Abstract
The spindle checkpoint ensures genome fidelity by temporarily halting chromosome segregation and the ensuing mitotic exit until the last kinetochore is productively attached to the mitotic spindle. At the interface between proper chromosome attachment and the metaphase-to-anaphase transition are the mammalian spindle checkpoint kinases. Compelling evidence indicates that the checkpoint kinases Bub1 and BubR1 have the added task of regulating kinetochore-microtubule attachments. However, the debate on the requirement of kinase activity is in full swing. This minireview summarizes recent advances in our understanding of the core spindle checkpoint kinases Bub1 and BubR1 and considers evidence that supports and opposes the role of kinase activity in regulating their functions during mitosis.
INTRODUCTION
Maintenance of genome stability is necessary to ensure the continued survival of progeny throughout multiple rounds of division. In mitosis, the shortest but most visually striking phase of the cell cycle, accurate distribution of chromosomes to the nascent progeny requires proper attachment of the duplicated chromosome (sister chromatid pair) to microtubules emanating from opposite poles of the mitotic spindle and their subsequent alignment to the spindle equator. The site of microtubule attachment is the kinetochore, a conserved, proteinaceous network that assembles onto chromosomes upon mitotic entry (45, 73, 82). In addition to its structural role, the enrichment of kinases, phosphatases, and other modifying enzymes to its various substructures support its function as a signaling hub during mitosis. Microtubule capture by kinetochores is a highly dynamic and stochastic process involving numerous protein complexes and a multitude of weak microtubule binding sites (11, 58, 90). Not surprisingly, errors in attachment do occur in early mitosis; these include syntelic attachments, which involve microtubules from a single pole binding both sister chromatids, and merotelic attachments, which occur when a kinetochore is attached to microtubules emanating from both poles. Most misattachments, however, are sensed and corrected, given sufficient time. The spindle checkpoint (also known as the spindle assembly checkpoint and the mitotic checkpoint) is a conserved surveillance mechanism that provides this extra time when necessary. Importantly, this checkpoint does not permanently arrest cells in mitosis. Rather, it delays mitotic progression until all kinetochores are attached (62, 63). Whether microtubule attachment itself or the tension generated at kinetochores as a result of this attachment satisfies the spindle checkpoint is vigorously debated and is the subject of a number of excellent recent reviews (57, 65, 72). The duration of a spindle checkpoint-mediated arrest is highly variable and appears to be cell type and organism dependent (24, 77). Moreover, the activity of certain checkpoint kinases (see below) may modulate the length of a checkpoint-mediated arrest. Cells that do not satisfy the checkpoint often die or exit mitosis into the next G1 as single tetraploid cells via poorly understood “slippage” or “adaptation” pathways (77). The importance of accurate and stable microtubule attachments to the regulation of checkpoint signaling is underscored by increasing evidence that points to an active role for the spindle checkpoint kinases during the establishment of attachments.
SPINDLE CHECKPOINT SIGNALING
The core components of the spindle checkpoint were originally identified in the budding yeast Saccharomyces cerevisiae and include the budding uninhibited by benzimidazole (Bub) proteins Bub1 and Bub3 (29, 49, 80) and the mitotic-arrest deficient (Mad) proteins Mad1, Mad2, and Mad3 (BubR1 in higher eukaryotes). Subsequently, the dual-specificity kinase monopolar spindle 1 (Mps1), which is required for spindle pole body (SPB) duplication in yeast, was also shown to be essential for spindle checkpoint function (26, 100). For most of these proteins, checkpoint function is conserved from yeast to humans as well as in plants (7). Checkpoint signaling, however, may be a more elaborate process in metazoans. In budding yeast for example, full attachment is achieved by the binding of a single microtubule to each kinetochore, whereas it is estimated that 25 to 30 microtubules attach per kinetochore in mammals (44, 78).
The only known target of the spindle checkpoint is Cdc20, a substrate binding subunit of the anaphase-promoting complex/cyclosome (APC/C) (71). The APC/C is a large, multisubunit E3 ubiquitin ligase that targets two key proteins during mitosis, cyclin B and securin. Cyclin B is an obligatory activating partner of the major mitotic kinase Cdk1, and its degradation allows for rapid Cdk1 inactivation and the ensuing spindle disassembly and mitotic exit. Loss of securin releases active separase, which cleaves the cohesin rings holding sister centromeres together (45, 62, 63). How the spindle checkpoint functions to attenuate APC/CCdc20 is an intense field of research. In particular, the role of posttranslational modifications of both the APC/CCdc20 and core checkpoint components remains controversial. Considerable evidence points to Mad2 and BubR1 as the ultimate arbitrators of the “wait anaphase” signal. Elegant structural and biochemical studies demonstrated that Mad2 exists in two distinct structural conformations, open O-Mad2 and closed C-Mad2. Mad1 at the kinetochore binds stably to C-Mad2, and soluble O-Mad2 dimerization with kinetochore-bound C-Mad2 catalyzes the release of C-Mad2, which is capable of Cdc20 binding and inhibition (52, 56) (Fig. 1A). More recently, the function of Mad3/BubR1 in Cdc20 inhibition and checkpoint signaling has garnered increasing attention. Mad3/BubR1 orthologues contain two KEN (Lys-Glu-Asn) motifs. Although these motifs usually mediate APC-substrate recognition and ubiquitination, two key studies showed that in S. cerevisiae Mad3, these motifs are essential for checkpoint function (5, 40) (Fig. 1B). These observations have been verified in fission yeast (83), flies (74), rodents (53), and human cells (19). While the N-terminal KEN box of Mad3/BubR1 orthologues binds directly to Cdc20 and may be involved in Cdc20 degradation, the C-terminal motif does not (19, 40, 83); rather, it has been proposed that this motif mediates an interaction between Mad3/BubR1 and the core APC/C (19), although this remains to be demonstrated. Remarkably, the molecular mechanisms of the spindle checkpoint may be conserved across kingdoms, as both KEN box motifs are present in the recently identified Arabidopsis thaliana Mad3 (7).
Fig. 1.
The “wait anaphase” signal is generated at improperly attached kinetochores. (A) Mad2 exists in two major conformations, open (O-Mad2, light red indented circles), which is mainly a free, cytoplasmic form, and closed (C-Mad2, bright red circles), which is either Mad1 or Cdc20 bound. Kinetochore-bound Mad1 dimers associate with Mad2 in the closed form at unattached kinetochores. Dimerization between O-Mad2 and C-Mad2 results in the release of a C-Mad2 molecule capable of binding to and inhibiting Cdc20; (inhibited Cdc20 is shown in green). The pathway for APC/CCdc20 inhibition is kinetochore dependent. (B) Mad3/BubR1-Bub3 can bind to and inhibit active Cdc20 (shown in blue) independent of Mad2 and kinetochores. Through its N-terminal KEN motif, BubR1 can bind directly to and inhibit Cdc20. It is not clear if Cdc20 changes structural con- formation upon Mad2 or Mad3/BubR1 binding. (C) Both Mad2 and Mad3/BubR1 are required for checkpoint function in vivo and may function cooperatively to mediate APC/CCdc20 inhibition. Prior binding to Mad2 may prime Cdc20 for the interaction with the Mad3/BubR1-Bub3 complex. Mad3/BubR1 inhibits APC/CCdc20 activity by acting as a pseudosubstrate and/or by mediating Cdc20 ubiquitination and degradation, as denoted by the dashed arrow. Mad2 may dissociate from the inhibited APC/CCdc20 complex once it is formed, being released into the cytoplasm again as free O-Mad2.
Both Mad2 and Mad3/BubR1 are required for the checkpoint in vivo, suggesting that they function cooperatively. The prevalent model of checkpoint signal transduction suggests that Mad3/BubR1 binding to Cdc20 may require prior priming of Cdc20 by Mad2 (14, 40, 68, 85), a process that is greatly expedited at unattached kinetochores that generate C-Mad2 (46) (Fig. 1C). Mad2 may be subsequently released from the inhibited APC/CCdc20 complex, leaving bound Mad3/BubR1 to inhibit securin and cyclin B polyubiquitination (68). How Mad2 stimulates Mad3/BubR1 binding to Cdc20 is clearly a question for future studies. Curiously, some evidence suggests that Mad2 and BubR1 Cdc20-inhibitory complexes may form and function to some extent independently. Mad2 and BubR1 can individually bind and inhibit APC/CCdc20 in vitro (22, 32, 91), and reconstitution of checkpoint signaling in vitro demonstrated that Mad2 inhibition of APC/CCdc20 is kinetochore-dependent, whereas BubR1 binding and inhibition of Cdc20 do not require kinetochores (46). Moreover, double small interfering RNA (siRNA)-mediated depletion of Mad2 and BubR1 from mammalian cells appears to accelerate progression through mitosis more than single depletions (60), which would not be expected if their functions were entirely interdependent. Clearly, a full understanding of APC/CCdc20-inhibitory mechanisms remains to be realized.
THE SPINDLE CHECKPOINT KINASES
The response of the spindle checkpoint to the state of ki-netochore-microtubule attachment is exquisitely fine-tuned such that a single unattached kinetochore generates sufficient “wait anaphase” signal to arrest a cell in mitosis (76). In addition, the establishment of productive kinetochore-microtubule interactions, the event monitored by the spindle checkpoint, is a dynamic process during which microtubules are rapidly captured and released to allow correct attachment formation. The expeditious and precise nature of these events implies that signals must be rapidly and efficiently turned on and off. Classical experiments have suggested that biophysical changes caused by microtubule attachment and tension generation translate into a biochemical signal reflected by the phosphorylation state of a kinetochore epitope recognized by the 3F3/2 monoclonal antibody (8, 66, 67). These observations laid the foundation for the hypothesis that kinase activity is required to maintain an active checkpoint and must be inhibited or counteracted for anaphase to proceed (8). This model is particularly attractive in light of the number of kinases involved in spindle checkpoint signaling either directly through APC/CCdc20 binding and inhibition or indirectly through modulating micro-tubule attachments (Table 1). Here I discuss the structurally related kinases Bub1 and BubR1, with emphasis on the implications of catalytic activity for the spindle checkpoint and chromosome alignment.
Table 1.
Mitotic kinases required for spindle assembly, congression, and the spindle checkpointa
| Family | Mammalian mitosis-specific member | Mitotic functions | Reference(s) |
|---|---|---|---|
| Cdk | Cdk1 (together with cyclins A and B) | Mitotic entry, nuclear envelope breakdown, chromosome condensation, bipolar spindle assembly, microtubule attachment, APC/C regulation, spindle checkpoint regulation | 20a, 67a |
| Polo kinase | Plk1 | Mitotic entry, centrosome maturation, cohesion removal, bipolar spindle assembly, microtubule attachment stability, cytokinesis | 1, 71a |
| Aurora kinase | Aurora A | Mitotic entry, Plk1 activation in late G2, centrosomes maturation, centrosome-dependent and -independent bipolar spindle formation | 8a, 47, 48b |
| Aurora B | Catalytic subunit of the chromosomal passenger complex, spindle assembly, correction of tensionless microtubule attachments, sister chromatid and centromeric cohesion removal, cytokinesis and furrow ingression, kinase activity may regulate a prolonged spindle checkpoint-mediated arrest | ||
| Aurora C | Meiotic functions, somatic role unclear | ||
| NIMA kinase | NEK2A | Mitotic and meiotic spindle assembly, centrosome splitting, microtubule attachment stability | 68a |
| NEK6 | Mitotic progression | 68b, 103a | |
| Mitotic checkpoint kinase | Bub1 | Regulation of end-on microtubule attachments at kinetochores, regulates the spindle checkpoint through recruitment of Mad1, Mad2, and Mad3/BubR1 | 2a, 96a, 96b |
| BubR1 | Regulation of microtubule attachment stability, core checkpoint component through APC/CCdc20 inhibition | 2a | |
| hMps1/TTK (Hs), Esk (Mm) | Correction of improper microtubule attachments, control of the spindle checkpoint through kinetochore recruitment of Mad1 and Mad2, kinetochore recruitment of the Rod-Zwilch-ZW10 complex | 27a, 34a, 48a, 52a, 82a | |
| MAPK | MAPK1/2, p38 | Phosphorylation of Cdc20, Mps1, and Bub1; mitotic progression; kinetochore-microtubule attachment | 13, 13a, 105 |
| Haspin | Haspin | Histone H3 phosphorylation, chromosome congression | 27b |
| Pre-mRNA processing | PRP4 | Kinetochore recruitment of Mps1, Mad1, and Mad2 | 61a |
| ILK | ILK | Spindle assembly | 23a |
This table is not meant to be exhaustive, and kinases not involved in the mitotic checkpoint or spindle assembly and stability are not included.
Bub1. (i) Scaffolding functions of Bub1.
Bub1 is one of the first checkpoint components to dock at the nascent kinetochore in early prophase (33) and is a true checkpoint protein; cells in which Bub1 function is ablated do not arrest in response to microtubule poisons (1a, 2, 29, 86, 96). Bub1 recruitment to the kinetochore occurs through a direct interaction between its N-terminal tetratricopeptide repeat (TPR) domain and blinkin (also known as hKNL1, AF15q14, D40, and CASC5), a member of the conserved KMN (KNL1/Mis12 complex/Ndc80 complex) network of kinetochore proteins (10, 11). Studies of fluorescence recovery after photobleaching in both yeast and human cells have indicated that Bub1 is a stable component of the kinetochore and may act as a scaffold for coordinating checkpoint signaling (28, 79, 84). Indeed, artificially tethering Bub1 to telomeres in yeast is sufficient for the ectopic recruitment of downstream checkpoint components in a kinase-independent manner (79). In this capacity, Bub1 determines the kinetochore recruitment of a number of targets, including centromere proteins E and F (Cenp-E and Cenp-F, respectively), Bub3, Mad3/BubR1, the mitotic centromere-associated kinesin (MCAK), Mad1, and Mad2 (3, 30, 35, 42, 61, 86, 98). In particular, a conserved region (CDI) between amino acids 458 and 467 of hBub1 is required for Mad1, Mad2, and BubR1 recruitment and consequently for checkpoint function (43). Moreover, Bub1 also regulates the targeting of the MEI-S332/shugoshin (Sgo) proteins to the centromere during both meiosis and mitosis (41, 93, 94), although this may be kinase dependent (69). Interestingly, recent observations in mammalian cells have suggested that cytoplasmic Bub1 is at least partially functional (43).
(ii) Bub1 kinase activity and the spindle checkpoint.
In budding yeast, expression of a stable truncated mutant of Bub1 entirely lacking the kinase domain supports a functional checkpoint (23, 98). In contrast, in fission yeast, while some studies indicated a robust checkpoint response in cells lacking Bub1 kinase activity (37, 79), others have suggested that kinase function is required but not sufficient for checkpoint signaling (103). Similarly, a kinase-inactive Bub1 in Xenopus egg extracts supports the checkpoint, albeit at high concentrations of the microtubule-depolymerizing drug nocodazole, and this effect was attenuated at low concentrations (86). In mammals, the general consensus is that Bub1 kinase activity is expendable for mounting a spindle checkpoint response. It may, however, play a subtle role in fine-tuning the arrest. Recent studies have addressed the function of Bub1 kinase using inactivation-complementation approaches in mammals. Using a floxed allele, Bub1 was specifically inactivated in mouse oocytes, resulting in premature APC/C activation in meiosis I, indicating that a Bub1-dependent checkpoint response can be generated in oocytes (59). The delay in APC/C activation normally imposed by endogenous Bub1 could be rescued by both active and inactive Bub1 kinase, although the latter was somewhat less efficient. In murine somatic cells however, Bub1 depletion was rescued to the same extent by both active and inactive Bub1 kinase, indicating that in this context Bub1 catalytic activity is not essential for the checkpoint signaling (69, 70). Similarly, In hTERT-RPE1 cells Bub1 kinase activity appeared to be dispensable for the checkpoint, whereas somewhat surprisingly, the same study indicated that checkpoint activity was more efficient in the presence of active Bub1 in HeLa cells (43). Therefore, while the kinase activity of Bub1 does not regulate the checkpoint in a switch-like manner, it may modulate the strength of the checkpoint signal and delay APC/CCdc20 activation and anaphase onset in certain cell lines or developmental contexts.
What are the potential targets of Bub1 kinase activity relevant to the spindle checkpoint? In vitro, human Bub1 can inhibit the APC/C when bound to Cdc20; in contrast, a catalytically inactive mutant cannot. This inhibition might be direct, as Bub1 can phosphorylate Cdc20 in vitro (92). Although in vivo phosphorylation sites identified on Cdc20 have been attributed to Bub1, this remains to be formally demonstrated. Nevertheless, a nonphosphorylatable Cdc20 mutant does not support mitotic arrest in response to nocodazole or taxol treatment to the same extent as wild-type Cdc20, arguing that phosphorylation may be critical for a checkpoint-mediated arrest. Importantly, Cdc20 has been reported to be a mitogen-activated protein kinase (MAPK) substrate in Xenopus, and mutation of the MAPK phosphorylation sites also weakens the checkpoint response (13). Whether Cdc20 is also a MAPK target in mammals is not known.
(iii) Bub1 kinase function and chromosome congression.
In all tested organisms, impairment of Bub1 function causes congression defects. In both budding and fission yeasts, Bub1 deletion results in severe chromosome missegregation at levels that are elevated compared to those observed after depletion of other checkpoint proteins such as Mad1, Mad2, and Mad3 (23, 97, 98). In human cells, knockdown of Bub1 by RNA interference (RNAi) also causes errors in chromosome alignment resulting from the accumulation of lateral attachments and the consequent delay in formation of stable end-on attachments (17, 25, 50, 61).
There is compelling evidence that the kinase activity of Bub1 contributes to its role in chromosome congression and alignment. Fission and budding yeasts expressing inactive Bub1 are remarkably sensitive to microtubule drugs and display defects in biorientation and chromosome missegregation (23, 97, 98). In an isogenic siRNA complementation system in human cells, Bub1 kinase activity was necessary for precise chromosome alignment (43), in agreement with observations in yeast. However, in murine cells, the equivalent mutants effectively restored proper alignment, suggesting that the kinase activity of Bub1 may not be required for biorientation in this context (69). The surprising discrepancy between the murine and human Bub1 findings may be due to inefficient levels of exogenous protein expression or may reflect the inherent variations in alignment efficiency between organisms and cells types. The question remains as to the targets of Bub1 that direct end-on attachment, although the Sgo proteins are attractive candidates (see below) (81).
(iv) Bub1 activity and Sgo1.
Following DNA replication, sister chromatid pairs are held together in part due to the cohesin protein complex, which is thought to form a ring around the newly replicated DNA (64, 87). At prophase, most of the cohesion is removed from chromosome arms as a consequence of phosphorylation by Plk1 and Aurora B (51, 89). The residual centromeric pool is protected from phosphorylation by Sgo. Whereas budding yeast and flies have only one Sgo protein, fission yeast, plants, frogs, and mammals have two Sgo-like proteins, the mitosis-specific Sgo1 and Sgo2, which is expressed during both meiosis and mitosis (99). Recruitment and maintenance of Sgo proteins at the centromere are complex. In prometaphase Bub1 directs centromeric localization of Sgo proteins, and attenuation of either Sgo or Bub1 results in loss of sister chromatid cohesion and chromosome missegregation (41, 94). Centromere recruitment of Sgo is dependent on Bub1 kinase activity in yeast during mitosis (23) and meiosis (103). Consistently, in frogs and in human cells, Bub1 kinase activity is necessary for Sgo centromere localization (3, 43, 69). Bub1 also directs the PP2A phosphatase to centromeres, where it maintains Sgo protection by counteracting Plk1-mediated phosphorylation (41, 75, 93). Phosphorylation of histone H2A may also facilitate Sgo localization. Bub1 phosphorylates the conserved S121 of fission yeast histone H2A in vitro and in vivo. In yeast and human cells, mutation of this residue phenocopies inactive Bub1, resulting in disruption of centromeric Sgo and microtubule attachments (37). In fission yeast and mouse embryonic fibroblasts (MEFs), Swi6/heterochromatin protein 1 (HP1α) has also been implicated in Sgo localization through a direct interaction between the chromo-shadow domain of Swi6/HP1 and Sgo1 (102). A potentially interesting twist comes from the observation that while Bub1 kinase activity appears to be necessary for Sgo recruitment to the centromere during mitosis, Sgo1's functions in late G2 rather than prophase may confer its protective activity toward centromeric cohesion (69). Indeed, Bub1 may protect centromeric cohesion by regulating the checkpoint, rather than through direct regulation of Sgo1 function (70). This intriguing discovery implies that Bub1-mediated Sgo recruitment in mitosis may serve an entirely different function, perhaps regulation of kinetochore-microtubule attachments. In concordance with this idea, Xenopus Sgo was identified by virtue of its ability to bind and polymerize microtubules (81).
BubR1.
BubR1 was initially identified by virtue of it its homology to Bub1 (6). It was shortly thereafter recognized as the mammalian orthologue of yeast Mad3, and its indispensable function in the checkpoint was confirmed in mammals (9, 95). BubR1 is found in higher eukaryotes, whereas Mad3, which clearly lacks a kinase domain, is expressed in yeasts and plants. This difference implies that BubR1 has acquired additional functions for which the kinase is required. Although the nature of these remains contentious, a role in both the spindle checkpoint and microtubule attachment stability has been proposed.
(i) BubR1 kinase activity and the spindle checkpoint.
Initial studies in frogs indicated that a lack of BubR1 kinase activity does not interfere with Cdc20 binding or APC/CCdc20 inhibition (21, 91). Indeed, BubR1 entirely lacking the kinase domain still supports the checkpoint (12, 14, 27, 53). These conclusions have been disputed by other Xenopus studies, however, which suggest that minimal BubR1 kinase function is indispensable for the checkpoint and that this activity is silenced as a result of spindle microtubule capture by Cenp-E (54, 55). A recent study reports an allosteric Cenp-E inhibitor (GSK923295) (101). Treatment of cells with this inhibitor mimicked the phenotype observed after Cenp-E depletion and antibody microinjection, characterized by an increase in the mitotic index and chromosomes lagging at the spindle poles. Importantly, this inhibitor locks Cenp-E in a microtubule-bound state; thus, in contrast to the situation in Xenopus extracts, binding of the Cenp-E motor domain to microtubules per se appears to be insufficient to satisfy the spindle checkpoint in human cells. Although the significance of the Cenp-E interaction with BubR1 remains unclear, the development of small-molecule inhibitors of both BubR1 and Cenp-E together with structural studies of BubR1-Cenp-E complexes will pave the way to answering some of the lingering questions.
The argument for a subtle BubR1 kinase function in the checkpoint has also been inferred from recent studies with mammalian cells. In MEFs both inactive BubR1 and the N-terminal Mad3-homologous region are capable of Cdc20 and APC/C binding and support growth and survival at the cellular level. However, the checkpoint response to a prolonged nocodazole challenge under these conditions is attenuated, suggesting that BubR1 kinase activity may contribute to the maintenance rather than the initiation of a checkpoint (27, 53). In keeping with this, flies expressing catalytically inactive BubR1 are viable and fertile and retain a functional checkpoint. Nevertheless, premature sister chromatid separation (PSCS), a sign of untimely mitotic exit and therefore checkpoint failure, is elevated in BubR1-KD flies (74). Taken together, these data support a model where BubR1 kinase activity is not essential during normal growth and development. The tight control over checkpoint function by the N-terminal Mad3 domain is sufficient to ensure viable progeny, at least during normal unperturbed mitoses. Kinase function may become important under conditions that prolong mitosis or when the checkpoint signal is weakened, as is expected when only a few kinetochores remain unattached, or under conditions that challenge attachment stability. Such a model leads to several important predictions. First, during a normal undisrupted mitosis, the BubR1 kinase domain and activity are not essential for the checkpoint. This is supported by the observations that mitotic timing in HeLa cells is not affected by BubR1 kinase activity, that cell viability does not require the kinase domain, and that BubR1-KD flies are viable and fertile (20, 53, 74). However, it will be important to verify these observations during development in knock-in mice, as the spindle checkpoint is not strictly essential in flies (4). Second, if the BubR1 kinase activity is indeed required for maintaining the checkpoint, it is reasonable to expect kinase targets to have checkpoint functionality. This has not yet been demonstrated for proposed substrates of BubR1 such as the microtubule plus-end protein adenomatous polyposis coli (36). Conversely, no genuine spindle checkpoint proteins have been shown to be definitively phosphorylated by BubR1. An important caveat is that the integrity of the BubR1 kinase domain is also critical for protein stability and may indirectly modulate the checkpoint through controlling BubR1 levels. Mutations in the kinase domain of BubR1 have been linked to the human cancer predisposition syndrome mosaic variegated aneuploidy, and cells from these patients display reduced BubR1 protein abundance and a defective checkpoint response to microtubule insult (88). It will be important to uncouple kinase activity from protein stability in order to definitively determine whether BubR1 kinase function is required for the checkpoint. Strikingly, we have also observed that mutations in the Bub1 kinase domain cause reduced Bub1 protein expression, suggesting that Bub1 protein stability may be regulated in a similar fashion (unpublished observations).
(ii) BubR1 kinase activity and chromosome alignment.
A dual role for BubR1 in both checkpoint signaling and chromosome alignment was first recognized by Taylor and colleagues, who reported a reduction in metaphase (and increase in prometaphase) cells when transition to anaphase was blocked in BubR1-depleted cells, suggesting that BubR1 is indeed required for chromosome alignment (17). High-resolution microscopy subsequently demonstrated that the microtubule attachments were unstable in BubR1-depleted cells (48), and several lines of evidence suggest that proper alignment may depend on BubR1 kinase activity. When overexpressed, inactive BubR1 prolonged prometaphase in HeLa cells, a phenotype commonly attributed to lack of stable attachments (27). Similarly, neuroblasts from flies expressing catalytically dead BubR1 displayed a tendency toward a prolonged prometaphase characterized by slow congression and difficulty remaining aligned (74). In MEFs and HeLa cells, most inaccurate kinetochore-microtubule attachments are corrected in the absence of BubR1 kinase function, albeit inefficiently; however, BubR1 inactivation did not cause a mitotic delay (20, 31, 53). One possible interpretation of these results is that BubR1 kinase activity is required for the checkpoint (31). An alternative view is that timely anaphase onset may have been initiated, as the type of defects observed upon loss of BubR1 activity, such as merotelic attachments, are not detected by the spindle checkpoint.
Outer-kinetochore and microtubule plus-end binding proteins constitute attractive candidate substrates for BubR1 in its capacity to regulate attachment stability. Adenomatous polyposis coli and its binding partner EB1 are plus-end binding proteins that localize to kinetochores. Their depletion results in little or no delay in chromosome congression, but cells displayed lagging strands during anaphase, a phenotype strikingly similar to that caused by BubR1 kinase inactivation (18). As EB1- and adenomatous polyposis coli-depleted cells arrest efficiently after treatment with microtubule poisons, their loss may create lesions that are not monitored correctly by the checkpoint. In Xenopus extracts, BubR1 forms a complex with the adenomatous polyposis coli-EB1 dimer that is potentially enhanced by microtubule attachment (104). This mechanism may be conserved in mammals, as adenomatous polyposis coli can be phosphorylated by human BubR1 in vitro (36). Importantly, adenomatous polyposis coli also interacts with and is a substrate for Bub1 (36), and careful dissection of the individual kinase contribution to adenomatous polyposis coli phosphorylation and function in mitosis will be necessary to resolve this issue.
Interestingly, instability in microtubule attachments caused by BubR1 depletion can be suppressed upon Aurora B inhibition, suggesting that BubR1 and Aurora B activities may counteract each other (48). A significant body of work has demonstrated that Aurora B activity is required for destabilizing erroneous attachments, such as merotelic configurations, through phosphorylation of microtubule binding factors such as MCAK and Hec1 (15, 16, 38, 47). In yeast, the Aurora B orthologue Ipl1 also phosphorylates Mad3 to regulate the checkpoint response to lack of tension at kinetochores (39). However, the Ipl1 phosphorylation sites on Mad3 are not conserved in higher eukaryotes, and the interplay between Aurora B and BubR1 remains to be fully explored. Nevertheless, as Aurora B and BubR1 kinase activities have opposing functions with respect to attachment stability, it will be interesting to test whether key Aurora B substrates such as MCAK and Hec1 are shared with BubR1.
CONCLUDING REMARKS
The progress achieved in our understanding of Bub1 and Mad3/BubR1 in recent years has revealed their remarkably pleiotropic behavior and has begun to unravel their role in APC/CCdc20 inhibition at the molecular level (see Fig. 2 for a summary). Numerous questions nonetheless remain unanswered. In particular, the significance of kinase activity to the checkpoint is unclear. Broadly speaking, the checkpoint is functional without either Bub1 or BubR1 kinase activity, but target phosphorylation may play a role in ensuring accurate chromosome attachment. Catalytic activity appears to be more important in certain cell lines and developmental contexts for prolonging the checkpoint response. While incomplete protein inactivation in complementation assays can explain these observations, we postulate that variations arise as a reflection of the inherent differences in the efficiency of the microtubule capture machinery in the various cell lines. This in turn is a function of the number of chromosomes that must be captured relative to cell volume and microtubule density in a particular cell or organism. In the future, the development of small-molecule inhibitors will be critical for advancing our understanding of Bub1 and BubR1, as it would enable unprecedented spatial and temporal control of kinase activity. In light of their dual role in both congression and the checkpoint, these compounds would also facilitate evaluation of Bub1 and BubR1 inhibition as a means to curb cancer cell proliferation (34). Ultimately, identification of biologically relevant substrates will be necessary to answer some of the lingering questions.
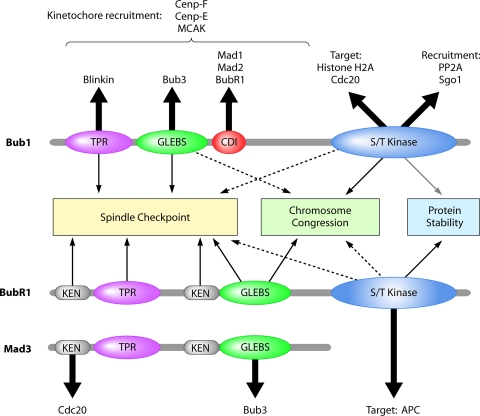
Fig. 2.
Summary of the functions, interacting partners, and targets of the spindle checkpoint kinases Bub1 and BubR1. The domain architectures of Bub1, BubR1, and its orthologue Mad3 are illustrated, and the contribution of each motif to the mitotic checkpoint or to chromosome congression and biorientation is shown. A solid arrow indicates a clear requirement for the motif for a particular function across evolution, whereas a dashed arrow indicates that the contribution of the domain remains controversial. The gray arrow indicates our own unpublished results. Bold arrows indicate the interaction partners identified to date for each of the domains and interaction motifs highlighted in Bub1 and Mad3/BubR1. In the case of Cenp-F, Cenp-E, and MCAK, their kinetochore recruitment is Bub1 kinase independent.
ACKNOWLEDGMENTS
I thank Anna Santamaria and Michael Schwab for critical reading of the manuscript. I am also indebted to Guy Poirier and his group for hosting my lab during its infancy.
Work in my lab is supported by a start-up grant from the Foundation of Stars, Québec, Canada.
Footnotes
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Barr F. A., Sillje H. H., Nigg E. A. 2004. Polo-like kinases and the orchestration of cell division. Nat. Rev. Mol. Cell Biol. 5:429–440 [DOI] [PubMed] [Google Scholar]
- 1a. Basu J., et al. 1999. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol. 146:13–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernard P., Hardwick K., Javerzat J. P. 1998. Fission yeast bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J. Cell Biol. 143:1775–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a. Bolanos-Garcia V. M., Blundell T. L. 2011. BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends Biochem. Sci. 36:141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyarchuk Y., Salic A., Dasso M., Arnaoutov A. 2007. Bub1 is essential for assembly of the functional inner centromere. J. Cell Biol. 176:919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buffin E., Emre D., Karess R. E. 2007. Flies without a spindle checkpoint. Nat. Cell Biol. 9:565–572 [DOI] [PubMed] [Google Scholar]
- 5. Burton J. L., Solomon M. J. 2007. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 21:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cahill D. P., et al. 1998. Mutations of mitotic checkpoint genes in human cancers. Nature 392:300–303 [DOI] [PubMed] [Google Scholar]
- 7. Caillaud M. C., et al. 2009. Spindle assembly checkpoint protein dynamics reveal conserved and unsuspected roles in plant cell division. PLoS One 4:e6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell M. S., Gorbsky G. J. 1995. Microinjection of mitotic cells with the 3F3/2 anti-phosphoepitope antibody delays the onset of anaphase. J. Cell Biol. 129:1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a. Carmena M., Ruchaud S., Earnshaw W. C. 2009. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr. Opin. Cell Biol. 21:796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan G. K., Jablonski S. A., Sudakin V., Hittle J. C., Yen T. J. 1999. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146:941–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheeseman I. M., Chappie J. S., Wilson-Kubalek E. M., Desai A. 2006. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127:983–997 [DOI] [PubMed] [Google Scholar]
- 11. Cheeseman I. M., Desai A. 2008. Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 9:33–46 [DOI] [PubMed] [Google Scholar]
- 12. Chen R. H. 2002. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen R. H. 2004. Phosphorylation and activation of Bub1 on unattached chromosomes facilitate the spindle checkpoint. EMBO J. 23:3113–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a. Chung E., Chen R. H. 2003. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 5:748–753 [DOI] [PubMed] [Google Scholar]
- 14. Davenport J., Harris L. D., Goorha R. 2006. Spindle checkpoint function requires Mad2-dependent Cdc20 binding to the Mad3 homology domain of BubR1. Exp. Cell Res. 312:1831–1842 [DOI] [PubMed] [Google Scholar]
- 15. DeLuca J. G., et al. 2006. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127:969–982 [DOI] [PubMed] [Google Scholar]
- 16. DeLuca K. F., Lens S. M., DeLuca J. G. 2011. Temporal changes in Hec1 phosphorylation control kinetochore-microtubule attachment stability during mitosis. J. Cell Sci. 124:622–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ditchfield C., et al. 2003. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161:267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Draviam V. M., Shapiro I., Aldridge B., Sorger P. K. 2006. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 25:2814–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elowe S., et al. 2010. Uncoupling of the spindle-checkpoint and chromosome-congression functions of BubR1. J. Cell Sci. 123:84–94 [DOI] [PubMed] [Google Scholar]
- 20. Elowe S., Hummer S., Uldschmid A., Li X., Nigg E. A. 2007. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev. 21:2205–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a. Enserink J. M., Kolodner R. D. 2010. An overview of Cdk1-controlled targets and processes. Cell Div. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fang G. 2002. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell 13:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fang G., Yu H., Kirschner M. W. 1998. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12:1871–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernius J., Hardwick K. G. 2007. Bub1 kinase targets Sgo1 to ensure efficient chromosome biorientation in budding yeast mitosis. PLoS Genet. 3:e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a. Fielding A. B., Dedhar S. 2009. The mitotic functions of integrin-linked kinase. Cancer Metastasis Rev. 28:99–111 [DOI] [PubMed] [Google Scholar]
- 24. Gascoigne K. E., Taylor S. S. 2008. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14:111–122 [DOI] [PubMed] [Google Scholar]
- 25. Gillett E. S., Espelin C. W., Sorger P. K. 2004. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J. Cell Biol. 164:535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hardwick K. G., Weiss E., Luca F. C., Winey M., Murray A. W. 1996. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273:953–956 [DOI] [PubMed] [Google Scholar]
- 27. Harris L., Davenport J., Neale G., Goorha R. 2005. The mitotic checkpoint gene BubR1 has two distinct functions in mitosis. Exp. Cell Res. 308:85–100 [DOI] [PubMed] [Google Scholar]
- 27a. Hewitt L., Tighe A., Santaguida S., White A. M., Jones C. D., Musacchio A., Green S., Taylor S. S. 2010. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J. Cell Biol. 190:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27b. Higgins J. M. 2010. Haspin: a newly discovered regulator of mitotic chromosome behavior. Chromosoma 119:137–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howell B. J., et al. 2004. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 14:953–964 [DOI] [PubMed] [Google Scholar]
- 29. Hoyt M. A., Totis L., Roberts B. T. 1991. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66:507–517 [DOI] [PubMed] [Google Scholar]
- 30. Huang H., et al. 2007. Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J. Cell Biol. 177:413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang H., et al. 2008. Phosphorylation sites in BubR1 that regulate kinetochore attachment, tension, and mitotic exit. J. Cell Biol. 183:667–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hwang L. H., et al. 1998. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279:1041–1044 [DOI] [PubMed] [Google Scholar]
- 33. Jablonski S. A., Chan G. K., Cooke C. A., Earnshaw W. C., Yen T. J. 1998. The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma 107:386–396 [DOI] [PubMed] [Google Scholar]
- 34. Janssen A., Kops G. J., Medema R. H. 2009. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc. Natl. Acad. Sci. U. S. A. 106:19108–19113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a. Jelluma N., Brenkman A. B., van den Broek N. J., Cruijsen C. W., van Osch M. H., Lens S. M., Medema R. H., Kops G. J. 2008. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell 132:233–246 [DOI] [PubMed] [Google Scholar]
- 35. Johnson V. L., Scott M. I., Holt S. V., Hussein D., Taylor S. S. 2004. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J. Cell Sci. 117:1577–1589 [DOI] [PubMed] [Google Scholar]
- 36. Kaplan K. B., et al. 2001. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat. Cell Biol. 3:429–432 [DOI] [PubMed] [Google Scholar]
- 37. Kawashima S. A., Yamagishi Y., Honda T., Ishiguro K., Watanabe Y. 2010. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327:172–177 [DOI] [PubMed] [Google Scholar]
- 38. Kelly A. E., Funabiki H. 2009. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr. Opin. Cell Biol. 21:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. King E. M., Rachidi N., Morrice N., Hardwick K. G., Stark M. J. 2007. Ipl1p-dependent phosphorylation of Mad3p is required for the spindle checkpoint response to lack of tension at kinetochores. Genes Dev. 21:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. King E. M., van der Sar S. J., Hardwick K. G. 2007. Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS One 2:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kitajima T. S., Hauf S., Ohsugi M., Yamamoto T., Watanabe Y. 2005. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol. 15:353–359 [DOI] [PubMed] [Google Scholar]
- 42. Kiyomitsu T., Obuse C., Yanagida M. 2007. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev. Cell 13:663–676 [DOI] [PubMed] [Google Scholar]
- 43. Klebig C., Korinth D., Meraldi P. 2009. Bub1 regulates chromosome segregation in a kinetochore-independent manner. J. Cell Biol. 185:841–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kline-Smith S. L., Walczak C. E. 2004. Mitotic spindle assembly and chromosome segregation: refocusing on microtubule dynamics. Mol. Cell 15:317–327 [DOI] [PubMed] [Google Scholar]
- 45. Kops G. J. 2008. The kinetochore and spindle checkpoint in mammals. Front. Biosci. 13:3606–3620 [DOI] [PubMed] [Google Scholar]
- 46. Kulukian A., Han J. S., Cleveland D. W. 2009. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev. Cell 16:105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lampson M. A., Cheeseman I. M. 2011. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 21:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lampson M. A., Kapoor T. M. 2005. The human mitotic checkpoint protein BubR1 regulates chromosome-spindle attachments. Nat. Cell Biol. 7:93–98 [DOI] [PubMed] [Google Scholar]
- 48a. Lan W., Cleveland D. W. 2010. A chemical tool box defines mitotic and interphase roles for Mps1 kinase. J. Cell Biol. 190:21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48b. Lens S. M., Voest E. E., Medema R. H. 2010. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat. Rev. Cancer 10:825–841 [DOI] [PubMed] [Google Scholar]
- 49. Li R., Murray A. W. 1991. Feedback control of mitosis in budding yeast. Cell 66:519–531 [DOI] [PubMed] [Google Scholar]
- 50. Logarinho E., Resende T., Torres C., Bousbaa H. 2008. The human spindle assembly checkpoint protein Bub3 is required for the establishment of efficient kinetochore-microtubule attachments. Mol. Biol. Cell 19:1798–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Losada A., Hirano M., Hirano T. 2002. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 16:3004–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luo X., Yu H. 2008. Protein metamorphosis: the two-state behavior of Mad2. Structure 16:1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a. Maciejowski J., George K. A., Terret M. E., Zhang C., Shokat K. M., Jallepalli P. V. 2010. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J. Cell Biol. 190:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Malureanu L. A., et al. 2009. BubR1 N terminus acts as a soluble inhibitor of cyclin B degradation by APC/C(Cdc20) in interphase. Dev. Cell 16:118–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mao Y., Abrieu A., Cleveland D. W. 2003. Activating and silencing the mitotic checkpoint through CENP-E-dependent activation/inactivation of BubR1. Cell 114:87–98 [DOI] [PubMed] [Google Scholar]
- 55. Mao Y., Desai A., Cleveland D. W. 2005. Microtubule capture by CENP-E silences BubR1-dependent mitotic checkpoint signaling. J. Cell Biol. 170:873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mapelli M., Musacchio A. 2007. MAD contortions: conformational dimerization boosts spindle checkpoint signaling. Curr. Opin. Struct. Biol. 17:716–725 [DOI] [PubMed] [Google Scholar]
- 57. Maresca T. J., Salmon E. D. 2010. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J. Cell Sci. 123:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McEwen B. F., Dong Y. 2010. Contrasting models for kinetochore microtubule attachment in mammalian cells. Cell. Mol. Life Sci. 67:2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McGuinness B. E., et al. 2009. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr. Biol. 19:369–380 [DOI] [PubMed] [Google Scholar]
- 60. Meraldi P., Draviam V. M., Sorger P. K. 2004. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell 7:45–60 [DOI] [PubMed] [Google Scholar]
- 61. Meraldi P., Sorger P. K. 2005. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 24:1621–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61a. Montembault E., Dutertre S., Prigent C., Giet R. 2007. PRP4 is a spindle assembly checkpoint protein required for MPS1, MAD1, and MAD2 localization to the kinetochores. J. Cell Biol. 179:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Musacchio A., Hardwick K. G. 2002. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol. 3:731–741 [DOI] [PubMed] [Google Scholar]
- 63. Musacchio A., Salmon E. D. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393 [DOI] [PubMed] [Google Scholar]
- 64. Nasmyth K., Haering C. H. 2009. Cohesin: its roles and mechanisms. Annu. Rev. Genet. 43:525–558 [DOI] [PubMed] [Google Scholar]
- 65. Nezi L., Musacchio A. 2009. Sister chromatid tension and the spindle assembly checkpoint. Curr. Opin. Cell Biol. 21:785–795 [DOI] [PubMed] [Google Scholar]
- 66. Nicklas R. B., Campbell M. S., Ward S. C., Gorbsky G. J. 1998. Tension-sensitive kinetochore phosphorylation in vitro. J. Cell Sci. 111:3189–3196 [DOI] [PubMed] [Google Scholar]
- 67. Nicklas R. B., Ward S. C., Gorbsky G. J. 1995. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J. Cell Biol. 130:929–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67a. Nigg E. A. 2001. Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2:21–32 [DOI] [PubMed] [Google Scholar]
- 68. Nilsson J., Yekezare M., Minshull J., Pines J. 2008. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nat. Cell Biol. 10:1411–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68a. O'Regan L., Blot J., Fry A. M. 2007. Mitotic regulation by NIMA-related kinases. Cell Div. 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68b. O'Regan L., Fry A. M. 2009. The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol. Cell Biol. 29:3975–3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Perera D., Taylor S. S. 2010. Sgo1 establishes the centromeric cohesion protection mechanism in G2 before subsequent Bub1-dependent recruitment in mitosis. J. Cell Sci. 123:653–659 [DOI] [PubMed] [Google Scholar]
- 70. Perera D., et al. 2007. Bub1 maintains centromeric cohesion by activation of the spindle checkpoint. Dev. Cell 13:566–579 [DOI] [PubMed] [Google Scholar]
- 71. Peters J. M. 2006. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 7:644–656 [DOI] [PubMed] [Google Scholar]
- 71a. Petronczki M., Lenart P., Peters J. M. 2008. Polo on the rise: from mitotic entry to cytokinesis with Plk1. Dev. Cell 14:646–659 [DOI] [PubMed] [Google Scholar]
- 72. Pinsky B. A., Biggins S. 2005. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 15:486–493 [DOI] [PubMed] [Google Scholar]
- 73. Przewloka M. R., Glover D. M. 2009. The kinetochore and the centromere: a working long distance relationship. Annu. Rev. Genet. 43:439–465 [DOI] [PubMed] [Google Scholar]
- 74. Rahmani Z., Gagou M. E., Lefebvre C., Emre D., Karess R. E. 2009. Separating the spindle, checkpoint, and timer functions of BubR1. J. Cell Biol. 187:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Riedel C. G., et al. 2006. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441:53–61 [DOI] [PubMed] [Google Scholar]
- 76. Rieder C. L., Cole R. W., Khodjakov A., Sluder G. 1995. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130:941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rieder C. L., Maiato H. 2004. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7:637–651 [DOI] [PubMed] [Google Scholar]
- 78. Rieder C. L., Salmon E. D. 1998. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 8:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rischitor P. E., May K. M., Hardwick K. G. 2007. Bub1 is a fission yeast kinetochore scaffold protein, and is sufficient to recruit other spindle checkpoint proteins to ectopic sites on chromosomes. PLoS One 2:e1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Roberts B. T., Farr K. A., Hoyt M. A. 1994. The Saccharomyces cerevisiae checkpoint gene BUB1 encodes a novel protein kinase. Mol. Cell. Biol. 14:8282–8291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Salic A., Waters J. C., Mitchison T. J. 2004. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118:567–578 [DOI] [PubMed] [Google Scholar]
- 82. Santaguida S., Musacchio A. 2009. The life and miracles of kinetochores. EMBO J. 28:2511–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82a. Santaguida S., Tighe A., D'Alise A. M., Taylor S. S., Musacchio A. 2010. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 190:73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sczaniecka M., et al. 2008. The spindle checkpoint functions of Mad3 and Mad2 depend on a Mad3 KEN box-mediated interaction with Cdc20-anaphase-promoting complex (APC/C). J. Biol. Chem. 283:23039–23047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shah J. V., et al. 2004. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 14:942–952 [DOI] [PubMed] [Google Scholar]
- 85. Shannon K. B., Canman J. C., Salmon E. D. 2002. Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell 13:3706–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sharp-Baker H., Chen R. H. 2001. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J. Cell Biol. 153:1239–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shintomi K., Hirano T. 2010. Sister chromatid resolution: a cohesin releasing network and beyond. Chromosoma 119:459–467 [DOI] [PubMed] [Google Scholar]
- 88. Suijkerbuijk S. J., et al. 2010. Molecular causes for BUBR1 dysfunction in the human cancer predisposition syndrome mosaic variegated aneuploidy. Cancer Res. 70:4891–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sumara I., et al. 2002. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell 9:515–525 [DOI] [PubMed] [Google Scholar]
- 90. Tanaka T. U., Desai A. 2008. Kinetochore-microtubule interactions: the means to the end. Curr. Opin. Cell Biol. 20:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tang Z., Bharadwaj R., Li B., Yu H. 2001. Mad2-independent inhibition of APCCdc20 by the mitotic checkpoint protein BubR1. Dev. Cell 1:227–237 [DOI] [PubMed] [Google Scholar]
- 92. Tang Z., Shu H., Oncel D., Chen S., Yu H. 2004. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol. Cell 16:387–397 [DOI] [PubMed] [Google Scholar]
- 93. Tang Z., et al. 2006. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev. Cell 10:575–585 [DOI] [PubMed] [Google Scholar]
- 94. Tang Z., Sun Y., Harley S. E., Zou H., Yu H. 2004. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc. Natl. Acad. Sci. U. S. A. 101:18012–18017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Taylor S. S., Ha E., McKeon F. 1998. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J. Cell Biol. 142:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Taylor S. S., McKeon F. 1997. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell 89:727–735 [DOI] [PubMed] [Google Scholar]
- 96a. Vanoosthuyse V., Hardwick K. G. 2005. Bub1 and the multilayered inhibition of Cdc20-APC/C in mitosis. Trends Cell Biol. 15:231–233 [DOI] [PubMed] [Google Scholar]
- 96b. Vanoosthuyse V., Hardwick K. G. 2003. The complexity of Bub1 regulation—phosphorylation, phosphorylation, phosphorylation. Cell Cycle 2:118–119 [DOI] [PubMed] [Google Scholar]
- 97. Vanoosthuyse V., Valsdottir R., Javerzat J. P., Hardwick K. G. 2004. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol. Cell. Biol. 24:9786–9801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Warren C. D., et al. 2002. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell 13:3029–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Watanabe Y. 2005. Shugoshin: guardian spirit at the centromere. Curr. Opin. Cell Biol. 17:590–595 [DOI] [PubMed] [Google Scholar]
- 100. Weiss E., Winey M. 1996. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132:111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wood K. W., et al. 2010. Antitumor activity of an allosteric inhibitor of centromere-associated protein-E. Proc. Natl. Acad. Sci. U. S. A. 107:5839–5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yamagishi Y., Sakuno T., Shimura M., Watanabe Y. 2008. Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455:251–255 [DOI] [PubMed] [Google Scholar]
- 103. Yamaguchi S., Decottignies A., Nurse P. 2003. Function of Cdc2p-dependent Bub1p phosphorylation and Bub1p kinase activity in the mitotic and meiotic spindle checkpoint. EMBO J. 22:1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103a. Yin M. J., Shao L., Voehringer D., Smeal T., Jallal B. 2003. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J. Biol. Chem. 278:52454–52460 [DOI] [PubMed] [Google Scholar]
- 104. Zhang J., Ahmad S., Mao Y. 2007. BubR1 and APC/EB1 cooperate to maintain metaphase chromosome alignment. J. Cell Biol. 178:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhao Y., Chen R. H. 2006. Mps1 phosphorylation by MAP kinase is required for kinetochore localization of spindle-checkpoint proteins. Curr. Biol. 16:1764–1769 [DOI] [PubMed] [Google Scholar]