Abstract
Tripropeptin C (TPPC) is a naturally occurring cyclic lipodepsipeptide antibiotic produced by a Lysobacter sp. TPPC exhibits potent antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and penicillin-resistant Streptococcus pneumoniae. This antibiotic also inhibits the incorporation of N-acetylglucosamine into the peptidoglycan of S. aureus at a 50% inhibitory concentration (IC50) of 0.7 μM, which is proportional to its MIC (0.87 μM; equivalent to 1.0 μg/ml). Treatment of exponential-phase S. aureus cells with TPPC resulted in accumulation of UDP-MurNAc-pentapeptide in the cytoplasm. The antimicrobial activity of TPPC was weakened by the addition of prenyl pyrophosphates but not by prenyl phosphates, UDP-linked sugars, or the pentapeptide of peptidoglycan. The direct interaction between TPPC and undecaprenyl pyrophosphate (C55-PP) was observed by mass spectrometry and thin-layer chromatography analysis, indicating that TPPC can potentially inhibit C55-PP phosphatase activity, which plays a crucial role in the lipid cycle of peptidoglycan synthesis. As expected, TPPC inhibits this enzymatic reaction at an IC50 of 0.03 to 0.1 μM in vitro, as does bacitracin. From the analysis of accumulation of lipid carrier-related compounds, TPPC was found to cause the accumulation of C55-PP in situ, leading to the accumulation of a glycine-containing lipid intermediate. This suggested that the TPPC/C55-PP complex also inhibits the transglycosylation step or flippase activity, adding to the inhibition of C55-PP dephosphorylation. This mode of action is different from that of currently available drugs such as vancomycin, daptomycin, and bacitracin.
INTRODUCTION
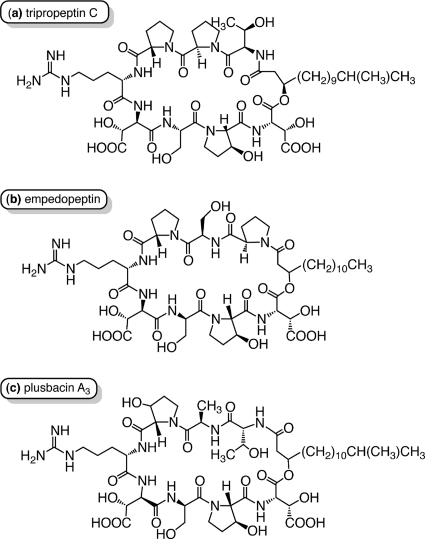
The lipopeptide antibiotic tripropeptin C (TPPC) is isolated from the fermentation broth of the soil bacterium Lysobacter sp. strain BMK333-48F3. TPPC consists of a cyclic octapeptide core and a fatty acyl side chain, 13-methyl-3-hydroxy-tetradecanoic acid (Fig. 1) (4, 6). TPPC has a characteristic structure in that five of the eight amino acid residues in the peptide core are unusual amino acids: d-allo-threonine, l-trans-3-hydroxy proline, threo-beta-hydroxy-l-aspartic acid, threo-beta-hydroxy-d-aspartic acid, and d-proline.
Fig. 1.
Structures of tripropeptin C (a) and related compounds empedopeptin (b) and plusbacin A3 (c).
Plusbacins (22, 23) and empedopeptin (11, 25), which are structurally related to TPPC, are reported to have potent activity against Gram-positive bacteria (Fig. 1). Characteristic features of this class of antibiotics include the presence and positions of an arginine residue and two hydroxy aspartic acids. This class of antibiotics shows potent in vitro antimicrobial activity against Gram-positive pathogens, including drug-resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE), and also shows excellent therapeutic efficacy, comparable to that of vancomycin (VAN), the last resort for MRSA treatment, in a mouse model of staphylococcal septicemia (11, 22). TPPC shows no cross-resistance to other important antibiotic-resistant strains (4, 5), strongly suggesting that TPPC has a unique mode of action. TPPC also exhibits a favorable toxicological profile that includes no acute toxicity and no 14-day repeated toxicities in mice when administered intravenously at 300 mg/kg of body weight and 100 mg/kg/day, respectively (7). These facts indicate that TPPC represents a promising novel class of antibiotics for the treatment of MRSA and VRE.
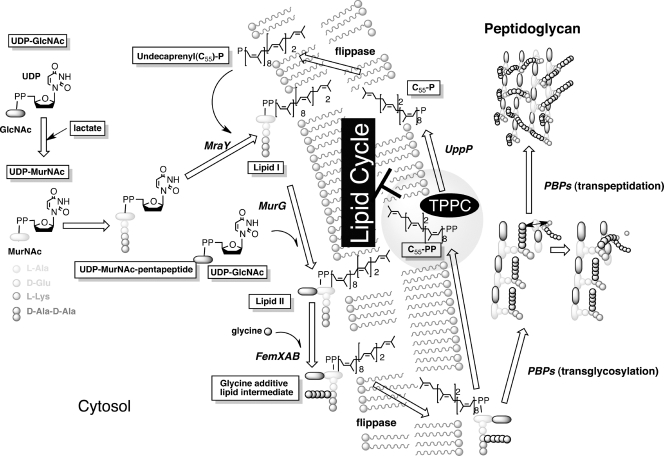
Within this class of antibiotics, only plusbacin A3 has been studied with regard to its mode of action (15). It has been suggested that plusbacin A3 inhibits peptidoglycan biosynthesis by blocking transglycosylation and the subsequent biosynthetic steps (Fig. 2). Although the exact target of plusbacin A3 is still not fully understood (15), its mode of action is distinct from that of VAN. The authors also speculated that the inhibition of nascent peptidoglycan and lipid intermediate biosynthesis by plusbacin A3 resulted from its binding to a lipid intermediate, and this hypothesis was supported by the antagonism of antibacterial activity with cell wall membrane fractions (15). Recent studies revealed that many cyclic peptides with potent antimicrobial activity inhibit enzymatic reactions by binding to substrates. For instance, VAN binds to the d-Ala-d-Ala terminus of a pentapeptide peptidoglycan precursor (10), resulting in inhibition of the transglycosylation of the peptidoglycan by penicillin binding proteins (PBPs); ramoplanin binds to lipid II as a dimer, resulting in inhibition of MurG-transglycosylases (8); and friulimicin B binds to undecaprenyl phosphate, resulting in the inhibition of the phospho-N-acetyl-muramoyl pentapeptide transferase activity catalyzed by MraY (20).
Fig. 2.
Staphylococcal peptidoglycan-synthetic pathway and proposed mode of action of tripropeptin C. We assume that tripropeptin C (TPPC) forms a tertiary complex with undecaprenyl pyrophosphate (C55-PP) and calcium ions (molar ratio, 1:1:1). Interaction between TPPC and C55-PP leads to disruption of the lipid cycle and results in inhibition of peptidoglycan synthesis.
In this study, we used radiolabeled precursors to examine the inhibition of macromolecular biosynthesis by TPPC, examined the enzymatic reactions of the peptidoglycan-synthetic pathway, and screened for TPPC-antagonizing molecules from envelope-related compounds. Based on these results, we examined the biological activities and mode of action of TPPC compared with those of key antibiotics such as VAN, bacitracin (BAC), and daptomycin (DAP).
(Portions of this work have been presented at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2010 [7a]).
MATERIALS AND METHODS
Bacterial strains.
S. aureus strain Smith (9) was used to raise in vitro drug-resistant strains by serial passage using the macrodilution method and to screen TPPC-antagonizing molecules using the microdilution assay. S. aureus strain N315 was used to assay the incorporation of [2-14C]mevalonolactone. pMUTIN mutants of Bacillus subtilis strain 168 were kindly provided by the National Institute of Genetics, Japan (National BioResource Project: B. subtilis).
Infection models.
The in vivo therapeutic efficacies of TPPC were assessed by experimental inoculation of mice with MRSA strain ATCC 33591 and VRE strain ATCC 51575. Mice were intraperitoneally injected with 1.0 × 107 CFU/0.5 ml/mouse (90 to 100% lethal dose [LD90–100]) of S. aureus ATCC 33591 or 3.2 × 108 CFU/0.5 ml/mouse (LD90–100) of Enterococcus faecalis ATCC 51575 suspended in brain heart infusion broth (Becton, Dickinson and Company, Franklin Lakes, NJ) with 7% fetal bovine serum (Invitrogen, Carlsbad, CA) containing 5% mucin (from porcine stomach, type II; Sigma, St. Louis, MO). Test substances and the saline were administered intravenously to the mice (n = 10) 1 h after bacterial challenge. TPPC was dissolved in saline with 10% dimethyl sulfoxide (DMSO) and 0.5% Tween 80. VAN was used as a reference antibiotic in a solution of sterilized water. The mice were observed for 7 days in order to determine the median effective dose (ED50) at day 7.
Materials.
TPPC was isolated by the method reported previously (4). VAN was purchased from Shionogi (Osaka, Japan). DAP was purchased from Funakoshi (Tokyo, Japan). BAC (zinc salt), ampicillin, levofloxacin, tunicamycin, and linezolid were purchased from Wako (Osaka, Japan). Geranyl phosphate, farnesyl phosphate, geranylgeranyl phosphate, geranyl pyrophosphate, farnesyl pyrophosphate, geranylgeranyl pyrophosphate, and nisin were purchased from Sigma. Undecaprenyl phosphate (C55-P) was purchased from Larodan (Malmö, Sweden). Undecaprenyl pyrophosphate (C55-PP) was purchased from the Polish Academy of Sciences (Warsaw, Poland). [methyl-3H]thymidine, [5,6-3H]uridine, l-[4,5-3H]leucine, [1-14C]acetate, N-acetyl-d-[1-3H]glucosamine (GlcNAc), and RS-[2-14C]mevalonate were purchased from GE Healthcare Bioscience (Fairfield, CT). Undecaprenyl [1-3H]monophosphate and undecaprenyl [1-3H]pyrophosphate were purchased from Biotrend (Cologne, Germany).
Divalent metal cation dependency assay.
The effects of divalent cations on the antimicrobial activities of TPPC, VAN, BAC, and DAP were assessed by standard microdilution methodology using Iso-Sensitest medium (Oxoid Ltd., Hampshire, England).
Precursor incorporation studies.
Inhibition of macromolecular synthesis in S. aureus strain Smith by TPPC was assayed by measuring the incorporation of radioactive precursors into the precipitate with 10% trichloroacetic acid for peptidoglycan, DNA, RNA, and protein synthesis as reported previously (3).
Accumulation of N-acetyl-muramoyl pentapeptide.
Accumulation of the cytoplasmic peptidoglycan nucleotide precursor was analyzed as described previously with slight modifications (20). First, 20 μg/ml of chloramphenicol (CHL) was added to mid-exponential-phase cells of S. aureus strain Smith (optical density at 600 nm [OD600], 0.4) grown in 50 μg/ml of Ca2+-supplemented Mueller–Hinton broth (MHBC). After incubation for 15 min at 37°C, antibiotics at four times the MIC were added, and the cultures were further incubated for 60 min at 37°C. Cells were harvested and extracted with boiling water. The cell extract was then centrifuged, and the supernatant was lyophilized. The UDP-linked cell wall precursor N-acetyl-muramoyl pentapeptide (UDP-MurNAc-P5) was analyzed using high-performance liquid chromatography (HPLC), and the analysis was confirmed by LC-mass spectrometry (LTQ Orbitrap; Thermo Fisher Scientific, Waltham, MA). Peak areas of UDP-MurNAc-P5 in mass chromatograms were used for the comparison of accumulated quantities, and their fold ratios were calculated using a standard curve.
In vitro lipid I/lipid II synthesis with isolated membranes.
In vitro lipid I/lipid II synthesis was assayed using the membrane of Micrococcus luteus strain IFO3333 as described previously with some modifications (21). The membrane was isolated from lysozyme-treated cells by centrifugation (40,000 × g for 30 min), washed twice with a mixture of 50 mM Tris-HCl and 10 mM MgCl2 (pH 7.4), and stored under liquid nitrogen until use. An in vitro lipid II synthesis assay (MraY/MurG) was performed in a final volume of 50 μl containing 12.5 nM purified UDP-MurNAc-P5, 6.3 nmol of C55-P, 0.5 nmol of [3H]C55-P (0.37 to 0.74 TBq/mmol; Biotrend), and 12.5 nM UDP-GlcNAc in a mixture of 60 mM Tris-HCl, 5 mM MgCl2, 1.25 mM CaCl2 (pH 8.0), and 0.5% (wt/vol) Triton X-100. UDP-MurNAc-P5 was purified from the cells, treated with CHL and VAN as described above, extracted with boiling water, and then used in reverse-phase HPLC. The reaction was initiated by the addition of 50 μg of the membrane. Each of the concentrated drugs was added to the reaction mixture before the addition of the M. luteus membrane. The reaction mixture was incubated for 1 h at 30°C. Lipids were extracted with the same volume of butanol–6 M pyridine acetate (2:1, vol/vol) (pH 4.2) and were analyzed by thin-layer chromatography (TLC) on plates precoated with silica gel 60 (Merck, Darmstadt, Germany) using a solvent system of chloroform-methanol-water-ammonia (88:48:10:1, vol/vol/vol/vol). Radioactive spots were detected by exposure to BioMax XAR film (Kodak, Tokyo, Japan) for 14 days after treatment with En3Hance spray (Perkin-Elmer) and were scanned with a Fuji Medical film processor, model FPM800 (Fujifilm, Tokyo, Japan). An in vitro lipid I synthesis assay (MraY) was performed in the same way as the lipid II synthesis assay except for the omission of UDP-GlcNAc from the reaction mixture.
C55-PP phosphatase assay.
An in vitro C55-PP phosphatase assay was performed as reported previously (2) with slight modifications. The assay was performed in a final volume of 50 μl containing 1.0 nmol [3H]C55-PP (0.37 to 0.74 TBq/mmol; Biotrend) in a mixture of 100 mM Tris-HCl, 10 mM MgCl2 and 3.9 mM n-dodecyl-β-maltoside (pH 7.5). For the TPPC and BAC assays, 1.25 mM CaCl2 and 0.77 mM ZnCl2, respectively, were also added to the medium. The reaction was initiated by addition of 50 μg of the membrane of M. luteus. The reaction mixture was incubated for 1 h at 30°C. The substrate (C55-PP) and the reaction product (C55-P) were observed by TLC on silica gel 60 plates (Merck) using a solvent system of chloroform–methanol–water–1 M ammonia (90:60:12:1, vol/vol/vol/vol). Radioactive spots were detected in the same way as for the in vitro lipid I/lipid II assay. IC50s for C55-PP phosphatase were determined by measuring the densities of C55-P and C55-PP bands of digitalized X-film data using free ImageJ software (http://rsbweb.nih.gov/ij/).
TLC analysis of metabolites from [2-14C]mevalonate.
[2-14C]mevalonate metabolites were analyzed as reported previously (13) with slight modifications. S. aureus strain N315 was cultivated in MHBC at 37°C until mid-log phase (OD600, 0.5). CHL was added to the culture medium to a final concentration of 50 μg/ml in order to terminate de novo protein synthesis, followed by the addition of 10 μCi/ml of [2-14C]mevalonate (55 mCi/mmol). This mixture was divided into 1-ml aliquots, and the cells were exposed to TPPC, VAN, or BAC. In the TPPC and BAC assays, 1.25 mM CaCl2 and 0.77 mM ZnCl2, respectively, were also added to the medium. After incubation at 37°C for the indicated times, the cells were harvested and washed three times with distilled water. The cell pellets were extracted with chloroform-methanol (1:1, vol/vol), dried in vacuo, and then resuspended in chloroform. The suspension was then separated on a high-performance TLC (HPTLC) silica gel 60 plate (10 by 10 cm, with a 2.5-by-10 cm concentrating zone; Merck) with chloroform-methanol-water-ammonia (88:48:10:1, vol/vol/vol/vol). The radioactive spots were detected by exposure to BioMax XAR film (Kodak) for 14 days and were scanned with a Fuji Medical film processor (Fujifilm).
Raising a drug-resistant strain using the sequential macrodilution assay.
To raise drug-resistant strains in vitro, sequential macrodilution assays were performed as reported previously with slight modifications (24). MHBC containing test drugs at 0.25, 0.5, 1, 2, and 4 times the MIC was inoculated with S. aureus strain Smith from a single colony. Cultures (1 ml) were incubated at 37°C overnight without shaking. From the second highest concentrations that supported growth, cultures were diluted 1:100 into fresh medium containing the test drug at 2-fold dilutions. They were then incubated at 37°C overnight without shaking. This cycle was carried out daily and was continued until the MICs became stable.
Testing of the susceptibilities of Bacillus subtilis 168 and its pMUTIN insertion mutants.
MICs for Bacillus subtilis 168 and its pMUTIN insertion mutants were assessed by standard microdilution methodology using Mueller-Hinton broth. Erythromycin (1 μg/ml) was added to the culture as a marker to prevent revertant growth, since pMUTIN contains the erm gene.
RESULTS
In vitro and in vivo activities of TPPC.
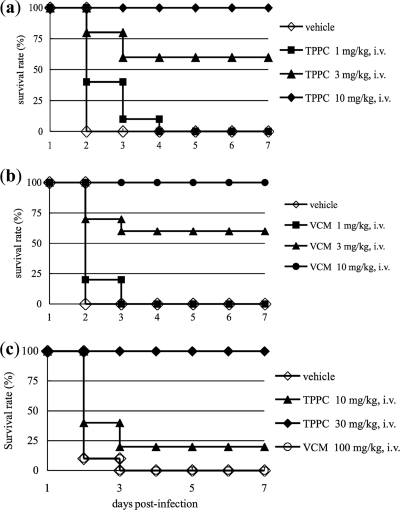
The MIC values of TPPC against MRSA (ATCC 33591) and VRE (E. faecalis ATCC 51575), as determined by the standard agar dilution assay, were 1.0 and 4.0 μg/ml, respectively, and those of VAN were 0.3 and >100 μg/ml, respectively. TPPC showed excellent therapeutic efficacy in a mouse septicemia model upon infection with either MRSA (ATCC 33591) or VRE (E. faecalis ATCC 51575) (Fig. 3), with ED50 values of 2.52 and 20.0 mg/kg, respectively. By comparison, the ED50 values for VAN were 2.95 and >100 mg/kg, respectively. Our experiments therefore revealed that TPPC exhibits potent activity against the drug-resistant pathogens MRSA and VRE both in vivo and in vitro. In our experiments, clinically isolated strains, including MRSA, VRE, and penicillin-resistant Streptococcus pneumoniae, showed no resistance to TPPC (4, 5), indicating that TPPC has a unique mode of action.
Fig. 3.
Therapeutic efficacies of tripropeptin C (TPPC) (a and c) and vancomycin (VCM) (b and c) in a mouse model of septicemia. The results of the survival analysis for groups of mice (n = 10) infected with MRSA (ATCC 33591) (a and b) or VRE (ATCC 51575) (c) and receiving either saline (vehicle), TPPC, or vancomycin are shown. TPPC and vancomycin were administered intravenously (i.v.).
The antimicrobial activities of TPPC and DAP were calcium dependent, whereas that of BAC was dependent on zinc and cobalt ions but not calcium and VAN activity was independent of the presence of the divalent metal cations tested (Table 1). Although these results indicated that TPPC might have a mode of action similar to that of DAP, TPPC activity against S. aureus strain Smith differs from DAP activity in that it exhibits little membrane potential disturbance with 3,3′-dipropylthiadicarbocyanine iodide (DiSC3[5]; Molecular Probes, Carlsbad, CA), shows no potassium efflux with potassium-binding benzofuran isophthalate (PBFI; Molecular Probes), and does not lead to loss of membrane integrity when To-Pro-3-iodide (Molecular Probes) is used (see Fig. S1 in the supplemental material).
Table 1.
Effects of divalent cations on the antimicrobial activities of TPPC, VAN, BAC, and DAPa
| Cation and concn (μg/ml) | MIC (μg/ml)b for S. aureus Smith |
|||
|---|---|---|---|---|
| TPPC | BAC | DAP | VAN | |
| Control | 4 | 4 | >64 | 1 |
| Ca2+ | ||||
| 25 | 0.5 | 4 | 4 | 1 |
| 50 | 0.25 | 4 | 2 | 1 |
| Zu2+ | ||||
| 25 | 4 | 1 | >64 | 1 |
| 50 | 4 | 0.5 | >64 | 1 |
| Co2+ | ||||
| 25 | 4 | 1 | NT | 1 |
| 50 | 4 | 0.5 | NT | 1 |
Tested on Iso-Sensitest medium at 37°C for 18 h. NT, not tested.
Determined by broth dilution.
Precursor incorporation studies.
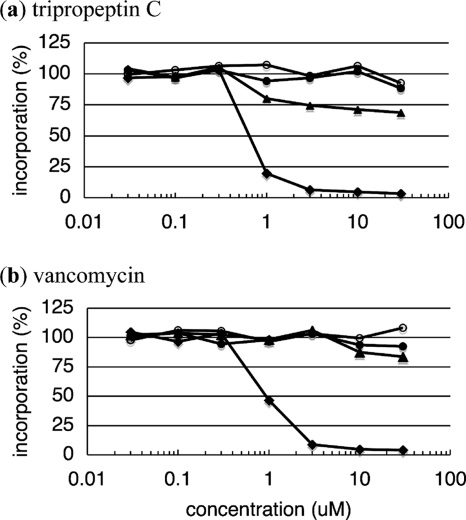
Both TPPC and VAN showed almost no effect on DNA, RNA, or protein synthesis in S. aureus strain Smith. TPPC and VAN inhibited cell wall biosynthesis with IC50s of 0.7 and 0.8 μM, respectively (Fig. 4), proportional to their MICs against S. aureus strain Smith.
Fig. 4.
Inhibition by tripropeptin C (a) and vancomycin (b) of macromolecular synthesis by S. aureus strain Smith. The percentages of incorporation of DNA ([methyl-3H]thymidine, open circles), RNA ([5,6-3H]uridine, filled triangles), protein (l-[4,5-3H]leucine, filled circles), and the cell wall ([1-3H]GlcNAc, filled diamonds) after incubation for 10 min are plotted against the drug concentration.
Effects of TPPC on in vitro cell wall biosynthesis.
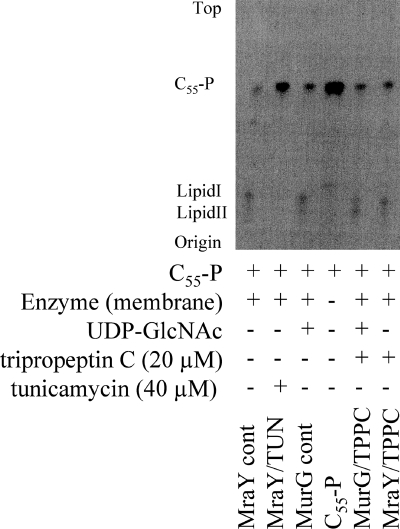
To reveal if TPPC interrupts one of the early enzyme reactions of murein biosynthesis, the accumulation of UDP-MurNAc-P5 in TPPC-treated cells was analyzed. Among the seven compounds tested, TPPC caused the highest intracellular accumulation of the cell wall precursor UDP-MurNAc-P5 in S. aureus strain Smith, with quantities around six times higher than those observed with VAN (Table 2; see also Fig. S2 in the supplemental material). This result indicated that TPPC is not involved in the biosynthesis of the final soluble precursor, UDP-MurNAc-P5, but might block one of the subsequent membrane-associated steps in peptidoglycan biosynthesis (Fig. 2). In this experiment, treatment with DAP caused no accumulation of UDP-MurNAc-P5, as reported previously (20). The difference in the quantities of accumulated UDP-MurNAc-P5 between TPPC- and VAN-treated cells also suggested that TPPC interrupts an earlier step of peptidoglycan biosynthesis than that affected by VAN; thus, MraY is a possible target of TPPC. Unexpectedly, however, TPPC had no effect on either lipid I or lipid II biosynthesis, catalyzed by MraY and MurG, respectively, as shown in Fig. 5.
Table 2.
Effects of TPPC and cell wall synthesis inhibitors on the accumulation of the intracellular precursor UDP-MurNAc pentapeptide in S. aureus strain Smith
| Cell wall synthesis inhibitor | Fold accumulation of UDP-MurNAc pentapeptidea | Target |
|---|---|---|
| Control | 1.0 | |
| Daptomycin | 1.1 ± 0.2 | Membrane |
| Teicoplanin | 7.4 ± 2.8 | Pbp (d-Ala-d-Ala) |
| Vancomycin | 10.3 ± 4.2 | Pbp (d-Ala-d-Ala) |
| Amphomycin | 42.6 ± 16.9 | MraY (C55-P) |
| Bacitracin | 45.6 ± 13.2 | C55-PP phosphatase |
| Caprazamycin B | 63.9 ± 12.4 | MraY |
| Tripropeptin C | 66.9 ± 16.6 |
Relative to that with the control.
Fig. 5.
Effects of TPPC on lipid I and lipid II biosynthesis.
Screening for TPPC-antagonizing compounds from cell wall-related molecules.
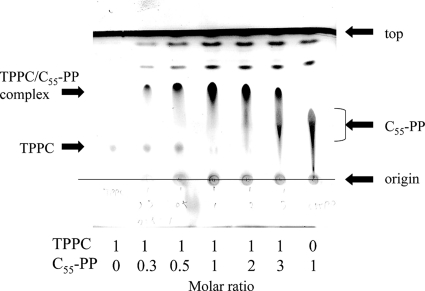
During the course of our study on the mode of action of TPPC, we found that the antimicrobial activity of TPPC was antagonized when cell wall preparations were added to the culture. As a result of the screening for TPPC-antagonizing compounds from cell wall-related compounds, prenyl pyrophosphates, including undecaprenyl pyrophosphate (C55-PP), showed antagonizing activities, which correlated with the lengths of the prenyl chains (Table 3). However, both sodium pyrophosphate and UDP, containing pyrophosphate, showed no effect on the antibacterial activity of TPPC. Unlike VAN, TPPC was not antagonized by molecules containing the tail structure of nascent peptidoglycan, such as acetyl (Ac)-Lys-d-Ala-d-Ala, UDP-MurNAc-P5, and peptidoglycan. To investigate the direct interaction between TPPC and C55-PP, each was loaded separately onto a silica gel 60 plate (containing a sufficient amount of calcium as gypsum; Merck) for TLC and was developed with a mixture of chloroform-methanol-water (5:5:1). The spots of TPPC and C55-PP were observed at relative flow (Rf) values of 0.21 and 0.37, respectively. A new spot appeared at an Rf of 0.60 when TPPC and C55-PP were coloaded onto TLC plates and developed. Interestingly, the spots corresponding to TPPC and C55-PP alone disappeared when equimolar amounts of each component were coloaded onto TLC plates (Fig. 6). This result strongly suggested that TPPC and C55-PP form a complex at a ratio of 1:1. The stoichiometry of this complex was confirmed by liquid chromatography-mass spectrometry only in the presence of 50 μg/ml of calcium ions, and the stoichiometry of the tertiary complex formed, TPPC (C51H83N11O19)/C55-PP (C55H92O7P2)/Ca2+, was observed at a molar ratio of 1:1:1 by time-of-flight mass spectrometry (m/z 2119.1886, a value identical to that of the adduct of [TPPC + Ca2+ + C55-PP − H+]+) (JMS-T100LC mass spectrometer; JEOL, Tokyo, Japan) (see Fig. S3 in the supplemental material). The mass signal detected indicated that this tertiary complex is formed not by a covalent bond but by a coordinate bond. Furthermore, when TPPC, calcium ions, and C55-P were mixed, the corresponding mass signal was not detected by mass spectrometry, indicating that TPPC recognizes pyrophosphate as an indispensable unit for the formation of the tertiary complex (data not shown).
Table 3.
Antagonism tests of antimicrobial activities with bacterial envelope-related compounds
| Test samplea and concn (μM) | MIC (μg/ml)b against S. aureus Smith |
|||
|---|---|---|---|---|
| TPPCc | VAN | DAPc | BAC | |
| Control | 0.5 | 1 | 0.25 | 2 |
| UDP (250) | 0.5 | I | 0.25 | NT |
| GlcNAc (250) | 0.5 | 1 | 0.25 | NT |
| UDP-GlcNAc (62.5) | 0.5 | 0.25 | NT | |
| C55-OH (125) | 0.5 | 1 | 0.25 | NT |
| C10-P (125) | 0.5 | 1 | 0.25 | NT |
| C15-P (125) | 0.5 | 1 | 0.25 | NT |
| C20-P | ||||
| 31.2 | 1 | 1 | 0.25 | NT |
| 125 | 1 | 1 | 0.25 | NT |
| C55-P | ||||
| 31.2 | 1 | 1 | 0.25 | NT |
| 125 | 1 | 1 | 0.25 | NT |
| C10-PP | ||||
| 31.2 | 1 | 1 | 0.25 | NT |
| 125 | 2 | 1 | 0.25 | NT |
| C15-PP | ||||
| 31.2 | 4 | 1 | 0.25 | NT |
| 125 | 16 | 1 | 0.25 | NT |
| C20-PP | ||||
| 31.2 | 8 | 1 | 0.25 | 128 |
| 125 | 32 | 1 | 0.25 | >256 |
| C55-PP | ||||
| 31.2 | 8 | 1 | 0.25 | 256 |
| 125 | 22 | 1 | 0.25 | >256 |
| Ac-Lys-d-Ala-d-Ala (250) | 0.5 | 16 | 0.25 | NT |
| UDP-MurNAc-P5 (250) | 0.5 | 8 | 0.25 | NT |
GlcNAc, N-acetylglucosamine; C55, undecaprenyl; C10, geranyl; C15, farnesyl; C20, geranylgeranyl; P, monophosphate; PP, pyrophosphate; P5, pentapeptide.
Determined by broth dilution. TPPC, tripropeptin C; VAN, vancomycin; DAP, daptomycin; BAC, bacitracin zinc salt; NT, not tested.
Calcium ions (50 μg/ml) were added to the medium.
Fig. 6.
Analyses of the interaction between tripropeptin C (TPPC) and undecaprenyl pyrophosphate (C55-PP) by silica gel TLC. The TLC plate used in this experiment contains calcium in the form of gypsum. One hundred nanomoles of TPPC and/or the indicated molar ratio of C55-PP was loaded separately onto a TLC plate and was dried for 5 min at room temperature before development. Compounds were developed with chloroform-methanol-water (5:5:1, vol/vol/vol). After drying, the TLC plate was treated with phosphomolybdic acid and was heated in order to visualize the presence of the loaded molecules. TPPC and C55-PP solutions were prepared separately, and a new spot (Rf, 0.60) emerged when TPPC and C55-PP were coloaded in the same lane. When equimolar amounts of TPPC and C55-PP were coloaded, neither component could be detected alone; only the postulated novel complex was detectable.
Effect of TPPC on cell wall biosynthesis both in vitro and in situ.
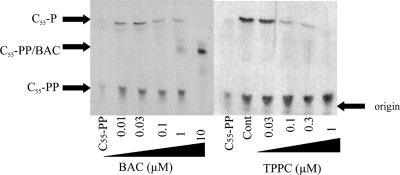
The results presented above strongly suggested that TPPC inhibits C55-PP phosphatase activity. TPPC indeed inhibited this enzymatic reaction at an IC50 of 0.03 to 0.1 μM, and this IC50 was comparable with that of BAC, used as a positive control in the C55-PP phosphatase assay (Fig. 7). The inhibition of C55-PP phosphatase activity by TPPC would cause the starvation of the available lipid carrier C55-P, and this could explain why TPPC causes the accumulation of a large amount of the cell wall precursor UDP-MurNAc-P5 in S. aureus strain Smith.
Fig. 7.
Inhibition of undecaprenyl pyrophosphate (C55-PP) phosphatase by tripropeptin C (TPPC) and bacitracin (BAC). Inhibition of C55-PP phosphatase activity was evaluated using 14C-labeled C55-PP with the membrane from M. luteus in the presence of TPPC or BAC. Plates were developed with a solvent system of chloroform–methanol–water–1 M ammonia (90:60:12:1, vol/vol/vol/vol).
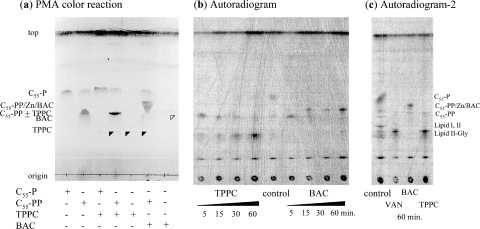
To investigate whether TPPC also inhibits C55-PP phosphatase in situ and causes the accumulation of C55-PP, we studied the accumulation of peptidoglycan precursors and lipid carriers through the mevalonate pathway by a [2-14C]mevalonolactone incorporation assay using HPTLC (silica gel 60 plate; Merck). Treatment with BAC or VAN caused the intracellular accumulation of C55-PP or the glycine-containing lipid intermediate, respectively, as reported previously (13) (Fig. 8c). TPPC caused the intracellular accumulation of C55-PP after 5 min of treatment, similarly to BAC, as expected from the results of the C55-PP phosphatase assay, and then also caused the accumulation of the glycine-containing lipid intermediate (Fig. 8b). This result suggested that TPPC inhibits the transglycosylation step or flippase activity after complex formation with calcium ions and C55-PP, adding to the inhibition of C55-PP dephosphorylation.
Fig. 8.
TLC analysis of lipid carrier-related metabolites from mevalonate. The plates were developed with a solvent system of chloroform-methanol-water-ammonia (88:48:10:1, vol/vol/vol/vol). (a) TLC analysis using cold compounds to confirm the Rf values of lipid carriers and their complexes with tripropeptin C (TPPC) or bacitracin (BAC). Solid and open arrowheads indicate the presence of TPPC and BAC, respectively. PMA, phosphomolybdic acid. (b and c) Mid-log-phase S. aureus N315 was labeled with [14C]mevalonate in the presence of 8 μg/ml TPPC, 8 μg/ml vancomycin (VAN), or 32 μg/ml BAC. After labeling, lipid extracts from treated cells were separated by HPTLC on silica gel 60 plates.
Antimicrobial activity of TPPC against mutants with defects in their bacitracin efflux systems.
As shown in Table 4, BAC exhibited 32-, 32-, and 8-fold more-potent antimicrobial activity against bceR, bceS, and ywoA (bcrC) pMUTIN insertion mutants, respectively, than against the parent strain B. subtilis 168. In contrast, TPPC was equally active against the parent strain and pMUTIN insertion mutants (MICs, 0.25 μg/ml).
Table 4.
Antimicrobial activities of TPPC and BAC against Bacillus subtilis 168 and its pMUTIN insertion mutants
| Test sample | Presence or absence of erythromycina | Strain | MIC (μg/ml) |
|---|---|---|---|
| TPPCb | − | B. subtilis 168 | 0.25 |
| ΔbceR mutant | 0.25 | ||
| ΔbceS mutant | 0.25 | ||
| ΔywoA (bcrC) mutant | 0.25 | ||
| + | B. subtilis 168 | Not grown | |
| ΔbceR mutant | 0.25 | ||
| ΔbceS mutant | 0.25 | ||
| ΔywoA (bcrC) mutant | 0.25 | ||
| BACc | − | B. subtilis 168 | 512 |
| ΔbceR mutant | 16 | ||
| ΔbceS mutant | 16 | ||
| ΔywoA (bcrC) mutant | 64 | ||
| + | B. subtilis 168 | Not grown | |
| ΔbceR mutant | 16 | ||
| ΔbceS mutant | 16 | ||
| ΔywoA (bcrC) mutant | 64 |
−, no erythromycin; +, 1 μg/ml of erythromycin was added to prevent revertant growth.
Calcium ions (50 μg/ml) were added to the test medium.
Zinc ions (50 μg/ml) were added to the test medium.
Acquisition of drug resistance using a sequential dilution assay.
The initial MICs of ofloxacin, ampicillin, gentamicin, linezolid, TPPC, and VAN are 0.01, 0.03, 0.05, 0.78, 0.39, and 0.78 μg/ml, respectively, in a macrodilution assay against S. aureus strain Smith. S. aureus strain Smith acquired more than 100-fold tolerance to ofloxacin, ampicillin, and gentamicin within 20 passages, and 16-fold tolerance to linezolid. For TPPC, S. aureus strain Smith acquired 4-fold tolerance (MIC, 1.56 μg/ml) by 20 passages, comparable to that of VAN (MIC, 3.12 μg/ml) (see Fig. S4 in the supplemental material).
DISCUSSION
In this study, we demonstrated that TPPC inhibited the lipid cycle of cell wall synthesis by complex formation with undecaprenyl pyrophosphate in the presence of calcium ions. Calcium ions were needed for TPPC to exhibit its full antimicrobial activity, since they were needed for this anionic peptide to approach the negatively charged bacterial envelope and bind to undecaprenyl pyrophosphate. TPPC inhibited [3H]GlcNAc incorporation at an IC50 of 0.7 μM, and this IC50 value corresponded to its MIC against S. aureus strain Smith, suggesting that the antimicrobial activity of TPPC is caused by inhibition of peptidoglycan synthesis. Treatment of S. aureus strain Smith with TPPC together with CHL led to the accumulation of the cytoplasmic peptidoglycan nucleotide precursor UDP-MurNAc-P5, as seen for other inhibitors in the late-stage of peptidoglycan synthesis, such as VAN and amphomycin. The quantity of accumulated UDP-MurNAc-P5 after TPPC treatment was six times higher than that after VAN treatment. VAN is known to inhibit peptidoglycan polymerization catalyzed by PBPs after the maturation of this peptidoglycan building block through the activities of MraY, MurG, and FemXAB and putative flippase reactions. After MraY has used UDP-MurNAc-P5 as a substrate, at least four more steps are involved before VAN interferes with the transglycosylation of PBPs. The difference in the quantity of substrate accumulated after treatment with TPPC versus VAN might be caused by the difference in the timing of their inhibitory activities. TPPC did not inhibit lipid I and lipid II biosynthesis through MraY and MurG enzymatic reactions, even at 20 μM, 20 times the MIC (Fig. 5). Although we did not take into account the possibility of primary inhibition of FemX or Pbp by TPPC, this is unlikely, since lipid II or the glycine-containing lipid intermediate would be selectively accumulated if TPPC directly inhibited FemX or PBP, respectively.
We identified C55-PP as the in vitro target of TPPC by screening for TPPC-antagonizing compounds and found that TPPC inhibits C55-PP phosphatase. BAC is also known to be an inhibitor of C55-PP phosphatase. Although BAC has been widely used for humans, food animals, and food crops for several decades, bacterial acquired resistance against this antibiotic is still rare in S. aureus (14, 26), suggesting that C55-PP is an ideal drug target for antibiotics. Like BAC, VAN binds to a small molecular substrate rather than an enzyme (which could be susceptible to mutations), and due to this characteristic feature of VAN, the emergence of VAN-resistant S. aureus was thought to be slow (12). Historically, it took more than 30 years for VAN-resistant strains to emerge after the introduction of VAN for clinical use. Therefore, drugs whose targets are small molecular substrates, such as VAN, BAC, and TPPC, would offer great advantages for preventing (or delaying) the emergence of resistant bacteria (12). Indeed, our experiments demonstrated that the acquisition of 8-fold resistance to TPPC in S. aureus strain Smith proceeded more slowly than the acquisition of resistance to linezolid, gentamicin, ofloxacin, and ampicillin in serial passage, as determined using macrodilution methodology, but was comparable to that of VAN (see Fig. S4 in the supplemental material).
Although the biological activities of TPPC and BAC are quite similar with regard to antagonism with prenyl pyrophosphates and the inhibition of C55-PP phosphatase, the antimicrobial spectra of these compounds are distinct. TPPC shows potent activity against staphylococci, enterococci, streptococci, and bacilli, while enterococci, some streptococci, and bacilli show resistance to BAC, because of their BAC efflux systems (1, 16–19). From our study using mutants with defects in the BAC efflux system, such as bceR, bceS, and ywoA (bcrC) mutants, it was revealed that TPPC is equally active against B. subtilis strain 168, despite the presence of these efflux systems (Table 4). This indicated that the TPPC/C55-PP complex is not recognized as a substrate for these efflux proteins.
In addition, the results of a [2-14C]mevalonolactone incorporation assay showed that levels of the glycine-containing lipid intermediate increased as levels of C55-PP (or the C55-PP/TPPC complex) decreased, possibly indicating that the TPPC/C55-PP complex causes interference with peptidoglycan polymerization or that disruption of membrane fluidity resulted in inhibition of flippase activity.
In summary, TPPC exhibits potent in vitro antimicrobial activities and excellent in vivo therapeutic efficacies against both MRSA and VRE in a mouse model of septicemia. Furthermore, the fact that TPPC displays a mode of action different from those of other drugs currently available strongly suggests that TPPC might represent a promising novel class of antibiotics for use against infectious diseases caused by Gram-positive bacteria.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to Kayoko Tsuchiya of Showa Pharmaceutical University (Tokyo, Japan) and Makoto Kuroda of the National Institute of Infectious Diseases (Tokyo, Japan) for constructive discussions and to Yoshimasa Ishizaki of the Institute of Microbial Chemistry (Tokyo, Japan) for technical advice. We thank Hironori Niki of the National Institute of Genetics (Mishima, Japan) for providing the B. subtilis 168 pMUTIN insertion mutants.
No funding was received for this study.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Bernard R., Joseph P., Guiseppi A., Chippaux M., Denizot F. 2003. YtsCD and YwoA, two independent systems that confer bacitracin resistance to Bacillus subtilis. FEMS Microbiol. Lett. 228:93–97 [DOI] [PubMed] [Google Scholar]
- 2. El Ghachi M., Derbise A., Bouhss A., Mengin-Lecreulx D. 2005. Identification of multiple genes encoding membrane proteins with undecaprenyl pyrophosphate phosphatase (UppP) activity in Escherichia coli. J. Biol. Chem. 280:18689–18695 [DOI] [PubMed] [Google Scholar]
- 3. Hashizume H., Adachi H., Igarashi M., Nishimura Y., Akamatsu Y. 2010. Biological activities of pargamicin A, a novel cyclic peptide antibiotic from Amycolatopsis sp. J. Antibiot. 63:279–283 [DOI] [PubMed] [Google Scholar]
- 4. Hashizume H., et al. 2001. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. I. Taxonomy, isolation and biological activities. J. Antibiot. 54:1054–1059 [DOI] [PubMed] [Google Scholar]
- 5. Hashizume H., Hattori S., Igarashi M., Akamatsu Y. 2004. Tripropeptin E, a new tripropeptin group antibiotic produced by Lysobacter sp. BMK333-48F3. J. Antibiot. 57:394–399 [DOI] [PubMed] [Google Scholar]
- 6. Hashizume H., et al. 2004. Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. II. Structure elucidation. J. Antibiot. 57:52–58 [DOI] [PubMed] [Google Scholar]
- 7. Hashizume H., et al. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1221 American Society for Microbiology, Washington, DC [Google Scholar]
- 7a. Hashizume H., et al. 2010. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1611 American Society for Microbiology, Washington, DC [Google Scholar]
- 8. Hu Y., Helm J. S., Chen L., Ye X.-Y., Walker S. 2003. Ramoplanin inhibits bacterial translocases by binding as a dimer to lipid II. J. Am. Chem. Soc. 125:8736–8737 [DOI] [PubMed] [Google Scholar]
- 9. Hunt G. A., Moses A. J. 1958. Acute infection of mice with Smith strain of Staphylococcus aureus. Science 128:1574–1575 [DOI] [PubMed] [Google Scholar]
- 10. Kannan R., et al. 1988. Function of the amino sugar and N-terminal amino acid of the antibiotic vancomycin in its complexation with the cell wall peptides. J. Am. Chem. Soc. 110:2946–2953 [Google Scholar]
- 11. Konishi M., et al. 1984. Empedopeptin (BMY-28117), a new depsipeptide antibiotic. I. Production, isolation and properties. J. Antibiot. 37:949–957 [DOI] [PubMed] [Google Scholar]
- 12. Koteva K., et al. 2010. A vancomycin photoprobe identified the histidine kinase VanSsc as a vancomycin receptor. Nat. Chem. Biol. 6:327–329 [DOI] [PubMed] [Google Scholar]
- 13. Kuroda M., Nagasaki S., Ohta T. 2007. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to beta-lactams in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 59:425–432 [DOI] [PubMed] [Google Scholar]
- 14. Losito P., Vergara A., Muscariello T., Ianieri A. 2005. Antimicrobial susceptibility of environmental Staphylococcus aureus strains isolated from a pigeon slaughterhouse in Italy. Poult. Sci. 84:1802–1807 [DOI] [PubMed] [Google Scholar]
- 15. Maki H., Miura K., Yamano Y. 2001. Katanosin B and plusbacin A3, inhibitors of peptidoglycan synthesis in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1823–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manson J. M., Keis S., Smith J. M., Cook G. M. 2004. Acquired bacitracin resistance in Enterococcus faecalis is mediated by an ABC transporter and a novel regulatory protein, BcrR. Antimicrob. Agents Chemother. 48:3743–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mascher T., Margulis N. G., Wang T., Ye R. W., Helmann J. D. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 50:1591–1604 [DOI] [PubMed] [Google Scholar]
- 18. Neumüller A. M., Konz D., Marahiel M. A. 2001. The two-component regulatory system BacRS is associated with bacitracin self-resistance of Bacillus licheniformis ATCC 10716. Eur. J. Biochem. 268:3180–3189 [DOI] [PubMed] [Google Scholar]
- 19. Ohki R., et al. 2003. The BceRS two-component regulatory system induces expression of the bacitracin transporter, BceAB, in Bacillus subtilis. Mol. Microbiol. 49:1135–1144 [DOI] [PubMed] [Google Scholar]
- 20. Schneider T., et al. 2009. The lipopeptide antibiotic friulimicin B inhibits cell wall biosynthesis through complex formation with bactoprenol phosphate. Antimicrob. Agents Chemother. 53:1610–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schneider T., et al. 2004. In vitro assembly of a complete, pentaglycine interpeptide bridge containing cell wall precursor (lipid II-Gly5) of Staphylococcus aureus. Mol. Microbiol. 53:675–685 [DOI] [PubMed] [Google Scholar]
- 22. Shoji J., et al. 1992. Isolation and characterization of new peptide antibiotics, plusbacins A1–A4 and B1–B4. J. Antibiot. 45:817–823 [DOI] [PubMed] [Google Scholar]
- 23. Shoji J., et al. 1992. Structure of new peptide antibiotics, plusbacins A1–A4 and B1–B4. J. Antibiot. 45:824–831 [DOI] [PubMed] [Google Scholar]
- 24. Silverman J. A., Oliver N., Li T. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sugawara K., Numata K., Konishi K., Kawaguchi H. 1984. Empedopeptin (BMY-28117), a new depsipeptide antibiotic. II. Structure determination. J. Antibiot. 37:958–964 [DOI] [PubMed] [Google Scholar]
- 26. Vinodhkumaradithyaa A., et al. 2009. Nasal carriage of methicillin-resistant Staphylococcus aureus among surgical unit staff. Jpn. J. Infect. Dis. 62:228–229 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.