Abstract
A G88C mutation in GyrA is one of the key alterations by which Mycobacterium tuberculosis mutants acquire DC-159a resistance in vitro. A novel double mutation in GyrA, G88C D94H, conferred high DC-159a resistance. Different mutation patterns in GyrA were demonstrated for DC-159a-resistant mutants and quinolone-resistant multidrug-resistant (QR-MDR) M. tuberculosis isolates, with a mutation either at position 90 or 94 and double mutations at 90 and 91 or at 90 and 94. DC-159a might be promising for QR M. tuberculosis treatment.
TEXT
Recently, DC-159a, representing a new spectrum of respiratory quinolones, was demonstrated to have potent in vitro and in vivo activity against quinolone-resistant (QR) Mycobacterium tuberculosis, as well as against multidrug-resistant (MDR) M. tuberculosis (3, 4). DC-159a exhibits notably high inhibitory activity against altered DNA gyrases with the substitution(s) Ala90Val and/or Asp94Gly in GyrA, as well as the wild-type enzyme of M. tuberculosis (11). However, the mechanism of resistance to DC-159a in M. tuberculosis is not yet fully understood. In this study, we investigated the molecular characteristics involved in DC-159a resistance in M. tuberculosis.
Pansusceptible M. tuberculosis H37Rv (JATA no. KK11-291) was used as the wild-type strain. Eleven clinical isolates of QR-MDR M. tuberculosis derived from Japanese patients were also used. All the strains were grown in 7H9 broth supplemented with glycerol and albumin-dextrose-catalase and on 7H10 agar supplemented with glycerol and oleic acid-albumin-dextrose-catalase. Ciprofloxacin (CIP; Bayer), gatifloxacin (GAT; Kyorin), DC-159a (Daiichi-Sankyo), and levofloxacin (LVX; Daiichi-Sankyo) were obtained from the respective pharmaceutical companies. The MICs of tested fluoroquinolones were determined by the agar dilution method (3). Approximately 104 CFU/2 μl of mycobacterial suspensions were inoculated with a microplanter (MIT-P; Sakuma Seisakusho) onto 7H10 agar plates containing 2-fold serial dilutions of each fluoroquinolone. All the plates were incubated at 37°C for 3 weeks.
Mutant strains of susceptible M. tuberculosis H37Rv were developed by the multistep resistance selection method on 7H10 agar plates containing DC-159a at 1×, 4×, 16×, and 64× MIC. Three primary mutants at 4× MIC, 13 secondary mutants at 16× MIC, and 5 tertiary mutants at 64× MIC were generated after exposure to DC-159a. The MIC ranges of DC-159a against the parent strain and the primary, secondary, and tertiary DC-159a mutants were 0.03, 1, 0.5 to 2, and 2 to 8 μg/ml (Table 1), respectively. They indicated a multistep resistance mechanism of DC-159a. The MIC range of DC-159a against QR-MDR M. tuberculosis isolates was 0.06 to 1 μg/ml, which was virtually equivalent to those of LVX, CIP, and GAT against H37Rv (0.03 to 0.5 μg/ml) (Table 1). DC-159a demonstrated the highest activity against QR-MDR M. tuberculosis isolates when compared with existing fluoroquinolones.
Table 1.
Properties of mutants of M. tuberculosis H37Rv selected for resistance by stepwise exposure in vitro to DC-159a
| Strain(s) | MIC (μg/ml) ofa: |
Mutation(s) in the QRDR of GyrA | |||
|---|---|---|---|---|---|
| LVX | CIP | GAT | DC-159a | ||
| Parent strain H37Rv | 0.5 | 0.5 | 0.06 | 0.03 | |
| DC-159a primary mutants (n = 3) | |||||
| DC-1, -2, -3 | 16 | 16 | 2 | 1 | Gly88→Cys (GGC→TGC) |
| DC-159a secondary mutants (n = 13) | |||||
| DC-1-1, -1-2, -1-3, -2-1, -2-2 | 8 | 16 | 2 | 1 | Gly88→Cys (GGC→TGC) |
| DC-2-5, -3-1, -3-2, -3-4, -3-5 | 16 | 16 | 2 | 1 | Gly88→Cys (GGC→TGC) |
| DC-2-3 | 8 | 8 | 2 | 0.5 | Gly88→Cys (GGC→TGC) |
| DC-2-4 | 8 | 8 | 2 | 1 | Gly88→Cys (GGC→TGC) |
| DC-3-3 | 16 | 16 | 4 | 2 | Gly88→Cys (GGC→TGC) |
| DC-159a tertiary mutants (n = 5) | |||||
| DC-2-1-1, -3-4-1 | 16 | 32 | 4 | 2 | Gly88→Cys (GGC→TGC) |
| DC-3-5-2, -3-5-3 | >32 | >32 | 16 | 8 | Gly88→Cys (GGC→TGC), Asp94→His (GAC→CAC) |
| DC-3-5-1 | >32 | 32 | 16 | 8 | Gly88→Cys (GGC→TGC), Asp94→His (GAC→CAC) |
| Clinical isolatesb (n = 11) | |||||
| MDR1 | 16 | 32 | 1 | 0.125 | Ala90→Val (GCG→GTG), Asp94→Ala (GAC→GCC) |
| MDR3 | 4 | 8 | 1 | 0.125 | Ala90→Val (GCG→GTG) |
| MDR4 | 8 | 8 | 1 | 0.125 | Asp94→Ala (GAC→GCC) |
| MDR5 | 8 | 8 | 1 | 0.5 | Ala90→Val (GCG→GTG), Ser91→Pro (TCG→CCG) |
| MDR7 | 4 | 4 | 0.25 | 0.06 | Ala90→Val (GCG→GTG) |
| MDR9 | 4 | 4 | 0.5 | 0.06 | Asp94→Val (GAC→GTC) |
| MDR12 | 4 | 8 | 1 | 0.125 | Asp94→Asn (GAC→AAC) |
| QR-3 | 2 | 4 | 0.5 | 0.06 | Ala90→Val (GCG→GTG) |
| QR-6 | 8 | >32 | 2 | 1 | Asp94→Gly (GAC→GGC) |
| QR-1 | 8 | >32 | 2 | 0.5 | Asp94→Tyr (GAC→TAC) |
| QR-9 | 8 | >32 | 4 | 0.5 | Asp94→Gly (GAC→GGC) |
LVX, levofloxacin; CIP, ciprofloxacin; GAT, gatifloxacin.
Except for MDR3 and MDR9, all clinical isolates had a natural polymorphism, Ser95→Thr, in GyrA. In the gyrB region, no alteration was detected in DC-159a-resistant mutants and QR-MDR M. tuberculosis isolates.
Two different DNA isolation methods were used to extract genomic DNA, as described previously (6, 16). The quinolone resistance-determining regions (QRDRs) of gyrA and gyrB were investigated as to whether an increased level of resistance to DC-159a was correlated with each gyrase alteration (Table 1). The QRDRs in M. tuberculosis were amplified by PCR. The PCR products were determined by the dideoxy chain termination method with an ABI Prism 3130 sequencer (Life Technologies Japan). Of the 21 DC-159a-resistant mutants, 18 had a substitution mutation, Gly88Cys, in GyrA, indicating that DC-159a resistance was accompanied by this replacement. The remaining three highly resistant mutants had a novel double mutation of Gly88Cys and Asp94His, suggesting that an additional Asp94His mutation might be necessary to develop high DC-159a resistance. On the contrary, in QR-MDR M. tuberculosis isolates, the Gly88Cys mutation in gyrA was not detected; out of 11 QR-MDR M. tuberculosis isolates, 10 had a mutation(s) at Ala90Val and/or Asp94Ala, -Asn, -Val, -Gly, or -Tyr and one had a double mutation at Ala90Val and Ser91Pro.
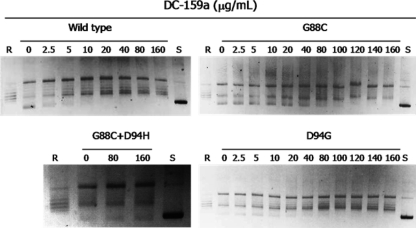
Expression plasmids encoding GyrA (wild type and Asp94Gly) and GyrB (wild type) were obtained from plasmid pTYB1 (New England BioLabs); the altered GyrA proteins (Gly88Cys and Gly88Cys Asp94His) were expressed by means of the plasmid (11). The wild-type GyrA, altered GyrA, and wild-type GyrB proteins of DNA gyrase were separately purified by chitin affinity chromatography according to the manufacturer's protocol (9). The supercoiling activity of DNA gyrase was measured by the previously described method (10). The inhibitory effects of DC-159a against recombinant gyrase complexes bearing GyrA wild type, Asp94Gly, Gly88Cys, and Gly88Cys Asp94His were measured. Gyrase-mediated supercoiling activity was detected in all the complexes in the absence of DC-159a (Fig. 1). The 50% inhibitory concentrations (IC50s) of DC-159a and LVX against the activity of each enzyme complex, i.e., GyrA Gly88Cys, GyrA Asp94Gly, and GyrA Gly88Cys Asp94His, were as follows: 5 and 13, 9 and >16, and >43 and >16 times higher, respectively, than those against the wild-type enzyme (Table 2). These results demonstrate that the GyrA Gly88Cys change confers DC-159a resistance, whereas the double mutation Gly88Cys Asp94His is responsible for the high resistance to DC-159a.
Fig. 1.
Inhibitory activity of DC-159a on the supercoiling activity of M. tuberculosis DNA gyrase. Relaxed pBR322 DNA (0.1 μg) was incubated at 37°C for 1 h in 15 μl of reaction mixture containing 35 mM Tris-HCl (pH 7.5), 4 mM MgCl2, 24 mM KCl, 2 mM dithiothreitol, 1 mM ATP, 1.8 mM spermidine, 0.1 mg/liter albumin, and 0.5 μg of GyrA and GyrB proteins. Reactions were terminated by the addition of a dye mix, and then the products were analyzed by electrophoresis in 1.0% agarose. R and S denote relaxed and supercoiled DNA, respectively.
Table 2.
DC-159a and levofloxacin activities against M. tuberculosis DNA gyrases
| Quinolone | IC50 (μg/ml)a for GyrA with indicated genotype |
|||
|---|---|---|---|---|
| Wild type | Asp94Gly | Gly88Cys | Gly88Cys Asp94His | |
| DC-159a | 3.7 | 32.2 | 17.3 | >160 |
| Levofloxacin | 9.8 | >160 | 129.2 | >160 |
The IC50 was defined as the drug concentration that reduced the enzymatic activity observed with drug-free controls by 50%.
DC-159a, representing a new spectrum of respiratory quinolones, is currently nominated at the end of the preclinical stage as a novel antituberculosis drug (18). The MIC90 of DC-159a against drug-susceptible M. tuberculosis clinical isolates is 0.06 μg/ml (3). We characterized here in vitro-selected DC-159a-resistant M. tuberculosis mutants having the mutation(s) Gly88Cys and Gly88Cys Asp94His in GyrA. DC-159a-resistant mutants showed an increased MIC range for DC-159a that was 16 to 256 times higher than that of the parent strain H37Rv (0.03 μg/ml) (Table 1). Regarding the drug inhibitory effects on the gyrase complexes of the mutants, all of the tested complexes were resistant to inhibition by DC-159a and LVX; however, the IC50 of DC-159a against the gyrase bearing GyrA Gly88Cys was 1.9-fold lower than that against the gyrase bearing GyrA Asp94Gly. In particular, the complex bearing GyrA with the novel double mutation Gly88Cys Asp94His led to high resistance to DC-159a. These data prove that the mutations Gly88Cys and Gly88Cys Asp94His are associated with DC-159a resistance in M. tuberculosis.
The principal mechanism of quinolone resistance in M. tuberculosis is the alteration of the QRDR in GyrA (19). A mutation at position 94 (Asp94Gly or Asp94Ala) is most frequently followed by a mutation at position 90 (Ala90Val) (1, 2, 15). Similar results were observed in our 11 clinical isolates of QR-MDR M. tuberculosis. In contrast, the Gly88Cys mutant is rarely detected in clinical practice (12). The mutant bearing GyrA Gly88Cys has higher resistance to broad-spectrum and fourth-generation drugs than to the older quinolones (8). Amino acid 88 involves the interaction of the R7 fluoroquinolone ring substituent with the quinolone binding pocket of M. tuberculosis DNA gyrase. Consequently, the replacement of glycine with cysteine at position 88 could create a steric hindrance to the bulky group at position R7 of new, broader-spectrum quinolones (8). It is not clear whether the low frequency of the Gly88Cys mutation will plateau or continue to rise with increasing use of new quinolones. The relationship between bacterial fitness and quinolone resistance depends not on the number of resistance mutations but, critically, on the nature of the resistance mutation (7, 14). An ad hoc study on improvement/reduction in fitness cost associated with gyrA Gly88Cys in M. tuberculosis may help to guide the application of DC-159a in the treatment of QR-MDR M. tuberculosis. In addition, our findings suggest that the mutation of DC-159a occurs in a stepwise manner. These findings agree with the previous report by Hooper (5). Therefore, the mutant prevention concentration (MPC) concept can be applied to DC-159a to prevent further growth of first-step mutants. The goal of maintaining the drug concentration above the MPC is desirable (13). Moreover, the fact that DC-159a has high penetration in the lungs also contributes to the potential usefulness of DC-159a in the treatment of QR-MDR M. tuberculosis (4).
In summary, we demonstrated the characteristic mechanism of DC-159a resistance in M. tuberculosis in vitro on the basis of a comparative study of mutation profiles of the QRDRs of GyrA and GyrB between DC-159a-resistant mutants and QR-MDR M. tuberculosis clinical isolates. The different resistance mutation patterns in GyrA between DC-159a-resistant mutants and QR-MDR M. tuberculosis isolates suggested that DC-159a may be a promising novel candidate for the treatment of QR-MDR M. tuberculosis cases.
Footnotes
Published ahead of print on 9 May 2011.
REFERENCES
- 1. Bozeman L., Burman W., Metchock B., Welch L., Weiner M. 2005. Fluoroquinolone susceptibility among Mycobacterium tuberculosis isolates from the United States and Canada. Clin. Infect. Dis. 40:386–391 [DOI] [PubMed] [Google Scholar]
- 2. Cheng A. F., et al. 2004. Multiplex PCR amplimer conformation analysis for rapid detection of gyrA mutations in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob. Agents Chemother. 48:596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Disratthakit A., Doi N. 2010. In vitro activities of DC-159a, a novel fluoroquinolone against Mycobacterium species. Antimicrob. Agents Chemother. 54:2684–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doi N., Disratthakit A., Ogiso S., Uoyama S., Kurosaka Y. 2006. In vivo efficacy of DC-159a, a new generation of respiratory quinolone, against experimental murine tuberculosis due to multi-drug-resistant Mycobacterium tuberculosis, abstr. F1-0492. Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother American Society for Microbiology, Washington, DC [Google Scholar]
- 5. Hooper D. C. 2001. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis. 7:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maeda S., Murase Y. Y., Mitarai S., Sugawara I. I., Kato S. 2008. Rapid, simple genotyping method by the variable numbers of tandem repeats (VNTR) for Mycobacterium tuberculosis isolates in Japan—analytical procedure of JATA (12)-VNTR. Kekkaku 83:673–678 (In Japanese.) [PubMed] [Google Scholar]
- 7. Marcusson L. L., Frimodt-Møller N., Hughes D. 2009. Interplay in the selection of fluoroquinolone resistance and bacterial fitness. PLoS Pathog. 5:e1000541 doi:10.1371/journal.ppat.1000541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matrat S., et al. 2006. Functional analysis of DNA gyrase mutant enzymes carrying mutation at position 88 in the A subunit found in clinical strains of Mycobacterium tuberculosis resistant to fluoroquinolones. Antimicrob. Agents Chemother. 50:4170–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. New England Biolabs 2006. Impact-CN instruction manual. New England Biolabs, Ipswich, MA: http://www.neb.com/nebecomm/ManualFiles/manualE6900.pdf [Google Scholar]
- 10. Onodera Y., Tanaka M., Sato K. 2001. Inhibitory activity of quinolones against DNA gyrase of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 47:447–450 [DOI] [PubMed] [Google Scholar]
- 11. Onodera Y., Hirata T., Hoshino K., Otani T. 2007. DC-159a, a novel quinolone, showed high inhibitory activity against altered topoisomerases of Streptococcus pneumoniae and Mycobacterium tuberculosis, abstr. F1-2126. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother., American Society for Microbiology, Washington, DC [Google Scholar]
- 12. Pitaksajjakul P., et al. 2005. Mutations in the gyrA and gyrB genes of fluoroquinolone-resistant Mycobacterium tuberculosis from TB patients in Thailand. Southeast Asian J. Trop. Med. Public Health 36(Suppl. 4):228–237 [PubMed] [Google Scholar]
- 13. Rodríguez J. C., et al. 2004. Mutant prevention concentration: comparison of fluoroquinolones and linezolid with Mycobacterium tuberculosis. J. Antimicrob. Chemother. 53:441–444 [DOI] [PubMed] [Google Scholar]
- 14. Rozen D. E., McGee L., Levin B. R., Klugman K. P. 2007. Fitness costs of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 50:412–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siddiqi N., et al. 2002. Molecular characterization of multi-drug-resistant isolates of Mycobacterium tuberculosis from patients in North India. Antimicrob. Agents Chemother. 46:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Embden J. D., et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Von Groll A., et al. 2009. Fluoroquinolone resistance in Mycobacterium tuberculosis and mutations in gyrA and gyrB. Antimicrob. Agents Chemother. 53:4498–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Working group on new TB drugs 2010. New respiratory quinolone DC-159a. Working group on new TB drugs. http://www.newtbdrugs.org/project.php?id=134
- 19. Zhang Y., Telenti A. 2000. Genetics of drug resistance in Mycobacterium tuberculosis, p. 235–254 In Hatfull G. F., Jacobs W. R. (ed.), Molecular genetics of mycobacteria. American Society for Microbiology, Washington, DC [Google Scholar]