Abstract
Trypanosoma cruzi infects millions of people in Latin America and often leads to the development of Chagas disease. T. cruzi infection can be acquired at or near the bite site of the triatomine vector, but per os infection is also a well-documented mode of transmission, as evidenced by recent microepidemics of acute Chagas disease attributed to the consumption of parasite-contaminated foods and liquids. It would also be convenient to deliver vaccines for T. cruzi by the oral route, particularly live parasite vaccines intended for the immunization of reservoir hosts. For these reasons, we were interested in better understanding immunity to T. cruzi following oral infection or oral vaccination, knowing that the route of infection and site of antigen encounter can have substantial effects on the ensuing immune response. Here, we show that the route of infection does not alter the ability of T. cruzi to establish infection in muscle tissue nor does it impair the generation of a robust CD8+ T cell response. Importantly, oral vaccination with attenuated parasites provides protection against wild-type (WT) T. cruzi challenge. These results strongly support the development of whole-organism-based vaccines targeting reservoir species as a means to alleviate the burden of Chagas disease in affected regions.
INTRODUCTION
Millions of people throughout Latin America are affected by Chagas disease. This condition is caused by persistent infection with the hemoflagellate protozoan parasite Trypanosoma cruzi, which sustains infection in mammalian hosts by replication of amastigotes in a cytoplasmic niche (61). Accounting for nearly 700,000 disability-adjusted life years (DALY) (39), Chagas disease is a prominent public health challenge, and current approaches to treatment and prevention are far from optimal (52). Metacyclic trypomastigotes in the feces of triatomine vectors are infective to a wide range of mammals, which primarily acquire T. cruzi through breaks in the skin, exposure to mucosal surfaces, or ingestion (61). Domestic spraying campaigns have had some degree of success in controlling transmission, most notably in Chile, Uruguay, and parts of Brazil (17). In this context, more recent attention has been given to outbreaks of T. cruzi infection acquired from food or drink tainted with T. cruzi-laden triatomine excreta (14, 28, 67). These incidences confirm that oral infection with T. cruzi is not only possible, but may be even a major route of T. cruzi infection in humans. The prominence of oral infection among reservoirs is supported by observations of opossums (50), raccoons (55), and dogs (51) ingesting triatomine bugs. It is not known how frequently humans acquire T. cruzi infection from contaminated food or ingestion of fomites. From an epidemiological perspective, it would be beneficial to distinguish people who acquired T. cruzi by an oral route from those who acquired it by other means of transmission, perhaps by a phenotypic signature of the T cell response. This aim necessitates a better understanding of the host immune response to T. cruzi following exposure via the gastrointestinal (GI) tract.
Oral infection may lead to distinct parasitological and/or immunological outcomes compared to other routes of infection. GI mucosal tissue forms an interface between the organism and its environment and constitutes an immense surface area constantly in contact with potential pathogens and commensals. Accordingly, the immune system associated with mucosae has evolved a unique capacity to determine when an aggressive response is appropriate, balancing regulation and activation (56). Antigen (Ag) encounter at the GI mucosa or in gut-associated lymphoid tissue often results in tolerance, particularly for T cell responses, a process largely mediated by the cytokines transforming growth factor β (TGFβ) and interleukin-10 (IL-10) (27, 41). We questioned whether parasite-specific CD8+ T cell responses may develop differently during oral infection and explored the possibility that T. cruzi may exhibit a tropism when infecting by the GI tract different than that previously observed with systemic routes of infection (68). T cell populations found in the mucosal tissue along the GI tract have several characteristics that separate them from T cells in peripheral circulation. For example, homing to the GI mucosa is controlled by expression of distinct adhesion molecules. T cells primed by dendritic cells (DC) from Peyer's patches (PP) or mesenteric lymph nodes (LN) (mesLN) express specific integrins on their surface that confer the ability to home to gut tissue (18, 45). We also asked if the mucosal route of infection would bias responding CD8+ T cells to accumulate in the intestines, possibly at the expense of a parasite-specific CD8+ response in other peripheral tissue.
If a robust immune response is generated with oral T. cruzi infection, one would hypothesize that vaccination by this route could be effective. Although not currently available, it is a major goal to develop a vaccine that protects against T. cruzi infection, especially one that elicits T cell-based immunity (42, 63). CD8+ T cells, which respond to foreign Ag processed from the intracellular compartment and presented on molecules from the class I major histocompatibility complex (MHC), are essential for controlling infection by T. cruzi (34). In mice, a population of immunodominant CD8+ T cells recognizes epitopes derived from the T. cruzi trans-sialidase gene family (35), and CD8+ T cells are strongly implicated in experimental vaccine-induced protection against this parasite (25, 43, 44, 62, 65). An attainable, practical approach to reducing the burden of Chagas disease would be to implement a transmission-reducing vaccine targeting reservoirs of T. cruzi. In areas where the disease is endemic, dogs are an integral component of domestic transmission of T. cruzi (13, 20, 22), and mathematical models have suggested that removing infected dogs from homes in some regions could almost completely prevent vector transmission to humans (13). To that end, our lab is working to generate genetically attenuated strains of T. cruzi as vaccine candidates. As this vaccine would likely be administered to reservoir dogs by feeding, we carried out protection studies with mice following oral immunization with an attenuated strain of T. cruzi.
Here, we address several specific questions. (i) Does entry via the oral route alter the capacity of T. cruzi to establish infection systemically in muscle tissue? (ii) Does the immune system recognize and respond to T. cruzi as a localized gut infection? (iii) Can the phenotype of T. cruzi-specific T cells be used to distinguish the route of exposure to T. cruzi in an already-infected host? (iv) Can oral vaccination with attenuated parasites induce protective immunity to systemic challenge with T. cruzi?
MATERIALS AND METHODS
Mice, parasites, infections, and vaccinations.
C57BL/6 (Ly5.2+) (B6) mice were purchased from either The Jackson Laboratory or National Cancer Institute at Frederick (Frederick, MD). Mice were maintained at the University of Georgia animal facility in microisolator cages under specific pathogen-free conditions. Mice were infected with metacyclic forms of Brazil or CL strain T. cruzi by oral gavage (p.o.), intraperitoneal (i.p.) injection, or subcutaneous infection in the footpad (f.p.). Mice could be infected by ad libitum ingestion of food that had been contaminated with T. cruzi metacyclic trypomastigotes (data not shown); however, a gavage needle was used in the majority of experiments to maximize infection efficiency and allow consistent dosing between animals. As previously described (6), epimastigote cultures were maintained in liver infusion tryptose (LIT) medium, and metacyclic trypomastigotes were generated by stressing cultures with triatomine artificial urine (TAU) medium. Mice were given 50,000 to 500,000 metacyclic trypomastigotes. Alternatively, for vaccination/challenge experiments, tissue culture trypomastigotes were obtained from passage through Vero cells, and mice were infected by i.p. or f.p. injection with 1,000 tissue culture trypomastigotes (TCT). Attenuated parasites (ECH1+/− ECH2−/−) were generated by targeting the tandem enoyl-coenzyme A (CoA) hydratase 1 and 2 genes in a high-throughput knockout system recently developed by our lab (66). These ECH1+/− ECH2−/− parasites fail to establish persistent infection in C57B6/J mice (as determined by immunosuppression) (7) or in immunodeficient mice. For vaccine protection studies, attenuated ECH1+/− ECH2−/− metacyclic trypomastigotes were administered by gavage. Mice received 3 doses separated by approximately 2 weeks. A total of 5 × 105 metacyclic trypomastigotes were given for doses 1 and 3, and 1.35 × 105 metacyclic trypomastigotes were given in dose 2. Control mice received equal volumes (100 μl) of phosphate-buffered saline (PBS) at each dose. Mice were sacrificed by CO2 inhalation, and cervical dislocation followed. All animal protocols were approved by the University of Georgia Institutional Animal Care and Use Committee.
Assessing protection with in vivo imaging.
For protection studies, T. cruzi parasites of the CL strain expressing the far-red tdTomato protein (9, 64) were subcutaneously inoculated into superficial subcutaneous tissue of the footpads. Mouse feet were imaged every other day using the Maestro 2 in vivo imaging system (CRi, MA) with the green set of filters (acquisition settings: 560 to 750 in 10-nm steps; exposure time of 88.18 ms and 2 × 2 binning). The total fluorescent signal was quantitated and normalized by exposure time and the area of the camera field corresponding to the source of the fluorescence, and values are reported as photons/cm2/second.
Isolation of lymphocytes from nonlymphoid tissues and adoptive transfers.
Before tissue removal, mice were perfused with 20 ml of PBS containing 0.8% sodium citrate as an anticoagulant. Perfusion was done by opening the abdominal and thoracic cavities, nicking the portal vein, and forcing PBS into the heart ventricles with a one-half-inch 35-gauge needle and 10-ml syringe. Tissue-derived lymphocytes were obtained by teasing tissues apart and vigorously pushing them through a 40-μm nylon mesh screen. Lymphocytes were obtained from lamina propria and intestinal epithelium as previously described (33). In brief, small intestines were isolated, cleaned, and cut into small segments. Pieces were stirred at 37°C for 20 min in Ca+/Mg+-free Hanks balanced salt solution containing 10% fetal calf serum (FCS) and 1 mM dithioerythritol to free intraepithelial lymphocytes (IEL). Gut pieces were further digested by stirring for 1 h at 37°C in RPMI 1640 medium containing 5% FCS, 1 mM CaCl2, 1 mM MgCl2, and 150 units/ml type II collagenase (Sigma). Tissue homogenate was then passed over a 40-μm nylon mesh screen, and both IEL and lamina propria (LP) cell populations were further purified by collection from the interface of 44% Percoll in RPMI medium underlain with 67% Percoll in PBS.
T cell phenotyping.
Spleens were homogenized, and red blood cells (RBCs) were lysed in a hypotonic ammonium chloride solution. Washes and staining were done in PAB (2% bovine serum albumin [BSA] and 0.02% azide in PBS). Peripheral blood was obtained by retro-orbital venipuncture or by nicking the tail and collecting blood with a capillary, collected in sodium citrate solution, and washed in PAB. Cells were obtained from peripheral tissue as described below. Cells were incubated with class I MHC tetramer-phycoerythrin (PE) complexes loaded with TSKb20 peptide (35) and the labeled antibodies (Abs). Cells were stained for 30 min at 4°C in the dark, washed in PAB, and fixed in 2% formaldehyde. For whole blood, RBCs were lysed after surface staining in a hypotonic ammonium chloride solution and washed twice in PAB. Data were acquired using a CyAn flow cytometer (DakoCytomation) and analyzed with FlowJo software (Tree Star). MHC I tetramer TSKb20 (ANYKFTLV)/Kb was synthesized at the Tetramer Core Facility (Emory University, Atlanta, GA). Antibodies for flow cytometric analysis were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), and Caltag Laboratories/Invitrogen (Carlsbad, CA).
PCR.
Quantitative real-time PCR was performed as previously described (16). Briefly, tissue was collected from mice and finely minced. Samples were incubated at 55°C for 4 h in SDS-proteinase K lysis buffer. DNA was extracted twice with phenol-chloroform-isoamyl alcohol (25:24:1), precipitated with 100% ethanol, and resuspended in nuclease-free water. Samples were analyzed on an iCycler (Bio-Rad). For real-time and standard PCR, the following primers were used to amplify a 182-bp product from genomic T. cruzi DNA: TCZ-F*, 5′-GCTCTTGCCCACAMGGGTGC-3′, where M = A or C, and TCZ-R 5′-CCAAGCAGCGGATAGTTCAGG-3′ (16).
Histology.
Heart and skeletal muscle was obtained from T. cruzi-infected mice and controls, fixed in 10% buffered formalin, and embedded in paraffin. Five-micrometer-thick sections were obtained and stained with hematoxylin-eosin (H&E). Inflammation was evaluated qualitatively according to the presence or absence of myocyte necrosis and the severity of leukocyte infiltration.
Statistical analysis.
We calculated statistical significance with a two-tailed Student t test in all cases, and a one-way, nonparametric analysis of variance (ANOVA) was used for data shown in Fig. 6C.
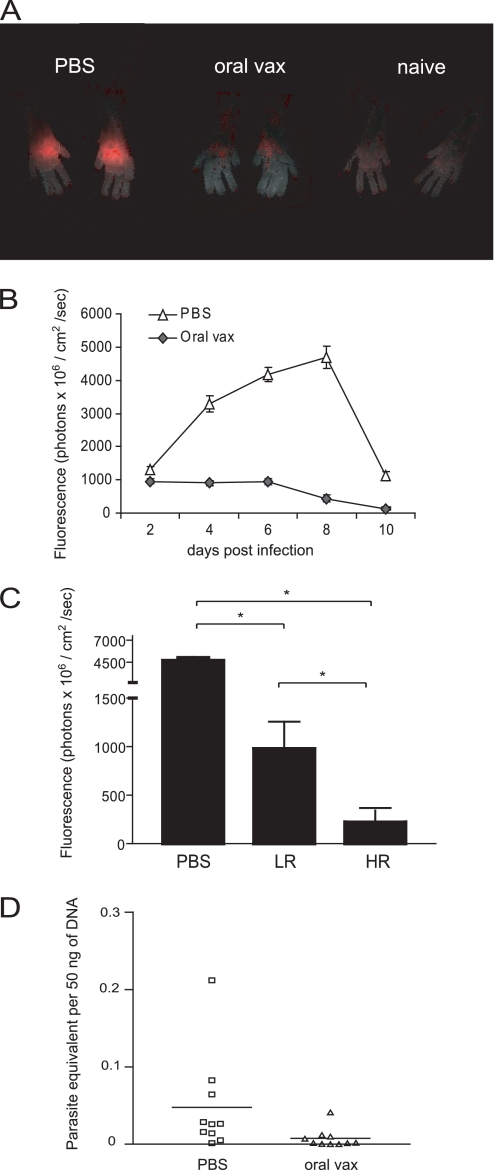
Fig. 6.
Oral vaccination with attenuated parasites protects mice from WT T. cruzi challenge. (A) Parasites are immediately controlled at infection site. Vaccinated mice (n = 10) and control mice (n = 10) were challenged with 2.5 × 103 WT fluorescent T. cruzi trypomastigotes injected in superficial subcutaneous tissue of each footpad. Parasite load in f.p. was assessed by quantitating the fluorescent signal with an in vivo imaging system. Pictures show left and right feet of individual mice representative of the indicated group at 8 days postchallenge. (B) Vaccine protection is evident at all times through the first 10 days of T. cruzi infection. Graph shows the fluorescent signal of all feet in each group at indicated time points (average ± SEM, P < 0.05 at all time points, n = 20 feet [10 mice]). (C) Extent of T cell activation is predictive of vaccine efficacy. Parasite load at 8 dpc is graphed for the 4 mice with the highest percentage of CD44hi CD8+ T cells (HR), and the 2 mice with the lowest percentage of CD44hi CD8+ T cells (LR) are graphed along with all mice from PBS group (average ± SEM, * indicates P < 0.0001 by ANOVA). (D) Oral vaccine mice have lower parasite burden in skeletal muscle following WT T. cruzi challenge. Parasite load was measured in DNA samples extracted from skeletal muscle of oral vaccine (both HR and LR) and control mice 25 dpc by real-time PCR. Bars show mean of group.
RESULTS
T. cruzi infects skeletal muscle following oral exposure.
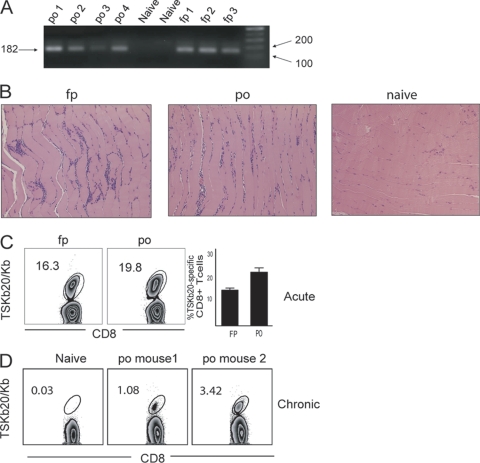
We first asked if parasites infecting via the oral route establish a systemic infection similar to that generated using other routes of infection. Metacyclic trypomastigotes were administered to mice by oral gavage (p.o.). Control infections were done by injecting metacyclics intraperitoneally (i.p.) or subcutaneously in the footpad (f.p.). PCR analysis revealed parasite DNA in the skeletal muscle at 21 to 25 days postinfection (dpi) in both p.o. and f.p.-infected mice (Fig. 1A). In addition, similar patchy mononuclear cell infiltrates were detectable in skeletal muscle at 35 dpi in mice infected by p.o. and f.p. routes (Fig. 1B). The emergence of a population of CD8+ T cells specific for the T. cruzi-derived immunodominant peptide TSKb20 (35) was also observed with mice infected p.o., comparable to mice infected in the f.p. (Fig. 1C). By 140 dpi, this robust TSKb20-specific CD8+ T cell response contracted and stabilized at frequencies typical of chronic T. cruzi infection in mice (Fig. 1D) (35). Thus, within ∼3 weeks after oral infection, T. cruzi disseminates, colonizes skeletal muscle, and induces a CD8+ T cell response that is indistinguishable from that induced in mice infected via i.p. or f.p. routes.
Fig. 1.
Oral infection with metacyclic trypomastigotes of T. cruzi leads to systemic infection. (A) Metacyclic trypomastigotes delivered by either p.o. or f.p. route are detectable in skeletal muscle. DNA was extracted from skeletal muscle of mice 21 to 25 dpi, and a 182-bp segment of satellite DNA was amplified by PCR. n = 10 for f.p. and n = 7 for p.o. The sizes of the PCR product and markers are shown (in base pairs) at the left and right, respectively. (B) Similar inflammatory infiltrates develop in skeletal muscle of mice infected by f.p. and p.o. H&E sections of skeletal muscle are shown for naïve and p.o. and f.p.-infected mice at 35 dpi and are representative of at least 3 more experiments that ended between 21 and 40 dpi. (C and D) T. cruzi infection via the oral route induces a robust and lasting systemic T cell response. Representative flow plots show the frequency of CD8+ T cells specific for TSKb20 in PBMC at 14 dpi (n = 10 for p.o. and n = 5 for f.p. [C]), and the means ± standard error of the mean (SEM) are displayed. (D) TSKb20-specific CD8+ T cells were measured in spleens of two individual mice infected p.o. at 140 dpi.
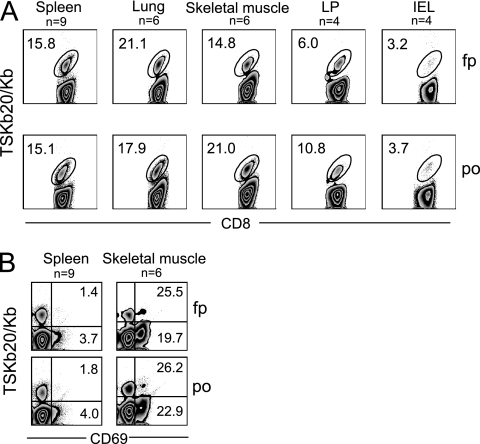
T. cruzi-specific CD8+ T cell traffic to tissues irrespective of infection route.
We hypothesized that parasite-specific CD8+ T cells would accumulate in gut tissue following p.o. but not i.p. infection with T. cruzi if parasite Ag accumulated or persisted at the infection site. To examine this possibility, lymphocytes isolated from lymphoid and nonlymphoid tissues of mice infected by these routes were analyzed. The frequency and distribution of TSKb20+ CD8+ T cells did not vary depending on infection route, as mice infected i.p. or by the f.p. developed parasite-specific CD8+ T cells able to traffic to gut tissue, and mice infected p.o. had proportions of TSKb20-specific cells in spleen, lung, and skeletal muscle comparable to those in mice infected i.p. (Fig. 2A). We have recently shown that T. cruzi-specific CD8+ T cells express CD69 selectively in sites of parasite persistence (M. H. Collins and R. L. Tarleton, unpublished data). CD8+ T cells isolated from skeletal muscle of p.o., i.p., and f.p.-infected mice expressed similar levels of this marker of recent activation, whereas little expression was detected with T cells isolated from the spleens of these animals (Fig. 2B). Taken together, these results indicate that regardless of infection route, T. cruzi achieves a systemic infection and thus induces T cells capable of accessing multiple tissue compartments.
Fig. 2.
T. cruzi-specific CD8+ T cells traffic to peripheral tissues and are activated in sites of parasite persistence independently of infection route. (A) Flow plots show the frequency of TSKb20-specific CD8+ cells in the indicated tissue of f.p.- or p.o. infected mice during acute T. cruzi infection. (B) Recently activated T. cruzi-specific CD8+ T cells are present in sites of persistence. Flow plots are gated on CD8+ T cells isolated from spleen or skeletal muscle of f.p.- or p.o. infected mice and show CD69 expression on TSKb20-specific cells. Numbers indicate the frequency of TSKb20+ CD8+ (top right) or TSKb20− CD8+ (bottom right) T cells expressing CD69. Numbers in flow plots are the average frequency obtained from mice sacrificed between 19 and 39 dpi. The number of data points (n) is shown for each group; some data points comprise cells isolated from the pooled tissue of two or three mice harvested on the same day.
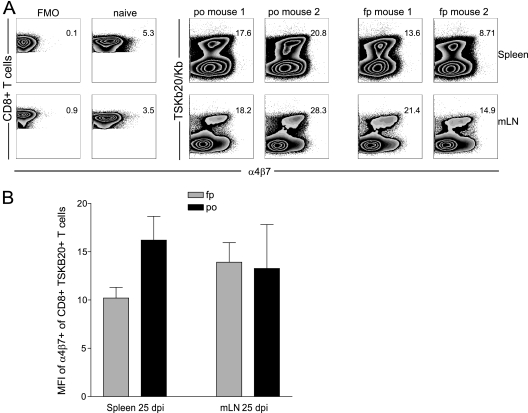
T cells primed during oral T. cruzi infection are not imprinted for gut homing.
T cells are thought to be educated to adopt a particular homing pattern when Ag is acquired in specific anatomical sites. Most notably, dendritic cells from GI tissue or GI-associated LN have been shown to confer a gut-homing program on T cells by induction of homing receptors such as CCR9 and α4β7 integrin (18, 45). To determine whether imprinting is a phenomenon associated with the initial T cell priming following oral exposure to T. cruzi, the expression of α4β7 was examined on parasite-specific CD8+ T cells following p.o. and f.p. infection. Expression of this homing molecule was similar among TSKb20+ CD8+ T cells from spleen and mesLN in mice infected p.o. and by the f.p. with T. cruzi (Fig. 3B and C), suggesting that p.o. infection did not evoke a mucosally targeted CD8+ T cell response.
Fig. 3.
Oral infection route does not skew T. cruzi-specific CD8+ T cells toward a gut homing phenotype. Lymphocytes were isolated from spleen (top) and mLN (bottom) of T. cruzi-infected mice at 25 dpi and naïve age-matched mice, and surface expression of α4β7 was assessed by flow cytometry. (A) Fluorescence minus one (FMO) and naïve CD8+ T staining controls for α4β7 expression are shown (left). Expression of α4β7 among TSKb20-specific CD8+ T cells is shown for 2 representative mice from a total of 5 mice (right). Flow plots are gated on CD8+ T cells. Numbers indicate the median fluorescence intensity (MFI) of CD8+ T cells for FMO and naïve samples and the MFI of TSKB20+ CD8+ T cells for the orally or footpad-infected mice. (B) Mean ± SEM of MFI for α4β7 expression on TSKB20+ CD8+ T cells. n = 5 mice per group.
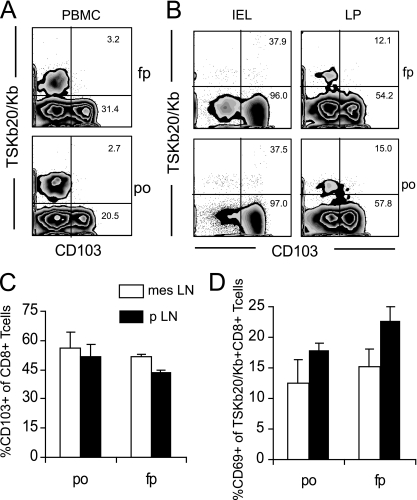
While expression of β7 integrin is key for CD8+ T cell homing to the LP and IEL compartments of the gut (30, 37), expression of CD103 (αE integrin) overlaps with that of β7 on Ag-specific CD8+ T cells in the gut (38) and has been implicated in the localization of T cells in the intestine (59). CD103 was not expressed on TSKb20+ CD8+ T cells in peripheral blood at 14 dpi (Fig. 4A). Many (50% and 95%, respectively) CD8+ T cells in LP and IEL populations are CD103+, but few TSKb20-specific CD8+ T cells express this integrin (Fig. 4B)—though the frequency of tetramer-positive cells is likely diluted by CD8αα+ T cells, especially in the IEL compartment (37). Importantly, the frequency of CD103-expressing cells is not affected by the route of infection. CD103 was also undetectable on TSKb20+ CD8+ T cells in LN (data not shown). Expression of CD103 among total CD8+ T cells was not enhanced in either peripheral (pLN) or gut-draining LN (mesLN) by an oral route of infection (Fig. 4C). Thus, no evidence of imprinting is observed after p.o. infection with T. cruzi.
Fig. 4.
Route of infection does not affect the distribution of TSKb20+ CD103+ CD8+ T cells or activation of TSKb20+ CD8+ T cells in gut-draining LN. (A) Gut-imprinted CD8+ T cells specific for T. cruzi are not detectable in blood early in infection. PBMC from mice at 14 dpi were isolated, and CD103 expression among TSKb20-specific cells was assessed by flow cytometry. Numbers in flow plots indicate the average percentage of TSKb20+ CD8+ (top) or TSKb20− CD8+ (bottom) T cells that express CD103 (f.p., n = 5; p.o., n = 10). (B) CD103 is expressed by gut CD8+ T cells, but by few T. cruzi-specific CD8+ T cells. IEL and LP lymphocytes were isolated from T. cruzi-infected mice between 19 and 39 dpi, and surface expression of CD103 was assessed by flow cytometry. Flow plots are gated on CD8+ T cells. Numbers indicate the average percentage of TSKb20+ CD8+ (top) or TSKb20− CD8+ (bottom) CD8+ T cells that express CD103, from 4 similar experiments. (C) Flow cytometry was used to assess the proportion of all CD8+ T cells expressing CD103 in pLN and mesLN of mice after f.p. and p.o. infection with T. cruzi. Bars show average percentage of CD103+ CD8+ cells ± SEM. (D) The proportion of recently activated TSKb20+ CD8+ T cells in different lymph nodes was assessed by flow cytometry. Bars show average percentage of CD103+ CD8+ cells ± SEM. n = 3 mice per group. Similar data were obtained in a replicate experiment.
Considering that CD69 is a marker of recent activation and that it associates with sphingosine-1-phosphate receptor 1 (S1P1) to retain lymphocytes in lymph nodes (60), we compared CD69 expression on CD8+ T cells in pLN and mesLN as an alternative method for investigating anatomically restricted antigen (Ag) presentation. The expression of CD69 on TSKb20-specfic CD8+ T cells in pLN or mesLN was not favored by one infection route over the other (Fig. 4D). Taken together, all these data suggest that T. cruzi enters its host and disseminates early in infection; it is not anatomically confined or immunologically recognized as an infection of any one tissue compartment.
An orally administered attenuated strain of T. cruzi provides protection against a challenge infection.
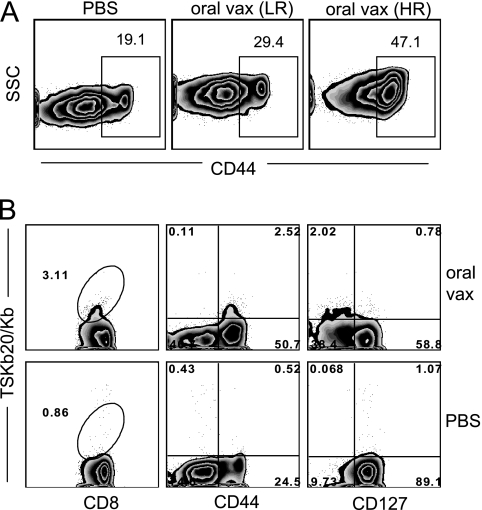
Given the robust CD8+ T cell response observed with spleen, blood, gut, and other nonlymphoid tissues following p.o. infection by T. cruzi, we hypothesized that oral vaccination could provide protection against a heterologous route of T. cruzi challenge. To test this hypothesis, we exposed mice to an attenuated strain of T. cruzi by the oral route and subsequently challenged them with virulent parasites. Mice received three doses of T. cruzi strain CL attenuated by the deletion of one allele of the enoyl-CoA-hydratase-1 (ECH1) gene and both alleles of the ECH2 gene (66). Both ECH genes encode an enzyme involved in fatty acid oxidation, a process thought to be important in amastigote energy metabolism (2). Parasites deficient in ECH have been shown to exhibit poor replication as intracellular amastigotes in vitro and fail to establish persistent infection in most immunocompetent mice (D. Xu and R. L. Tarleton, unpublished data). Mice received ECH1+/− ECH2−/− parasites by oral gavage (oral vaccine) or control gavage solution (PBS). Two weeks after the final vaccination, peripheral blood mononuclear cells (PBMCs) from the majority of mice exhibited upregulation of CD44, with over 50% of CD8+ T cells becoming CD44hi in some cases (Fig. 5A). Vaccinated mice could be divided into two groups based on the presence of antigen-experienced CD8+ T cells (with a 35% frequency of CD44+ cells as the cutoff). CD8+ T cells from “high responder” (HR) mice (n = 7) expressed this activation marker at significantly greater frequencies than “low responder” (LR) mice (n = 3) (47.1 versus 29.7, P = 0.029). Additionally, TSKb20+ CD8+ populations were apparent in the blood taken from one of the HR mice (Fig. 5B), further indicating the induction of T. cruzi-specific T cell responses in these mice. In contrast, LR mice were difficult to distinguish from PBS controls based on the CD44 expression profile of CD8+ T cells (Fig. 5A) and did not have measurable levels of TSKb20-specific CD8+ T cells in the peripheral blood.
Fig. 5.
Oral vaccination (vax) with attenuated parasites stimulates T. cruzi-specific CD8+ T cells. (A) Variable degrees of T cell stimulation result from oral vaccination. CD44 expression was assessed 2 weeks after final vaccination on CD8+ T cells in PBMC. Of 10 vaccinated mice, 3 were designated LR and 7 were designated HR. Flow plots are gated on CD8+ T cells, and numbers and gates indicate the average percentage of CD44hi cells in each group. (B) Oral vaccination generates activated T. cruzi-specific CD8+ T cells. Flow plots are from an individual mouse chosen from HR group to screen for TSKb20+ responses and CD127 downregulation. All plots are gated on CD8+ T cells, and numbers indicate the percentage of CD8+ T cells in a gate or quadrant.
The orally vaccinated mice were challenged in each footpad with 2.5 × 105 CL trypomastigotes transfected with a gene encoding TdTomato fluorescent protein, and parasite load at the site of infection was monitored from 2 to 10 days postchallenge (dpc) by in vivo imaging (9). Orally vaccinated mice displayed a significant reduction in the fluorescence signal compared to control unvaccinated mice at all times measured (Fig. 6A and B). While the fluorescence signal progressively increased in PBS mice until 8 dpc, a consistently low signal was displayed from 2 to 6 dpc in oral vaccine mice, and measurements were statistically indistinguishable from those for the background (naïve) signal by 8 dpc. As expected, HR mice controlled f.p. infection with T. cruzi more efficiently than LR mice, but LR mice still displayed a significantly lower fluorescence signal than PBS mice at this time (Fig. 6C). At 25 dpc, oral vaccine mice exhibited lower parasite loads in skeletal muscle than the controls did, although both groups controlled parasites well after these early time points (Fig. 6D). Together, these results indicate that oral vaccination with attenuated parasites stimulated effective adaptive immunity that successfully protected mice from challenge with WT T. cruzi.
DISCUSSION
Mucosal surfaces are the target of infection for many human pathogens (46), including those that cause HIV, tuberculosis, respiratory tract infections, and diarrheal illnesses, and account for an enormous portion of global disease burden (39). Immune responses to infection or immunization may evolve differently relative to whether antigen exposure is initially or exclusively at mucosal or nonmucosal sites. For example, the distribution of rotavirus and the responding cytotoxic T lymphocytes (CTL) varies according to route of infection (29, 47), and CD8+-dependent protection against mucosal HIV challenge can be generated by mucosal vaccination, whereas subcutaneous vaccination failed to elicit protective CTL at mucosal sites (5). Even simultaneously within the same animal, CD8+ T cells specific for the same tumor Ag can acquire different homing phenotypes if the Ag is introduced in multiple distinct environments (8).
Oral T. cruzi infection in humans is an increasingly prominent public health issue (14, 28, 67), and the oral route may be the primary means of infection in many animal species. Unlike most strictly mucosal pathogens that remain localized to the airways, gut lumen, mucosal surface epithelium, or lamina propria, T. cruzi transits these superficial compartments and establishes a systemic infection. These characteristics of T. cruzi and the data from other infections predict that the immune system may respond differently to T. cruzi infection initiated orally compared to f.p. or i.p. infection. In this study, we investigated whether the CD8+ T cell response to T. cruzi is altered by an oral route of parasite entry.
The nature of the immune response to parasitic infection can be heavily influenced by the earliest events of the host-parasite encounter, and there have recently been exciting advances in this area of parasitology (1, 12, 15, 49). The precise cells first infected and/or acting as antigen-presenting cells (APCs) that signal the adaptive immune system during oral T. cruzi infection are not known, but one can envisage at least two possible scenarios as to how the immune system recognizes T. cruzi as it progresses from mucosal exposure to a systemic infection. Dissemination could occur early after infection, with trypomastigotes entering the bloodstream soon after invasion or following the first round of replication in cells at the site of initial infection. If this were the case, the phenotype, frequency, and distribution of parasite-specific CD8+ T cells would be expected to be similar irrespective of the infection route. Alternatively, T. cruzi introduced at mucosal surfaces may be restricted to local GI cells for the first cycles of replication and host cell invasion. In this case, Ag presentation would be initially confined to mucosally associated lymphoid tissue, possibly leading to the imprinting of primed T cells for trafficking back to the mucosa. Others have documented local proliferation of parasites following mucosal T. cruzi infection, with the early involvement of draining LN, but inflammatory lesions and parasites can be observed at distal sites as early as 14 dpi (21, 26). Our data (Fig. 6) (48) support local parasite amplification following f.p. infection, but parasite DNA is also detectable in dLN within hours of infection (48). These observations suggest that either some parasites migrate out of the infection site prior to replication or that parasites are shuttled to the dLN in lymph or associated with host cells such as DC.
Herein we show that the CD8+ T cell response to T. cruzi infection develops similarly, regardless of infection route. T. cruzi-specific CD8+ T cells were found in all assayed lymphoid and nonlymphoid (including gut) tissues following either f.p. or p.o. infection. In a different model of oral infection, T. cruzi has been shown to replicate in gastric mucosa en route to systemically infecting mice (26). Our initial hypothesis was that replication of parasites in gut-associated tissue such as gastric or intestinal mucosa following oral infection but not f.p. infection may program primed CD8+ T cells to traffic to gut tissues via expression of α4β7 (45). However, we were not able to detect differences in the expression of this homing integrin in different routes of infection. The localization of TSKb20-specific cells to GI tissue following f.p. infection was intriguing, given that this compartment often displays stringent “gating” that is permissive only to certain subsets of T cells with a specific homing signature (32). One explanation for the presence of gut-homing T. cruzi-specific T cells in f.p.-infected mice is that intestinal tissue is being infected by parasites in the bloodstream. Parasite-induced local inflammation could override the need for α4β7 expression for T cells to enter gut tissue (3, 53). Alternatively, recent work in a viral infection model suggests that there is a window early in systemic infections during which CD8+ T cells adopt a gut homing phenotype and populate mucosal sites (36). The protracted course of T. cruzi infection may mean that there is not one particular time during acute infection when a substantial proportion of T cells are expressing α4β7. The role of CD103 in the CD8+ T cell-mediated, adaptive immune response to GI infections is unclear. CD103 appears to be important for retaining T cells in epithelial tissue and dispensable for homing to these sites (19, 36, 59). Though we did not detect a large population of T. cruzi-specific CD103+ CD8+ T cells in the IEL compartment, it is tempting to speculate that cells with this signature are important for mucosal immunity to T. cruzi infection and may contribute to protection against mucosal T. cruzi challenge observed by others (23–25, 58). Another possibility is that immunity and protection against mucosal T. cruzi challenge are mediated by CD8+ T cells that transiently survey the gut along with other peripheral tissues. Thus, regardless of route, systemic infection by T. cruzi elicits a parasite-specific CD8+ T cell response in many tissue compartments, including the GI mucosa.
T. cruzi also showed tropism for skeletal muscle after oral administration (Fig. 1A) and recently activated (CD69+) TSKb20+ CD8+ T cells selectively accumulate at that site—an observation also true for f.p.-infected mice (Fig. 2B). Lastly, comparable proportions of T. cruzi-specific CD8+ T cells were activated in lymph nodes draining skeletal muscle or the gut regardless of infection route (Fig. 2D). Overall, these results support a model in which the T. cruzi-specific CD8+ T cell response is initiated by APC that have acquired Ag in sites dispersed throughout the host, consistent with a systemic distribution of parasites beyond the point of parasite entry very shortly after infection.
One reason for undertaking this study was to determine whether we could identify the initial route of infection of hosts by assessing the phenotype of the anti-T. cruzi T cells. Although a number of small epidemics of T. cruzi infection have clearly been linked to ingestion of contaminated food or drink (14, 28, 67), it is difficult to know the contribution of the oral route of infection when this parasite is acquired in and around houses infested with T. cruzi-infected triatomines. Our results suggest that immune parameters will not be particularly useful in determining the mode of transmission in individuals with T. cruzi infection, presumably because the infection rapidly becomes systemic irrespective of the route of the initial infection. Another principle reason for studying the immune response following oral infection with T. cruzi is that an oral route would be an attractive means of delivery of a vaccine, especially for the protection of reservoir hosts that are integral to the transmission of T. cruzi to insect vectors. A number of studies have shown that the interruption of T. cruzi transmission to humans could be achieved by decreasing the ability of dogs to transmit T. cruzi to insects, a primary factor promoting household infections in many areas where T. cruzi is endemic (13, 20, 22). Canine vaccination is a long-standing idea and has a proven role in controlling zoonotic diseases, including rabies and leishmaniasis (40). Reservoirs could conveniently be vaccinated by the oral route, and previous studies support the immunogenic potential of mucosally delivered T. cruzi Ag (10, 11, 21, 23–25, 58). It has also been shown that vaccination can generate effective immunity against mucosal T. cruzi challenge (23, 24, 58). A key question that had not been addressed is whether a genetically attenuated strain of T. cruzi could be used as an oral vaccine.
Here, we show that ECH1+/− ECH2−/− metacyclic trypomastigotes given by oral gavage provide protective immunity to WT T. cruzi challenge. Protection was evident as early as 2 dpc using a new method for tracking the early success of infection by tracking fluorescently tagged parasites (31). Parasite burden at the challenge site was well controlled in vaccinated mice, whereas it increased steadily in controls over the first 8 dpc (Fig. 6B) before declining with the local control of the infection and the dissemination of parasites to other tissues (A. Padilla and R. L. Tarleton, unpublished data). Thus, vaccination reduces the initial “take” of T. cruzi infection as well as replication of parasites that successfully invade host cells. The level of CD8+ T cell activation measured in peripheral blood prior to challenge predicted the degree of protection observed among vaccinated animals. Despite the relative absence of T cell activation in the LR mice, these also exhibit a degree of protection. This result demonstrates that while induction of parasite-specific T cell immunity may be a convenient indicator of vaccine effectiveness, its absence should not absolutely discount a protocol. We chose a heterologous route of challenge in these studies as a more rigorous test of vaccine efficacy. Although there is a discrepancy in the literature as to whether mucosal vaccination is absolutely necessary to induce protection at mucosal sites (4, 31), there is no doubt that mucosal vaccination can stimulate mucosal immunity (27). Additionally, mucosal infection or immunization with T. cruzi parasites or Ag leads to IgA production (10, 11, 21, 26, 57) and confers resistance to mucosal T. cruzi challenge (23, 24, 58). Thus, given the protection against systemic challenge observed after oral vaccination with T. cruzi in these studies, we also predict that an oral route of immunization will effectively protect against mucosal challenge. While strategies such as heterologous prime-boost regimens may maximize immunogenicity for a particular Ag (54), live parasites may best induce a broad-based, protective immune response (63) and be the practical choice to diminish the potential of reservoirs to infect triatomine bugs (22). This work provides support for the further development of attenuated parasite lines for vaccination against T. cruzi and defines a system in which to test vaccine candidates and establish correlates of protection.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-022070 and AI-33106 from NIAID/NIH. M.H.C. was supported in part by NIH training grant T32 AI-060546 to the Center for Tropical and Emerging Global Diseases.
The technical assistance of Dan Xu and the help of Angel Padilla in assembly of the figures are gratefully acknowledged.
Footnotes
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Amino R., et al. 2006. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat. Med. 12:220–224 [DOI] [PubMed] [Google Scholar]
- 2. Atwood J. A., III, et al. 2005. The Trypanosoma cruzi proteome. Science 309:473–476 [DOI] [PubMed] [Google Scholar]
- 3. Bell L. V., Else K. J. 2008. Mechanisms of leucocyte recruitment to the inflamed large intestine: redundancy in integrin and addressin usage. Parasite Immunol. 30:163–170 [DOI] [PubMed] [Google Scholar]
- 4. Belyakov I. M., Ahlers J. D. 2009. Comment on “trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination.” J. Immunol. 182:1779–1780 [DOI] [PubMed] [Google Scholar]
- 5. Belyakov I. M., et al. 1998. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Invest. 102:2072–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourguignon S. C., de Souza W., Souto-Padron T. 1998. Localization of lectin-binding sites on the surface of Trypanosoma cruzi grown in chemically defined conditions. Histochem. Cell Biol. 110:527–534 [DOI] [PubMed] [Google Scholar]
- 7. Bustamante J. M., Bixby L. M., Tarleton R. L. 2008. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat. Med. 14:542–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calzascia T., et al. 2005. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22:175–184 [DOI] [PubMed] [Google Scholar]
- 9. Canavaci A. M., et al. 2010. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLoS Negl. Trop. Dis. 4:e740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cazorla S. I., et al. 2008. Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect. Immun. 76:324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cazorla S. I., et al. 2008. Prime-boost immunization with cruzipain co-administered with MALP-2 triggers a protective immune response able to decrease parasite burden and tissue injury in an experimental Trypanosoma cruzi infection model. Vaccine 26:1999–2009 [DOI] [PubMed] [Google Scholar]
- 12. Chakravarty S., et al. 2007. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 13:1035–1041 [DOI] [PubMed] [Google Scholar]
- 13. Cohen J. E., Gurtler R. E. 2001. Modeling household transmission of American trypanosomiasis. Science 293:694–698 [DOI] [PubMed] [Google Scholar]
- 14. Coura J. R., Junqueira A. C., Fernandes O., Valente S. A., Miles M. A. 2002. Emerging Chagas disease in Amazonian Brazil. Trends Parasitol. 18:171–176 [DOI] [PubMed] [Google Scholar]
- 15. Courret N., et al. 2006. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings K. L., Tarleton R. L. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 129:53–59 [DOI] [PubMed] [Google Scholar]
- 17. Dias J. C., Silveira A. C., Schofield C. J. 2002. The impact of Chagas disease control in Latin America: a review. Mem. Inst. Oswaldo Cruz 97:603–612 [DOI] [PubMed] [Google Scholar]
- 18. Dudda J. C., et al. 2005. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur. J. Immunol. 35:1056–1065 [DOI] [PubMed] [Google Scholar]
- 19. El-Asady R., et al. 2005. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J. Exp. Med. 201:1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Estrada-Franco J. G., et al. 2006. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg. Infect. Dis. 12:624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giddings O. K., Eickhoff C. S., Smith T. J., Bryant L. A., Hoft D. F. 2006. Anatomical route of invasion and protective mucosal immunity in Trypanosoma cruzi conjunctival infection. Infect. Immun. 74:5549–5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gürtler R. E., et al. 2007. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology 134:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoft D. F., Eickhoff C. S. 2005. Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen. Infect. Immun. 73:4934–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoft D. F., Eickhoff C. S. 2002. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infect. Immun. 70:6715–6725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoft D. F., Eickhoff C. S., Giddings O. K., Vasconcelos J. R., Rodrigues M. M. 2007. Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J. Immunol. 179:6889–6900 [DOI] [PubMed] [Google Scholar]
- 26. Hoft D. F., Farrar P. L., Kratz-Owens K., Shaffer D. 1996. Gastric invasion by Trypanosoma cruzi and induction of protective mucosal immune responses. Infect. Immun. 64:3800–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmgren J., Czerkinsky C. 2005. Mucosal immunity and vaccines. Nat. Med. 11:S45–S53 [DOI] [PubMed] [Google Scholar]
- 28. Igreja R. P. 2009. Chagas disease 100 years after its discovery. Lancet 373:1340. [DOI] [PubMed] [Google Scholar]
- 29. Jiang J. Q., He X. S., Feng N., Greenberg H. B. 2008. Qualitative and quantitative characteristics of rotavirus-specific CD8 T cells vary depending on the route of infection. J. Virol. 82:6812–6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johansson-Lindbom B., Agace W. W. 2007. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Rev. 215:226–242 [DOI] [PubMed] [Google Scholar]
- 31. Kaufman D. R., et al. 2008. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J. Immunol. 181:4188–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klonowski K. D., et al. 2004. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20:551–562 [DOI] [PubMed] [Google Scholar]
- 33. Laky K., Lefrancois L., Puddington L. 1997. Age-dependent intestinal lymphoproliferative disorder due to stem cell factor receptor deficiency: parameters in small and large intestine. J. Immunol. 158:1417–1427 [PubMed] [Google Scholar]
- 34. Martin D., Tarleton R. 2004. Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection. Immunol. Rev. 201:304–317 [DOI] [PubMed] [Google Scholar]
- 35. Martin D. L., et al. 2006. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masopust D., et al. 2010. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207:553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masopust D., Jiang J., Shen H., Lefrancois L. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348–2356 [DOI] [PubMed] [Google Scholar]
- 38. Masopust D., Vezys V., Marzo A. L., Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413–2417 [DOI] [PubMed] [Google Scholar]
- 39. Mathers C. D., Ezzati M., Lopez A. D. 2007. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl. Trop. Dis. 1:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meeusen E. N., Walker J., Peters A., Pastoret P. P., Jungersen G. 2007. Current status of veterinary vaccines. Clin. Microbiol. Rev. 20:489–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mestecky J., Russell M. W., Elson C. O. 2007. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J. Immunol. 179:5633–5638 [DOI] [PubMed] [Google Scholar]
- 42. Miyahira Y. 2008. Trypanosoma cruzi infection from the view of CD8+ T cell immunity: an infection model for developing T cell vaccine. Parasitol. Int. 57:38–48 [DOI] [PubMed] [Google Scholar]
- 43. Miyahira Y., et al. 1999. Induction of CD8+ T cell-mediated protective immunity against Trypanosoma cruzi. Int. Immunol. 11:133–141 [DOI] [PubMed] [Google Scholar]
- 44. Miyahira Y., et al. 2005. Immune responses against a single CD8+-T-cell epitope induced by virus vector vaccination can successfully control Trypanosoma cruzi infection. Infect. Immun. 73:7356–7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mora J. R., et al. 2005. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201:303–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neutra M. R., Kozlowski P. A. 2006. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 6:148–158 [DOI] [PubMed] [Google Scholar]
- 47. Offit P. A., Cunningham S. L., Dudzik K. I. 1991. Memory and distribution of virus-specific cytotoxic T lymphocytes (CTLs) and CTL precursors after rotavirus infection. J. Virol. 65:1318–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Padilla A. M., Simpson L. J., Tarleton R. L. 2009. Insufficient TLR activation contributes to the slow development of CD8+ T cell responses in Trypanosoma cruzi infection. J. Immunol. 183:1245–1252 [DOI] [PubMed] [Google Scholar]
- 49. Peters N. C., et al. 2008. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321:970–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rabinovich J., Schweigmann N., Yohai V., Wisnivesky-Colli C. 2001. Probability of Trypanosoma cruzi transmission by Triatoma infestans (Hemiptera: Reduviidae) to the opossum Didelphis albiventris (Marsupialia: Didelphidae). Am. J. Trop. Med. Hyg. 65:125–130 [DOI] [PubMed] [Google Scholar]
- 51. Reithinger R., Ceballos L., Stariolo R., Davies C. R., Gurtler R. E. 2005. Chagas disease control: deltamethrin-treated collars reduce Triatoma infestans feeding success on dogs. Trans. R. Soc. Trop. Med. Hyg. 99:502–508 [DOI] [PubMed] [Google Scholar]
- 52. Reithinger R., Tarleton R. L., Urbina J. A., Kitron U., Gurtler R. E. 2009. Eliminating Chagas disease: challenges and a roadmap. BMJ 338:b1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rivera-Nieves J., et al. 2005. l-Selectin, alpha 4 beta 1, and alpha 4 beta 7 integrins participate in CD4+ T cell recruitment to chronically inflamed small intestine. J. Immunol. 174:2343–2352 [DOI] [PubMed] [Google Scholar]
- 54. Rodrigues M. M., et al. 2003. Importance of CD8 T cell-mediated immune response during intracellular parasitic infections and its implications for the development of effective vaccines. An. Acad. Bras. Cienc. 75:443–468 [DOI] [PubMed] [Google Scholar]
- 55. Roellig D. M., Ellis A. E., Yabsley M. J. 2009. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J. Parasitol. 95:360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Round J. L., Mazmanian S. K. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schnapp A. R., Eickhoff C. S., Scharfstein J., Hoft D. F. 2002. Induction of B- and T-cell responses to cruzipain in the murine model of Trypanosoma cruzi infection. Microbes Infect. 4:805–813 [DOI] [PubMed] [Google Scholar]
- 58. Schnapp A. R., Eickhoff C. S., Sizemore D., Curtiss R., III, Hoft D. F. 2002. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect. Immun. 70:5065–5074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schön M. P., et al. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J. Immunol. 162:6641–6649 [PubMed] [Google Scholar]
- 60. Shiow L. R., et al. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544 [DOI] [PubMed] [Google Scholar]
- 61. Stuart K., et al. 2008. Kinetoplastids: related protozoan pathogens, different diseases. J. Clin. Invest. 118:1301–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tarleton R. L. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 144:717–724 [PubMed] [Google Scholar]
- 63. Tarleton R. L. 2005. New approaches in vaccine development for parasitic infections. Cell. Microbiol. 7:1379–1386 [DOI] [PubMed] [Google Scholar]
- 64. Winnard P. T., Jr., Kluth J. B., Raman V. 2006. Noninvasive optical tracking of red fluorescent protein-expressing cancer cells in a model of metastatic breast cancer. Neoplasia 8:796–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wizel B., Garg N., Tarleton R. L. 1998. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 66:5073–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu D., Brandan C. P., Basombrio M. A., Tarleton R. L. 2009. Evaluation of high efficiency gene knockout strategies for Trypanosoma cruzi. BMC Microbiol. 9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yoshida N. 2008. Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity. Parasitol. Int. 57:105–109 [DOI] [PubMed] [Google Scholar]
- 68. Zhang L., Tarleton R. L. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J. Infect. Dis. 180:480–486 [DOI] [PubMed] [Google Scholar]