Abstract
The exceptional in vitro potency of the hepatitis C virus (HCV) NS5A inhibitor BMS-790052 has translated into an in vivo effect in proof-of-concept clinical trials. Although the 50% effective concentration (EC50) of the initial lead, the thiazolidinone BMS-824, was ∼10 nM in the replicon assay, it underwent transformation to other inhibitory species after incubation in cell culture medium. The biological profile of BMS-824, including the EC50, the drug concentration required to reduce cell growth by 50% (CC50), and the resistance profile, however, remained unchanged, triggering an investigation to identify the biologically active species. High-performance liquid chromatography (HPLC) biogram fractionation of a sample of BMS-824 incubated in medium revealed that the most active fractions could readily be separated from the parental compound and retained the biological profile of BMS-824. From mass spectral and nuclear magnetic resonance data, the active species was determined to be a dimer of BMS-824 derived from an intermolecular radical-mediated reaction of the parent compound. Based upon an analysis of the structural elements of the dimer deemed necessary for anti-HCV activity, the stilbene derivative BMS-346 was synthesized. This compound exhibited excellent anti-HCV activity and showed a resistance profile similar to that of BMS-824, with changes in compound sensitivity mapped to the N terminus of NS5A. The N terminus of NS5A has been crystallized as a dimer, complementing the symmetry of BMS-346 and allowing a potential mode of inhibition of NS5A to be discussed. Identification of the stable, active pharmacophore associated with these NS5A inhibitors provided the foundation for the design of more potent inhibitors with broad genotype inhibition. This culminated in the identification of BMS-790052, a compound that preserves the symmetry discovered with BMS-346.
INTRODUCTION
Hepatitis C virus (HCV) is the major causative agent of non-A, non-B hepatitis worldwide, which affects more than 3% of the world's population. Of those infected with HCV, ∼70% proceed to a chronic state which can lead to severe liver diseases, including fibrosis, cirrhosis, or hepatocellular carcinoma (1, 7). There is currently no vaccine against HCV and no generally effective therapy for all HCV genotypes. The current optimal therapy is pegylated alpha interferon in combination with ribavirin, a regimen associated with significant side effects and limited efficacy in the most prevalent patient population, consisting of genotype 1 (4). Therefore, there is an urgent need for the development of more effective, HCV-specific antiviral therapies with fewer side effects.
In the search for more efficacious, safer HCV therapies, the most actively pursued antiviral targets have been the NS3 protease and NS5B RNA-dependent RNA polymerase, both essential enzymes for the replication of HCV (2, 11, 12). Exciting progress has been demonstrated in clinical trials with multiple HCV NS3 serine protease inhibitors, as well as with both nucleoside and nonnucleoside polymerase inhibitors. However, due to the error-prone nature of the HCV polymerase, HCV is a highly heterogeneous virus and resistance variants exist as part of the viral quasispecies. It is widely recognized that combinations of drugs with different resistance profiles are likely to be required to effectively suppress the emergence of resistant virus and achieve a sustained viral response. Thus, agents that inhibit HCV replication via novel targets are of considerable interest.
With the development of HCV replicon and virus systems, it is now possible to identify inhibitors targeting nonenzymatic proteins via cell-based screens. The use of a cell-based replication assay includes essential functions that previously could not be evaluated with in vitro enzyme assays. Inhibitors that target HCV NS5A, a protein with no known enzymatic function, provide an interesting example of this approach. NS5A is a multifunctional protein required for several stages of the viral life cycle. It is a membrane-associated phosphoprotein (9, 18) thought to be involved in interferon resistance that also has been shown to interact with a number of host proteins, although its precise role in HCV replication is unknown (14). NS5A has recently been validated as a clinically relevant target (6), and inhibitors targeting this protein are actively being pursued in clinical trials.
We recently reported the identification of compounds that inhibit HCV replication in cell-based assays and target NS5A (10). One such compound, BMS-824, is a potent and specific inhibitor of HCV RNA replication with a 50% effective concentration (EC50) of ∼10 nM. Studies to further characterize this compound revealed that BMS-824 was not stable in medium yet anti-HCV activity was maintained. In this report, we describe the use of an HCV bioactivity chromatogram assay (referred to here as a “biogram” [5]) to isolate and identify two trace constituents from incubations of BMS-824 in assay medium that demonstrate exceptionally potent HCV inhibition in replicons.
MATERIALS AND METHODS
Cell culture and compound.
Both bovine viral diarrhea virus (BVDV) and HCV replicon cell lines were isolated as previously described (10, 15) and were maintained in Dulbecco's modified Eagle medium (DMEM) with 100 U/ml penicillin-streptomycin, 10% fetal bovine serum (FBS), and 0.3 to 0.5 mg/ml Geneticin (G418). Huh-7 cells cured of a Con1 replicon were generated as previously described (10) and were propagated in DMEM with penicillin-streptomycin and 10% FBS. The compounds used in this study were synthesized at Bristol-Myers Squibb.
Cell culture cytotoxicity and HCV inhibition assays.
To assess HCV-inhibitory activity, HCV replicon cells were plated at a density of 104 per well in 96-well plates in DMEM medium containing 10% FBS (assay medium). Following incubation overnight, compounds or high-performance liquid chromatography (HPLC) fractions (detailed below) were added to cell plates and incubated at 37°C for 3 days prior to assaying for cytotoxicity and HCV inhibition. Cell viability was measured using an alamarBlue assay, and the CC50, the concentration of compound which caused a 50% reduction in cell viability, was calculated using the median-effect equation.
HCV inhibition was measured using a fluorescence resonance energy transfer (FRET) assay which was performed as previously described (15). Briefly, after staining with alamarBlue, replicon cell plates were washed with phosphate-buffered saline and then used for the FRET assay by the addition of 30 μl of the FRET peptide assay reagent per well. The assay reagent consisted of 1× luciferase cell culture lysis buffer, 150 mM NaCl, and 20 μM FRET peptide. The plate containing the assay reagent was then read in kinetic mode in a Cytofluor 4000 instrument which had been set to 340-nm excitation and 490-nm emission wavelengths in automatic mode for 20 cycles. EC50s were calculated as the compound concentrations which caused a 50% reduction in HCV FRET activity.
Isolation of resistant replicons.
Selection of resistant replicon cells was performed by growing genotype 1b replicon cells in medium containing a concentration of 5 μM BMS-346. Medium containing compound was added to monolayers of HCV 1b-377-neo replicon cells at ∼25% confluence in the presence of 0.5 mg/ml G418. Replicon cells maintained in the presence of DMSO were used as a control. After 5 to 6 weeks, control DMSO-selected replicon cells and compound-selected cells were tested for compound sensitivity using the HCV replicon FRET assay.
cDNA cloning.
Total RNA was isolated from both DMSO- and compound-selected cell lines using Trizol (Gibco-BRL) according to the manufacturer's protocol. To generate HCV cDNAs, the NS5A gene was amplified using the SuperScript One-Step reverse transcription-PCR kit (Gibco-BRL) and primers targeting the NS4B and NS5B genes. Reaction products were cloned directly into pCR2.1-TOPO using a TOPO TA cloning kit (Invitrogen), and the DNA sequence of the NS5A coding region was determined for multiple clones.
Transient-replication assays.
RNA transcripts of HCV replicons containing a luciferase reporter gene were synthesized in vitro using ScaI-digested DNAs and the T7 MegaScript transcription kit (Ambion) according to the manufacturer's directions. For transient-replication assays, subconfluent cured Huh-7 cells in a 35-mm dish were transfected with 2.5 μg of RNA transcript using DMRIE-C (Invitrogen) according to the manufacturer's directions. Four hours later, transfectant was removed and replaced with DMEM–10% FBS with or without compound and then incubated at 37°C. At various time points, cells were harvested and luciferase activity was determined using the Renilla luciferase assay kit (Promega).
Extraction and HPLC biogram.
To begin isolation of active components derived from BMS-824, BMS-824 (5 μM final concentration) was incubated in assay medium at 37°C for 48 h in an initial 6-ml pilot experiment. After 48 h, 6 ml acetonitrile was added to the incubation mixture and the resulting suspension was centrifuged. A 100-μl aliquot of the supernatant was subjected to HPLC fractionation. The HPLC (C18) conditions used included an Agilent HP-1100, a Waters X-Terra 5-μm (C18) column (4.6 by 150 mm), a mobile phase consisting of a 0.01% trifluoroacetic acid–acetonitrile gradient (8), a flow rate of 1.2 ml/min, and 254-nm UV detection. For the HPLC biogram (replicon assay) analyses, fractions were collected in Beckman 96-deep-well plates using a Gilson 215 liquid handler and dried under vacuum using a Savant SpeedVac. The dried material was resuspended in medium, and a portion of it was tested for inhibition in the replicon assay. The procedure was repeated using an enriched acetonitrile extract, which was prepared by freezing the aqueous medium-acetonitrile supernatant at −20°C, followed by recovery of the upper, acetonitrile phase, evaporation to dryness, and reconstitution in 200 μl methanol. In this manner, bioassay of all fractions revealed activity that correlated with a distinct yet minor late-eluting UV-detectable peak. The incubation procedure was scaled up (2 liters of 5 μM BMS-824 in assay medium at 37°C, 5% CO2, and 95% humidity for 48 h with the bottle cap closed). The incubation mixture was extracted with 2 liters of acetonitrile, followed by centrifugation (Beckman GS-6R, 5,000 rpm, 20 min). The centrifuged aqueous medium-acetonitrile solutions were frozen at −20°C, and the resulting upper acetonitrile extract was recovered. The crude acetonitrile extract was dissolved in methanol-water at 65:35 (20 ml) and extracted twice with equal volumes of chloroform that had been presaturated with methanol-water at 65:35. The biogram fractionation on the enriched chloroform extract was conducted with an Agilent HP-1100, a YMC Pro-C18 5-μm column (4.6 by 150 mm), a mobile phase consisting of a 0.01% trifluoroacetic acid–acetonitrile linear gradient of 60:40 to 10:90 (vol/vol) over 20 min, holding at 0.01% trifluoroacetic acid–acetonitrile at 10:90 for 5 min, a 1.2-ml/min flow rate, and UV detection at 254 nm. In this manner, bioassay of all fractions revealed two active UV peaks (17.8 min [peak 4] and 19.0 min [peak 6]). The chloroform extract was subjected to preparative HPLC with a Beckman System Gold workstation, a YMC Pro-C18 5-μm column (20 by 150 mm), a mobile phase consisting of a 0.01% trifluoroacetic acid–acetonitrile linear gradient of 60:40 to 10:90 (vol/vol) over 20 min, holding at 0.01% trifluoroacetic acid–acetonitrile at 10:90 for 5 min, a 20-ml/min flow rate, and UV detection at 254 nm. Replicon active peaks 4 and 6 were manually collected and submitted for biological evaluation.
A second scale-up incubation was conducted at a higher concentration of BMS-824 (100 μM) in 1 liter of assay medium. A solution of BMS-824 (60 mg/60 ml DMSO) was added to the medium, and the medium was divided into four 500-ml Erlenmeyer shake flasks, sealed with a semipermeable membrane (Biowrap), and placed in an incubator-orbital shaker at 37°C and 100 rpm for 67 h. Workup and isolation as described above yielded sufficient amounts of replicon active components for structure elucidation (peak 4, 1.1 mg; peak 6, 1.1 mg).
High-resolution MS, NMR and HPLC.
Electrospray ionization–high-resolution mass spectrometry (ESI-HR-MS) data were obtained with a Micromass QTOF-2 mass spectrometer in positive-ion mode with a full-width half-maximal resolution of 9,500, a LEU-enkephalin m/z 556 (M + H)+ lock mass, and tandem MS (MS/MS) with a collision energy of 22 eV. NMR spectra were obtained on a Bruker DRX 500-MHz spectrometer equipped with a 5-mm TXI CryoProbe. Proton and carbon chemical shifts are reported in ppm relative to tetramethylsilane (compound 1, acetonitrile-d3; compound 4, acetone-d6 solvent) (see Fig. 4). HPLC coinjection analyses were conducted using a (i) Waters Sunfire C18 column (5 μm, 4.6 by 150 mm), a mobile phase consisting of 10 mM ammonium acetate-acetonitrile at 95:5 (solvent A)–10 mM ammonium acetate-acetonitrile at 5:95 (solvent B) at an 85:15 to a 0:100 ratio of solvent A to solvent B over 25 min, holding at 100% B solvent for 5 min, a flow rate of 1.2 ml/min, and UV detection at 254 nm or (ii) a Waters Acquity UPLC BEH C18 or a Shield RP18 (1.7 μm, 2.1 by 50 mm) column, a mobile phase consisting of 10 mM ammonium acetate-acetonitrile or methanol at 95:5 (solvent A)–10 mM ammonium acetate-acetonitrile or methanol at 5:95 (solvent B) at a 100:0 to a 0:100 ratio of solvent A to solvent B over 4 min, holding at 100% B solvent for 1 min, a flow rate of 0.5 ml/min (methanol method) or 0.83 ml/min (acetonitrile method), and UV detection at 220 nm.
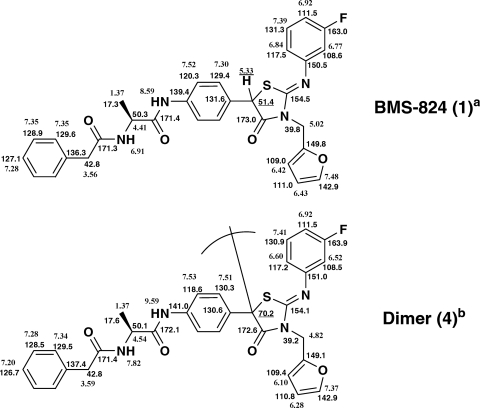
Fig. 4.
1H and 13C NMR chemical shifts for BMS-824 (compound 1) and dimer (compound 4). NMR solvents: a, acetonitrile-d3; b, acetone-d6.
RESULTS
Characterization of HCV NS5A inhibitors.
As part of the characterization of the NS5A inhibitor BMS-824 (Fig. 1) (10), it became apparent that under certain conditions this compound exhibited some chemical reactivity, leading to experiments designed to ascertain its chemical behavior in medium. Toward this end, BMS-824 was incubated in cell-free assay medium for 72 h under the conditions used for the replicon assay. After incubation, medium containing the compound was extracted with acetonitrile and examined by HPLC fractionation. A control sample of BMS-824 which was not incubated in medium gave a single UV-detectable peak in the HPLC trace. In contrast, the parent compound could not be detected by HPLC in the sample of BMS-824 incubated in medium, providing evidence of parent compound reactivity under assay conditions (data not shown). The major products formed from BMS-824 (compound 1) were identified as an oxidation product (compound 2) and a thiourea derivative (compound 3) derived from compound 2 by hydrolysis (Fig. 1). When tested in the HCV replicon assay, these products exhibited poor activity (EC50, >10 μM; data not shown), raising the possibility that undetected amounts of another highly potent component(s) were present. To assess this possibility, BMS-824 was incubated with medium for various times (0 to 120 h) and the medium was subjected to the 3-day HCV replicon assay to determine if the HCV-inhibitory activity remained. The EC50 remained constant at all time points, indicating that after 5 days of incubation, potency was maintained even though the parent compound was no longer detectable (data not shown). This suggested that an active, stable component(s) was generated from the parent compound during incubation in medium and that the resulting new species was a potent HCV inhibitor that could potentially be isolated and characterized.
Fig. 1.
Structures of BMS-824 (compound 1) and medium-induced products (compounds 2, 3, and 4 to 6).
Identification of fractions with HCV activity.
To identify the source of the potent inhibition, we first used a replicon HPLC biogram assay to determine which fractions contained anti-HCV activity. The fraction collection utilized a time-based protocol, resulting in a direct relationship between an anti-HCV active well's position on the plate and a corresponding area on the HPLC chromatogram. Following incubation of BMS-824 and extraction, the sample was subjected to HPLC fractionation (Fig. 2) and 80 fractions were collected and evaluated in the HCV replicon assay. To ensure that any activity we observed was specific for HCV, the fractions were tested against the Y93H BMS-824-resistant replicon cell line and evaluated for cytotoxicity. BMS-824 and an HCV NS3 protease inhibitor were used as controls for the assay and yielded the expected inhibitory profiles. Incubation of cells with 50 nM BMS-824 gave 72 and 0% inhibition on the wild-type and Y93H replicon cells, respectively, while the protease inhibitor gave similar levels of inhibition on both cell lines. When all 80 fractions were tested, HCV inhibition (>35% inhibition) was reproducibly detected in fractions 56, 61, and 62 (Fig. 2). These fractions showed no significant activity toward the Y93H replicon, suggesting cross-resistance to BMS-824 with no overt cytotoxicity. Under the fractionation conditions used, a distinct UV peak is not detectable in the region of interest (fractions 61 and 62); however it is clear from the biogram results that the active component(s) eluted later than the parent compound, BMS-824 (compound 1) (fraction 56).
Fig. 2.
Biogram analysis to identify regions containing active components. BMS-824 was incubated in medium for 48 h and then subjected to extraction and HPLC fractionation. Eighty fractions were collected and tested for anti-HCV activity; the regions containing the peak inhibitory activity are indicated by arrows. Solid line, HPLC trace; dotted line, percent inhibition of HCV replicon activity; mAU, milliabsorbance units; Fr., fraction.
Isolation of active components.
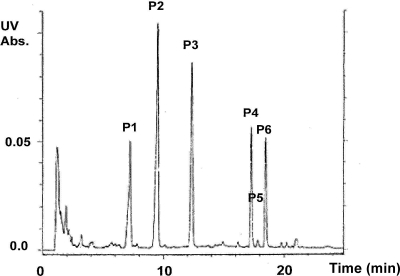
Following the incubation of BMS-824 in medium, material was extracted using specific modifications to facilitate separation (see Materials and Methods) and the enriched sample was subjected to HPLC fractionation. A critical enrichment step prior to HPLC involved extraction of the complex medium matrix with acetonitrile, followed by centrifugation and freezing at −20°C to facilitate phase separation. The unfrozen acetonitrile extract was recovered and further refined using a chloroform-methanol-water partitioning step (i.e., modified Folch method [3]). The HPLC-UV peaks in the enriched material consisted of starting material (peak 3), plus four major peaks and one minor peak between peak 4 and peak 6 (Fig. 3). Each fraction was tested for anti-HCV activity on both the wild-type and Y93H resistant cells, which show reduced susceptibility to BMS-824 (Table 1). Peak fractions were compared to those of control BMS-824, which yielded an EC50 ∼10-fold higher in this experiment than that normally observed (10). This was interpreted as a reduced ability to convert to an active species in the 3-day replicon assay. As a control, a medium-only extraction-separation was performed as described above and tested in parallel. The control extraction did not yield peaks of similar intensity, nor did the gradient contain any significant inhibitory activity (data not shown). As shown in Table 1, peaks 3, 4, and 6 from the sample containing the incubated compound exhibited significant anti-HCV activity, with no detectable cytotoxicity. The EC50s of these peaks ranged from 43 nM to 600 pM. Importantly, these three peaks showed 30- to ∼3,800-fold resistance on the Y93H cell line, suggesting that they contained components related to the parent compound, BMS-824. Additional fractions representing the entire gradient were tested and did not contain any significant antiviral activity (data not shown). The parent compound, BMS-824 (compound 1), was identified in UV peak 3, the same peak in which control nonincubated BMS-824 also eluted. An oxidized form (compound 2) of BMS-824 was identified in UV peak 2, and a thiourea component (compound 3) was identified in UV peak 1. These peaks were of less interest due to their reduced HCV-inhibitory activity compared to that of the other peaks and were not pursued further. Peaks 4 and 6 did not correspond to parent compound BMS-824 yet contained very potent anti-HCV activity; in fact, peak 6 had more potent HCV activity than the parent compound, which is indicative of conversion to a more active species.
Fig. 3.
Fractionation of BMS-824 incubated in medium. BMS-824 was incubated in medium for 48 h and then subjected to extraction with acetonitrile and chloroform, followed by preparative HPLC. The six key peaks identified are labeled P1 to P6. Assignment of peak components indicated: P1, thiourea; P2, oxidized BMS-824; P3, BMS-824. Other peaks did not contain enough material for structure determination. Abs., absorbance.
Table 1.
Anti-HCV activities of HPLC fractions
| Sample | EC50 (μM)a |
Fold resistanceb | WT CC50 (μM)c | |
|---|---|---|---|---|
| WT | Y93H | |||
| P1 | >5 | >5 | 1 | >5 |
| P2 | 4.8 | >5 | 1 | >5 |
| P3 | 0.005 | 5 | 1,000 | >5 |
| P4 | 0.043 | 1.3 | 30 | >5 |
| P5 | 2.8 | >5 | >1.8 | >5 |
| P6 | 0.0006 | 2.3 | 3,833 | >5 |
| BMS-824 | 0.061 | 6.1 | 100 | >50 |
| PI | 0.200 | 0.209 | 1 | >5 |
Major peak fractions identified by HPLC fractionation were titrated on wild-type (WT) and resistant (Y93H) replicon cells and tested for antiviral activity and toxicity. Nonfractionated BMS-824 and an HCV protease inhibitor (PI) were included as controls.
Fold resistance = Y93H EC50/WT EC50.
Due to the amount of P1 to P6, the highest concentration used for CC50 determination was 5 μM.
Structure elucidation.
A second scale-up was performed in order to generate a sufficient quantity of the BMS-824-derived active species to allow structure determination. From ESI-HR-MS, the molecular formula of compounds 4 and 6 was determined to be C62H52N8O8F2S2 [observed mass of compounds 4 and 6, m/z 1,139.3379 (M + H)+; calculated mass, m/z 1,139.3396]. The NMR spectrum (500 MHz, acetone-d6) of compound 4 revealed that all of the substituents; phenacetyl, alanine, fluorobenzene, furan, and the thiazolidinone ring, that were in the starting material, BMS-824 (compound 1), were present. Key differences, however, included the loss of the benzylic methine proton (δ 5.33, BMS-824 [compound 1]) and a 13C resonance shift from δ 51.4 (compound 1) to δ 70.2 (compound 4) (Fig. 4). From these data, it was surmised that compound 4 is a symmetrical (homo)dimer of BMS-824, with dimerization occurring at the benzylic carbon, and this was confirmed by mass spectral data (Fig. 5). In the course of optimizing NMR experimental conditions (i.e., solvent, temperature), we observed that upon prolonged NMR data acquisition with compound 6 in acetonitrile-d3 at 55°C, this compound, similarly shown to be a homodimer of BMS-824 (compound 1), converted to compound 4. This was concluded based on coinjections on HPLC with orthogonal methods, by proton NMR, and mass spectral data. We therefore propose that compounds 4 and 6 are atropisomers rather than diastereomers. The gross structure of compound 4 is depicted in Fig. 6A, and further studies regarding its formation and the stereochemistry at the dimer linkage are currently under way.
Fig. 5.
MS analysis of dimers 4 and 6 (positive-ion electrospray LC-MS and LC-MS/MS).
Fig. 6.
Dimeric structures. (A) Proposed structure of the active component in peaks 4 and 6 based on NMR and MS. (B) Structure of dimeric compound BMS-346.
Biological profile of a dimeric inhibitor.
Due to the dimeric structure of compound 4, it appeared that the symmetry of the molecule was important for achieving potent HCV-inhibitory activity. To test this hypothesis, a simplified dimeric stilbene derivative, BMS-346 (Fig. 6B), was synthesized based on the precise structure-activity relationship (SAR) associated with the amino acid moiety (17). When tested in the HCV replicon assay, BMS-346 had an EC50 of 86 pM (Table 2), an approximately 70-fold enhancement of potency compared to that of BMS-824. In contrast, the EC50 of BMS-346 on the BVDV replicon was >10 μM, demonstrating that the dimeric inhibitor has excellent potency against, as well as selectivity for, HCV. Resistance of the Y93H replicon to BMS-346 indicated the mechanistic relatedness of this compound to BMS-824 and suggested that NS5A is also the target of this compound (Table 2).
Table 2.
Biological activity of BMS-346a
| Replicon | EC50 (μM) | CC50 (μM) |
|---|---|---|
| WT HCV | 0.000086 | >10 |
| Y93H | 4 | >10 |
| BVDV | >10 | >10 |
EC50s were derived from this single experiment. On multiple test occasions, the average EC50s and standard deviations of BMS-346 were as follows: wild type (WT), 0.000052 ± 0.0000076 μM; Y93H, 3.5 ± 3.1 μM.
To further explore the antiviral activity of BMS-346, we used the compound to select for resistance on genotype 1b HCV replicon cells. Mapping of the BMS-346-resistant cell line revealed an L31V NS5A substitution in 6 out of 6 clones, with two of the clones having L31V linked with a Q54L substitution in NS5A. These are the same substitutions previously identified from selection with BMS-824 (10), suggesting that BMS-346 binds in a manner similar to that of the active component of BMS-824. When tested in a transient-replication assay, the single Q54L and L31V mutants conferred 30- to 80-fold resistance to BMS-346, respectively (Fig. 7). However, when the L31V and Q54L mutations were present together, resistance to BMS-346 increased significantly to >400-fold, suggesting that both changes are required to maximally affect compound potency. As expected, none of the NS5A mutants conferred resistance to a control HCV protease inhibitor (data not shown).
Fig. 7.
Resistance analysis of BMS-346 selected substitutions. Huh-7 cells were transfected with wild-type (WT) or mutant replicon RNAs and incubated in the presence or absence of various concentrations of BMS-346. Luciferase activities were determined in lysates of cells harvested at 72 h after transfection, and EC50s were determined. The n-fold resistance of the mutant RNAs relative to that of wild-type RNA (mutant EC50/wild-type EC50) is depicted.
The chemical stability of BMS-346 was also examined by incubating the compound in cell medium for 72 h at 37°C, followed by HPLC fractionation. Under these conditions, less than 3% degradation of the parent structure was observed, indicating that BMS-346 is stable in medium and demonstrating that this novel dimeric species has potent and selective anti-HCV activity.
In summary, the active components in replicon medium were identified whose symmetry afforded the necessary insight to prepare inhibitor BMS-346, a compound chemically inert under assay conditions, and the NS5A-inhibitory activity of BMS-346 was clearly established. Subsequently, this compound provided the basis for the design of BMS-790052, the first NS5A inhibitor to show clinical efficacy.
DISCUSSION
A multiplexed HCV replicon screen was used to identify specific, nontoxic, low-molecular-weight inhibitors such as BMS-824 that target the NS5A protein. Careful evaluation of potency and specificity in a number of additional assays revealed that BMS-824 was chemically reactive under the assay conditions used and transformed into other species in tissue culture medium that were responsible for inhibitory activity. It was the surprising finding that anti-HCV activity was maintained in the absence of detectable parent compound that led us to pursue the identification of the exceptionally active components. To do so, we used a sensitive replicon HPLC biogram assay which allowed us to monitor fractions that contained anti-HCV activity. Inclusion of an NS5A-resistant cell line in the experiments to demonstrate cross-resistance was key to confirming relatedness to BMS-824 and allowing us to identify the peak fractions of interest.
A powerful aspect of the HPLC biogram methodology lies in the ability to use a functional assay to detect biologically active substances in crude matrices that are not readily detectable by physical methods such as, for example, HPLC-UV (5, 8). We began the present study by incubating BMS-824 in HCV replicon assay medium prior to isolation experiments. Two essential sample enrichment steps enabled the HPLC-UV detection and isolation of two minor peaks that correlated with HCV replicon inhibitory activity and allowed us to conduct structure determination using conventional spectroscopic techniques. In this manner, the active species was determined to be a larger, dimeric form of the parent molecule derived from a presumed intermolecular reaction rather than a smaller degraded derivative. The dimerization of BMS-824 is currently believed to occur through a radical mechanism, and this process is the subject of continuing examination in order to determine the precise reaction pathway and define the stereochemical relationships.
The synthesis of BMS-346, a compound that symmetrizes elements of BMS-824 thought to be critical for HCV-inhibitory activity, yielded a compound with excellent activity against HCV (EC50, ∼86 pM), confirming the hypothesis that potent antiviral activity could be derived from a symmetrical dimeric molecule. This was further demonstrated by extensive SAR studies (17). In addition, resistance generation and mapping yielded NS5A resistance substitutions similar to those identified by selection with BMS-824, implying a common binding site for the BMS-824-derived active species and BMS-346. Based on resistance mapping, these NS5A inhibitors appear to interact, either directly or indirectly, with the N terminus of NS5A. The NS5A protein consists of three putative domains (I, II, and III), with the resistance mutations residing in domain I. Domain I consists of the first 213 amino acids of the protein and contains a membrane-anchoring α-helix in the N-terminal 30 amino acids (16). The solid-state structure of domain I was recently determined, and it was shown to form a dimeric complex via contacts near the N-terminal ends of the molecules, which can adopt different conformations (13, 19). The dimeric structure of BMS-346 complements the symmetry observed in domain I, suggesting a functional role that allows association with the NS5A protein across the dimer interface. The mechanism of action of NS5A inhibitors, like the role of the NS5A protein during the life cycle of HCV, is poorly understood. The coincidence of dimeric structures for both the inhibitor and the NS5A protein, as well as the exceptional potency of the inhibitors, provides an opportunity to speculate on and test different working models. Given that the resistance substitutions for BMS-346 lie in the vicinity of the dimer interface, it is possible that these inhibitors interfere with NS5A dimer formation. The exceptional potency of NS5A inhibitors suggests that the anti-HCV effect may be amplified. It is conceivable that NS5A proteins form multimers during the formation of the replication complex and active viral RNA replication. A single inhibitor may not only disrupt the formation of a single NS5A dimer but also affect adjacent NS5A dimers, thereby inactivating the function of an entire replication complex. Alternatively, the compounds may disrupt the proximal α-helix that promotes essential membrane association or interaction of NS5A with an unknown host or viral factor(s) required for HCV RNA replication. Studies are in progress to gain a better understanding of the multiple functions of NS5A and the mode of inhibition of these NS5A inhibitors.
Efforts to identify the active component of compound BMS-824 revealed dimeric molecules generated from an intermolecular dimerization reaction, leading to the synthesis of a novel class of symmetrical molecules that demonstrate excellent potency against HCV and which target the NS5A protein. These discoveries provided the catalyst for an extensive investigation of further structural modifications of BMS-346 in which optimization efforts focused on broadening its genotype coverage and incorporating pharmacokinetic properties suitable for oral administration. BMS-346 formed the foundation for the discovery of BMS-790052, an HCV NS5A inhibitor that exhibits picomolar EC50s toward a broad range of HCV genotypes in vitro and has shown excellent clinical efficacy following oral administration to subjects chronically infected with HCV genotype 1 (6).
ACKNOWLEDGMENTS
We thank our Bristol-Myers Squibb colleagues for helpful discussions during the course of this work and Mark Cockett for his continued support.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Alter M. J. 2007. Epidemiology of hepatitis C virus infection. World J. Gastroenterol. 13:2436–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blight K. J., Kolykhalov A. A., Rice C. M. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972–1974 [DOI] [PubMed] [Google Scholar]
- 3. Folch J., Lees M., Stanley, Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509 [PubMed] [Google Scholar]
- 4. Foster G., Mathurin P. 2008. Hepatitis C virus therapy to date. Antivir. Ther. 13(Suppl. 1):3–8 [PubMed] [Google Scholar]
- 5. Fura A., Shu, Zhu Y.-Z. M., Hanson R. L., Roongta V., Humphreys W. G. 2004. Discovering drugs through biological transformation: role of pharmacologically active metabolites in drug discovery. J. Med. Chem. 47:4339–4351 [DOI] [PubMed] [Google Scholar]
- 6. Gao M., et al. 2010. An inhibitor of NS5A with potent antiviral activity in hepatitis C virus-infected subjects. Nature 465:96–10020410884 [Google Scholar]
- 7. Hoofnagle J. H. 2002. Course and outcome of hepatitis C. Hepatology 36:S21–S29 [DOI] [PubMed] [Google Scholar]
- 8. Hook D. J., More C. F., Yacobucci J. J., Dubay G., O'Connor S. 1987. Integrated biological-physicochemical system for the identification of antitumor compounds in fermentation broths. J. Chromatogr. 385:99–108 [DOI] [PubMed] [Google Scholar]
- 9. Kaneko T., et al. 1994. Production of two phosphoproteins from the NS5A region of the hepatitis C viral genome. Biochem. Biophys. Res. Commun. 205:320–326 [DOI] [PubMed] [Google Scholar]
- 10. Lemm J. A., et al. 2010. Identification of hepatitis C virus NS5A inhibitors. J. Virol. 84:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lohmann V., Korner F., Dobierzewska A., Bartenschlager R. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lohmann V., et al. 1999. Replication of subgenomic hepatitis C virus RNA in a hepatoma cell line. Science 285:110–113 [DOI] [PubMed] [Google Scholar]
- 13. Love R. A., Brodsky O., Hickey M. J., Wells P. A., Cronin C. N. 2009. Crystal structure of a novel dimeric form of NS5A domain I protein from hepatitis C virus. J. Virol. 83(9):4395–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacDonald A., Harris M. 2004. Hepatitis C virus NS5A: tale of a promiscuous protein. J. Gen. Virol. 85:2485–2502 [DOI] [PubMed] [Google Scholar]
- 15. O'Boyle D. R., II, et al. 2005. Development of a cell-based high-throughput specificity screen using a hepatitis C virus-bovine viral diarrhea virus dual replicon assay. Antimicrob. Agents Chemother. 49:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Penin F., et al. 2004. Structure and function of the membrane anchor domain of hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 279:40835–40843 [DOI] [PubMed] [Google Scholar]
- 17. Romine J. L., et al. 2011. Inhibitors of HCV NS5A: from iminothiazolidinones to symmetrical stilbenes. ACS Med. Chem. Lett. 2:224–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanji Y., Kaneko T., Satoh S., Shimotohno K. 1995. Phosphorylation of hepatitis C virus-encoded nonstructural protein 5A. J. Virol. 69:3980–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tellinghuisen T. L., Marcotrigiano J., Rice C. M. 2005. Structure of the zinc-binding domain of an essential replicase component of hepatitis C virus reveals a novel fold. Nature 435:374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]