Abstract
Mycobacterium tuberculosis is the causative agent of a pulmonary epidemic that is estimated to infect one-third of the world's population and that has an increased incidence of multidrug resistance. The evaluation of new chemical entities against M. tuberculosis is hampered by the lack of biological tools to help predict efficacy, from early drug development to clinical trials. As the rat is the animal species of choice in the pharmaceutical industry, we have developed a rat model of acute and chronic phases of M. tuberculosis infection for drug efficacy testing. In this model, we have evaluated the impact of tuberculosis drugs on T cell response using the enzyme-linked immunospot assay methodology. Infected rats treated with isoniazid (INH) or rifampin (RIF) responded to therapy, the potency of which was comparable to that seen in the mouse. Peripheral blood mononuclear cells from infected rats produced gamma interferon (IFN-γ) in response to RD-1 antigens, such as the 6-kDa early secretory antigen target (ESAT-6) and the 10-kDa culture filtrate protein (CFP-10). A decrease in IFN-γ spot-forming cells (SFCs) was consistently observed in response to drug treatment. In both the acute- and chronic-phase models, the T cell response was more sensitive to ESAT-6 than to CFP-10. The SFC count in response to ESAT-6 appears to be an indicator of bacterial killing in the rat. Collectively, our data suggest that the ESAT-6 response could be used as a potential surrogate of drug efficacy in the rat and that such a readout could help shorten drug testing during preclinical development.
INTRODUCTION
Mycobacterium tuberculosis infection is among the world's leading infectious diseases, causing about 2 million deaths annually. The emergence of multidrug-resistant M. tuberculosis strains along with the increase of HIV coinfected cases worsens the situation (42). In countries with a high incidence of tuberculosis (TB), TB control programs rely on a diagnostic methods and drugs that have been developed decades ago and that are inadequate to effectively control the epidemic. The urgent need to develop new diagnostic tools as well as new therapeutic interventions is hampered by long clinical trials, where markers of infection and drug response are lacking (38).
For decades, the tuberculin skin test (TST) has been used to diagnose TB (18). The TST measures cell-mediated immunity in the form of a delayed-type hypersensitivity response to the purified protein derivative (PPD), a crude mixture of antigens shared among M. tuberculosis, Mycobacterium bovis BCG, and several nontuberculous mycobacteria (NTM). As a result, the TST has lower specificity in populations with high BCG coverage and NTM exposure and shows poor sensitivity in immunocompromised individuals (30). The gamma interferon (IFN-γ) enzyme-linked immunospot (ELISpot) assay has emerged as an alternative to the TST. The assay consists of in vitro stimulation of peripheral blood mononuclear cells (PBMCs) using RD-1 antigens, the 6-kDa early secretory antigen target (ESAT-6) and the 10-kDa culture filtrate protein (CFP-10), which are early secretory antigens specific to M. tuberculosis (41). The presence of specific IFN-γ spot-forming cells (SFCs) is indicative of an M. tuberculosis infection and seems to correlate better than the TST with the level of exposure to M. tuberculosis (3). In this context, the IFN-γ ELISpot assay is considered to be a major advance in TB diagnostics.
The IFN-γ ELISpot assay has also been tested as a means to monitor the response to TB therapy, where efficacy is classically evaluated by measuring rates of relapse 6 to 12 months after treatment completion (11, 43). On the basis of the hypothesis that the IFN-γ ELISpot assay could function as a bacterial load sensor, a comparatively smaller amount of SFCs in response to ESAT-6 and/or CFP-10 over time would be indicative of a good response to treatment, leading to a lower probability of relapse. Though encouraging, the readings of recent exploratory studies were confounded by immune status, stage of infection, and previous drug treatment; and no clear conclusion was reached with regard to the predictive value of the ELISpot assay as a marker of therapy response (1, 5, 13, 19, 28). Relapse as a clinical trial endpoint is a key issue in drug development. Under optimal treatment conditions and compliance, relapse rates for nonmultidrug-resistant patients lie at about 2 to 4%. Therefore, efficacy trials aimed at significantly improving relapse rates require the recruitment of large patient cohorts and 4 to 5 years for completion. Hence, the identification of early markers of drug response would have a tremendous impact on therapeutic interventions and on the shortening of long clinical trials (40).
With a widely accepted predictive value in toxicology and pharmacokinetics, the rat has been a species of choice for early drug development. It has also helped to identify markers of disease and surrogate markers of drug response in chronic inflammation and age-related diseases (2, 17, 26). Recent studies, including some from our group, have also shown that M. tuberculosis-infected rats develop organized granulomas within the lungs while controlling the infection over time (32). This suggests that the rat may be a good alternative to the mouse model for in vivo drug efficacy studies (4, 34). The rat offers the additional advantage of providing larger volumes of body fluids and larger numbers of PBMCs than the mouse, which makes the model attractive to study the response of circulating immune cells as it is performed in tuberculosis patients.
In this context, we have established a model of acute and chronic phases of M. tuberculosis infection in the rat to test the pharmacokinetic and pharmacodynamic properties of antituberculosis compounds in early drug discovery stages. Here we report on the development of a rat version of the ELISpot assay used in clinical settings. We observe that this immunological assay appears to be suitable to understand some aspects of drug-mediated killing in the rat models of acute and chronic phases of M. tuberculosis infection. Our results show that while the current ELISpot assay has limitations in predicting drug efficacy, the rat model is a suitable tool to optimize immunological assays which could then be used in clinical studies for monitoring drug efficacy.
MATERIALS AND METHODS
Animals.
Female Wistar rats 8 to 10 weeks of age were purchased from the Biological Resources Centre Singapore and housed in individually ventilated cages within the biosafety level 3 facility. Studies described in this report were approved and performed according to the guidelines and policies of the Institutional Animal Care and Use Committee (IACUC).
Peptides and bacterial strains.
Synthetic peptides were synthesized by Mimotopes Pte. Ltd. (Clayton, Victoria, Australia) on the basis of the amino acid sequences. Each peptide contains 20 amino acid (aa) residues, with 10 residues overlapping adjacent peptides covering the full length of the corresponding proteins. The H37Rv M. tuberculosis strain (catalog no. 27294; ATCC) was cultured in 7H9 medium enriched with 10% ADS (albumin, dextrose, saline) and 0.05% Tween 80 (Sigma-Aldrich) for 4 to 5 days at 37°C. Mycobacteria were grown to an optical density at 600 nm (OD600) of 0.3 to 0.5 in 7H9-oleic acid-albumin-dextrose-catalase (OADC) medium and centrifuged at 2,200 × g for 10 min, and the pellet was washed once in warm 7H9-OADC medium. The washed pellet was resuspended at an OD600 of 1 (equivalent to 1.47 × 108 CFU) in 7H9-OADC medium supplemented with 15% glycerol and frozen at −80°C. On the day of infection, 1 ml of culture stock was thawed and added to 9 ml of 7H9 medium, before being subjected to sonication for 30 s. It was then serially diluted to obtain a final inoculum concentration of 103 CFU/ml. For the culture of M. tuberculosis, both 7H9 medium and 7H11 medium were obtained from Difco (Becton Dickinson). OADC (Becton Dickinson) and Tween 80 (Sigma-Aldrich) were used as supplements. For the in vitro culture of rat immune cells, serum-free medium (Cellular Technology Ltd.) was used. Rifampin (RIF) and isoniazid (INH) (Sigma-Aldrich) were formulated in 0.5% carboxymethyl cellulose (CMC).
Bacterial infection and drug treatment of rats.
The cocktail used for anesthetizing rats comprises ketamine (87.5 mg/kg of body weight), diazepam (2.5 mg/kg), and atropine (0.4 mg/kg) diluted in 0.4 ml of 1× phosphate-buffered saline (PBS; Invitrogen). Using a 1-ml syringe, the inoculum (100 μl) was administered via the endotracheal route at a concentration of 104 CFU/ml. Drugs were administered orally once a day for 28 days in 0.5% CMC at 10 mg/kg or 25 mg/kg for INH and 25 mg/kg for RIF. Treatment started 7 days postinoculation in the acute-phase model and 28 days postinoculation in the chronic-phase model.
CFU count.
Rats were euthanized by CO2 inhalation at selected time points. Lungs were removed under aseptic conditions, placed in 5 ml of PBS enriched with 0.05% Triton X-100, and homogenized using a gentleMACS dissociator (Miltenyi Biotec, Germany). The homogenates were serially diluted using 7H9 medium and plated on 7H11 agar enriched with 10% OADC. Plates were incubated at 37°C with 5% CO2 supplementation for 3 weeks before CFU counts were determined. The remaining lung homogenates were stored at −80°C until the end of the experiment, once all CFU data had been collected and analyzed.
IFN-γ ELISpot assay and enzyme-linked immunosorbent assay (ELISA).
At selected time points, 5 ml of blood was collected from a group of 3 to 5 rats and PBMCs were isolated from EDTA-treated blood by standard Ficoll-gradient centrifugation (GE Healthcare, United Kingdom). Cells were washed with PBS, resuspended in serum-free medium (Cellular Technology Ltd.), and counted prior to being assayed with a rat IFN-γ ELISPOT assay kit according to the manufacturer's instructions (Mabtech AB, Sweden). Briefly, PBMCs were seeded in duplicate at 2.0 × 105 cells/wells with different M. tuberculosis peptides at a concentration of 10 μg/ml. Plates were incubated for 24 h at 37°C with 5% CO2 supplementation. After incubation, plates were washed with PBS and biotin-labeled anti-rat IFN-γ antibody was added at 1 μg/ml. Then, streptavidin-alkaline phosphatase was added and the plates were further incubated for 1 h at room temperature. Color development was performed. The number of IFN-γ spot-forming cells was determined using an automated CTL-ImmunoSpot S5 microanalyzer (Cellular Technology Ltd.) designed to detect spots with predetermined criteria based on size, shape, and colorimetric density and expressed per 1 million cells.
The circulating level of IFN-γ was evaluated with the IFN Duoset ELISA according to the manufacturer (R&D).
Statistical analysis.
A two-tailed Student t test was used for statistical analysis. A P value of <0.05 was considered significant. The average of duplicates for SFC counts was calculated for each stimulant and each rat, and the mean for each group of animals was normalized to 106 PBMCs ± standard deviation (SD).
RESULTS
Rat IFN-γ ELISpot assay as an indirect readout of M. tuberculosis infection.
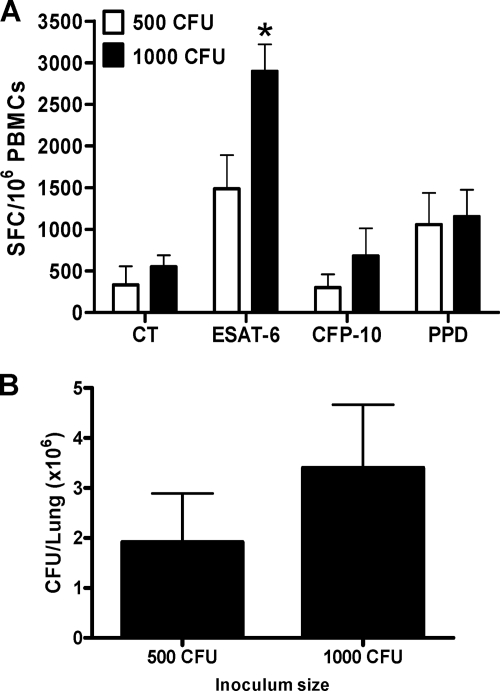
The IFN-γ ELISpot assay is based on IFN-γ release after PBMC stimulation with specific M. tuberculosis-derived antigens. Hence, the level of IFN-γ response is thought to reflect the antigen load available to the immune system. We determined if the SFC number in response to stimulation with a pool of synthetic peptides derived from ESAT-6 and CFP-10 was proportional to the bacterial load and could therefore be used to monitor the response to drug treatment in the TB rat model. Two M. tuberculosis H37Rv starting inocula of 5 × 102 CFU and 1 × 103 CFU were used, and PBMCs were isolated during the peak of infection or 1 month postinoculation (Fig. 1A). At that time, we observed that the number of CFU/lung was higher in rats that had received the higher inoculum (Fig. 1B). Correspondingly, the number of SFCs produced in response to ESAT-6 was significantly higher in high-inoculum than in low-inoculum animals (P < 0.05; Fig. 1A). The CFP-10 response was low in both cases. Overall, the ESAT-6 response was higher with a starting inoculum of 103 CFU and appeared to be more sensitive to the bacterial load than the CFP-10 response. Wistar rats were then infected with an inoculum of 103 CFU for the remainder of the study.
Fig. 1.
Stimulation of rat PBMCs with TB antigens. (A) PBMCs from infected rats (3 rats) are stimulated in vitro for 24 h with ESAT-6, CFP-10, or PPD at 10 μg/ml. IFN-γ ELISpot assay was performed at 1 month postinfection with 500 CFU (white bars) or 103 CFU (black bars) of the H37Rv M. tuberculosis strain. (B) The bacterial burden (CFU) was enumerated at 1 month postinfection. Three rats per group were used, and mean numbers of SFCs/106 PBMCs or CFU/lungs ± SDs are shown *, P < 0.05 (Student's t test), comparison between unstimulated and stimulated cells. The experiment was carried out twice. CT, control (unstimulated) group.
Drug efficacy and ELISpot assay response in the rat model of acute phase of M. tuberculosis infection.
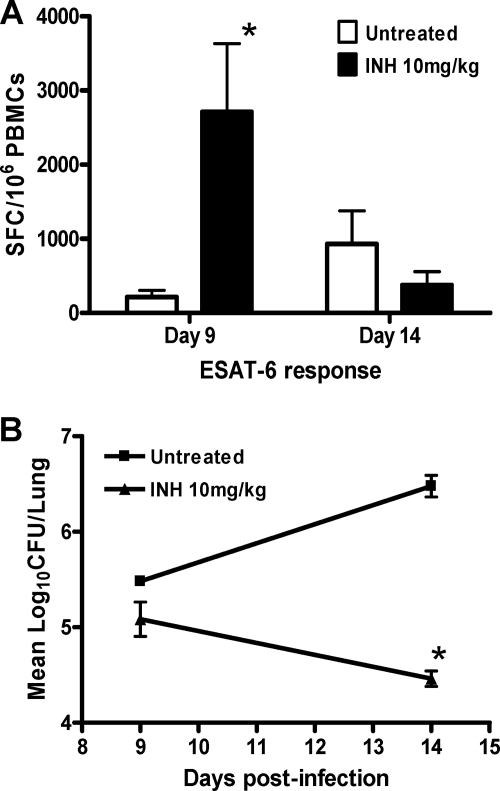
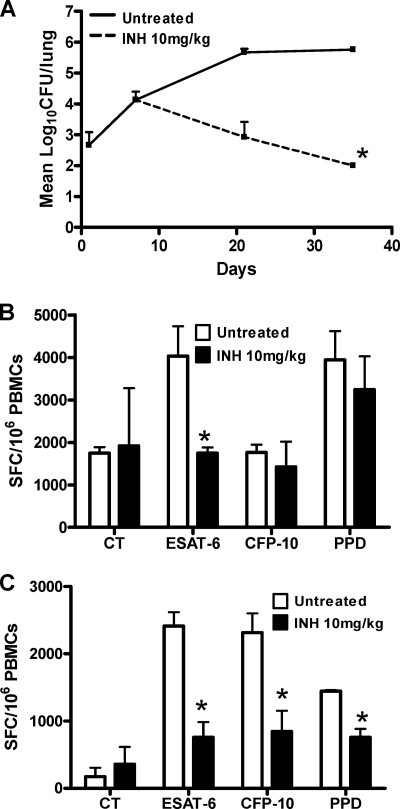
In order to determine whether the IFN-γ ELISpot assay could be used to monitor chemotherapy and drug-mediated killing, we first treated rats with 10 mg/kg INH during the acute phase of infection and investigated the early specific ESAT-6 response at 48 h and 7 days posttreatment. The number of SFCs observed after 48 h of INH treatment (day 9 postinfection) is significantly higher than that in untreated infected animals (P = 0.024; Fig. 2A). After 1 week of treatment (day 14 postinfection), we did observe a significant decrease of bacterial load in the INH-treated group (Fig. 2B), with concomitant drops in the IFN-γ response and SFC counts which were below those observed in untreated infected animals. These results suggest that, in the TB rat model, INH-mediated killing appears to produce a burst of antigen release which in turn is reflected in transiently high SFC counts. Rats were then treated for a month with 10 mg/kg INH during the acute phase of infection, from day 7 postinoculation onwards. As expected, INH at 10 mg/kg was very effective at killing M. tuberculosis within 4 weeks (Fig. 3A), as previously described in the mouse model of the acute phase of M. tuberculosis infection (16). Using PBMCs isolated from M. tuberculosis-infected rats, we tested the impact of INH on the number of IFN-γ-producing cells specific to ESAT-6 and CFP-10 at 2 and 4 weeks posttreatment. A significant decrease in the number of IFN-γ-producing cells after ESAT-6 stimulation was observed in the treated group compared to the nontreated group after 2 weeks of treatment (P = 0.04; Fig. 3B), but no significant difference was observed following CFP-10 stimulation. After 4 weeks of treatment, the SFC number was reduced when PBMCs were stimulated with either ESAT-6, CFP-10, or PPD (P = 0.019, P = 0.025, and P = 0.02, respectively; Fig. 3C). These observations suggested that the IFN-γ ELISpot assay can be used as a tool to monitor drug efficacy during the acute phase of infection and that the ESAT-6 response is the most sensitive to drug-mediated killing.
Fig. 2.
Early ESAT-6 response after isoniazid treatment during the acute phase of infection. Female Wistar rats (n = 4) were infected with 103 CFU of M. tuberculosis strain H37Rv. At day 7, rats were orally treated with isoniazid at 10 mg/kg for 1 week. The ELISpot assay was performed at day 9 (48 h after treatment initiation) and at day 14, and PBMCs were stimulated in vitro in duplicate. ESAT-6 peptides were used at 10 μg/ml. (A) Mean numbers of SFCs/106 PBMCs and SDs per group and per time point are shown. (B) Bacterial burden was enumerated for each time point, and mean numbers of CFU and SDs are shown. *, P < 0.05 (Student's t test), statistical difference between treated and untreated groups. The experiment was carried out twice independently.
Fig. 3.
IFN-γ ELISpot assay following isoniazid treatment during the acute phase of infection. Female Wistar rats were infected with 103 CFU of M. tuberculosis strain H37Rv. At day 7, rats were orally treated with isoniazid for 4 weeks. Lungs were harvested at days 1, 7, 21, and 35 for CFU counts. (A) Bacterial burden was enumerated for each time point, and mean numbers of CFU and SDs are shown. (B and C) The ELISpot assay was performed at day 21 (B) and at day 35 (C), and PBMCs were stimulated in vitro in duplicate. ESAT-6, CFP-10, and PPD were used at 10 μg/ml. Four animals were used at each time point, and the mean numbers of SFCs/106 PBMCs ± SDs are shown. *, P < 0.05 (Student's t test), comparison between treated and untreated groups. The experiment was repeated independently twice. CT, control (unstimulated) group.
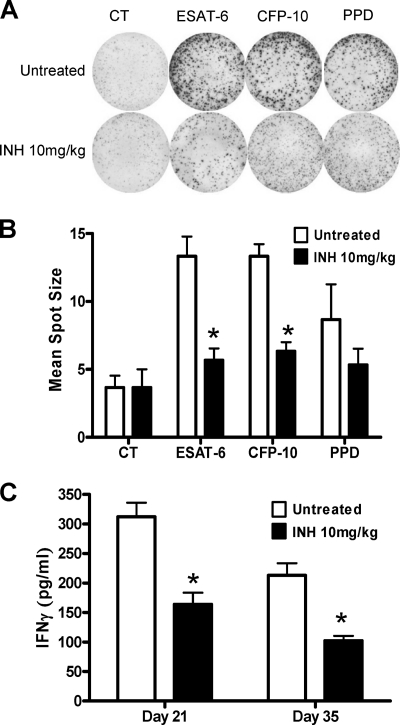
We also observed that the average diameter of PBMC spots was smaller for INH-treated animals than for infected untreated rats. This suggests that the amount of IFN-γ produced by a single immune cell from a treated rat is smaller than the one produced by PBMCs of untreated infected rats (Fig. 4A). When we assessed the impact of INH treatment on the average spot diameter after 4 weeks of treatment, we observed a dramatic reduction in spot size diameter in response to both ESAT-6 and CFP-10 (Fig. 4B). We also evaluated the serum levels of IFN-γ during treatment, and we observed that serum IFN-γ decreased when infected rats were treated with INH (Fig. 4C). Taken together, our results indicated that INH treatment reduced the proportion of SFCs in response to ESAT-6 and CFP-10 and that these specific circulating cells were producing less cytokine, resulting in a decrease of secreted IFN-γ in serum.
Fig. 4.
Impact of isoniazid treatment on IFN-γ response in vitro and in vivo. (A) The densities of spot-forming cells before and after isoniazid treatment at day 35 are shown for ESAT-6, CFP-10, and PPD stimulation in duplicate at 10 mg/ml. (B) The mean spot sizes (in square millimeters) in the IFN-γ ELISpot assay are compared between stimulated PBMCs from the isoniazid-treated (black bars) and untreated (white bars) infected animals. The mean spot sizes (from duplicates of each animal) and SDs are shown. (C) Serum IFN-γ measured by ELISA 2 weeks and 4 weeks after isoniazid treatment start. Four animals were used at each time point, and the mean spot sizes or serum IFN-γ concentrations are shown. *, P < 0.05 (Student's t test), comparison between treated and untreated groups. The experiment was carried out twice independently. CT, control (unstimulated) group.
Drug efficacy and ELISpot assay response in the rat model of chronic phase of M. tuberculosis infection.
We then tested the ELISpot assay in a model of the chronic phase of infection where the rats were infected and treated for 4 weeks starting at 1 month postinfection. We observed a slow drop of lung CFU counts following treatment with 25 mg/kg of INH once daily (Fig. 5A) that was significant only after 4 weeks of treatment (P < 0.05). This is in agreement with what is seen in the mouse model, where INH is less efficient during the chronic phase of infection. Interestingly, a significant decrease in ESAT-6 response was detected after 2 weeks of INH therapy (P = 0.023; Fig. 5B), a time point at which the CFU drop was not yet affected significantly. After 4 weeks of therapy, both lung CFU and ESAT-6 responses were significantly lower (P = 0.0286 and P = 0.015, respectively). The CFP-10 response was significantly lower only after 4 weeks of therapy (P = 0.038; Fig. 5C). These observations show that a significant decrease in ESAT-6 response preceded the significant drop in lung CFU, suggesting that the ESAT-6 response is very sensitive to INH-mediated killing.
Fig. 5.
IFN-γ ELISpot assay following isoniazid treatment during the chronic phase of infection. Female Wistar rats (n = 4) were inoculated with 103 CFU of M. tuberculosis strain H37Rv. At day 28, rats were treated with isoniazid at 25 mg/kg for 4 weeks. Lungs were harvested at 2 and 4 weeks after treatment initiation to evaluate in vivo efficacy. (A) CFU counts. (B and C) The numbers of SFCs/106 PBMCs in the IFN-γ ELISpot assay were evaluated in duplicate for each stimulation at day 44 (B) and at day 58 (C). Average numbers of CFU and SFCs ± SDs per group and per time point are shown. *, P < 0.05 (Student's t test), statistically significant difference between treated and untreated groups. Treatment during the chronic phase of infection was carried out twice. CT, control (unstimulated) group.
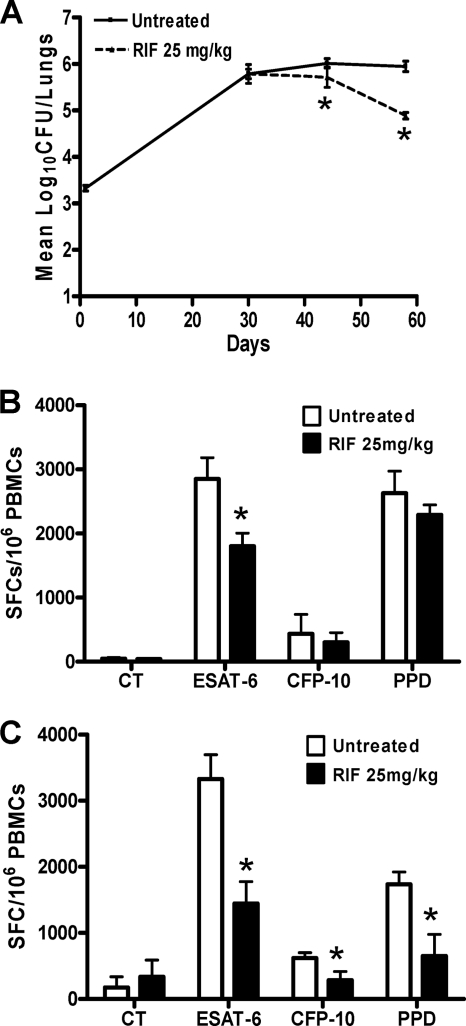
Treatment with RIF at 25 mg/kg daily for a month was more efficient in reducing lung CFU over time than treatment with INH (P = 0.0003; Fig. 6A), which is in agreement with RIF generally being recognized as more active during the chronic phase of infection in the mouse model (15, 20, 25). When the T-cell response was tested 2 weeks after RIF therapy (Fig. 6B), we detected a significant drop following ESAT-6 stimulation, which was confirmed at 4 weeks with CFP-10 stimulation as well (P = 0.009 and P = 0.011, respectively; Fig. 6C). This mirrored the reduction of lung bacterial load in response to RIF treatment. Overall, treatment with either INH or RIF caused a CFU reduction, which is reflected by a drop in the number of specific IFN-γ-secreting cells in response to stimulation with RD-1 antigens, with the ESAT-6 response being the most sensitive to therapy.
Fig. 6.
Impact of rifampin treatment on T cell response during the chronic phase of infection. Female Wistar rats (n = 4) were infected with 103 CFU of M. tuberculosis strain H37Rv. After 1 month of infection, the rats were orally treated with 25 mg/kg of rifampin for 4 weeks. (A) Bacterial burden was enumerated at day 58 after 2 and 4 weeks of treatment, and mean numbers of CFU and SDs are shown. (B and C) PBMCs were stimulated in duplicate in vitro with ESAT-6, CFP-10, and PPD at 10 μg/ml at 2 weeks (B) and 4 weeks (C) posttreatment. Four animals per time point of each group were used, and the mean numbers of SFCs/106 PBMCs ± SDs are shown. *, P < 0.05 (Student's t test), statistically significant difference between treated and untreated groups. The experiment was carried out twice independently. CT, control (unstimulated) group.
DISCUSSION
In this study, we have shown that the efficacy of isoniazid and rifampin in the TB rat model was comparable to what has been reported in the mouse. We have then used this rat model to compare the ELISpot assay readout before and after treatment and evaluate whether there is a link between drug-mediated killing and T cell response. A direct correlation between ELISpot assay and CFU counts was not established in this study, as we had a limited set of data points for only one drug dose. However, the reduction of bacterial counts within the lung translated into a reduction of peripheral T cell response to ESAT-6 and CFP-10 stimulation, with the ESAT-6 response being more sensitive to treatment. The apparently less sensitive and slower CFP-10-mediated response appears to be in agreement with results reported in several clinical studies, where PBMCs from active TB cases responded more specifically to ESAT-6 (19, 31, 36). While the ESAT-6 response was shown to be dependent on CD4+ T cells (7, 23), it is not clear how T cells react to CFP-10 epitopes (14, 45). In our rat model, analysis of cell recruitment into the lungs has shown that CD4+/IFN-γ+ T cells seem to be recruited rapidly within 2 weeks postinfection, which could favor an early response to ESAT-6 (A. Singhal, unpublished data). Therefore, drug-mediated killing of M. tuberculosis in rat lung could be reflected in the early peripheral T cell response to ESAT-6. We also detected a specific T cell burst after 2 days of INH treatment. This rapid and transient increase could potentially be exploited as an indicator of drug-mediated killing in good responders. Accordingly, a clinical study by Wilkinson et al. (43) revealed an increase in T cell response immediately after the onset of treatment, likely as a result of antigen release following bacterial killing and lysis (8, 39). The ELISpot assay has also been used to investigate B cell response following TB chemotherapy, where a rapid change of antibody production only a few days after the beginning of treatment was indicative of bacterial killing and antigen release (33). We observed that the number of peripheral IFN-γ SFCs as well as the circulating levels of IFN-γ in serum was reduced at 1 month postinfection. The recruitment of IFN-γ-positive T cells to the site of infection might be responsible for the apparent reduction of T cell response which we observed in the blood (27, 29). However, regulatory T cells which develop during active disease may also prevent the specific IFN-γ SFC response to RD-1 antigens (7, 21). Direct stimulation of pulmonary immune cells in our assay should confirm whether there is a concomitant increase in lung-specific IFN-γ SFC numbers, as recently shown in the monkey model (22). As the RD-1 antigens are secreted by the bacilli, a decrease of bacterial load due to drug-mediated killing would translate into a decrease of T cell stimulation and thus fewer IFN-γ SFCs specific to ESAT-6/CFP-10. The reactivity of effector T cells would then decrease as a result of antigen withdrawal as the level of production of IFN-γ from a single immune cell decreased after treatment. It has been shown that IFN-γ production after 24 h of stimulation fades upon treatment and correlates with the development of memory T cells (10). Cytokine production might also change after treatment, as recently suggested (24). Further studies would help to evaluate the impact of treatment on different T cell populations and on the role of newly described polyfunctional T cells (44). During the chronic phase of infection, the ESAT-6 response was significantly affected after 2 weeks of INH therapy, while a significant drop in lung CFU was seen only 4 weeks later. A recent study challenged the theory of static equilibrium—i.e., no killing, no growth—during the chronic phase of infection and showed that (i) M. tuberculosis does replicate during this stationary phase and (ii) the apparently static number of bacteria found in the lungs is thought to be a balance between growth and immune-mediated killing (9). In light of these observations, the replicating subpopulation of bacteria could be rapidly affected by INH therapy, causing a decrease in the ESAT-6 response, while the total lung CFU count is only slightly affected. In this context, the ESAT-6 response could be used to predict drug efficacy, as it precedes a significant decrease in the numbers of lung CFU. While the TB mouse model is by far the most convenient and widespread experimental tool, mice do not develop organized, necrotic, and hypoxic lesions (6, 35, 37). It is only recently that the rat has been investigated as a potentially predictive model of TB infection (4, 34). Our group has recently shown that Wistar rats can form organized granulomas. We have also observed that the Wistar rat can develop subclinical manifestations of human disease with no detectable CFU in the lung, where bacilli can be reactivated upon immunosuppression (32). Furthermore, we tested the hypothesis that during the chronic phase of infection the Wistar rat could develop hypoxic lesions that may favor dormancy and phenotypic drug tolerance. We did observe a pimonidazole staining within rat granulomas, indicative of low oxygen tension (12). Therefore, the Wistar rat could be a relevant preclinical model to test new chemical entities and evaluate drug response through new innovative biological assays based on the ELISpot assay concept.
In summary, we have shown that the efficacy of INH and RIF in the Wistar rat model of TB infection is comparable to what is seen in the mouse model and that a reduction of the ESAT-6 response accompanies CFU reduction in the lung. Such an assay could reduce the timelines of preclinical drug testing in animal models and could be used to evaluate how future treatment-shortening drugs would affect the assay readout. Importantly, our results suggest that the rat model is a useful tool to modify and optimize the ELISpot assay and to develop a predictive biomarker of drug response to be tested in clinical studies.
ACKNOWLEDGMENTS
We thank Suresh B. Lakshminarayana, Anne Goh, and the whole pharmacology team for their technical support. We also thank Antonio Bertoletti and his lab members for technical assistance and R. J. Wilkinson for critical review of the manuscript.
The authors declare no competing financial interest.
Footnotes
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Aiken A. M., et al. 2006. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect. Dis. 6:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aleixandre de Artinano A., Miguel Castro M. 2009. Experimental rat models to study the metabolic syndrome. Br. J. Nutr. 102:1246–1253 [DOI] [PubMed] [Google Scholar]
- 3. Andrade D. R., Jr., Santos S. A., Andrade D. R. 2008. Measurement of peripheral blood mononuclear cells producing IFN-gamma in patients with tuberculosis. Braz. J. Infect. Dis. 12:123–127 [DOI] [PubMed] [Google Scholar]
- 4. Elwood R. L., et al. 2007. The American cotton rat: a novel model for pulmonary tuberculosis. Tuberculosis (Edinb.) 87:145–154 [DOI] [PubMed] [Google Scholar]
- 5. Ferrand R. A., Bothamley G. H., Whelan A., Dockrell H. M. 2005. Interferon-gamma responses to ESAT-6 in tuberculosis patients early into and after anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis. 9:1034–1039 [PubMed] [Google Scholar]
- 6. Flynn J. L. 2006. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 8:1179–1188 [DOI] [PubMed] [Google Scholar]
- 7. Gallegos A. M., Pamer E. G., Glickman M. S. 2008. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J. Exp. Med. 205:2359–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garbe T. R., Hibler N. S., Deretic V. 1996. Isoniazid induces expression of the antigen 85 complex in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:1754–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gill W. P., et al. 2009. A replication clock for Mycobacterium tuberculosis. Nat. Med. 15:211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goletti D., et al. 2006. Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J. Infect. Dis. 194:984–992 [DOI] [PubMed] [Google Scholar]
- 11. Goletti D., et al. 2007. Isoniazid prophylaxis differently modulates T-cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis: a pilot study. Respir. Res. 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heng Y., et al. 31 May 2011. Mycobacterium tuberculosis infection induces hypoxic lung lesions in the rat. Tuberculosis [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 13. Kalsdorf B., et al. 2009. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med. 180:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamath A. B., et al. 2004. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J. Exp. Med. 200:1479–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karakousis P. C., Williams E. P., Bishai W. R. 2008. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J. Antimicrob. Chemother. 61:323–331 [DOI] [PubMed] [Google Scholar]
- 16. Khor M. S., Lowrie D. B., Mitchison D. A. 1988. Gamma interferon in experimental murine tuberculosis. Bull. Int. Union Tuber. Lung Dis. 63:11–15 [PubMed] [Google Scholar]
- 17. Kucharewicz I., Bodzenta-Lukaszyk A., Buczko W. 2008. Experimental asthma in rats. Pharmacol. Rep. 60:783–788 [PubMed] [Google Scholar]
- 18. Lalvani A., Pareek M. 2010. A 100 year update on diagnosis of tuberculosis infection. Br. Med. Bull. 93:69–84 [DOI] [PubMed] [Google Scholar]
- 19. Lalvani A., et al. 2001. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163:824–828 [DOI] [PubMed] [Google Scholar]
- 20. Lenaerts A. J., et al. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li L., Wu C. Y. 2008. CD4+ CD25+ Treg cells inhibit human memory gammadelta T cells to produce IFN-gamma in response to M tuberculosis antigen ESAT-6. Blood 111:5629–5636 [DOI] [PubMed] [Google Scholar]
- 22. Lin P. L., et al. 2009. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect. Immun. 77:4631–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lugos M. D., et al. 2009. Evaluation of the contribution of major T cell subsets to IFN-gamma production in TB infection by ELISPOT. Immunol. Invest. 38:341–349 [DOI] [PubMed] [Google Scholar]
- 24. Millington K. A., et al. 2007. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 178:5217–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchison D. A. 1985. Mechanisms of the action of drugs in the short-course chemotherapy. Bull. Int. Union Tuberc. 60:36–40 (In French.) [PubMed] [Google Scholar]
- 26. Moisan M. P., Ramos A. 2010. Rat genomics applied to psychiatric research. Methods Mol. Biol. 597:357–388 [DOI] [PubMed] [Google Scholar]
- 27. Nemeth J., et al. 2009. Recruitment of Mycobacterium tuberculosis specific CD4+ T cells to the site of infection for diagnosis of active tuberculosis. J. Intern. Med. 265:163–168 [DOI] [PubMed] [Google Scholar]
- 28. Nicol M. P., et al. 2005. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin. Infect. Dis. 40:1301–1308 [DOI] [PubMed] [Google Scholar]
- 29. Ordway D., et al. 2007. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. J. Immunol. 179:2532–2541 [DOI] [PubMed] [Google Scholar]
- 30. Pai M., Riley L. W., Colford J. M., Jr 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761–776 [DOI] [PubMed] [Google Scholar]
- 31. Ravn P., et al. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637–645 [DOI] [PubMed] [Google Scholar]
- 32. Singhal A., et al. 2011. Experimental tuberculosis in the Wistar rat: a model for protective immunity and control of infection. PLoS One 6:e18632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sousa A. O., et al. 2000. Kinetics of circulating antibodies, immune complex and specific antibody-secreting cells in tuberculosis patients during 6 months of antimicrobial therapy. Tuber. Lung Dis. 80:27–33 [DOI] [PubMed] [Google Scholar]
- 34. Sugawara I., Udagawa T., Yamada H. 2004. Rat neutrophils prevent the development of tuberculosis. Infect. Immun. 72:1804–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsai M. C., et al. 2006. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell. Microbiol. 8:218–232 [DOI] [PubMed] [Google Scholar]
- 36. Ulrichs T., et al. 1998. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur. J. Immunol. 28:3949–3958 [DOI] [PubMed] [Google Scholar]
- 37. Via L. E., et al. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wallis R. S., et al. 2009. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect. Dis. 9:162–172 [DOI] [PubMed] [Google Scholar]
- 39. Wallis R. S., et al. 2001. Inhibition of isoniazid-induced expression of Mycobacterium tuberculosis antigen 85 in sputum: potential surrogate marker in tuberculosis chemotherapy trials. Antimicrob. Agents Chemother. 45:1302–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walzl G., Ronacher K., Djoba Siawaya J. F., Dockrell H. M. 2008. Biomarkers for TB treatment response: challenges and future strategies. J. Infect. 57:103–109 [DOI] [PubMed] [Google Scholar]
- 41. Warier A., Gunawathi S., Venkatesh, John K. R., Bose A. 2010. T-cell assay as a diagnostic tool for tuberculosis. Indian Pediatr. 47:90–92 [DOI] [PubMed] [Google Scholar]
- 42. Wells C. D., et al. 2007. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J. Infect. Dis. 196(Suppl. 1):S86–S107 [DOI] [PubMed] [Google Scholar]
- 43. Wilkinson K. A., et al. 2006. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J. Infect. Dis. 193:354–359 [DOI] [PubMed] [Google Scholar]
- 44. Wilkinson K. A., Wilkinson R. J. 2010. Polyfunctional T cells in human tuberculosis. Eur. J. Immunol. 40:2139–2142 [DOI] [PubMed] [Google Scholar]
- 45. Woodworth J. S., Fortune S. M., Behar S. M. 2008. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect. Immun. 76:4199–4205 [DOI] [PMC free article] [PubMed] [Google Scholar]