Abstract
Bacillus cereus G9241 was isolated from a welder with a pulmonary anthrax-like illness. The organism contains two megaplasmids, pBCXO1 and pBC218. These plasmids are analogous to the Bacillus anthracis Ames plasmids pXO1 and pXO2 that encode anthrax toxins and capsule, respectively. Here we evaluated the virulence of B. cereus G9241 as well as the contributions of pBCXO1 and pBC218 to virulence. B. cereus G9241 was avirulent in New Zealand rabbits after subcutaneous inoculation and attenuated 100-fold compared to the published 50% lethal dose (LD50) values for B. anthracis Ames after aerosol inoculation. A/J and C57BL/6J mice were comparably susceptible to B. cereus G9241 by both subcutaneous and intranasal routes of infection. However, the LD50s for B. cereus G9241 in both mouse strains were markedly higher than those reported for B. anthracis Ames and more like those of the toxigenic but nonencapsulated B. anthracis Sterne. Furthermore, B. cereus G9241 spores could germinate and disseminate after intranasal inoculation into A/J mice, as indicated by the presence of vegetative cells in the spleen and blood of animals 48 h after infection. Lastly, B. cereus G9241 derivatives cured of one or both megaplasmids were highly attenuated in A/J mice. We conclude that the presence of the toxin- and capsule-encoding plasmids pBCXO1 and pBC218 in B. cereus G9241 alone is insufficient to render the strain as virulent as B. anthracis Ames. However, like B. anthracis, full virulence of B. cereus G9241 for mice requires the presence of both plasmids.
INTRODUCTION
The Bacillus cereus group, of which Bacillus anthracis, Bacillus thuringiensis, and B. cereus are members, are Gram-positive, spore-forming, rod-shaped bacteria that reside in the soil. The most pathogenic of the species toward humans is B. anthracis, the etiologic agent of inhalational, cutaneous, and gastrointestinal anthrax. B. thuringiensis is an insect pathogen that is used worldwide as a pesticide. However, in rare cases, B. thuringiensis has been identified as the etiological agent in cases of food poisoning, wound infection, and catheter-associated bacteremia (11, 20, 24). B. cereus is primarily associated with food-borne illness, and these cases usually arise from improper food preparation and storage. In addition, B. cereus is an opportunistic human pathogen that has been implicated in wound infections, endocarditis, osteomyelitis, endophthalmitis, and urinary tract infections in humans (2). Strains of B. cereus have also been associated with more severe “inhalational anthrax-like” infections in humans (12, 14, 15, 25, 37, 38) and chimpanzees (18). The abundance and distribution of B. cereus isolates that cause severe inhalational disease in humans are not clear, in part due to the genetic similarity among members of the B. cereus group. One of these B. anthracis-like B. cereus strains, G9241, was isolated from a welder in Louisiana who survived a life-threatening, inhalational anthrax-like disease (14).
Members of the B. cereus group are closely related phylogenetically and can retain megaplasmids that encode virulence genes (30). The fully virulent B. anthracis Ames strain contains the 189-kb pXO1 and the 96-kb pXO2, and both are required for full virulence (4, 41). The megaplasmid pXO1 contains the genes that encode the toxin components protective antigen (PA), edema factor (EF), and lethal factor (LF). The operon required for synthesis of the unique poly-γ-d-glutamic acid capsule is encoded on pXO2. The widely used laboratory strain B. anthracis Sterne contains pXO1 but not pXO2. B. anthracis Sterne is avirulent in rabbits (34), unlike B. anthracis Ames (34), but is of moderate but lower virulence than a pXO1- and pXO2-containing B. anthracis strain in most mouse models of anthrax disease (39, 41). B. anthracis Sterne is used as a live spore vaccine for cattle, and a similar attenuated derivative of wild-type B. anthracis was used as a vaccine in humans in the former Soviet Union (19, 34).
Megaplasmids with high similarity to pXO1 and, in some cases, pXO2, have been identified in B. cereus isolates from pulmonary anthrax-like disease (12, 14, 18). B. cereus G9241 contains three endogenous plasmids: the megaplasmids pBCXO1 and pBC218 and the smaller pBClin29. The plasmid pBCXO1 is >99% similar to pXO1 (14). The genes that encode PA (pag), EF (cya), and LF (lef) are all present on pBCXO1; the gene products are 99.7% (PA), 96% (EF), and 99% (LF) identical to the B. anthracis proteins. In addition, homologs of pag and lef are present on pBC218; however, the pBC218-encoded PA and LF are only 60% and 36% identical to their B. anthracis homologs (14). In addition, the pBC218-encoded LF does not contain the zinc metalloprotease domain. Fieldhouse et al. recently used computational models to propose that the pBC218-encoded LF, which they named Certhrax, contains a domain that is predicted to have ADP-ribosyltransferase activity; however, no experimental evidence of activity was presented (8). (Note that the plasmid pBC218 name is derived from its originally reported size of ∼218 kb [14]; however, subsequent studies have shown that it is 210 kb, and it is thus designated pBC210 [31]. In this report we will refer to this plasmid by its original designation, pBC218.) While pBC218 is not similar in sequence to the capsule-encoding pXO2, pBC218 encodes a putative polysaccharide capsule operon, and B. cereus G9241 has been shown to produce a capsule (14, 36). The operon contains all of the genes that are predicted to encode the glycosyltransferases, translocase, polymerase, and regulatory elements required for polysaccharide capsule biosynthesis in Gram-positive bacteria (14). The third plasmid, pBClin29, contains genes that encode putative phage proteins (14). In B. anthracis, the toxin-encoding genes and the capsule operon are positively regulated by the pXO1-encoded AtxA; expression of atxA is increased in the presence of CO2 (29). Homologs of atxA are found on both pBCXO1 and pBC218. Furthermore, Passalacqua et al. found that expression of the pBCXO1 atxA gene was upregulated in CO2, as was the putative pBC218 polysaccharide capsule operon (28).
B. anthracis has been used as a bioweapon in the United States and elsewhere (9, 16, 17). Because B. cereus G9241 and B. anthracis share phenotypic traits and can cause similar diseases in humans, we assessed the virulence of B. cereus G9241 in well-characterized animal models of B. anthracis infection (New Zealand White rabbits and A/J and C57BL/6J mice). Moreover, since both the toxin- and capsule-encoding plasmids (i.e., pXO1 and pXO2) are required for full virulence of B. anthracis, we generated plasmid-cured strains of B. cereus G9241 and assessed the contribution of each plasmid toward virulence, toxin production, and polysaccharide capsule production. Taken together, the data presented here demonstrate that, as with B. anthracis, both toxin expression and capsule production are required for full virulence of B. cereus G9241. However, we conclude that the presence of these B. anthracis-like plasmids is not sufficient to produce a strain of B. cereus that is as virulent as wild-type B. anthracis in rabbits or mice.
MATERIALS AND METHODS
Bacteria strains and spore preparation.
All strains used in this work are listed in Table 1. Bacteria were routinely cultured in brain heart infusion (BHI) broth (BD Biosciences, San Jose, CA) at 35 to 37°C with aeration. Spores were prepared for rabbit challenge experiments by the shaker flask method (3), and spores for the mouse challenge experiments were produced on solid sporulation medium (26) as previously described (6, 33).
Table 1.
Bacterial strains used in this study
Rabbit challenge models.
Because the virulence of B. cereus G9241 had not yet been established when these studies were initiated, all rabbit experiments were performed with animal biosafety level 3 (ABSL-3) containment in accordance with the Battelle Biomedical Research Center Institutional Animal Care and Use Committee regulations. Rabbit virulence was determined in specific-pathogen-free New Zealand White rabbits (Covance, Inc., Princeton, NJ) that were individually housed and ear tagged for identification. Rabbits (50% male, 50% female) weighed 2.3 to 3.5 kg at the beginning of the study. Six animals each were inoculated subcutaneously with ∼104 or ∼105 B. cereus G9241 spores (10 or 100 times the reported B. anthracis Ames 50% lethal dose [LD50] of 1.6 × 103 spores for rabbits inoculated subcutaneously [43]). Two rabbits were challenged with ∼104, three rabbits with ∼106, and two rabbits with ∼107 B. cereus G9241 spores by aerosol exposure (0.1 to 100 times the published B anthracis Ames LD50 of 1.1 × 105 spores for rabbits challenged by an inhalational route [43]). A modified Microbiological Research Establishment type three jet collision nebulizer (BGI, Waltham, MA) with a precious-well fluid jar was used to generate controlled delivery of an aerosol of spores in a water suspension. Rabbits were placed into a muzzle-only inhalation exposure chamber and subjected to B. cereus G9241 spores for 10 to 20 min. Rabbits were monitored twice daily for 14 days postexposure for morbidity, respiratory distress, and changes in appetite, activity, and temperature. In addition, blood was collected daily to assess bacteremia.
Mouse challenge models.
All mouse experiments were conducted after rabbit data demonstrated that BSL-3 containment was unnecessary; as such, mouse studies were done under ABSL-2 conditions in accordance with the Uniformed Services University Institutional Animal Care and Use Committee regulations. Six-week-old female A/J and C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were housed in filter-top cages and provided food and water ad libitum. Spores were diluted to the desired dose in sterile water and inoculated into mice either intranasally or subcutaneously. For intranasal inoculation, mice were lightly anesthetized with isoflurane delivered through the XGI-8 gas anesthesia system (Caliper Life Sciences, Hopkinton, MA). Animals were then held upright, and 20 to 25 μl of a spore suspension or water was introduced directly onto both nares. Mice were held upright until the inoculum was inhaled. For subcutaneous inoculation, mice were injected behind the right foreleg with 0.1 ml spores diluted in sterile water or water alone. Mice were monitored daily for morbidity and mortality for 14 days postinoculation. The LD50 and 95% confidence limits were calculated by probit analysis with SAS version 9.2 when permitted by the data; otherwise, the LD50 was calculated by using Reed-Muench analysis (32).
For B. cereus dissemination studies, A/J mice were inoculated intranasally with 3 × 106 B. cereus G9241 spores (10× the LD50) in 25 to 50 μl sterile water. To assess dissemination, mice (n = 5 per time point) were sacrificed 6, 24, and 48 h postinoculation, and organs (lungs, spleen, and heart) were collected. Blood was collected from the tail vein of mice prior to sacrifice. Organs were also collected from a group of five mice that succumbed naturally to infection. Organs were homogenized in 1 ml phosphate-buffered saline (PBS) with a tissue homogenizer (Omni International, Kennesaw, GA). Half of each blood or tissue sample was heat treated at 65°C to kill vegetative cells. All samples were serially diluted and plated for bacterial enumeration. Vegetative CFU/ml was calculated by subtraction of the spore CFU/ml (heat treated) from the total CFU/ml (not heat treated). The dissemination experiment was performed twice.
Generation of plasmid-cured B. cereus G9241 strains.
Strains were cured of pBCXO1 and/or pBC218 by growth either in the presence of novobiocin or at 41°C by slight modifications of a previously published protocol (10). For generation of the pBCXO1+/pBC218− strain, a liquid culture of B. cereus G9241 was grown overnight in Luria-Bertani (LB) broth at 41°C with shaking at 225 rpm. On each of three consecutive days, the culture was diluted 1/1,000 into fresh LB broth and grown as above. Dilutions of the final culture were plated onto NBY-bicarbonate agar (nutrient broth, 8 g/liter; yeast extract, 3 g/liter; agar, 15 g/liter; 0.8% NaHCO3) and grown at 37°C in 5% CO2 to promote capsule production. Individual colonies were passaged several times onto fresh NBY-bicarbonate plates and incubated as described above. Colonies with a rough appearance were isolated and tested for loss of pBC218 and presence of pBCXO1 by PCR as described below. For generation of the pBCXO1−/pBC218+ strain, B. cereus G9241 was grown in LB broth supplemented with 2 μg/ml novobiocin at 37°C with shaking at 225 rpm for 3 days. Dilutions were plated as described above. Smooth colonies were streaked for isolation and screened by PCR for loss of pBCXO1 and maintenance of pBC218. The pBCXO1−/pBC218− strain was directly derived from the pBCXO1−/pBC218+ strain by the same novobiocin method except that rough colonies were screened by PCR.
For PCR screening of candidate strains, individual colonies were lysed in sterile, nuclease-free water at 98°C for 10 min. The lysate was used as the template for PCRs that included GoTaq Green master mix (Promega, Madison, WI) and a primer set unique to different regions on each of the B. cereus G9241 plasmids pBCXO1 and pBC218 (Table 2). Control reactions designed to amplify a region of the B. cereus G9241 chromosome and pBClin29 plasmid were included. PCR assays were done in either a Tetrad 2 (Bio-Rad) or a PTC-200 (MJ Research, Waltham, MA) thermocycler under the following conditions: 95°C for 5 min; 40 cycles of 95°C for 1 min, 61°C for 1 min, and 72°C for 1.5 min; 72°C final extension for 7 min. For primers pBC218-14 and pBC218-18, the annealing temperature was increased to 65°C to prevent nonspecific product formation. The initial PCR screen consisted of only one primer set for each plasmid to minimize reagent consumption and maximize screening throughput. Once candidate clones were obtained, they were further screened with the full panel of primers (Table 2). PCR products were separated by 1% agarose gel electrophoresis, and intercalated ethidium bromide was visualized with UV light.
Table 2.
Sequences of plasmid test primers
| Target | Gel lane | Amplified region (bp) | Primer pair sequences (5′→3′) |
|---|---|---|---|
| Chromosome | 1 | fabG gene, GI 47564343 | ATGAGATTGGCAAACGACGGTGCATTAG |
| CTATAAACAAAACCCTCCAGAAACATCTATAATCTG | |||
| pBClin29 | 2 | 19026–20219 | GTGCATGAAATGATTTTAGGTACGGAAAAACAGC |
| TCAATTATCACCCAAGTACAATCTAGGTAGATTGGC | |||
| pBCXO1 | 3 | 16930–18119 | GGAGGTCCATAGAATAATATAGAACGGGATGC |
| CAGCACTATCAACACTGGAGCGATTC | |||
| 4 | 32346–46593 | TATCAAACAGATGTCAACCGTATCG | |
| CAGCATCTTTAACCCTAGACCTAAC | |||
| 5 | 46593–47364 | CTACATTGCTGACATTCAAAGGTAG | |
| GTACGGTGATACAACACAAATTGAC | |||
| 6 | 61760–62583 | GTTTGCCAGCTCCATTCCCAATAAG | |
| GGCTGAAAGCGTTAAGGCATATTCC | |||
| 7 | 77380–78264 | CCTTCTGCGGTGTACTTGTTAATGG | |
| AATGCCTGACGGCACAACAATGTTC | |||
| 8 | 93388–94239 | TACGTGATTCAGCAGCACATAGTAG | |
| TACAGCGATACCAGATACTCCTGTC | |||
| 9 | 123095–124127 | AAGGTTCATCTTCAGGCACAGATTC | |
| CAGCTCTTGATCCACTATAGGATTC | |||
| 10 | 139725–140879 | CTTCTACAGTCACTTGGTCTCTAGTGAGTGGAC | |
| CATCCATTGAATTAGGTTCATCTTGGCATATGGATC | |||
| 11 | 155955–156946 | GTACAAGAGGAAGAGCAAGTAAATC | |
| CTCATTCCCATTCATTCTCCTTATC | |||
| 12 | 170672–171559 | GTGAAGATTAGAAAAGCGATTATCCCAGCAG | |
| TTATTTATTGATAAGTTTATCTCGAGTGATATTTCTCAAATAACTC | |||
| pBC218 | 13 | 14756–15936 | GTTACGCAGCATCTCGAATATTAAC |
| AAATCTTGCCCATGTCCAACTAAGG | |||
| 14 | 29999–30609 | CACTCATATCTGCTATATAAGCCCCATGTA | |
| GTTGGAACTGGATCAGATGAGATTTAAGG | |||
| 15 | 44761–45728 | CGACTAAATTGCTGAAGGCAGTAAC | |
| TTAGGAGGAAGGTAAATGAGGAAAG | |||
| 16 | 60017–60948 | CGCTTATATGCCAATATCCCTAATG | |
| AAATCTCTTTCGGTAGGGAAATCTG | |||
| 17 | 83364–84403 | ACCCACTATATTCGGACTAAATACC | |
| ATGACCTTTGGGCACAGTATGTAAC | |||
| 18 | 107041–107811 | ATTCTCCTCGTCTCCCTCCTGATTC | |
| GCACGGGACTTTCCGATAGACATGC | |||
| 19 | 123026–123817 | ATACATACCGTGACTTCGTGTTCTC | |
| TAGGTGTTTATGGGACTACGGTTAC | |||
| 20 | 139043–139856 | ACCGATCAACTCCTCAAGAAACTGATCC | |
| TCCGGCTTCAACCTATTCCATCTTCAGTC | |||
| 21 | 155428–156313 | CAGTATTCGTGCTAAAGGTTATAGG | |
| ACCGGTAATATCCAATCATTAGGTG | |||
| 22 | 171874–172635 | AGCGTGCTGTAAAGGTGTTAGAATC | |
| CATTTGTCTTCATCAGGTCAATAGG | |||
| 23 | 187803–189013 | ATTATCAACCACGAGTGGTACGTAG | |
| AGGATTTCGGTGTTTAATAGCTGAC | |||
| 24 | 208076–208938 | GTATTAGCGAAATGAGACGGAGAAG | |
| CTTTGTAATTTCTCCACCGACAGAC |
Phenotypic characterization of B. cereus G9241 plasmid-cured strains.
The growth rates of each derivative of B. cereus G9241 and B. anthracis Sterne in BHI broth were determined in triplicate according to standard protocols. To assess germination rates of the B. cereus G9241 derivatives and B. anthracis Sterne, the decrease in optical density at 562 nm (OD562) of triplicate cultures was followed over time in an EL800 absorbance microplate reader (BioTek, Winooski, VT). Spores were diluted to either the same OD562 (≈1.0) or to the same final CFU/ml (1 × 107 CFU/ml), and germination was initiated with 100 μM inosine and 50 μM l-alanine. The ratio (ODintial − ODtime)/(ODfinal − ODinitial) was plotted versus time, and the germination rate was calculated from the slope of the linear portion of the curve (1, 23).
For detection of the toxin subunits PA, EF, and LF, overnight HI cultures of each strain were diluted 1/10,000 into HI broth that contained 0.8% sodium bicarbonate and grown at 37°C in 5% CO2 for 24 h. Each bacterial culture was pelleted by centrifugation, and culture supernatants were further clarified by vacuum filtration with 0.22-μm cellulose acetate filters (Corning, Acton, MA). Volumes of cleared supernatant were normalized prior to concentration based on the final OD600 of the culture. Filtrates were concentrated with Amicon Ultra-4 10,000 MWCO regenerated cellulose centrifugal filter units (Millipore, Billerica, MA). Toxin subunits in culture supernatant filtrates were detected by Western blotting. Recombinant PA, LF, or EF (List Biologicals, Campbell, CA) and B. anthracis Sterne culture filtrate served as positive controls for Western blot assays. Culture filtrate of B. cereus 569 UM20 (10), a nontoxigenic and nonencapsulated B. cereus strain, served as a negative control for Western blot assays. The PA and LF blots were blocked with 5% skim milk (SM) and 0.1% Tween 20 (Fisher Scientific, Pittsburgh, PA) in PBS (PBST), and the EF blot was blocked with 5% normal rabbit serum in protein-free T20 blocking buffer (Thermo Fisher Scientific, Rockford, IL). Rabbit polyclonal anti-PA antibodies (Abcam, Cambridge, MA) were diluted 1/1,000 in SM-PBST, mouse monoclonal anti-PA and mouse monoclonal anti-LF (BEI Resources, Manassas, VA) were diluted 1/10,000 in SM-PBST, and goat polyclonal anti-EF (Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1/1,000 in protein-free T20 buffer. The secondary antibodies goat anti-rabbit Ig-horseradish peroxidase (HRP; 1/10,000), goat anti-mouse Ig-HRP (1/10,000), and rabbit anti-goat Ig-HRP (1/20,000; Bio-Rad Laboratories, Hercules, CA) were diluted in PBST. The ECL Plus Western blotting detection system (GE Healthcare, Fairfield, CT) and X-Omat film (Kodak, Rochester, NY) were used to visualize the toxin subunits.
RESULTS
B. cereus G9241 spores are nearly avirulent in rabbits but evoke lethal anthrax-like disease in mice.
In preliminary virulence studies, Hoffmaster et al. demonstrated that intraperitoneal inoculation of A/J mice with 104 or 106 B. cereus G9241 spores or B. anthracis Sterne spores caused 100% lethality (14). To more thoroughly assess the virulence of B. cereus G9241, we inoculated groups of New Zealand White rabbits with B. cereus G9241 at doses that ranged from 0.1 to 100 times the reported LD50 for B. anthracis Ames delivered via subcutaneous (1.6 × 103 CFU) or inhalational (1.1 × 105 CFU) routes (43). Of the rabbits inoculated with B. cereus G9241 spores, all animals survived subcutaneous challenge (12/12) and 6/7 survived aerosol exposure; the rabbit that succumbed to infection was inoculated with ∼107 spores.
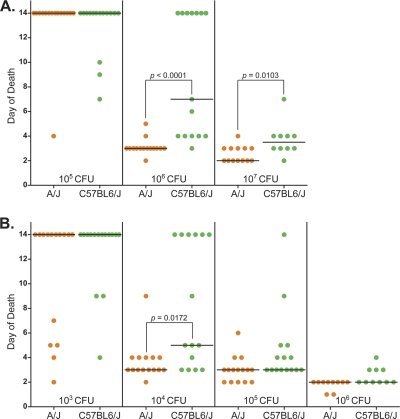
To further investigate the virulence of B. cereus G9241, we inoculated A/J and C57BL/6J mice with various doses of B. cereus G9241 via intranasal and subcutaneous routes. The LD50 for B. cereus G9241 delivered intranasally was calculated to be 3.2 × 105 spores for A/J mice and 6.3 × 105 spores for C57BL/6J mice (Table 3). Each of these LD50s is about 50-fold higher than the reported LD50 for B. anthracis Ames in these strains of mice (21). While the LD50s for B. cereus G9241 were similar in both strains of mice, the median times to death for C57BL/6J mice inoculated intranasally with 106 or 107 spores of B. cereus G9241 were significantly longer than for A/J mice (Fig. 1 A). The LD50s for B. cereus G9241 administered subcutaneously were calculated to be 1.3 × 103 spores for A/J mice and 5.0 × 103 spores for C57BL/6J mice (Table 3). These doses are approximately 400-fold (A/J) and 300-fold (C57BL/6) higher than the reported LD50s for B. anthracis Ames (4, 21, 41). We observed that, as above for mice inoculated intranasally, C57BL/6J mice inoculated subcutaneously with B. cereus G9241 had a significantly longer median time to death than did A/J mice at a dose of 104 spores (Fig. 1B). Taken together, our data show that B. cereus G9241 is significantly attenuated compared to reported B. anthracis Ames LD50 values in identical rabbit and mouse models of infection (4, 21, 41).
Table 3.
Virulence of B. anthracis Ames, B. anthracis Sterne, and B. cereus G9241 wild-type and plasmid-cured derivatives in mice
| Mouse strain | Bacterial strain | Subcutaneous LD50a | Source(s) | Intranasal LD50a | Source(s) |
|---|---|---|---|---|---|
| A/J | B. anthracis | ||||
| Ames (pXO1+/pXO2+) | 0.7 | 21 | 3.8 | 21 | |
| Sterne (pXO1+/pXO2−) | 3.2 | 5, 33, 41 | 4.8 | 5, 33 | |
| B. cereus G9241 | |||||
| pBCXO1+/pBC218+ | 3.1 (2.8–3.4)b | This study | 5.5c | This study | |
| pBCXO1+/pBC218− | 6.8 (6.5–7.2)b | This study | >7 | This study | |
| pBCXO1−/pBC218+ | >7 | This study | >7 | This study | |
| pBCXO1−/pBC218− | >7 | This study | >7 | This study | |
| C57BL/6J | B. anthracis | ||||
| Ames (pXO1+/pXO2+) | 1.4 | 4, 21 | 4.1 | 21 | |
| Sterne (pXO1+/pXO2−) | 5.9 | 41 | >7 | This study | |
| B. cereus G9241 | |||||
| pBCXO1+/pBC218+ | 3.7 (3.3–4.1)b | This study | 5.8 (5.4–6.2)b | This study |
LD50 values are the log10 CFU, with 95% confidence intervals indicated in parentheses.
LD50 values and confidence intervals were determined with the probit method.
The LD50 value was determined with the Reed-Muench method (32), as these data did not permit analysis by probit.
Fig. 1.
Dose response of A/J and C57BL/6J mice to B. cereus G9241. A/J and C57BL/6J mice were inoculated by the intranasal (A) or subcutaneous (B) route with B. cereus G9241 spores to determine the LD50 for each route. Each symbol (•) represents one A/J (orange) or one C57BL/6J (green) mouse. The median time to death at each dose is represented by the black horizontal line; a time to death of 14 days was assigned to all mice that survived the experiment. Significant differences in the median time to death were calculated with the Mann-Whitney rank sum test, and the P values are shown on the graph where applicable.
B. cereus G9241 disseminates from the lungs to the spleen.
Previous studies of mice showed that fully virulent B. anthracis disseminates from the lungs to distal organs during the course of acute infection (7, 21). To determine if B. cereus G9241 exhibits similar dissemination patterns, we challenged A/J mice intranasally with 3 × 106 B. cereus G9241 spores (10× the LD50) and enumerated both heat-sensitive (vegetative) and heat-resistant (spores) CFU from the lungs, spleen, and blood. We observed both spores and vegetative cells in the lungs within the first 6 h, a finding which suggests that germination occurred in the lungs (Fig. 2 A). Both forms of B. cereus G9241 continued to be present in the lungs 24 and 48 h postinoculation in the majority of mice. No spores were detected in the blood or spleen at any of the times that we tested, but increasing numbers of vegetative bacilli were found in both compartments beginning 48 h postinoculation (Fig. 2B and C). In mice that succumbed to infection (open symbols in Fig. 2), we detected both spores and vegetative cells in the lungs, as well as high numbers of vegetative cells in the blood and spleen. In aggregate, these data demonstrate that spores germinate in the lungs early in infection and the resultant vegetative cells disseminate systemically within 48 h postchallenge.
Fig. 2.
B. cereus G9241 dissemination following intranasal inoculation. A/J mice were intranasally inoculated with 3 × 106 B. cereus G9241 spores. Mice were sacrificed at 6 h (orange), 24 h (green), or 48 h (blue), or they succumbed to infection (purple). At each time point, lungs (A), blood (B), and spleen (C) were harvested, homogenized, and plated for bacterial enumeration with and without heat treatment. Spore CFU (■) were enumerated directly from the heat-treated samples, and vegetative CFU (•) were enumerated by subtraction of CFU in heat-treated samples from total CFU in non-heat-treated samples. Data shown are from two independent experiments (n = 5 mice per group), with a total of 10 mice per time point, and each mouse is represented by an individual symbol. A solid black line connects the spore and vegetative CFU for each mouse in panel A. The horizontal lines in panels B and C represent the geometric means for each group, and the dashed lines in all panels are the limits of detection.
Plasmid-cured B. cereus G9241 strains are attenuated for virulence in mice.
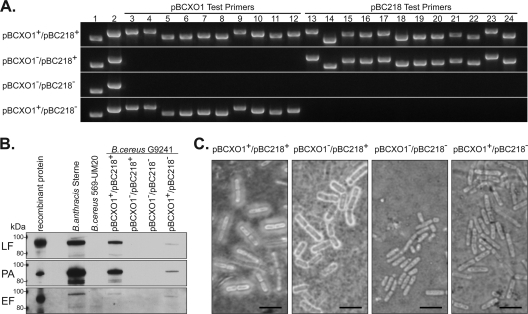
To assess the involvement of the toxins and capsule in the virulence of B. cereus G9241, we cured B. cereus G9241 of either the toxin-encoding plasmid pBCXO1, the putative polysaccharide capsule operon-encoding plasmid pBC218, or both plasmids. We screened and confirmed megaplasmid loss in each of the cured strains by PCR (Fig. 3 A). Thorough screening was required, because temperature and/or novobiocin treatment often resulted in only partial curing of the plasmids in which fragments/regions of the parent plasmid were detected by PCR (data not shown). Lastly, we determined that B. cereus G9241 and its plasmid-cured derivatives all displayed similar growth rates and germination rates in vitro (data not shown).
Fig. 3.
Characterization of B. cereus G9241 cured strains. (A) PCR screening of the B. cereus G9241 strains was done with primers specific for regions on pBCXO1 (lanes 3 to 12) and pBC218 (lanes 13 to 24) to verify the presence or absence of these plasmids; lysed bacterial cells were used as the PCR template. Primers for chromosomal DNA (lane 1) and pBClin29 (lane 2) were included as controls. (B) Western blot analysis of supernatants from cultures grown under toxin-inducing conditions. Recombinant B. anthracis toxin subunit proteins and B. anthracis Sterne culture supernatants were included as positive controls, and B. cereus 569 UM20 culture supernatant was a negative control. Cultures were normalized to the same OD600 value prior to concentration of the culture supernatant. (C) India ink stain of the B. cereus G9241 strains. Bar, 5 μm. A negatively stained halo around the bacteria is indicative of capsule expression.
To determine whether the secreted toxin components PA, EF, and LF were produced by B. cereus G9241 and the plasmid-cured derivatives, we analyzed supernatants of cultures grown under toxin-inducing conditions for toxin antigens by immunoblotting. As shown in Fig. 3B, we detected PA, EF, and LF in culture supernatants from B. anthracis Sterne and from pBCXO1+ B. cereus G9241 strains. However, the relative abundance of the three toxin subunits was lower in the pBCXO1+/pBC218− derivative than in the wild-type strain. In addition, we did not detect PA or LF in the pBCXO1−/pBC218+ derivative despite the presence of pag and lef homologs on pBC218. None of the commercially available antibodies against PA from B. anthracis that were tested in this study were cross-reactive with recombinant pBC218-encoded PA (data not shown). The epitope recognized by the monoclonal anti-LF antibody is not predicted to be part of the truncated LF carried on pBC218 (8), so lack of reactivity with this antibody does not necessarily indicate lack of expression of the toxin subunit.
We stained vegetative bacilli grown under conditions that promote capsule production with India ink to qualitatively determine whether capsule was produced by each of the B. cereus G9241 strains (Fig. 3C). We observed a negatively stained halo indicative of capsule expression around both pBC218+ strains and no halo around the pBC218− strains. These data provide experimental evidence that pBC218 is required for capsule production by B. cereus G9241.
To assess the virulence of the B. cereus G9241 plasmid-cured derivatives, we inoculated A/J mice via intranasal and subcutaneous routes with various doses of spores from the plasmid-cured strains. Of all the plasmid-cured strains, only the B. cereus G9241 pBCXO1+/pBC218− derivative caused mortality after subcutaneous inoculation at a dose >3 logs higher than the B. cereus G9241 LD50 (Table 3). These results indicate that both the polysaccharide capsule and the toxins are necessary for full virulence of B. cereus G9241 in these murine models of anthrax-like disease.
DISCUSSION
In this study, we showed that B. cereus G9241 is attenuated for virulence in rabbits and mice compared to published data for B. anthracis Ames, despite the facts that B. cereus G9241 was isolated from a patient who survived an inhalational anthrax-like disease and that the strain possesses virulence plasmids analogous to those of B. anthracis Ames. In our hands, B. cereus G9241 was avirulent when delivered to New Zealand White rabbits via subcutaneous injection. When delivered via aerosol droplets, the primary mechanism by which B. anthracis spores are introduced into the human airway, B. cereus G9241 was nearly avirulent and required a dose approximately 100× the LD50 for B. anthracis Ames before the sole death was observed. In both A/J and C57BL/6J mice, B. cereus G9241 caused lethal anthrax-like disease after subcutaneous and intranasal inoculation. However, the number of B. cereus G9241 spores that caused 50% mortality was 50 times and 300 to 400 times greater than the LD50s for B. anthracis Ames delivered intranasally and subcutaneously, respectively (4, 21, 41). Therefore, we conclude that B. cereus G9241 is significantly less virulent than wild-type B. anthracis in both strains of mice. This conclusion is further supported by comparison of the virulence of B. cereus G9241 and B. anthracis Sterne, an attenuated strain of B. anthracis that lacks pXO2 and is nonpathogenic toward humans. In A/J mice, the LD50 for B. cereus G9241 is similar for mice inoculated subcutaneously and only approximately 5 times greater for those inoculated intranasally than the reported LD50 values for B. anthracis Sterne (5, 33, 41). Since the pathological features of anthrax disease in humans are well mimicked in rabbit infection models and A/J and C57BL/6J mouse models indicate decreased susceptibility relative to virulent B. anthracis Ames, we conclude that B. cereus G9241 is less virulent than B. anthracis Ames.
One similarity between B. cereus G9241 and B. anthracis Ames is the presence of a capsule surrounding the bacterium that is known in the case of Ames to protect the organism from opsonophagocytosis (22). C57BL/6J mice are more resistant than A/J mice to nonencapsulated B. anthracis strains, such as B. anthracis Sterne (41). The increased resistance has been attributed in part to the fact that C57BL/6J mice have an intact complement system, while A/J mice are C5 deficient (41). The C5 deficiency in A/J mice causes reduced recruitment of neutrophils and macrophages to the site of infection, which allows the spores to germinate and the bacilli to replicate and disseminate more efficiently. The protective effect of the capsule for B. cereus G9241 is suggested by the slight increase in LD50 values of approximately 2× for intranasal and 4× for subcutaneous routes of inoculation in C57BL/6J mice compared to A/J mice (Table 3) and a statistically significant difference in the median time to death between the two mouse strains (Fig. 1); a similar modest increase in the LD50 is also observed with B. anthracis Ames (4, 21, 41). In contrast, the LD50 values for the nonencapsulated B. anthracis Sterne are ∼150× and ∼500× higher in C57BL/6J mice than in A/J mice inoculated via intranasal and subcutaneous routes, respectively (Table 3) (5, 33, 41). These observations suggest that the polysaccharide capsule of B. cereus G9241 may protect the organism from the host immune response, as does the polyglutamic acid capsule of B. anthracis Ames.
We also demonstrated that the megaplasmids pBCXO1 and pBC218 of B. cereus G9241 are required for full virulence of the organism in mice. In fact, curing of either plasmid rendered the organism nearly avirulent in A/J mice and curing of both plasmids resulted in complete avirulence. We showed that pBCXO1 is required for production of the anthrax toxin components PA, EF, and LF. However, we could not confirm with commercially available antibodies against B. anthracis PA and LF that the PA and LF genes carried on pBC218 were expressed under toxin-inducing conditions. Thus, the roles of the pBC218 PA and LF genes in the virulence of B. cereus G9241 remain unclear. In addition, we confirmed experimentally that pBC218 is required for capsule production; previous studies provided in silico but not in vivo evidence for a capsule biosynthetic operon on pBC218 (14, 36). Studies of the relative contributions of pXO1 and pXO2 to the pathogenesis of B. anthracis showed that strains that expressed pXO1 only caused lethality in immunocompromised mice and, to a lesser degree, in immunocompetent mice (41). In contrast, most strains that contained pXO2 only were as virulent as those that contained pXO1 and pXO2; the notable exception was B. anthracis Pasteur 6602 (pXO1−/pXO2+), which was avirulent in A/J mice (41, 42). Further, the chromosomal background in which pXO2 was expressed contributed significantly to the virulence of a B. anthracis strain (42). We attribute the reduced virulence of the plasmid-cured strains to the loss of toxins and/or capsule, which is analogous to the reduced virulence of B. anthracis Sterne (pXO1+/pXO2−) and B. anthracis Pasteur (pXO1−/pXO2+) compared to B. anthracis Ames (21); however, we acknowledge that additional factors carried on pBCXO1, pBC218, and/or the chromosome may contribute to the virulence of B. cereus G9241.
Neither of the single plasmid-cured strains produced wild-type levels of the toxin components or capsule, as determined by Western blotting (Fig. 3B) and India ink staining (Fig. 3C), respectively, despite the fact that growth rates of all of the B. cereus G9241 strains were similar. These data suggest that one or more positive regulators of virulence factor expression are encoded on pBCXO1 and pBC218. Hoffmaster et al. identified homologs of atxA and pagR on pBCXO1 and a homolog of atxA on pBC218 (14); both are encoded in B. anthracis on pXO1, and the expression of both genes is upregulated in the presence of CO2. The B. anthracis AtxA is a positive regulator of the toxin subunit genes pagA, lef, and cya (39) as well as the capsule operon (40), while PagR is a negative regulator of pagA and atxA (13). Passalacqua et al. demonstrated that the expression of the B. cereus G9241 pBCXO1 atxA is upregulated in the presence of CO2 but the expression of the pBC218 atxA is unaffected by growth in O2 or CO2 (28). These results, taken together with our findings in Fig. 3B, suggest that the absence of atxA carried on pBC218 is not the cause of reduced toxin production in the B. cereus G9241 pBCXO1+/pBC218− derivative and that additional pBC218-carried factors may be involved. Similarly, the pBCXO1−/pBC218+ derivative exhibited reduced capsule production, as qualitatively observed by India ink staining (Fig. 3C); this observation suggests that the regulator(s) of capsule production, including atxA, may be carried on pBCXO1. Uchida et al. reported a similar reduction in capsule production by B. anthracis cured of pXO1 (40). Regulation of toxin and capsule expression in B. cereus G9241 is clearly multifactorial and likely involves other effectors that have yet to be described.
In conclusion, while B. cereus G9241 possesses two virulence plasmids required for toxin and capsule production that are analogous to those in the highly virulent B. anthracis Ames, our results strongly suggest that the virulence of B. cereus G9241 is similar to that of the attenuated vaccine strain B. anthracis Sterne. Furthermore, our data demonstrate that toxin production and encapsulation alone are not predictive of virulence in Bacillus organisms and that the individual species and genetic background are important determinants of the pathogenicity of any given Bacillus species. Lastly, our animal data and the human data from individuals with the potential for impaired lung function due to occupation (12, 14) imply that B. cereus G9241, like most B. cereus isolates, is an opportunistic rather than a frank pathogen.
ACKNOWLEDGMENTS
We thank Theresa Koehler (University of Texas Health Sciences Center) for B. cereus 569 UM20 and Angela Melton-Celsa for critical review of the manuscript.
This study was funded by the Biological Defense Research Directorate, Naval Medical Research Center, United States Navy. Rabbit virulence studies were performed at Battelle Biomedical Research Center as part of a contract with the Biological Defense Research Directorate.
The opinions and assertions in this paper are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Navy or the Department of Defense.
Footnotes
Published ahead of print on 16 May 2011.
ADDENDUM
While the manuscript was under review, a study of the role of capsular polysaccharides in B. cereus G9241 pathogenesis was published that suggested a role for a second polysaccharide capsule operon encoded on pBCXO1 in the virulence of B. cereus G9241 (27).
REFERENCES
- 1. Akoachere M., et al. 2007. Identification of an in vivo inhibitor of Bacillus anthracis spore germination. J. Biol. Chem. 282:12112–12118 [DOI] [PubMed] [Google Scholar]
- 2. Bottone E. J. 2010. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 23:382–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmbhatt T. N., et al. 2007. Recombinant exosporium protein BclA of Bacillus anthracis is effective as a booster for mice primed with suboptimal amounts of protective antigen. Infect. Immun. 75:5240–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chand H. S., et al. 2009. Discriminating virulence mechanisms among Bacillus anthracis strains by using a murine subcutaneous infection model. Infect. Immun. 77:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cybulski R. J., Jr., et al. 2009. Four superoxide dismutases contribute to Bacillus anthracis virulence and provide spores with redundant protection from oxidative stress. Infect. Immun. 77:274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cybulski R. J., Jr., et al. 2008. Recombinant Bacillus anthracis spore proteins enhance protection of mice primed with suboptimal amounts of protective antigen. Vaccine 26:4927–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drysdale M., et al. 2005. Capsule synthesis by Bacillus anthracis is required for dissemination in murine inhalation anthrax. EMBO J. 24:221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fieldhouse R. J., Turgeon Z., White D., Merrill A. R. 2010. Cholera- and anthrax-like toxins are among several new ADP-ribosyltransferases. PLoS Comput. Biol. 6:e1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frischknecht F. 2003. The history of biological warfare. Human experimentation, modern nightmares and lone madmen in the twentieth century. EMBO Rep. 4:S47–S52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green B. D., Battisti L., Koehler T. M., Thorne C. B., Ivins B. E. 1985. Demonstration of a capsule plasmid in Bacillus anthracis. Infect. Immun. 49:291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez E., Ramisse F., Ducoureau J. P., Cruel T., Cavallo J. D. 1998. Bacillus thuringiensis subsp. konkukian (serotype H34) superinfection: case report and experimental evidence of pathogenicity in immunosuppressed mice. J. Clin. Microbiol. 36:2138–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmaster A. R., et al. 2006. Characterization of Bacillus cereus isolates associated with fatal pneumonias: strains are closely related to Bacillus anthracis and harbor B. anthracis virulence genes. J. Clin. Microbiol. 44:3352–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffmaster A. R., Koehler T. M. 1999. Autogenous regulation of the Bacillus anthracis pag operon. J. Bacteriol. 181:4485–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmaster A. R., et al. 2004. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc. Natl. Acad. Sci. U. S. A. 101:8449–8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ihde D. C., Armstrong D. 1973. Clinical spectrum of infection due to Bacillus species. Am. J. Med. 55:839–845 [DOI] [PubMed] [Google Scholar]
- 16. Inglesby T. V., et al. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252 [DOI] [PubMed] [Google Scholar]
- 17. Jernigan J. A., et al. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klee S. R., et al. 2010. The genome of a Bacillus isolate causing anthrax in chimpanzees combines chromosomal properties of B. cereus with B. anthracis virulence plasmids. PLoS One 5:e10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knop A. G., Abalakin V. A. 1986. Anthrax (Siberian plague), p. 100–109 Epidemic process as a socio-ecological system. Handbook of scientific works. Central Scientific Research Institute of Epidemiology, Moscow, Soviet Union [Google Scholar]
- 20. Kuroki R., et al. 2009. Nosocomial bacteremia caused by biofilm-forming Bacillus cereus and Bacillus thuringiensis. Intern. Med. 48:791–796 [DOI] [PubMed] [Google Scholar]
- 21. Lyons C. R., et al. 2004. Murine model of pulmonary anthrax: kinetics of dissemination, histopathology, and mouse strain susceptibility. Infect. Immun. 72:4801–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makino S., Uchida I., Terakado N., Sasakawa C., Yoshikawa M. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCormick N. G. 1965. Kinetics of spore germination. J. Bacteriol. 89:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McIntyre L., Bernard K., Beniac D., Isaac-Renton J. L., Naseby D. C. 2008. Identification of Bacillus cereus group species associated with food poisoning outbreaks in British Columbia, Canada. Appl. Environ. Microbiol. 74:7451–7453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller J., et al. 1997. Fulminating bacteremia and pneumonia due to Bacillus cereus. J. Clin. Microbiol. 35:504–507 (Erratum, 35:1294.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicholson W. L., Setlow P. 1990. Sporulation, germination, and outgrowth, p. 391–450In Harwood C. R., Cutting S. M. (ed.), Molecular biological methods for Bacillus. Wiley, Chichester, United Kingdom [Google Scholar]
- 27. Oh S. Y., Budzik J. M., Garufi G., Schneewind O. 2011. Two capsular polysaccharides enable Bacillus cereus G9241 to cause anthrax-like disease. Mol. Microbiol. 80:455–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Passalacqua K. D., Varadarajan A., Byrd B., Bergman N. H. 2009. Comparative transcriptional profiling of Bacillus cereus sensu lato strains during growth in CO2-bicarbonate and aerobic atmospheres. PLoS One 4:e4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perego M., Hoch J. A. 2008. Commingling regulatory systems following acquisition of virulence plasmids by Bacillus anthracis. Trends Microbiol. 16:215–221 [DOI] [PubMed] [Google Scholar]
- 30. Rasko D. A., Altherr M. R., Han C. S., Ravel J. 2005. Genomics of the Bacillus cereus group of organisms. FEMS Microbiol. Rev. 29:303–329 [DOI] [PubMed] [Google Scholar]
- 31. Rasko D. A., et al. 2007. Complete sequence analysis of novel plasmids from emetic and periodontal Bacillus cereus isolates reveals a common evolutionary history among the B. cereus-group plasmids, including Bacillus anthracis pXO1. J. Bacteriol. 189:52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reed L. J., Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- 33. Sanz P., et al. 2008. Detection of Bacillus anthracis spore germination in vivo by bioluminescence imaging. Infect. Immun. 76:1036–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shlyakhov E. N., Rubinstein E. 1994. Human live anthrax vaccine in the former USSR. Vaccine 12:727–730 [DOI] [PubMed] [Google Scholar]
- 35. Sterne M., Nicol J., Lambrechts M. C. 1942. The effect of large scale active immunisation against anthrax. J. S. Afr. Med. Vet. Assoc. 13:53 [Google Scholar]
- 36. Sue D., Hoffmaster A. R., Popovic T., Wilkins P. P. 2006. Capsule production in Bacillus cereus strains associated with severe pneumonia. J. Clin. Microbiol. 44:3426–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Turnbull P. C., Jorgensen K., Kramer J. M., Gilbert R. J., Parry J. M. 1979. Severe clinical conditions associated with Bacillus cereus and the apparent involvement of exotoxins. J. Clin. Pathol. 32:289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turnbull P. C., Kramer J. M. 1983. Non-gastrointestinal Bacillus cereus infections: an analysis of exotoxin production by strains isolated over a two-year period. J. Clin. Pathol. 36:1091–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uchida I., Hornung J. M., Thorne C. B., Klimpel K. R., Leppla S. H. 1993. Cloning and characterization of a gene whose product is a trans-activator of anthrax toxin synthesis. J. Bacteriol. 175:5329–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uchida I., Makino S., Sekizaki T., Terakado N. 1997. Cross-talk to the genes for Bacillus anthracis capsule synthesis by atxA, the gene encoding the trans-activator of anthrax toxin synthesis. Mol. Microbiol. 23:1229–1240 [DOI] [PubMed] [Google Scholar]
- 41. Welkos S. L., Keener T. J., Gibbs P. H. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 51:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Welkos S. L., Vietri N. J., Gibbs P. H. 1993. Non-toxigenic derivatives of the Ames strain of Bacillus anthracis are fully virulent for mice: role of plasmid pX02 and chromosome in strain-dependent virulence. Microb. Pathog. 14:381–388 [DOI] [PubMed] [Google Scholar]
- 43. Zaucha G. M., Pitt L. M., Estep J., Ivins B. E., Friedlander A. M. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982–992 [PubMed] [Google Scholar]