cleavage of anti-sigma(E), RseA. Genes Dev. 16:2147–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keel M. K., Songer J. G. 2006. The comparative pathology of Clostridium difficile-associated disease. Vet. Pathol. 43:225–240 [DOI] [PubMed] [Google Scholar]
- 29. Kelly C. P., LaMont J. T. 1998. Clostridium difficile infection. Annu. Rev. Med. 49:375–390 [DOI] [PubMed] [Google Scholar]
- 30. Kovacikova G., Skorupski K. 2002. The alternative sigma factor sigma(E) plays an important role in intestinal survival and virulence in Vibrio cholerae. Infect. Immun. 70:5355–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuehne S., et al. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713 [DOI] [PubMed] [Google Scholar]
- 32. Kyne L., Hamel M. B., Polavaram R., Kelly C. P. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34:346–353 [DOI] [PubMed] [Google Scholar]
- 33. Loo V. G., et al. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442–2449 [DOI] [PubMed] [Google Scholar]
- 34. Lyras D., et al. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manganelli R., et al. 2002. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 45:365–374 [DOI] [PubMed] [Google Scholar]
- 36. Manganelli R., Voskuil M. I., Schoolnik G. K., Smith I. 2001. The Mycobacterium tuberculosis ECF sigma factor sigmaE: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423–437 [DOI] [PubMed] [Google Scholar]
- 37. Mathur J., Davis B. M., Waldor M. K. 2007. Antimicrobial peptides activate the Vibrio cholerae sigmaE regulon through an OmpU-dependent signalling pathway. Mol. Microbiol. 63:848–858 [DOI] [PubMed] [Google Scholar]
- 38. McFarland L. V. 2008. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 3:563–578 [DOI] [PubMed] [Google Scholar]
- 39. McFarland L. V., Beneda H. W., Clarridge J. E., Raugi G. J. 2007. Implications of the changing face of Clostridium difficile disease for health care practitioners. Am. J. Infect. Control 35:237–253 [DOI] [PubMed] [Google Scholar]
- 40. Miller V. L., Mekalanos J. J. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Connor J. R., et al. 2006. Construction and analysis of chromosomal Clostridium difficile mutants. Mol. Microbiol. 61:1335–1351 doi:10.1111/j.1365-2958.2006.05315.x [DOI] [PubMed] [Google Scholar]
- 42. Ozaki E., et al. 2004. Clostridium difficile colonization in healthy adults: transient colonization and correlation with enterococcal colonization. J. Med. Microbiol. 53:167–172 [DOI] [PubMed] [Google Scholar]
- 43. Pepin J., et al. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ 171:466–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pietiainen M., et al. 2005. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type sigma factors and two-component signal transduction systems. Microbiology 151:1577–1592 [DOI] [PubMed] [Google Scholar]
- 45. Sambol S. P., Merrigan M. M., Tang J. K., Johnson S., Gerding D. N. 2002. Colonization for the prevention of Clostridium difficile disease in hamsters. J. Infect. Dis. 186:1781–1789 [DOI] [PubMed] [Google Scholar]
- 46. Schobel S., Zellmeier S., Schumann W., Wiegert T. 2004. The Bacillus subtilis sigmaW anti-sigma factor RsiW is degraded by intramembrane proteolysis through YluC. Mol. Microbiol. 52:1091–1105 [DOI] [PubMed] [Google Scholar]
- 47. Sebaihia M., et al. 2006. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 38:779–786 [DOI] [PubMed] [Google Scholar]
- 48. Shaw L. N., et al. 2008. Identification and characterization of sigma, a novel component of the Staphylococcus aureus stress and virulence responses. PLoS One 3:e3844–e3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Slauch J. M., Silhavy T. J. 1991. cis-Acting ompF mutations that result in OmpR-dependent constitutive expression. J. Bacteriol. 173:4039–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Staron A., et al. 2009. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) sigma factor protein family. Mol. Microbiol. 74:557–581 [DOI] [PubMed] [Google Scholar]
- 51. Sun R., et al. 2004. Mycobacterium tuberculosis ECF sigma factor sigC is required for lethality in mice and for the conditional expression of a defined gene set. Mol. Microbiol. 52:25–38 [DOI] [PubMed] [Google Scholar]
- 52. Testerman T. L., et al. 2002. The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol. Microbiol. 43:771–782 [DOI] [PubMed] [Google Scholar]
- 53. Ulrich L. E., Zhulin I. B. 2007. MiST: a microbial signal transduction database. Nucleic Acids Res. 35:D386–D390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Voth D. E., Ballard J. D. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walsh N. P., Alba B. M., Bose B., Gross C. A., Sauer R. T. 2003. OMP peptide signals initiate the envelope-stress response by activating DegS protease via relief of inhibition mediated by its PDZ domain. Cell 113:61–71 [DOI] [PubMed] [Google Scholar]
- 56. Wilken C., Kitzing K., Kurzbauer R., Ehrmann M., Clausen T. 2004. Crystal structure of the DegS stress sensor: How a PDZ domain recognizes misfolded protein and activates a protease. Cell 117:483–494 [DOI] [PubMed] [Google Scholar]
- 57. Wilson G. A., Bott K. F. 1968. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J. Bacteriol. 95:1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Youngman P., Perkins J. B., Losick R. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 12:1–9 [DOI] [PubMed] [Google Scholar]
- 59. Zellmeier S., Hofmann C., Thomas S., Wiegert T., Schumann W. 2005. Identification of sigma(V)-dependent genes of Bacillus subtilis. FEMS Microbiol. Lett. 253:221–229 [DOI] [PubMed] [Google Scholar]
- 60. Zhong J., Karberg M., Lambowitz A. M. 2003. Targeted and random bacterial gene disruption using a group II intron (targetron) vector containing a retrotransposition-activated selectable marker. Nucleic Acids Res. 31:1656–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zweers J., Wiegert T., van Dijl J. 2009. Stress-responsive systems set specific limits to the overproduction of membrane proteins in Bacillus subtilis. Appl. Environ. Microbiol. 75:7356–7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.