Abstract
Vaccines that could effectively prevent Pseudomonas aeruginosa pulmonary infections in the settings of cystic fibrosis (CF) and nosocomial pneumonia could be exceedingly useful, but to date no effective immunotherapy targeting this pathogen has been successfully developed for routine use in humans. Evaluations using animals and limited human trials of vaccines and their associated immune effectors against different P. aeruginosa antigens have suggested that antibody to the conserved surface polysaccharide alginate, as well as the flagellar proteins, often give high levels of protection. However, alginate itself does not elicit protective antibody in humans, and flagellar vaccines containing the two predominant serotypes of this antigen may not provide sufficient coverage against variant flagellar types. To evaluate if combining these antigens in a conjugate vaccine would be potentially efficacious, we conjugated polymannuronic acid (PMA), containing the blocks of mannuronic acid conserved in all P. aeruginosa alginates, to type a flagellin (FLA) and evaluated immunogenicity, opsonic killing activity, and passive protective efficacy in mice. The PMA-FLA conjugate was highly immunogenic in mice and rabbits and elicited opsonic antibodies against mucoid but not nonmucoid P. aeruginosa, but nonetheless rabbit antibody to PMA-FLA showed evidence of protective efficacy against both types of this organism in a mouse lung infection model. Importantly, the PMA-FLA conjugate vaccine did not elicit antibodies that neutralized the Toll-like receptor 5 (TLR5)-activating activity of flagellin, an important part of innate immunity to flagellated microbial pathogens. Conjugation of PMA to FLA appears to be a promising path for developing a broadly protective vaccine against P. aeruginosa.
INTRODUCTION
Vaccination to prevent infection by Pseudomonas aeruginosa would be a welcome advance in many regards, but particularly for preventing the chronic infection that is the major cause of morbidity and mortality in cystic fibrosis (CF) patients (20, 24). Colonization of the CF lung by P. aeruginosa occurs early in life (23, 42) predominantly by low-alginate-producing, nonmucoid, motile strains expressing a single polar flagellum. Components of the P. aeruginosa flagella as well as the constituent flagellin proteins are also involved in adhesion to host tissues, binding to mucins and also possibly to the glycolipid asialo-GM1 and to Toll-like receptor 5 (TLR5) (1, 39, 40), where host innate immune effectors are activated. The onset of increased yearly declines in CF lung function is associated with the conversion of P. aeruginosa from a nonmucoid and motile to a mucoid and nonmotile phenotype in the lung (42). Mucoid strains overproduce alginate, a random polymer of partially O-acetylated d-mannuronic acid and l-guluronic acid residues linked β1→4 (22, 46). Alginate plays many roles in the pathogenesis of the respiratory tract infection in CF. It serves as a mechanism for the formation of microcolonies or biofilms, confers antiphagocytic properties to mucoid strains, and protects P. aeruginosa from the consequences of inflammation, such as lethal oxygen radicals (17).
While early antibiotic treatment of the initial colonizing population of P. aeruginosa might prevent or at least delay chronic pulmonary infection (14, 42), vaccination that could prevent the establishment of chronic infection in the CF lung would have many advantages. Epidemiologic studies have linked opsonic antibodies specific to alginate in the sera of CF patients with a lack of chronic mucoid P. aeruginosa colonization and better overall lung function (13, 23, 31, 35, 37), but these antibodies are rarely induced by infection in most CF patients (31, 37). Purified P. aeruginosa alginate is safe when administered to humans (35) but alginate overall is poorly immunogenic in most humans, failing to elicit high titers of opsonic, protective antibodies. In an attempt to increase the immunogenicity of alginate, the molecule has been conjugated to carrier proteins (10, 13, 20, 47) by using detoxified exotoxin A, keyhole limpet hemocyanin (KLH), and tetanus toxoid (TT) as carrier proteins. However, a major challenge to this approach is that alginate from different mucoid strains has variable ratios of mannuronic to guluronic acids (∼10:1 to close to 1:1) (35), making it difficult to find a preparation of alginate that gives rise to antibodies reactive with multiple strains of mucoid P. aeruginosa. Nonetheless, within most P. aeruginosa alginates are blocks of O-acetylated polymannuronic acid (PMA) (44), suggesting that a preparation of alginate rich in PMA residues could induce broadly reactive antibodies. O-acetyl groups on P. aeruginosa alginate affect the physical properties including the ability of P. aeruginosa to form biofilms in vivo (30). Acetate residues confer bacterial resistance to phagocytosis and complement by preventing activation of the alternative pathway of complement (34). It has been previously demonstrated that the O-acetyl groups of alginate are the epitopes recognized by antibody raised to purified alginate, alginate conjugates, or murine monoclonal antibodies (MAbs) that were opsonic and promoted protection against mucoid strains of P. aeruginosa in mice (15, 34, 47). However, a human MAb to alginate that protected against infection by both mucoid and nonmucoid strains recognized a different epitope on the PMA molecule (33), encompassing the uronic acid on C-6 of nonacetylated PMA blocks (33). Additional human MAbs generated for this prior study that bound to acetylated PMA blocks mediated opsonic killing of mucoid but not nonmucoid strains, leading to the conclusion that the optimal epitope for antibodies to P. aeruginosa alginate able to protect against both phenotypes of this organism were directed to the nonacetylated blocks of PMA contained with the alginate.
As for being a good carrier protein for a conjugate vaccine, P. aeruginosa flagellin and whole flagellar protein have potential desirable properties, as these molecules have been shown to be protective antigens (5, 11, 18, 19, 25, 27, 29, 38, 49), and the interaction of flagellin with TLR5 can also provide an adjuvant effect for vaccines containing TLR5-active flagellins (6, 26, 45). Additionally, a bivalent P. aeruginosa flagellar vaccine administered to CF patients in a phase III clinical trial was well tolerated, elicited high IgG titers, and achieved a 34% reduction in the number of patients with a first positive culture for P. aeruginosa (12). In another study, a fusion protein vaccine containing OprF epitope 8, OprI, and type a and b flagellins administered to young African green monkeys generated high-affinity antibodies that, after passive transfer to mice, protected against nonmucoid P. aeruginosa lung infection (49). We have found in animal studies that whole flagella are a superior vaccine compared with flagellin (8), but the large, polymeric, and unstable nature of whole flagella makes them difficult to use as a carrier protein in conjugate vaccines. Therefore, we used the flagellin protein monomer as a carrier for conjugation as it was considerably more suitable for making this type of vaccine. However, use of flagellin in a vaccine has potential negative effects, wherein antibodies that inhibit the binding of both flagellin from P. aeruginosa and other organisms to TLR5 might be generated, thus interfering with an interaction that contributes to innate immunity to P. aeruginosa (41, 45) and other pathogens.
In this study, we describe the synthesis and characterization of a polymannuronic acid-flagellin (PMA-FLA) conjugate, using purified PMA and type a flagellin to evaluate the immunogenic response in rabbits and mice and the protective efficacy of passive immunization with antibody to PMA-FLA against mucoid as well as nonmucoid, low-alginate-producing P. aeruginosa strains (3, 36) in a murine model of lung infection. We also investigate whether this conjugate avoids potentially deleterious induction of antibodies that could inhibit the TLR5 activation that occurs with P. aeruginosa infection.
MATERIALS AND METHODS
Bacterial strains.
The P. aeruginosa strains used for these studies were as follows: PAK, a serogroup O6, type a flagellated strain; PAO1, a serogroup O2/O5, type b flagellated strain; and PAO1 ExoU+, a PAO1 strain expressing the ExoU cytotoxin (2). Clinical isolates obtained from CF patients included nonmucoid strains N6 and N13, which are type a flagellated strains, and nonflagellated mucoid strains 2192, FRD1, and 8050. Also, FRD1 ΔalgD is an FRD1 strain defective for alginate production (9).
Animals.
C57BL/6 and C3H/HeN mice were obtained from Charles River Laboratories. New Zealand White rabbits were from Millbrook Breeding Labs, Amherst, MA. All animal studies were conducted in accordance with protocols approved by the Harvard Medical Area Institutional Animal Care and Use Committee.
Reagents.
Nonacetylated polymannuronic acid derived from Pseudomonas fluorescens was provided by Gudmund Skajk-Braek (Trondheim, Norway). Protanal LF 120 M sodium alginate was obtained from FMC Biopolymers, Philadelphia, PA. These polysaccharides had <0.01% endotoxin as determined by Limulus amebocyte assay (Cape Cod Associates, Hyannis, MA). 1-Ethyl-3(3-dimethylaminopropyl) carbodiimide (EDC), dithiothreitol (DTT), dithionitrobenzoic acid (Ellman's reagent), cystamine, and cysteine were from Sigma-Aldrich, St. Louis, MO. The Limulus amebocyte lysate assay was from Cape Cod Associates, Woods Hole, MA. Superose 6 prep-grade and PD-10 columns (Sephadex G-25 M) were from GE Healthcare. GMBS [N-(γ-maleimidobutyryloxy)succinimide ester] was from Thermo Scientific Pierce Protein Research Products. Alkaline phosphatase conjugates of goat anti-rabbit IgG (whole molecule) were from Sigma. Bio-Rad protein assay dye reagent concentrate was from Bio-Rad Laboratories, Inc., Hercules, CA.
Purification of flagellin.
Recombinant type a flagellin was purified from Escherichia coli BL21(DE3) carrying the pET15BVP vector with His-tagged type a fliC gene as previously described (48).
Ultrasonic depolymerization of PMA.
Polymannuronic acid was depolymerized by using a sonicator (60 sonic dismembrator; Fisher Scientific) as previously described (32). Briefly, PMA was dissolved in deionized water at a concentration of 1 mg/ml and placed on ice. The sonication cycle consisted of 10 min of exposure at 3 W followed by 10 min of rest. PMA was sonicated until a cumulative time of 200 min was achieved.
PMA-flagellin conjugation.
PMA was conjugated to flagellin as previously described with some modifications (47). Sonicated PMA (2 mg/ml) and cystamine (35 mM) were dissolved in 0.05 M MES [2-(N-morpholino) ethanesulfonic acid] buffer, and the pH was adjusted to 5.0. EDC was added (0.4 M final concentration), and the reaction mixture was stirred at room temperature for 2 h while the pH was maintained between 4.9 and 5.1. The polysaccharide was then passed through a PD-10 column equilibrated with the conjugation buffer (50 mM phosphate-buffered saline [PBS], 0.9 M NaCl, 5 mM EDTA; pH 7.4) for removal of low-molecular-weight material. For conjugation to flagellin, PMA-cystamine was reduced with 0.05 M DTT for 2 h. The mixture was then desalted with a PD-10 column equilibrated with conjugation buffer, and volume fractions that assayed positive for free sulfhydryl groups and uronic acid were pooled and concentrated to a final concentration of 2.5 mg/ml.
To derivatize the type a flagellin protein, the protein (2 mg/ml) was dissolved in conjugation buffer, 2 mg of GMBS [N-(γ-maleimidobutyryloxy)succinimide ester] in 100 μl of dimethyl sulfoxide (DMSO) were added, and the reaction mixture was stirred at room temperature for 1 h. The GMBS-derivatized flagellin was then passed through a PD-10 column equilibrated with the conjugation buffer.
For conjugation, GMBS-derivatized flagellin was added to the reduced cystamine derivative of PMA, and the mixture was stirred at room temperature for 1 h. After incubation, the conjugate was passed through a 1.6 by 95-cm Superose 6 column with phosphate-buffered saline (PBS) used as running buffer. Void-volume fractions that assayed positive for both protein and uronic acid were designated polysaccharide-protein conjugate and were pooled, dialyzed against deionized water, and lyophilized in aliquots. The amounts of protein and PMA present in the conjugate were quantified by assaying for protein by the Bio-Rad Protein assay using bovine serum albumin (BSA) as the standard and for uronic acid by the carbazole assay with sodium alginate as the standard (Protanal LF 120 M; FMC Biopolymers).
Immunization of animals.
C3H/HeN mice (8/group), 6 to 8 weeks old, were immunized subcutaneously (s.c.) three times at weekly intervals with 3 μg of sugar in the PMA-FLA conjugate (determined in preliminary experiments to be the optimal dose) or a noncovalent mixture of PMA/FLA suspended in the adjuvant Specol. Serum samples were obtained on days 0, 28, 35, 42, and 56 for analysis.
A female New Zealand White rabbit was immunized with 120 μg of PMA on days 1 (in complete Freund's adjuvant) and 8 (in incomplete Freund's adjuvant [Sigma]). The rabbit was boosted intravenously with three 120-μg doses the following week on alternate days. A second rabbit was immunized with 100 μg of flagellin on days 1 and 8 in incomplete Freund's adjuvant. The rabbit was boosted intravenously with three 100-μg doses the following week. A third rabbit was immunized with 10 μg of conjugated PMA-FLA in incomplete Freund's adjuvant on days 1 and 8. The rabbit was boosted intravenously with three 10-μg doses the following week. Further booster doses were given to each rabbit intravenously at intervals of 2 to 4 months.
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed by standard methods as described previously (8). Briefly, microtiter plates were coated with 1 μg flagellin/ml of 0.2 M carbonate buffer (pH 9.6) or 10 μg PMA/ml of 0.04 M phosphate buffer (pH 7.4) and kept overnight at 4°C. Between incubation steps, plates were washed three times with PBS containing 0.05% Tween 20 (PBST). Blocking was performed with 1% BSA in PBS overnight at 4°C. Serum samples diluted 2-fold in 1% BSA in PBST were incubated for 1 h at 37°C. An alkaline phosphatase conjugate diluted 1:1,000 was used as secondary antibody, and p-nitrophenyl phosphate was used as a substrate (Sigma; 1 mg/ml in diethanolamine buffer, 0.5 mM MgCl2 [pH 9.8]). After 60 min of incubation at 37°C, the absorbance was measured at 405 nm. ELISA titers were calculated by linear regression analysis of the average of duplicate measurements; the titer was the serum dilution giving a final optical density value at 405 nm (OD405) of 0, as calculated from the linear regression curve.
An ELISA for inhibition, based on the competitive inhibition by PMA or PMA-FLA antigens of the binding of the antibodies to PMA to PMA antigen, was used to evaluate the modification of epitopes in the conjugated polysaccharide. Briefly, an ELISA was performed as described above, and serum samples were added at the same time with PMA as the inhibitor.
Opsonophagocytic assay.
An opsonophagocytic assay was used as previously described (8), with only one modification. To prepare mucoid bacteria for use in the assay, 5 ml of tryptic soy broth (TSB) was inoculated with bacteria from a tryptic soy agar (TSA) plate grown overnight at 37°C, with the inoculum placed into the TSB tubes and grown at 37°C by tumbling the tubes end over end on a rotator until an OD650 of 0.4 was obtained.
Motility inhibition assays.
Motility assays were performed as previously described (8). Briefly, bacteria were grown statically in TSB at 37°C. Approximately 105 log-phase organisms were inoculated onto plates made with lysogeny broth (LB) and 0.3% agar in the presence of antisera raised to flagellin or PMA-FLA at a dilution of 1:10 and incubated at 30°C for 18 h. Normal rabbit serum (NRS) was used as a negative control. Results were photographically recorded after 18 h.
Flagellar typing of CF strains.
Flagellar typing of CF strains was accomplished by PCR amplification of the central region of the flagellin gene, as previously described (8).
Mouse model of clearance of lung infection.
Antibody-promoted clearance of mucoid P. aeruginosa 4 h postinfection was used to measure vaccine efficacy against this phenotype of P. aeruginosa strains, as previously described (33). This clearance model was used due to the fact that the lipopolysaccharide (LPS) rough, mucoid P. aeruginosa strains are readily cleared by nonimmune mice unless very high challenge doses are used (33). After anesthetizing mice by intraperitoneal (i.p.) injection of 0.2 ml of xylazine (1.3 mg/ml) and ketamine (6.7 mg/ml), 6- to 8-week-old female C3H/HeN mice were given 50 μl of anti-PMA or anti-PMA-FLA or NRS intranasally (i.n.) 48 h and 24 h before infection.
P. aeruginosa mucoid strains were grown on TSA plates overnight, and bacteria from these plates were inoculated into TSB at an OD650 of 0.1 and grown to an OD650 of 0.4 at 37°C. Bacteria were recovered by centrifugation, resuspended in PBS to the desired dose for infection, and washed 3 times in this buffer. Prior to administration to animals, bacterial cells were plated on MacConkey agar plates to determine the inoculum. For infection, anesthetized mice were given 25 μl of P. aeruginosa i.n., prepared as described above. Infected mice were sacrificed 4 h postinfection, both lungs were removed, weighed, and homogenized, and the homogenate was diluted and plated for determinations of CFU per gram of lung tissue.
Protection against infection in a setting of acute pneumonia.
An acute pneumonia model of infection was used to measure efficacy against nonmucoid strains of P. aeruginosa. After anesthesia, 6- to 8-week-old female C57BL/6 mice were given 50 μl of antibody i.n. raised to either PMA, PMA-FLA, flagellin type a, or NRS 48, 24, and 4 h prior to infection. For infection, bacteria were prepared and inoculated i.n. as described above, and animals were observed for survival twice a day for up to 5 days. Animals found moribund were sacrificed and counted as deceased for the purposes of these experiments.
Cell culture and Toll-like receptor 5 inhibition assay.
A TLR5-expressing A549 lung epithelial cell line stably transfected with a nuclear factor-kappa B (NF-κB) luciferase reporter plasmid (NFκB-luc, Panomics, Freemont, CA) was used to detect cellular activation by P. aeruginosa flagellin or PMA-FLA conjugate as previously described (8). Briefly, the cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% bovine calf serum and selected by 100 μg hygromycin B/ml in T75 flasks. After ∼90% of confluence was reached, A549/NFkB-luc cells were transferred to 96-well solid white plates (Costar) at a concentration of 5 × 104 cells/well. Antisera were diluted 1:50 and then added along with flagellin or PMA-FLA to the plates, which were then incubated for 5 h. After 5 h, a Steady-Glo luciferase reagent (Promega) was added and the resultant luminescence read in a luminometer after 10 min of incubation at room temperature.
RESULTS
Composition of conjugates.
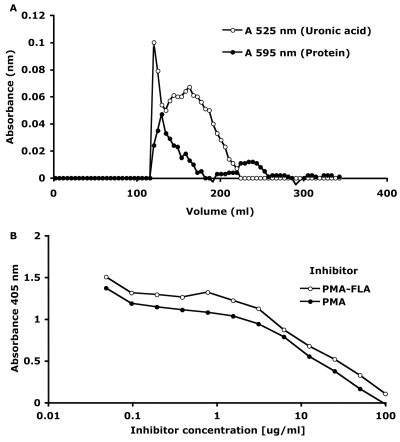
To synthesize the PMA-FLA conjugate vaccine, we used nonacetylated PMA from P. fluorescens, as it is a polymeric version of the epitope found to bind highly protective human MAb F429 (33), and P. aeruginosa type a flagellin, as it is the most prevalent type of flagellin expressed by P. aeruginosa isolates (4, 50). Initial attempts to conjugate native, high-molecular-weight (>250,000) PMA to type a flagellin were unsuccessful; we therefore reduced the molecular size of the polysaccharide in order to facilitate the conjugation process. Repeated sonication of PMA for short periods of time significantly reduced its molecular weight (∼60,000). Gel filtration of the PMA-FLA conjugate on a Superose 6 prep-grade column yielded a peak at void volume containing both protein and polysaccharide, a second minor protein peak at higher elution volumes, and a broad major peak containing material reacting positively for the presence of uronic acid (Fig. 1A). Since sonicated PMA elutes from this resin over a broad range of molecular sizes, only fractions eluting at the void volume were presumed to be free of nonconjugated polysaccharide and used as the PMA-FLA conjugate. The conjugate failed to enter a 4%-to-12% polyacrylamide gel. In an ELISA for inhibition, it was demonstrated that the conjugation process did not modify critical epitopes of the polysaccharide, as both purified PMA and the PMA-FLA conjugate equally inhibited the binding of antibodies to PMA to the PMA antigen (Fig. 1B). The PMA-FLA conjugate contained 75% (wt/wt) polysaccharide and 25% (wt/wt) protein.
Fig. 1.
Superose 6 prep-grade gel filtration profile of sonicated PMA conjugated to type a flagellin. (A) Fractions were assayed for protein by the Bradford assay (595 nm) and for uronic acid by the carbazole assay (525 nm). (B) ELISA for inhibition based on the competitive inhibition by PMA or PMA-FLA of the binding of the anti-PMA antibodies to PMA.
ELISA determination of immune responses.
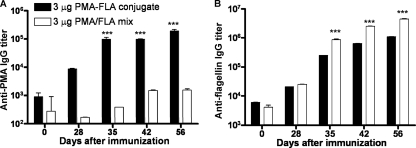
We evaluated the ability of the PMA-FLA conjugate to elicit a humoral response in mice. Mice were immunized s.c. three times at weekly intervals with 3 μg of PMA conjugated to type a flagellin (PMA-FLA) or a mixture of purified PMA and flagellin. Mice were bled weekly starting 1 week after the last immunization, and sera were evaluated by ELISA for IgG titers to PMA and type a flagellin antigens. As expected, vaccination with the PMA flagellin mixture failed to induce a significant rise in IgG titers to the PMA antigen, whereas immunization with the PMA-FLA conjugate elicited high levels of PMA-specific IgG (Fig. 2A), suggesting that the enhanced IgG response to PMA is a result of the covalent conjugation between flagellin and PMA. Immunization of mice with the PMA-FLA conjugate also induced high antibody titers to alginate purified from P. aeruginosa strains 2192 and FRD1 (data not shown).
Fig. 2.
Titers of IgG antibodies to PMA and flagellin in the sera of mice immunized s.c. with 3 μg of PMA-FLA vaccine three times at weekly intervals. (A) Titer to the homologous immunizing antigen PMA. (B) Titer to the homologous immunizing type a flagellin protein. Values represent means of triplicate determinations in sera pooled from 4 animals, and error bars represent standard errors of the mean (SEM). Statistical analysis was performed by two-way analysis of variance (ANOVA) using the Bonferroni correction for multiple comparisons. ***, P < 0.001.
We noted that the immune response to type a flagellin elicited by both the PMA-FLA conjugate and the PMA-flagellin mixture was high, with titers of >250,000 by 35 days post- initiation of the immunization (Fig. 2B). The PMA-flagellin mix did, however, elicit small but significantly higher levels of IgG titers to type a flagellin. Similar results, but lower total levels of antibodies, were observed in preliminary studies in mice that received 0.3 or 1 μg of PMA conjugated to or mixed with type a flagellin (data not shown). When examining the proportions of mouse IgG antibody isotypes elicited, we found only low levels of IgG2a, while IgG1 antibodies accounted for >80% of the binding activity (data not shown).
After immunizing three different rabbits with either type a flagellin, PMA, or the PMA-FLA conjugate in adjuvants, antibody titers to those antigens were high (Table 1), indicating that the adjuvants used with the rabbits promoted the immunogenicity of the purified PMA in contrast to the immune response of mice that received a milder adjuvant. Antibody to PMA-FLA showed reactivity to PMA similar to that of the antiserum raised to unconjugated PMA. The antiserum to PMA-FLA also reacted with type a flagellin, but to a lower level than the antiserum raised to type a flagellin alone.
Table 1.
Titers of antibodies to type a flagellin or to PMA in rabbit sera raised to type a flagellin, PMA or PMA-FLA
| Serum raised to: | Titer to indicated target antigen |
|
|---|---|---|
| Type a flagellin | PMA | |
| Type a flagellin | 920,000a | N/A |
| PMA | N/Ab | 112,635 |
| PMA-FLA | 204,320 | 130,916 |
Titer calculated as the serum dilution giving a final optical density value of 0 using least-squares linear regression.
N/A, not available.
Motility inhibition assays.
To determine the functional activity of the antisera raised either to P. aeruginosa type a flagellin or to the PMA-FLA conjugate, we evaluated the ability of the antisera to inhibit the motility of P. aeruginosa type a flagellin strains PAK, N6, and N13 and type b flagellin strain PAO1. Before this assay was performed, the flagellin type of the clinical isolates from CF patients was identified by PCR as described previously (8). In the motility inhibition assays, NRS was used as a negative control. At a serum dilution of 1:10, the rabbit antiserum to flagellin was a better inhibitor of the motility of strain PAK than was the rabbit antiserum raised to the PMA-FLA conjugate. The antisera either to flagellin or to the PMA-FLA conjugate were comparable in their abilities to inhibit the motility of P. aeruginosa type a flagellin strains N6 and N13. Both antisera also showed some cross-inhibition of the motility of flagellin type b strain PAO1 (data not shown).
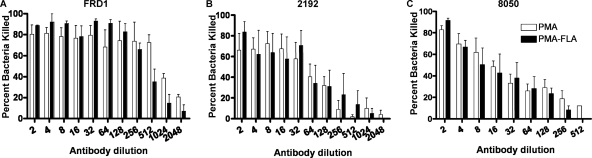
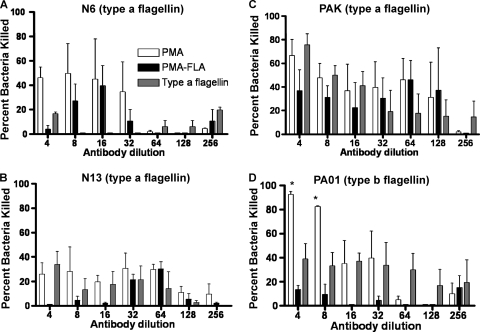
Opsonic killing activity of antisera raised to either PMA, PMA-FLA, or type a flagellin against P. aeruginosa.
In an opsonophagocytic killing assay using antibodies raised to PMA, PMA-FLA conjugate, or type a flagellin, we determined the overall activity and estimated the serum dilution mediating killing of 50% of the bacterial cells (EC50) (Table 2; Fig. 3 and 4). When testing the opsonic killing activity of the antisera against P. aeruginosa mucoid strains (Fig. 3A to C), antisera either to PMA or to PMA-FLA were active in opsonic killing against strains FRD1 (EC50 = 545 and 382 respectively) and 2192 (EC50 = 34 and 36 respectively), while they had a modest opsonic killing activity against mucoid strain 8050, with EC50 values of 15 and 13, respectively. Antiserum to type a flagellin was not tested against these nonmotile mucoid strains. The specificity of the antisera for alginate was shown by testing the opsonic killing activity against strain FRD1 ΔalgD, which cannot produce alginate. No opsonic killing was promoted by antisera to PMA or to PMA-FLA against this strain (Table 2). When testing the antibody to PMA, PMA-FLA, or type a flagellin against nonmucoid P. aeruginosa strains, the three antisera showed similar patterns of opsonic killing, promoting little to no killing of type a flagellin strains N6, N13, and PAK or of type b flagellin strain PAO1 (Table 2; Fig. 4A to D). Mouse sera raised to PMA-FLA conjugate also mediated the opsonic killing of P. aeruginosa mucoid strains 2192 and FRD1 but not the nonmucoid strain PAK (data not shown). These patterns of opsonic killing are the same as those observed with other P. aeruginosa alginate-based vaccines wherein activity is generated to mucoid but not nonmucoid strains (15, 33, 35, 47).
Table 2.
Estimated dilution of serum needed to mediate opsonophagocytic killing of 50% (EC50) of the target bacterial strain
| Target strain | Titer (95% confidence interval) mediating EC50 in antiserum raised to: |
||
|---|---|---|---|
| Flagellin | PMA | PMA-FLA | |
| PAK (type a) | 10 (6-17) | 14 (6-32) | 5 (2-13) |
| N6 (type a) | <4a | 7 (3-16) | <4 |
| N13 (type a) | <4 | <4 | <4 |
| PA01 (type b) | 6 (3-12) | 16 (9-28) | <4 |
| FRD1 | N/A | 545 (273-1,088) | 382 (275-529) |
| 2192 | N/A | 34 (21-56) | 36 (18-70) |
| 8050 | N/A | 15 (11-22) | 13 (8-22) |
| FRD1 ΔalgD | N/A | <4 | <4 |
Opsonic killing of <30% in the 1:4 serum dilution was considered to be within the range of the controls lacking an essential component needed for opsonization and/or killing, and thus titers are reported as less than 4.
Fig. 3.
Phagocyte-dependent killing activity of rabbit antibody to PMA or to PMA-FLA conjugate vaccine against P. aeruginosa mucoid strains. (A) P. aeruginosa strain FRD1. (B) P. aeruginosa strain 2192. (C) P. aeruginosa strain 8050. Bars represent means of quadruplicate-hextuplet determinations, and error bars represent standard errors of the mean (SEM). Statistical analysis was performed by two-way analysis of variance (ANOVA) using the Bonferroni correction for multiple comparisons, and results showed to be not significantly different (P > 0.05).
Fig. 4.
Phagocyte-dependent killing activity of rabbit antibody to PMA, PMA-FLA, and type a flagellin against P. aeruginosa nonmucoid strains. (A) P. aeruginosa type a flagellin strain N6. (B) P. aeruginosa type a flagellin strain N13. (C) P. aeruginosa type a flagellin strain PAK. (D) P. aeruginosa type b flagellin strain PAO1. Bars represent means of duplicate-quadruplicate determinations, and error bars represent standard errors of the mean (SEM). Statistical analysis was performed by two-way analysis of variance (ANOVA) using the Bonferroni correction for multiple comparisons. *, P < 0.05.
Promotion of pulmonary clearance of mucoid P. aeruginosa strains.
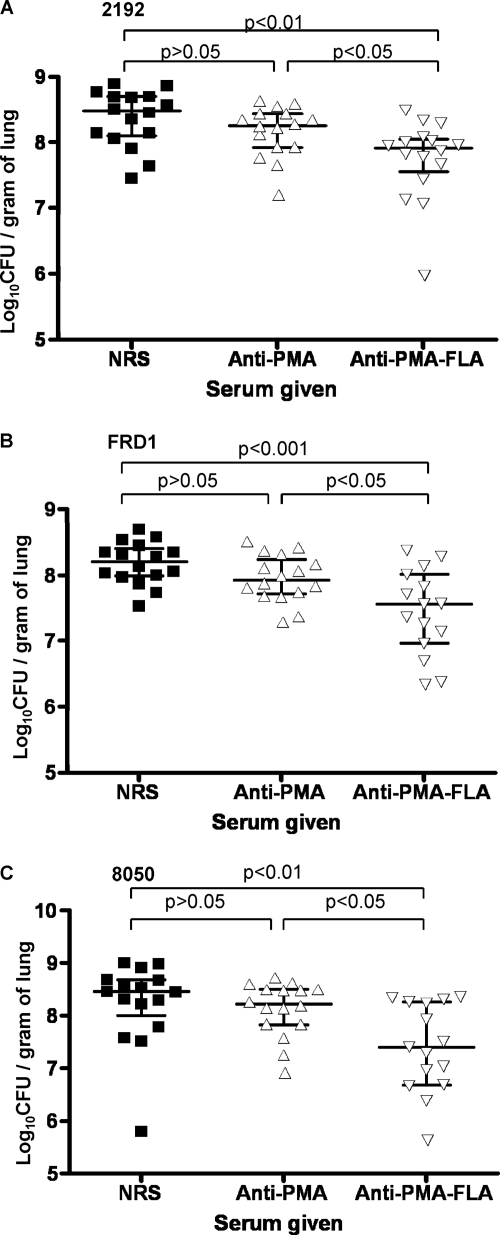
We used a clearance model to evaluate the efficacy of the antisera to PMA or PMA-FLA for effects on mucoid P. aeruginosa strains (Fig. 5A to C), since most mucoid isolates do not cause a lethal lung infection except at very high inocula, due to the fact they are LPS rough strains with a poor ability to disseminate systemically. Also, other models of mucoid P. aeruginosa infection such as embedding the organisms in agar or alginate beads are limited due to the poor ability of most mucoid strains to establish infections in mice by using these techniques (33). Intranasal delivery of antisera raised to the PMA-FLA conjugate 48 and 24 h before i.n. infection significantly enhanced the clearance of P. aeruginosa mucoid strains 2192, FRD1, and 8050 after 4 h of infection compared with mice given antisera to PMA or control NRS. The 4-h time period postinfection was chosen, as clearance mediated by a monoclonal antibody to alginate showed similar results at promoting clearance of mucoid strains at 4 and 18 h, but better than at 2 h after infection in mice (33). Antisera to the PMA-FLA conjugate yielded a 71.5% reduction in the CFU of strain 2192 (P = 0.0008 versus NRS), an 80% reduction in the CFU of strain FRD1 (P = 0.0021 versus NRS), and an 85.5% reduction in the CFU of strain 8050 (P = 0.0032 versus NRS) compared with control NRS. When comparing the CFU recovered from the lungs of mice given antisera to PMA with those recovered from control mice given NRS, the antiserum to PMA reduced bacterial burdens by 39%, 39.5%, and 23.5% after challenge with strains 2192, FRD1, and 8050, respectively (P values of 0.045, 0.0751, and 0.1045 versus NRS, respectively).
Fig. 5.
Pulmonary clearance of P. aeruginosa mucoid strains from lungs of infected mice by antisera to PMA and PMA-FLA. (A) Clearance of strain 2192 (≈6.65 × 107 CFU/mouse) 4 h after infection (anti-PMA-FLA versus anti-PMA, P < 0.05; anti-PMA-FLA versus NRS, P < 0.01; anti-PMA versus NRS, P > 0.05). (B) Clearance of strain FRD1 (≈3.175 × 107 CFU/mouse) 4 h after infection (anti-PMA-FLA versus anti-PMA, P < 0.05; anti-PMA-FLA versus NRS, P < 0.001; anti-PMA versus NRS, P > 0.05). (C) Clearance of strain 8050 (≈3.175 × 107 CFU/mouse) 4 h after infection (anti-PMA-FLA versus anti-PMA, P < 0.05; anti-PMA-FLA versus NRS, P < 0.01; anti-PMA versus NRS, P > 0.05). Values for P were determined by one-way analysis of variance (ANOVA) with Bartlett's test for equal variances and Tukey's multiple comparison test.
Protection against acute pneumonia by nonmucoid clinical isolates.
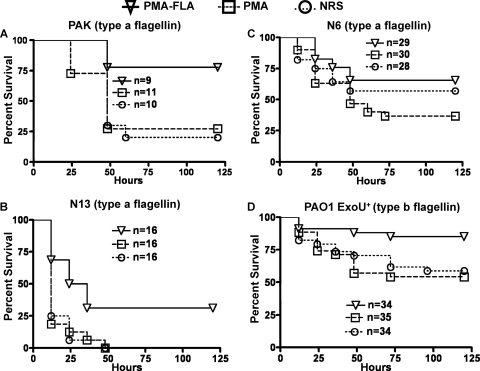
As nonmucoid, flagellum-positive, LPS smooth isolates of P. aeruginosa are relatively virulent in an acute lung infection model (2), we evaluated whether the antisera raised to the PMA-FLA conjugate or PMA (Fig. 6A to D) or type a flagellin (Fig. 7A to D) were protective against these strains in vivo. Passive immunization with antibody raised to the PMA-FLA conjugate vaccine resulted in protection against lethal pneumonia due to P. aeruginosa strains PAK (77.7% survival) (Fig. 6A), N13 (31.3% survival) (Fig. 6B), and PAO1 ExoU+ (85.3% survival) (Fig. 6D), and these levels of survival were all significantly greater than that in mice given either antibody raised to PMA alone or NRS. The antiserum to the PMA-FLA conjugate resulted in 65% survival of mice challenged with P. aeruginosa strain N6 (Fig. 6C), which was significantly greater (P = 0.03) than survival in mice given antibody to PMA but not mice given NRS, indicating a low efficacy of these antibodies against this strain at the challenge dose used. Antisera raised to purified PMA did not protect against any of the nonmucoid strains (Fig. 6A to D) when compared to mice receiving NRS.
Fig. 6.
Survival of mice passively immunized with antibody to PMA or PMA-FLA. (A) Mice challenged with P. aeruginosa type a strain PAK (≈5 × 107 CFU/mouse; log-rank test: PMA-FLA versus PMA, P = 0.021; PMA-FLA versus NRS, P = 0.0141; PMA versus NRS, P = 0.6714; median survival: PMA-FLA, undefined; PMA or NRS, 48 h). (B) Mice challenged with P. aeruginosa type a strain N13 (≈1.27 × 107 CFU/mouse; log-rank test: PMA-FLA versus PMA, P = 0.0034; PMA-FLA versus NRS, P = 0.0024; PMA versus NRS, P = 0.9349; median survival: PMA-FLA, 30 h; PMA or NRS, 12 h). (C) Mice challenged with P. aeruginosa type a strain N6 (≈2.66 × 107 CFU/mouse; log-rank test: PMA-FLA versus PMA, P = 0.0293; PMA-FLA versus NRS, P = 0.4009; PMA versus NRS, P = 0.2330; median survival: PMA-FLA and NRS, undefined; PMA, 48 h). (D) Mice challenged with P. aeruginosa type b strain PAO1 ExoU+ (≈5 × 107 CFU/mouse; log-rank test: PMA-FLA versus PMA, P = 0.0062; PMA-FLA versus NRS, P = 0.0168; PMA versus NRS, P = 0.6980; median survival: undefined for either group).
Fig. 7.
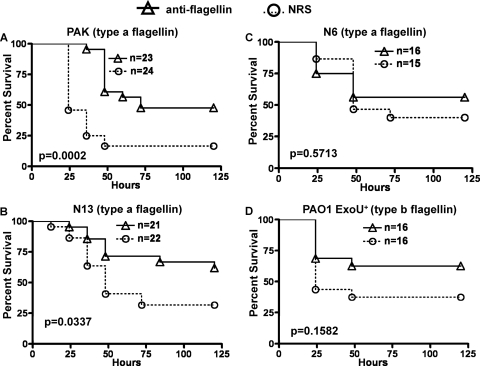
Survival of mice passively immunized with antibody to type a flagellin. (A) Mice challenged with P. aeruginosa type a strain PAK (≈4.125 × 107 CFU/mouse; log-rank test: anti-flagellin versus NRS, P = 0.0002; median survival: anti-flagellin, 72 h; NRS, 24 h). (B) Mice challenged with P. aeruginosa type a strain N13 (≈5.27 × 106 CFU/mouse; log-rank test: anti-flagellin versus NRS, P = 0.0337; median survival: anti-flagellin, undefined; NRS, 48 h). (C) Mice challenged with P. aeruginosa type a strain N6 (≈3 × 107 CFU/mouse; log-rank test: anti-flagellin versus NRS, P = 0.5713; median survival: anti-flagellin, undefined; NRS, 48 h). (D) Mice challenged with P. aeruginosa type b strain PAO1 ExoU+ (≈8.9 × 106 CFU/mouse; log-rank test: anti-flagellin versus NRS, P = 0.1582; median survival: anti-flagellin, undefined; NRS, 24 h).
Antibody to type a flagellin provided for 47.8% survival of mice infected with strain PAK (P = 0.0002 versus the NRS group) (Fig. 7A) and 61.9% survival against infection with strain N13 (P = 0.0337 versus the NRS group) (Fig. 7B). There was no protection, however, against type a flagellin P. aeruginosa strain N6 (Fig. 7C) or against flagellin type b strain PAO1 ExoU+ (Fig. 7D).
Immune serum inhibition of P. aeruginosa-flagellin mediated TLR5 activation.
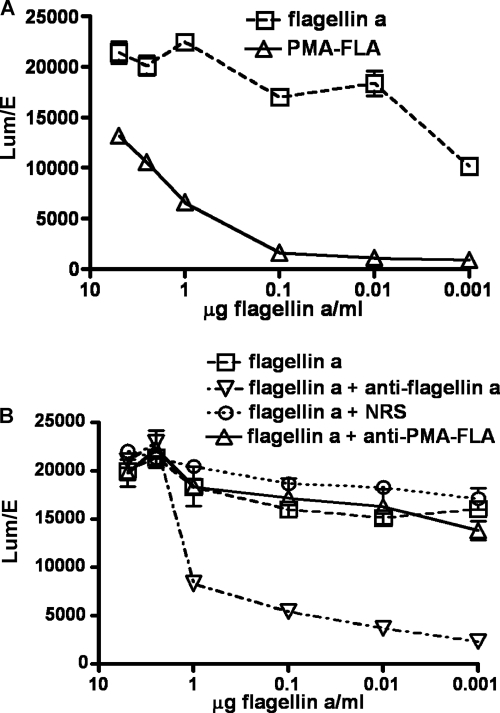
We next compared the ability of the flagellin component of the PMA-FLA conjugate to activate TLR5 with that of purified flagellin, using equal amounts of flagellin in the assays, and also evaluated whether antibody elicited by the PMA-FLA conjugate vaccine or type a flagellin alone inhibited TLR5 activation (Fig. 8). Unconjugated flagellin activated the cells to a higher level than did the flagellin component of the PMA-FLA conjugate at every concentration tested (Fig. 8A). This suggests that some of the epitopes in the flagellin protein responsible for TLR5 activation could have been modified or hidden by the conjugation. When antibodies to flagellin or PMA-FLA were used to inhibit TLR5 activation by purified P. aeruginosa flagellin, antibody to purified flagellin neutralized TLR5 activation, as has been previously described (8), whereas antisera to PMA-FLA were not capable of inhibiting the activation of TLR5 (Fig. 8B). Thus, conjugation of flagellin to PMA did not result in antibody that could interfere with the innate immune response to flagellin and make hosts potentially more susceptible to infection.
Fig. 8.
Inhibition of P. aeruginosa flagellin-mediated activation of TLR5 by antisera to flagellin or PMA-flagellin conjugate vaccine. (A) Activation of TLR5 by P. aeruginosa type a flagellin or PMA-FLA conjugate vaccine. (B) Effect of antibody to type a flagellin or PMA-FLA conjugate on TLR5 activation. The points are the results of triplicate determinations, and error bars represent standard errors of the mean (SEM). Normal rabbit serum (NRS) was also included for comparison.
DISCUSSION
Many studies have indicated the importance of the prevention of acquisition of nonmucoid P. aeruginosa and its subsequent conversion to the mucoid phenotype in the CF lung (12, 16, 21, 42, 49). In this regard, we found that a conjugate vaccine containing PMA, targeting a conserved antigenic epitope on the alginate antigen, along with type a flagellin as a vaccine carrier protein, showed protective efficacy against both mucoid and nonmucoid P. aeruginosa lung infection. Conjugation of PMA to flagellin enhanced its immunogenicity without eliciting antibodies that inhibit TLR5, indicating that desirable protective antibodies were elicited but not at the price of possibly inhibiting an important and conserved mechanism of innate immune resistance to pathogens (6).
In mice, the PMA-FLA conjugate vaccine was more effective at eliciting antibody to PMA than was the PMA-flagellin mixture, and although the IgG titers to PMA obtained from sera of rabbits immunized with either the PMA-FLA conjugate or PMA alone were similar, the amount of PMA required to obtained this same response was more than 10 times higher than the amount of PMA contained in the conjugate. Also, the results showing that unconjugated PMA was highly immunogenic in rabbits were not surprising, as such high-molecular-weight polysaccharides injected along with strong adjuvants are immunogenic on their own, as documented previously for other P. aeruginosa alginates (15, 35).
The immunization of mice and rabbits with the PMA-FLA conjugate induced a significant humoral immune response to flagellin, but in this case it was clearly lower than the one induced by the PMA-flagellin mix in mice or with flagellin when given alone to rabbits. Thus, the conjugation process might have modified some epitopes in the flagellin protein or reduced its immunogenicity overall. However, conjugated flagellin appears to retain sufficient immunogenicity to induce functional antibodies, as demonstrated in the motility inhibition assays, where antibody to the PMA-FLA conjugate had comparable activity at inhibiting motility of three type a and one type b flagellin P. aeruginosa strains as did that of the antibody to purified flagellin. This suggests that the flagellin component of the PMA-FLA conjugate provides sufficient immune responses to inhibit an important aspect of P. aeruginosa pathogenesis. The finding of some cross-reactivity between antibody to type a flagellin and a type b flagellin strain was not unexpected, as amino acid segments of the two flagellin types are partially similar, as previously described (8).
When we evaluated the effects of rabbit antibody to PMA-FLA and PMA on opsonic killing of mucoid P. aeruginosa in vitro, we found that antibody to PMA alone was able to promote opsonic killing of three mucoid CF clinical isolates, as were antibodies raised by the PMA-FLA conjugate. Specificity of antisera to PMA and PMA-FLA for alginate was shown by its inability to promote opsonic killing of an algD mutant strain of FRD1. Previously published data demonstrated that immunization with native P. aeruginosa alginate can induce opsonic antibodies to mucoid P. aeruginosa (15, 20, 35), and other groups found that conjugation of alginate to carrier proteins significantly increased the opsonic killing activity of mucoid strains (10, 20, 47). Likely, the conjugation of PMA to flagellin enhanced its immunogenicity, as lower doses of the conjugate were needed compared with the purified carbohydrate antigen alone in order to induce opsonic killing antibody.
The finding that antibody to flagellin, PMA, or the PMA-FLA conjugate had little to no opsonic killing activity against nonmucoid strains of P. aeruginosa was not unexpected. It has been previously described that antibody to flagellin is not opsonic against nonmucoid P. aeruginosa strains (8), and results with polyclonal rabbit antibodies to conjugated alginate vaccines that promoted opsonic killing of mucoid strains also showed poor killing of nonmucoid strains (47). These findings suggest that although many nonmucoid strains have been shown to produce low levels of alginate in vitro (3, 36), the level of alginate expression may not be sufficient for it to serve as a target for opsonic killing by polyclonal serum. In contrast, a fully human monoclonal antibody to P. aeruginosa alginate has demonstrated opsonic killing of nonmucoid CF clinical isolates (33), and its specificity was shown to be toward the PMA epitopes used in the PMA-FLA conjugate vaccine. However, it is not known if the PMA-FLA conjugate generated a high population of antibodies with the specificity for the uronic acid on the C-6 carbon of mannuronic acid, as was shown for the more broadly opsonic monoclonal antibody to alginate.
The results showing that antibody to flagellin protects against some nonmucoid, homologous type a flagellin P. aeruginosa strains in the mouse pneumonia model of infection were unexpected based on prior results using flagellin as a vaccine (8), wherein this antigen was poorly protective. Yu et al. (51) have recently shown that immunization with flagellin protected mice against P. aeruginosa lung infection, but they used active immunization, which may provide better results than passive immunization, and they used native, not recombinant, flagellin. Native flagellin, in contrast with the recombinant flagellin used in our studies, retains glycan groups, which stimulate an inflammatory response (41) that could contribute to the protection they observed. Instead, our prior results with antibody to recombinant flagellin showed no protection against acute P. aeruginosa lung infection with nonmucoid strains in mice (8). A possible explanation for this discordance could be the different route used to passively immunize the challenged mice (i.n. versus i.p. used previously), as it has been previously described that in order to achieve sufficient levels of a human monoclonal antibody to alginate within the lungs of mice, the antibody had to be delivered i.n. Our results with antibody to flagellin also differ from the ones obtained after immunization with a flagellin DNA vaccine that protected against heterologous but not homologous P. aeruginosa lung infection, suggesting that the DNA vaccine may be superior at promoting cross-protective antibody compared to the flagellin protein or that active immunization with a DNA vaccine may be superior to passive antibody transfer at promoting protection against infection (45).
Given the role that flagellin has in innate immunity through activation of TLR5 (18), it was critical to evaluate the consequences of immunization with PMA-FLA on TLR5 activation. Because chronic P. aeruginosa airway infection and the accompanying inflammatory responses are clearly the major clinical problems for CF patients today (23), it has been proposed that inhibition of TLR5 may reduce the damaging inflammatory response generated by the immune response triggered following exposure to P. aeruginosa (7). In addition, it has been demonstrated that the recognition of either lipoproteins or lipopolysaccharide by TLR2 and TLR4, or flagellin by TLR5, is sufficient to activate TLR-dependent signaling and control P. aeruginosa in the murine lung (41, 43), suggesting that if TLR5 activation is blocked, P. aeruginosa could still be recognized by TLR2 and TLR4. However, it has also been reported that the TLR5 mRNA expression is increased in CF airway epithelial cells and, as a consequence, these cells almost exclusively rely upon TLR5 to sense P. aeruginosa through its flagellin protein (7). Other studies have shown that TLR5-deficient mice are more susceptible to challenge with P. aeruginosa (28, 45), showing an inoculum-dependent defect in bacterial clearance associated with dysregulated early cytokine responses and delayed accumulation of bronchoalveolar neutrophils, suggesting that TLR5 plays an important role in the early innate immune response to P. aeruginosa (28). Taken together, it seems that blocking of TLR5 activation may impede the induction of protective immunity against P. aeruginosa and may increase the risk of acquiring infections from other flagellated bacteria that activate TLR5.
When testing the activation of TLR5 by PMA-FLA, we could demonstrate that conjugation of flagellin to PMA prevented the induction of antibody that could interfere with the innate immune response to flagellin. Saha et al. (45) established that antibody responses directed against the TLR5 activation domain of the flagellin protein hinder the induction of protective immunity and that modifying that domain to prevent the stimulation of those antibodies improves the host's ability to generate a protective immune response against P. aeruginosa (45). This suggests that during the conjugation process, the TLR5 activation domain of flagellin could have been modified or obscured and thus not immunogenic.
In summary, we have synthesized and characterized a PMA-FLA conjugate vaccine that elicited high titers of specific antibodies to PMA and flagellin that were able to protect mice against mucoid and nonmucoid strains of P. aeruginosa without interfering with TLR5-mediated immunity. Immunization of CF patients early in life with PMA-FLA conjugate vaccine may prevent the initial P. aeruginosa colonization and may also lower the incidence of chronic mucoid P. aeruginosa infection. However, it may be necessary to include other P. aeruginosa flagellin types in a future vaccine preparation, since some strains of P. aeruginosa expressing flagellin antigens not included in the PMA-FLA conjugate vaccine may arise, as has been previously described (12).
ACKNOWLEDGMENTS
This work was supported by NIH grants AI 15294 and I 155531.
Footnotes
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Adamo R., Sokol S., Soong G., Gomez M. I., Prince A. 2004. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am. J. Respir. Cell Mol. Biol. 30:627–634 [DOI] [PubMed] [Google Scholar]
- 2. Allewelt M., Coleman F. T., Grout M., Priebe G. P., Pier G. B. 2000. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68:3998–4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anastassiou E. D., Mintzas A. C., Kounavis C., Dimitracopoulos G. 1987. Alginate production by clinical nonmucoid Pseudomonas aeruginosa strains. J. Clin. Microbiol. 25:656–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arora S. K., Bangera M., Lory S., Ramphal R. 2001. A genomic island in Pseudomonas aeruginosa carries the determinants of flagellin glycosylation. Proc. Natl. Acad. Sci. U. S. A. 98:9342–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bates J. T., Honko A. N., Graff A. H., Kock N. D., Mizel S. B. 2008. Mucosal adjuvant activity of flagellin in aged mice. Mech. Ageing Dev. 129:271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bates J. T., Uematsu S., Akira S., Mizel S. B. 2009. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J. Immunol. 182:7539–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blohmke C. J., et al. 2008. Innate immunity mediated by TLR5 as a novel antiinflammatory target for cystic fibrosis lung disease. J. Immunol. 180:7764–7773 [DOI] [PubMed] [Google Scholar]
- 8. Campodonico V. L., et al. 2010. Evaluation of flagella and flagellin of Pseudomonas aeruginosa as vaccines. Infect. Immun. 78:746–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chitnis C. E., Ohman D. E. 1993. Genetic analysis of the alginate biosynthetic gene cluster of Pseudomonas aeruginosa shows evidence of an operonic structure. Mol. Microbiol. 8:583–590 [DOI] [PubMed] [Google Scholar]
- 10. Cryz S. J., Jr., Furer E., Que J. U. 1991. Synthesis and characterization of a Pseudomonas aeruginosa alginate-toxin A conjugate vaccine. Infect. Immun. 59:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cuadros C., Lopez-Hernandez F. J., Dominguez A. L., McClelland M., Lustgarten J. 2004. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect. Immun. 72:2810–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doring G., Meisner C., Stern M. 2007. A double-blind randomized placebo-controlled phase III study of a Pseudomonas aeruginosa flagella vaccine in cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 104:11020–11025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doring G., Pier G. B. 2008. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine 26:1011–1024 [DOI] [PubMed] [Google Scholar]
- 14. Frederiksen B., Koch C., Hoiby N. 1997. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 23:330–335 [DOI] [PubMed] [Google Scholar]
- 15. Garner C. V., DesJardins D., Pier G. B. 1990. Immunogenic properties of Pseudomonas aeruginosa mucoid exopolysaccharide. Infect. Immun. 58:1835–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gibson R. L., Burns J. L., Ramsey B. W. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918–951 [DOI] [PubMed] [Google Scholar]
- 17. Govan J. R., Deretic V. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Honko A. N., Mizel S. B. 2004. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect. Immun. 72:6676–6679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Honko A. N., Sriranganathan N., Lees C. J., Mizel S. B. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 74:1113–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kashef N., et al. 2006. Synthesis and characterization of Pseudomonas aeruginosa alginate-tetanus toxoid conjugate. J. Med. Microbiol. 55:1441–1446 [DOI] [PubMed] [Google Scholar]
- 21. Li Z., et al. 2005. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–588 [DOI] [PubMed] [Google Scholar]
- 22. Linker A., Jones R. S. 1966. A new polysaccharide resembling alginic acid isolated from pseudomonads. J. Biol. Chem. 241:3845–3851 [PubMed] [Google Scholar]
- 23. Lyczak J. B., Cannon C. L., Pier G. B. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathee K., et al. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 145:1349–1357 [DOI] [PubMed] [Google Scholar]
- 25. McSorley S. J., Ehst B. D., Yu Y., Gewirtz A. T. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 169:3914–3919 [DOI] [PubMed] [Google Scholar]
- 26. Miao E. A., Andersen-Nissen E., Warren S. E., Aderem A. 2007. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin. Immunopathol. 29:275–288 [DOI] [PubMed] [Google Scholar]
- 27. Mizel S. B., et al. 2009. Flagellin-F1-V fusion protein is an effective plague vaccine in mice and two species of nonhuman primates. Clin. Vaccine Immunol. 16:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris A. E., Liggitt H. D., Hawn T. R., Skerrett S. J. 2009. Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am. J. Physiol. Lung Cell. Mol. Physiol. 297:L1112–L1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neville L. F., et al. 2005. Antibodies raised against N′-terminal Pseudomonas aeruginosa flagellin prevent mortality in lethal murine models of infection. Int. J. Mol. Med. 16:165–171 [PubMed] [Google Scholar]
- 30. Nivens D. E., Ohman D. E., Williams J., Franklin M. J. 2001. Role of alginate and its O acetylation in formation of Pseudomonas aeruginosa microcolonies and biofilms. J. Bacteriol. 183:1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parad R. B., Gerard C. J., Zurakowski D., Nichols D. P., Pier G. B. 1999. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infect. Immun. 67:4744–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pawlowski A., Svenson S. B. 1999. Electron beam fragmentation of bacterial polysaccharides as a method of producing oligosaccharides for the preparation of conjugate vaccines. FEMS Microbiol. Lett. 174:255–263 [DOI] [PubMed] [Google Scholar]
- 33. Pier G. B., et al. 2004. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J. Immunol. 173:5671–5678 [DOI] [PubMed] [Google Scholar]
- 34. Pier G. B., Coleman F., Grout M., Franklin M., Ohman D. E. 2001. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect. Immun. 69:1895–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pier G. B., et al. 1994. Human immune response to Pseudomonas aeruginosa mucoid exopolysaccharide (alginate) vaccine. Infect. Immun. 62:3972–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pier G. B., Desjardins D., Aguilar T., Barnard M., Speert D. P. 1986. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J. Clin. Microbiol. 24:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pier G. B., et al. 1987. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N. Engl. J. Med. 317:793–798 [DOI] [PubMed] [Google Scholar]
- 38. Pino O., Martin M., Michalek S. M. 2005. Cellular mechanisms of the adjuvant activity of the flagellin component FljB of Salmonella enterica serovar Typhimurium to potentiate mucosal and systemic responses. Infect. Immun. 73:6763–6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prince A. 2006. Flagellar activation of epithelial signaling. Am. J. Respir. Cell Mol. Biol. 34:548–551 [DOI] [PubMed] [Google Scholar]
- 40. Ramphal R., Arora S. K. 2001. Recognition of mucin components by Pseudomonas aeruginosa. Glycoconj. J. 18:709–713 [DOI] [PubMed] [Google Scholar]
- 41. Ramphal R., et al. 2008. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J. Immunol. 181:586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramsey D. M., Wozniak D. J. 2005. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 56:309–322 [DOI] [PubMed] [Google Scholar]
- 43. Raoust E., et al. 2009. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4:e7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Russell N. J., Gacesa P. 1988. Chemistry and biology of the alginate of mucoid strains of Pseudomonas aeruginosa in cystic fibrosis. Mol. Aspects Med. 10:1–91 [DOI] [PubMed] [Google Scholar]
- 45. Saha S., et al. 2007. Blocking of the TLR5 activation domain hampers protective potential of flagellin DNA vaccine. J. Immunol. 179:1147–1154 [DOI] [PubMed] [Google Scholar]
- 46. Schurks N., Wingender J., Flemming H. C., Mayer C. 2002. Monomer composition and sequence of alginates from Pseudomonas aeruginosa. Int. J. Biol. Macromol. 30:105–111 [DOI] [PubMed] [Google Scholar]
- 47. Theilacker C., et al. 2003. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect. Immun. 71:3875–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verma A., Arora S. K., Kuravi S. K., Ramphal R. 2005. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect. Immun. 73:8237–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weimer E. T., Ervin S. E., Wozniak D. J., Mizel S. B. 2009. Immunization of young African green monkeys with OprF epitope 8-OprI-type A- and B-flagellin fusion proteins promotes the production of protective antibodies against nonmucoid Pseudomonas aeruginosa. Vaccine 27:6762–6769 [DOI] [PubMed] [Google Scholar]
- 50. Winstanley C., et al. 2005. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. J. Med. Microbiol. 54:519–526 [DOI] [PubMed] [Google Scholar]
- 51. Yu F. S., et al. 2010. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J. Immunol. 185:1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]