Abstract
MX-2401 is an expanded-spectrum lipopeptide antibiotic selective for Gram-positive bacteria that is a semisynthetic analog of the naturally occurring lipopeptide amphomycin. It was active against Enterococcus spp., including vancomycin-sensitive Enterococcus (VSE), vanA-, vanB-, and vanC-positive vancomycin-resistant Enterococcus (VRE), linezolid- and quinupristin-dalfopristin-resistant isolates (MIC90 of 4 μg/ml), methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive S. aureus (MSSA) (MIC90 of 2 μg/ml), coagulase-negative staphylococci, including methicillin-sensitive Staphylococcus epidermidis (MSSE) and methicillin-resistant S. epidermidis (MRSE) (MIC90 of 2 μg/ml), and Streptococcus spp. including viridans group streptococci, and penicillin-resistant, penicillin-sensitive, penicillin-intermediate and macrolide-resistant isolates of Streptococcus pneumoniae (MIC90 of 2 μg/ml). MX-2401 demonstrated a dose-dependent postantibiotic effect varying from 1.5 to 2.4 h. Furthermore, MX-2401 was rapidly bactericidal at 4 times the MIC against S. aureus and Enterococcus faecalis, with more than 99.9% reduction in viable bacterial attained at 4 and 24 h, respectively. The MICs of MX-2401 against MRSA, MSSA, VSE, and VRE strains serially exposed for 15 passages to sub- to supra-MICs of MX-2401 remained within three dilutions of the original MIC. In contrast to that of the lipopeptide daptomycin, the antibacterial activity of MX-2401 was not affected in vitro by the presence of lung surfactant, and MX-2401 was active in vivo in the bronchial-alveolar pneumonia mouse model, in which daptomycin failed to show any activity. Moreover, the activity of MX-2401 was not as strongly dependent on the Ca2+ concentration as is the activity of daptomycin. In conclusion, MX-2401 is a promising new-generation lipopeptide for the treatment of serious infections with Gram-positive bacteria, including hospital-acquired pneumonia.
INTRODUCTION
Hospital- and community-acquired antibiotic resistance in Gram-positive bacteria is of increasing concern. Methicillin-resistant Staphylococcus aureus (MRSA) continues to be widespread and is now responsible for more deaths in the United States than AIDS (17). MRSA infections have increasingly been found to be a cause of pneumonia in the United States, with the MRSA-related infections resulting in higher rates of mortality in cases of pneumonia and septic shock than other types of infection (17). The lipopeptide daptomycin was found to be ineffective for treating pneumonia during clinical trials since its binding to lung surfactant made it unavailable to act against the infecting bacteria (29). Quinupristin-dalfopristin (Synercid) is active against MRSA, but it is not recommended for treating pneumonia. Clinical trials suggested that vancomycin, which does not achieve significant concentrations in the alveolar lining fluid, may be inferior to linezolid for treatment of MRSA pneumonia (32). At present, linezolid and tigecycline are indicated for the treatment of pneumonia, but neither of these agents is bactericidal. Thus, a bactericidal agent that is effective for treating MRSA pneumonia is needed.
We examined the structure-activity relationships of a series of semisynthetic amphomycin analog lipopeptides with regard to their spectra of activity, bactericidal effect against Gram-positive bacteria, in vivo efficacy, pharmacokinetics, and safety in rodent species and selected MX-2401 as a promising candidate for further development (9, 10, 22). MX-2401 is a novel semisynthetic lipopeptide antibiotic that comprises a chemically modified amphomycin core to which an aromatic linker and C12 lipid side chain are added (22). Here we describe the antimicrobial characteristics of MX-2401 against clinically important Gram-positive pathogens, including MRSA, vancomycin-resistant enterococci (VRE), and penicillin-resistant Streptococcus pneumoniae (PRSP). To discriminate it from the lipopeptide daptomycin, the effect of lung surfactant on MX-2401 antimicrobial activity was also characterized.
MATERIALS AND METHODS
Antibiotics and bacterial strains.
Vancomycin and oxacillin were purchased from Sigma Chemical Company (St. Louis, MO). MX-2401 and amphomycin were produced by BioWest Therapeutics Inc. (formerly MIGENIX Inc.), and daptomycin was either produced by BioSource Pharm Inc. or purchased from Cubist Pharmaceutical Inc. The majority of the resistant Gram-positive clinical isolates tested came from the American Type Culture Collection (ATCC) or surveillance studies conducted mostly between 2000 and 2007 in the United States and Canada.
Antimicrobial susceptibility testing.
MIC testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines for broth microdilution (5). In summary, microtiter plates containing 10 μl of 2-fold serially diluted antimicrobials were inoculated with 90 μl of inoculum prepared in growth media at 105 to 106 CFU/ml. The plates were then incubated for 18 to 24 h at 36 ± 1°C in ambient air. MICs of daptomycin and MX-2401 were determined using cation-adjusted Mueller-Hinton broth (CAMHB) (Becton-Dickinson, Sparks, MD) supplemented with an additional 25 μg/ml of Ca2+ (CAMHBc). MIC testing for Streptococcus spp. was performed in CAMHB (vancomycin and oxacillin) or CAMHBc (MX-2401 and daptomycin) supplemented with 3% sterile laked horse blood (Cedarlane Laboratories Ltd., Hornby, Canada). The effect of calcium concentrations on MX-2401, vancomycin, and daptomycin activities was determined by comparing the MICs in Mueller-Hinton broth (MHB), CAMHB, and CAMHBc. The effect of inoculum on MICs was assessed using the agar dilution antibiotic susceptibility test, where two inocula were tested: 105 and 1010 CFU per agar plate. The MICs were interpreted in accordance with the recommendations of the CLSI. CLSI breakpoints were used for susceptibility interpretation of vancomycin and oxacillin (6). Vancomycin, tested in CAMHB against Enterococcus faecalis ATCC 29212, was used as a quality control.
MBC determination.
The minimum bactericidal concentrations (MBCs) were determined from the MIC plates after 18 to 24 h of incubation at 36 ± 1°C by plating 10 μl collected from each well onto a 150-mm CAMHB agar (Oxoid, Basingstoke, Hampshire, United Kingdom) plate. The agar plate was then incubated for 24 h at 36 ± 1°C, and the number of colonies for each antimicrobial dilution was recorded. The MBC was defined as the lowest concentration of the antimicrobial that killed 99.9% (3 log10 reduction) of bacteria from the initial inoculum used in the microdilution broth method.
Time-kill studies.
An isolated colony of E. faecalis (ATCC 29212) or S. aureus (ATCC 19636) was grown overnight in CAMHBc at 36 ± 1°C with agitation. One hundred microliters of this culture was then transferred to 5 ml of fresh CAMHBc, and the culture was incubated at 36 ± 1°C with shaking until an optical density at 600 nm (OD600) of 0.2 to 0.5 (∼108 CFU/ml) was reached. These log-phase cultures of E. faecalis or S. aureus were then diluted 1 in 100 in CAMHBc to a concentration of ∼106 CFU/ml. Antimicrobial solutions (0.1 ml) at 10 times the desired final drug concentrations were added to 0.9 ml of the 106 CFU/ml bacterial inocula in CAMHBc, and the bacteria were incubated at 36 ± 1°C with agitation. A positive control without the drug and a negative control without both the drug and bacteria were also included in each experiment. At several time points between 0 and 24 h of incubation, a 20-μl sample from each culture was serially 10-fold diluted to 10−5 to minimize potential drug carryover, and 50 μl of each dilution was plated on tryptic soy agar (Becton-Dickinson, Sparks, MD) plates. These plates were then incubated at 36 ± 1°C overnight, and the colonies were counted to determine the bacterial titer in each sample. The lower limit of detection was 20 CFU/ml.
PAE measurements.
Approximately 106 CFU/ml of S. aureus ATCC 29213 inoculum in 5 ml CAMHBc were exposed for 1 h to MX-2401, daptomycin, or vancomycin at 1 to 4 times the MIC of each drug. To examine the postantibiotic effect (PAE), the antimicrobial-treated cells were collected by centrifugation at 7,500 × g for 10 min, washed twice with 5 ml of phosphate-buffered saline (pH 7.4), and resuspended in the initial volume of warm (35°C) CAMHBc. The effect of the various antimicrobials on bacterial growth was then monitored by measuring the titers of the bacteria in the antimicrobial-exposed samples every hour until growth was observed visually in the tube with the highest concentration of the antimicrobial. Untreated control cells were handled in parallel in an identical fashion, and the PAE was calculated by the following equation: PAE = T − C, where T was the time to achieve 1 log10 CFU/ml growth for the antimicrobial-exposed sample and C was the time to achieve 1 log10 CFU/ml growth for the untreated control sample (8).
Frequency of spontaneous resistance.
A 100-μl volume of a high inoculum (∼1010 to 1011 CFU/ml) of S. aureus ATCC 19636 was inoculated onto 10 CAMHBc agar plates containing MX-2401, daptomycin, or vancomycin at 1 to 8 times the MIC of each drug. These plates were then incubated at 36 ± 1°C overnight, and the colonies representing the spontaneous mutants were counted to determine the frequency of spontaneous resistance. The frequency of spontaneous resistance was determined by dividing the number of spontaneous mutants on antimicrobial-containing plates by the total CFU inoculated.
Emergence of resistance by serial passaging.
A broth microdilution method for MIC testing was performed as described in “Antimicrobial susceptibility testing.” MX-2401, daptomycin, vancomycin, and fusidic acid were tested against vancomycin-sensitive enterococci (VSE; ATCC 29212), VRE (ATCC 51559), methicillin-sensitive S. aureus (MSSA; ATCC 19636 and ATCC 29213), and MRSA (ATCC 43300) strains. After an overnight incubation, the MIC values were determined. The well with the highest antimicrobial concentration that allowed for growth was diluted in test media to obtain a concentration of 1 × 105 to 5 × 105 CFU/ml, which was then used as the inoculum for the next (2nd) passage. A freshly prepared set of antibacterial dilutions was then tested against these inoculum preparations using the broth microdilution method (5). The procedure was repeated for a total of 14 times to yield a total of 15 passages.
Effect of lung surfactant on antimicrobial activity and membrane depolarization effect of MX-2401.
MICs in the presence of lung surfactant were determined as described above except that a bovine lung extract-based surfactant (Survanta; Abbot, St-Laurent, Canada) was added to CAMHBc at 1.5% or 3% (vol/vol) and inoculated with approximately 5 × 105 CFU/ml of bacteria for 18 to 24 h at 36 ± 1°C. The MICs were interpreted in accordance with the recommendations of the CLSI.
Membrane depolarization was determined by using a fluorescence assay based on the method described previously (28), with some modifications. An inoculum of ∼107 CFU/ml of Staphylococcus epidermidis ATCC 12228 at exponential phase was incubated with MX-2401, daptomycin, or amphomycin at various concentrations in CAMHBc in the presence or absence of lung surfactant Survanta (Abbot Laboratories) for 15 to 240 min. A membrane potential-sensitive dye, 3,3′-dipropylthiadicarbocyanine iodide [DiSC3(5); Invitrogen, CA] was added into the cells. To compare levels of membrane depolarization, the relative fluorescence unit (RFU) over 120 s was measured for each treatment group following addition of the dye and normalized against its corresponding control. An aliquot of cells was removed from each sample before the addition of the fluorescent dye to determine cell viability/bactericidal activity by counting CFU.
S. pneumoniae bronchial-alveolar pneumonia model.
The bronchial-alveolar pneumonia model was performed as described by Silverman and colleagues (29). Briefly, female CD-1 mice (n = 5 in treatment groups, n = 10 in the control group) were infected intranasally with 5 × 106 CFU of S. pneumoniae (ATCC 6303) and treated with MX-2401 at 1 and 4 h postinfection by subcutaneous injection. At 24 h postinoculation, mice were euthanized. Lungs were aseptically removed and homogenized. Serially diluted homogenates were plated, and bacterial counts were determined following plate incubation.
Effect of calcium on the secondary structure of MX-2401 by solution circular dichroism and nuclear magnetic resonance (NMR).
In order to determine whether Ca2+ has an effect on the secondary structure of MX-2401, solution state circular dichroism (CD) experiments were performed and results were compared to results from identical experiments carried out on daptomycin. A 1 mM stock solution of MX-2401 or daptomycin and a 1.5 mM stock solution of CaCl2 were prepared in 1 mM phosphate buffer, pH 6.8. A stock solution of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt) (POPG) (1:1 molar ratio) was prepared in the following manner. Appropriate volumes of POPC and POPG dissolved in chloroform were placed in a round-bottom flask. The chloroform was evaporated under a stream of nitrogen. The sample was then placed in a vacuum desiccator overnight, and, finally, the lipid was resuspended in 1 mM phosphate buffer, pH 6.8, and sonicated for 30 min to eliminate any turbidity. Appropriate amounts of the stock solutions were combined to yield CD samples consisting of 500 μM POPC-POPG and 100 μM MX-2401 or daptomycin, with Ca2+ being present in ratios of lipopeptide to Ca2+ of 1:0, 1:0.5, 1:1, and 1:1.5.
Solution CD experiments were carried out using a J-810 spectropolarimeter (JASCO, Victoria, BC, Canada) as previously described (4, 16). Briefly, the spectra were obtained over a wavelength range of 185 to 250 nm using the continuous scanning mode with a response of 1 s with 0.5-nm steps, a bandwidth of 1.5 nm, and a scan speed of 20 nm/min. The signal-to-noise ratio was increased by acquiring each spectrum over an average of three scans. Finally, each spectrum was corrected by subtracting the background from the sample spectrum. Solution CD samples were placed in a cell (0.1 cm in length) in 200-μl portions,
The following NMR samples were prepared: (i) 2 mM MX-2401 in 1 mM phosphate buffer, pH 6.8, and 10% D2O and (ii) 2 mM MX-2401 plus 4 mM CaCl2 in 1 mM phosphate buffer, pH 6.8, and 10% D2O. One-dimensional 1H spectra were recorded on a 500-MHz Bruker Avance spectrometer, operating at a frequency of 499.98 MHz. The spectra were acquired at 308 K. Water suppression was achieved using a presaturation pulse.
RESULTS
Activity against Gram-positive organisms.
The spectrum of activity of MX-2401 against resistant and sensitive Gram-positive bacterial isolates was determined. The majority of the resistant Gram-positive clinical isolates tested came from surveillance studies conducted in the United States and Canada. MIC50, MIC90, and ranges for Enterococcus spp., S. aureus, coagulase-negative staphylococci, Streptococcus spp., and anaerobes are provided in Table 1. The data indicate that MX-2401 is active against a broad spectrum of resistant and sensitive Gram-positive pathogens, with MICs ranging from ≤0.125 μg/ml against streptococci to 4 μg/ml against enterococci. The resistant pathogens tested include multidrug-resistant, vancomycin-resistant Enterococcus spp. (VRE), methicillin-resistant S. aureus (MRSA), methicillin-resistant S. epidermidis (MRSE), vancomycin-intermediate S. aureus (VISA), mupirocin-resistant S. aureus, linezolid- and quinupristin-dalfopristin-resistant Enterococcus spp., penicillin-intermediate and -resistant S. pneumoniae (PISP and PRSP, respectively), and macrolide-resistant S. pneumoniae. Resistance to other antibiotics had no observable impact on the MICs of MX-2401, with similar MICs against sensitive and resistant organisms observed. For example, the ranges of MICs against MSSA (n = 5) and MRSA (n = 10) were found to be 0.25 to 2 and 0.25 to 4 μg/ml, respectively. The ranges of MICs against vancomycin-sensitive Enterococcus spp. (n = 2) and VRE (n = 16) were 0.25 to 2 and 1 to 4 μg/ml, respectively.
Table 1.
MICs of MX-2401, vancomycin, and oxacillin against isolates of Gram-positive bacteria
| Organism | Drug (no. of isolates tested) | MIC (μg/ml) |
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| S. aureusa | MX-2401 (15) | 0.25–4 | 0.5 | 2 |
| Vancomycin (15) | ≤0.125–4 | 0.25 | 2 | |
| Oxacillin (10) | ≤0.125–>64 | >64 | >64 | |
| Coagulase-negative | MX-2401 (13) | 0.25–4 | 1 | 2 |
| staphylococcib | Vancomycin (13) | ≤0.125–4 | 2 | 2 |
| Oxacillin (10) | ≤0.125–>64 | 32 | 64 | |
| Enterococcus spp.c | MX-2401 (18) | 0.25–4 | 2 | 4 |
| Vancomycin (18) | 0.25–>64 | >64 | >64 | |
| Oxacillin (11) | 32–>64 | >64 | >64 | |
| Streptococcus spp.d | MX-2401 (12) | ≤0.125–2 | 0.25 | 2 |
| Vancomycin (12) | ≤0.125–2 | 0.25 | 1 | |
| Oxacillin (10) | ≤0.125–32 | 0.5 | 16 | |
| Anaerobese | MX-2401 (6) | 0.5–1 | NAf | NA |
| Vancomycin (6) | 0.25–1 | NA | NA | |
| Oxacillin (6) | 0.125–4 | NA | NA | |
Includes methicillin-sensitive and -resistant, vancomycin-intermediate, tetracycline-resistant, and mupirocin-resistant S. aureus.
Includes methicillin-sensitive and -resistant coagulase-negative staphylococci (including 11 strains of S. epidermidis).
Includes vancomycin-sensitive and -resistant (VanA-, VanB-, and VanC-positive strains), linezolid-sensitive, -intermediate, and -resistant, quinupristin-dalfopristin-sensitive, -intermediate, and resistant, and tetracycline-resistant Enterococcus spp. (E. faecalis, E. faecium, and E. gallinarum).
Includes penicillin-sensitive, -intermediate, and -resistant and erythromycin-sensitive and -resistant S. pneumoniae and azithromycin-resistant viridans group streptococci.
Includes Peptostreptococcus magnus, Clostridium difficile, and erythromycin-resistant and -sensitive Propionibacterium acnes.
NA, not available.
With respect to Enterococcus spp., the activity against E. faecium isolates (n = 8, MIC range of 0.25 to 4 μg/ml) was comparable to that against E. faecalis (n = 8, MIC range of 2 to 4 μg/ml). All of the Gram-positive strains tested were sensitive to MX-2401 compared to 74.1% for vancomycin. The majority of tested strains were resistant to oxacillin. MX-2401 was not active (MIC ≥ 256 μg/ml) against Gram-negative bacteria (Escherichia coli and Haemophilus influenzae) or yeasts (Candida albicans and Candida krusei).
Effect of calcium and inoculum on MX-2401 activity.
The calcium effect on the in vitro activity of MX-2401 and daptomycin against S. aureus, E. faecalis, and E. faecium was determined by comparing MICs in MHB, CAMHB (Ca2+ concentration of 0.625 mM), and CAMHBc (Ca2+ concentration of 1.25 mM), with the last medium representing the final concentration of calcium in the susceptibility testing method for daptomycin and MX-2401. The data indicate (Table 2) that the MICs of MX-2401 and daptomycin were reduced in the presence of higher calcium concentrations although in the case of MX-2401 the calcium dependence appeared to be somewhat less than that for daptomycin. As expected, the MIC of vancomycin was not affected by the increase in calcium concentrations.
Table 2.
Comparison of the MICs of MX-2401 at different calcium concentrations against S. aureus, E. faecalis, and E. faecium
| Organism | Compound | MIC (μg/ml) ina: |
Fold decrease in MIC | ||
|---|---|---|---|---|---|
| MHB | CAMHB | CAMHBc | |||
| MSSA (clinical isolate) | Daptomycin | 32 | NDb | 0.5 | 64 |
| MX-2401 | 32 | 1 | 32 | ||
| MRSA (ATCC 43300) | Daptomycin | 32 | ND | 0.5 | 64 |
| MX-2401 | 64 | 2 | 32 | ||
| E. faecium (VSE; clinical isolate) | Daptomycin | 128 | ND | 4 | 32 |
| MX-2401 | 64 | 4 | 16 | ||
| E. faecalis (VSE; ATCC 29212) | Daptomycin | ND | 32 | 2 | 16 |
| MX-2401 | ND | 8 | 2 | 4 | |
| Vancomycin | ND | 1 | 1 | None | |
MHB, Mueller-Hinton broth; CAMHB, cation-adjusted Mueller-Hinton broth; CAMHBc, cation-adjusted Mueller-Hinton broth supplemented with 25 mg/liter of Ca2+.
ND, not done.
In an additional study, the effect of various calcium concentrations (0.125, 0.375, 0.625, 1.25, and 1.875 mM) on MX-2401 activity against S. aureus ATCC 29213 and S. pneumoniae ATCC 49619 was tested: the MICs of MX-2401 decreased in a dose-dependent manner with increasing calcium levels, with the lowest MICs of 1 and 0.25 μg/ml, respectively, observed at the physiological calcium concentration of 1.25 mM. The same MIC values were observed at the highest calcium concentration of 1.875 mM (data not shown).
The effect of changing the inoculum size from 105 to 1010 CFU/ml on the solid-phase MICs of MX-2401, daptomycin, and vancomycin against S. aureus was also determined. MX-2401 and vancomycin were minimally affected by the 100,000-fold increase in inoculum size, with a 4-fold increase in MIC, while the MIC of daptomycin increased by 16-fold.
Effect of calcium on MX-2401 secondary structure.
The effect of calcium on the secondary structure of MX-2401 was compared to its effect on that of daptomycin to determine whether the changes in MIC observed above might be related to changes in structure of the lipopeptides or the arrangement of the lipopeptide in a model membrane with a bacterium-like composition (POPC-POPG). As previously observed, increasing the Ca2+ concentration caused a dramatic shift in the circular dichroism (CD) profile of daptomycin (Fig. 1A), consistent with the proposed effects of Ca2+ on membrane permeability. The addition of one or more equivalents of Ca2+ completely inverted the positive ellipticity at 230 nm in the absence of calcium. This effect has been interpreted as being due to a Ca2+-dependent change in structure (15, 19) or daptomycin-mediated fusion of POPC-POPG liposomes in the presence of calcium (16) without a structural change (12).
Fig. 1.
CD spectra of 100 μM daptomycin (A) and 100 μM MX-2401 (B) in 500 μM POPC-POPG (1:1 molar ratio) in the presence of increasing amounts of Ca2+: no calcium (black solid line), 1:0.5 molar ratio of lipopeptide to calcium (black dashed line), 1:1 molar ratio of lipopeptide to calcium (black dotted line), and 1:1.5 molar ratio of lipopeptide to calcium (gray line).
In contrast the effect of addition of Ca2+ on the CD spectrum of MX-2401 was much more subtle, resulting in a modest increase in the measured ellipticity at ∼200 nm and a shift in the maximum ellipticity to lower wavelengths (Fig. 1B). A similar observation was made with 100 μM MX-2401 in the presence of increasing amounts of Ca2+, in the absence of lipid (data not shown). Such a moderate change was previously observed for the natural lipopeptide antibiotic friulimicin (23) and was interpreted as indicating that the addition of calcium stabilizes the secondary structure. However, in other examples in the literature where increases in ellipticity and shifts are observed with increasing concentrations of Ca2+, the degrees of secondary structural change have been quantified and are typically small, for example, on the order of 2% and 13% for chromogranin A (CGA) (1) and calmodulin (20), respectively. Regardless of the exact interpretation, it was clear that calcium had a relatively subtle effect on MX-2401 compared its effect on daptomycin. This finding is further supported by the one-dimensional NMR spectra recorded for MX-2401 (data not shown), which were practically identical for the samples prepared with and without calcium.
Bactericidal activity.
The bactericidal activity of MX-2401 was initially evaluated by determining the MBC to MIC ratio using S. aureus (ATCC 19636). Compounds with MBCs within 2 dilutions of their MICs are generally considered bactericidal. The MBC/MIC ratio of MX-2401 against S. aureus was 0.5/0.25, while the ratios of bactericidal compounds vancomycin and daptomycin were 1/0.5 and 0.5/0.125, respectively, indicating that all compounds were bactericidal.
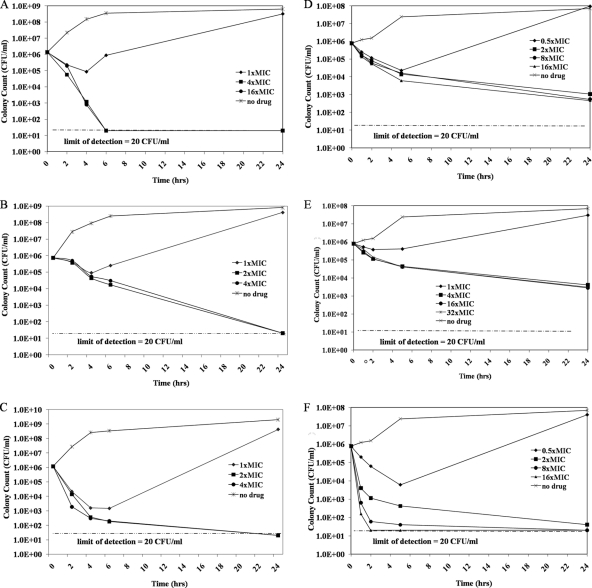
The bactericidal activity of MX-2401, vancomycin, and daptomycin was further explored with time-kill studies using S. aureus (Fig. 2A to C) and E. faecalis (Fig. 2D to F). The untreated controls showed growth from 106 (starting inoculum) to 108 to 109 CFU/ml after 24 h of incubation for both strains (Fig. 2A to F). At the MICs, MX-2401 showed some bactericidal activity against S. aureus and E. faecalis by initially reducing the bacterial load from ∼106 to ∼104 to 105 CFU/ml. However, the bacterial counts rebounded close to that for the untreated control at 24 h. At 4 times the MIC, MX-2401 was rapidly bactericidal against S. aureus, decreasing bacterial numbers by 3 log10 after 4 h of incubation and down to undetectable levels after 6 h (Fig. 2A) compared to 14 and 24 h, respectively, for vancomycin (Fig. 2B) and 4 h and 24 h, respectively, for daptomycin (Fig. 2C). At 2, 8, and 16 times the MIC, MX-2401 was also bactericidal against E. faecalis, with bacterial numbers decreasing by 99.9% after 24 h of incubation (Fig. 2D), while vancomycin was not bactericidal at concentrations up to 32 times the MIC (Fig. 2E). Against E. faecalis, daptomycin at 2, 8, and 16 times the MIC was more rapidly bactericidal than MX-2401 and vancomycin, reducing bacterial counts by 3 log10 after approximately 1 to 5 h of incubation (Fig. 2F).
Fig. 2.
Time-kill curves for MX-2401 (A), vancomycin (B), and daptomycin (C) versus S. aureus (MRSA strain ATCC 19636) and of MX-2401 (D), vancomycin (E), and daptomycin (F) versus E. faecalis (strain ATCC 29212). MICs of MX-2401, vancomycin, and daptomycin against S. aureus were 0.25, 0.5, and 0.5 μg/ml, respectively; MICs against E. faecalis were 2, 1, and 2 μg/ml, respectively.
PAE.
The postantibiotic effect (PAE) represents the continued suppression of antibacterial growth after antibiotic is removed and reflects the ability of a drug to continue killing bacteria after tissue or serum concentrations fall below the MIC. The PAE of MX-2401 against S. aureus (MSSA) was determined, and, like that of daptomycin, increased with increasing drug concentrations, while the PAE of vancomycin did not improve under these conditions. At 4 times the MIC, the PAE of MX-2401 was 2.4 h compared to 1.9 h for daptomycin and 1.4 h for vancomycin (Table 3).
Table 3.
Postantibiotic effect of MX-2401 against S. aureus ATCC 29213
| Concn | Postantibiotic effect (h) ofa: |
||
|---|---|---|---|
| Daptomycin (1) | Vancomycin (1) | MX-2401 (2) | |
| MIC | 0.9 | Not tested | 1.5 |
| 2× MIC | 1.4 | 1.7 | 1.9 |
| 4× MIC | 1.9 | 1.4 | 2.4 |
Values in parentheses are MICs in μg/ml.
Frequency of spontaneous resistance.
Single-step frequencies of spontaneous mutation leading to resistance to MX-2401, vancomycin, or daptomycin were determined using the MSSA strain ATCC 19636. Inocula of up to 2.1 × 1011 CFU were exposed to 4 or 8 times the MIC of vancomycin or daptomycin, while an inoculum of 1.2 × 1010 CFU was exposed to 4 or 8 times the MIC of MX-2401. No spontaneous S. aureus mutants resistant to these drugs were observed, indicating a spontaneous rate of <8.2 × 10−11.
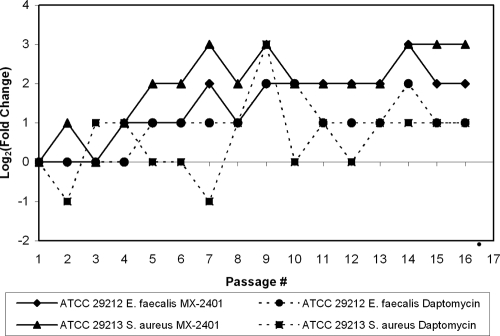
Emergence of resistance by serial passage.
Various organisms (∼1 × 105 to 5 × 105 CFU/ml) were serially passaged up to 15 passages in the presence of increasing concentrations of (subinhibitory) MX-2401, daptomycin, vancomycin, or fusidic acid. Fusidic acid was used as a control antibiotic since it selects for mutants relatively easily (Table 4). Resistance to the lipopeptides developed slowly (Fig. 3), peaking at 4 to 8 times the MIC for MX-2401 and 2 to 16 times the MIC for daptomycin (Table 4). These data indicated that MX-2401, similar to daptomycin, had a low potential for emergence of resistance. The MRSA or the VRE phenotypes, compared to the MSSA or VSE phenotypes, did not increase the potential for MX-2401 resistance to occur.
Table 4.
Results of serial passaging studies
| Straina | Antibiotic | MIC (μg/ml) |
MIC increase (fold) | |
|---|---|---|---|---|
| Initial | After 15 passages | |||
| S. aureus ATCC 19636 (MSSA) | MX-2401 | 1 | 8 | 8 |
| Daptomycin | 0.5 | 2 | 4 | |
| Vancomycin | 1 | 2 | 2 | |
| Fusidic acid | 0.25 | 0.5 | 2 | |
| S. aureus ATCC 29213 (MSSA) | MX-2401 | 2 | 16 | 8 |
| Daptomycin | 1 | 2 | 2 | |
| Vancomycin | NAb | NA | NA | |
| Fusidic acid | NA | NA | NA | |
| S. aureus ATCC 43300 (MRSA) | MX-2401 | 1 | 8 | 8 |
| Daptomycin | 0.25 | 4 | 16 | |
| Vancomycin | 0.5 | 2 | 4 | |
| Fusidic acid | 0.25 | 16 | 64 | |
| E. faecium ATCC 51559 (VRE) | MX-2401 | 2 | 8 | 4 |
| Daptomycin | 0.5 | 4 | 8 | |
| Vancomycin | >32 | >32 | NA | |
| Fusidic acid | 4 | >32 | >8 | |
| E. faecalis ATCC 29212 (VSE) | MX-2401c | 4 | 16–32 | 4–8 |
| Daptomycinc | 1–4 | 8 | 2–8 | |
| Vancomycin | 2 | 4 | 2 | |
| Fusidic acid | 4 | >32 | >8 | |
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; VRE, vancomycin-resistant enterococci; VSE, vancomycin-sensitive enterococci.
NA, not available.
Results of two experiments are shown.
Fig. 3.
Serial passaging of VSE (E. faecalis; ATCC 29212) and MSSA (ATCC 29213) in the presence of MX-2401 and daptomycin.
Effect of lung surfactant on antimicrobial activity and membrane depolarization effect of MX-2041.
The effect of lung surfactant on MICs of MX-2401, vancomycin, and daptomycin was investigated using S. aureus (MRSA strain; MICs of 2, 1, and 0.5 μg/ml, respectively) and S. pneumoniae (PRSP strain; MICs of 0.5, 0.5, and 0.125 μg/ml, respectively). In the presence 1.5 or 3.0% surfactant, the MICs of MX-2401 and vancomycin against S. aureus or S. pneumoniae were within 1 dilution of their MICs without lung surfactant (Fig. 4A). In contrast, the MICs of daptomycin against these bacteria were dramatically increased in the presence of lung surfactant (Fig. 4A and B). Specifically, in the presence of 1.5 or 3% surfactant, the MIC against S. aureus increased 32-fold and 64-fold, respectively. Against S. pneumoniae, the MIC of daptomycin increased 128-fold.
Fig. 4.
Effect of the lung surfactant on MICs of MX-2401, daptomycin, and vancomycin against MRSA (A) and PRSP (B). The changes of antimicrobial activity in the presence of surfactant are expressed as MICsurfactant/MICno surfactant ratios.
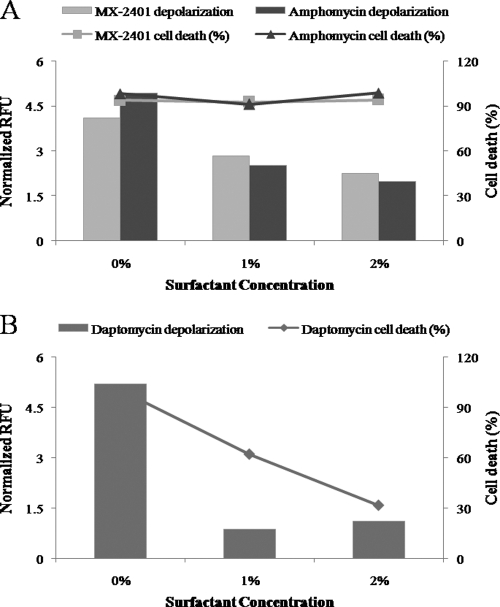
Our earlier studies indicated that MX-2401, similar to daptomycin, was able to induce membrane depolarization, albeit at a very low rate and only at high concentrations (25). To further evaluate the surfactant effect, membrane depolarization of MX-2401 and that of its parent compound amphomycin in the presence of lung surfactant were determined and compared to that of daptomycin. The bactericidal activity of the drugs was evaluated in parallel. Addition of surfactant resulted in a dose-dependent inhibition of the weak membrane depolarization caused by MX-2401 and amphomycin, yet the bactericidal effect of the drugs remained unchanged (>90% cell death) (Fig. 5A). These data confirmed our earlier finding (25) that the bactericidal mechanism of MX-2401 and related compounds is not associated with membrane depolarization. Inhibition of depolarization by the surfactant was also observed in the cells incubated with daptomycin, but this inhibition was coupled with a dramatic reduction of daptomycin bactericidal activity (Fig. 5B).
Fig. 5.
Membrane depolarization and cell death in S. epidermidis ATCC 12228 following incubation with supra-MICs of antibiotics in the presence or absence of surfactant. (A) Cells incubated for 60 min with 4 times the MIC of MX-2401 or amphomycin. (B) Cells incubated for 60 min with 4 times the MIC of daptomycin.
Activity in an S. pneumoniae bronchial-alveolar pneumonia model.
To further investigate how surfactant affects MX-2401 activity, the effect of the drug in a murine bronchial-alveolar model of pneumonia was determined. When daptomycin was tested in this model under the same experimental conditions, no detectable reduction of the bacterial burden was observed at 24 h postinfection, even at 100 mg/kg of body weight (29). In contrast, MX-2401 treatment resulted in a dose-dependent killing of S. pneumoniae in the lungs of infected mice, with a maximum effect characterized by a 5.1 log10 reduction in S. pneumoniae counts (Fig. 6). A positive control, cefotaxime, was tested at 6.25 mg/kg and demonstrated a 5.7 log10 reduction at this dose.
Fig. 6.
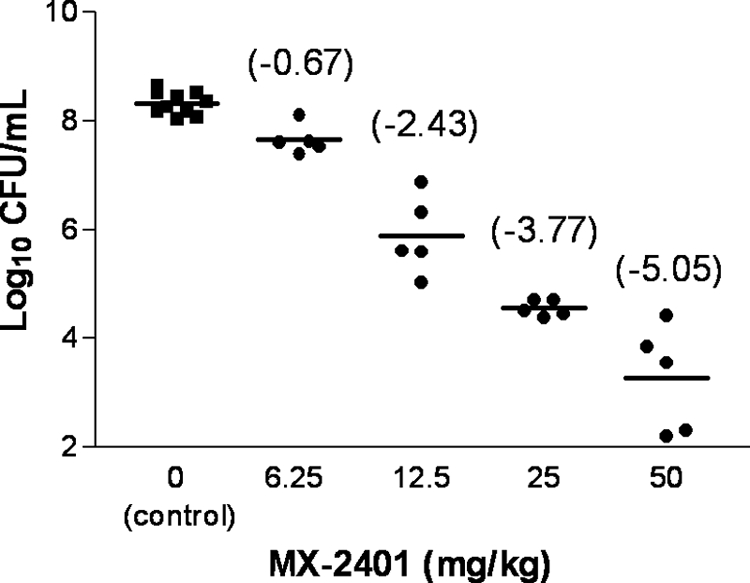
Activity of MX-2401 in a bronchoalveolar pneumonia model (S. pneumoniae ATCC 6303). Total doses of MX-2401 are shown (the drug was administered at 1 and 4 h postinfection). Log10 CFU/ml reductions versus control group are shown in parentheses.
DISCUSSION
MX-2401 is a novel lipopeptide antibiotic that was broadly active against a variety of Gram-positive bacteria associated with serious infections, including MRSA, VRE, and PRSP, with MIC90 ranges of 2 to 4 μg/ml. MX-2401 was also potent against the few anaerobes tested, with MICs between 0.5 and 1 μg/ml. No evidence of cross-resistance to MX-2401 was observed with oxacillin-resistant S. aureus; oxacillin-resistant S. epidermidis; vancomycin-resistant enterococci, including vanA-, vanB-, and vanC-positive strains; linezolid- and quinupristin-dalfopristin-resistant enterococci; macrolide-resistant S. pneumoniae; and penicillin-resistant or penicillin-intermediate S. pneumoniae isolates. These results are consistent with and expand on previous observations (13) that MX-2401 was potent and bactericidal against a range of S. aureus isolates, including sensitive strains as well as community- and health care-associated MRSA and vancomycin-intermediate and -resistant S. aureus.
The studies presented here suggest that MX-2401 exhibited a better bactericidal profile than vancomycin, killing more rapidly and demonstrating bactericidal activity against E. faecalis. Bactericidal activity is important in antimicrobial chemotherapy especially in cases of endocarditis (14) and osteomyelitis (31), as well as for the treatment of the immunocompromised patients (27), where the immune system of the host is unlikely to assist in bacterial eradication. Other observed microbiological properties of MX-2401 include the low frequency of spontaneous resistance, the low potential for emergence of resistance, and a long in vitro PAE, consistent with bacterial cell damage during exposure that impacts the growth fitness of bacteria. The PAE of MX-2401 was similar to or slightly longer than those of vancomycin and daptomycin as reported in our study and in keeping with literature values for the latter two antibiotics (7, 21). The long PAE was also observed in in vivo studies (9). In addition, MX-2401 exhibited a longer half-life than daptomycin and vancomycin in rodent species (i.e., 5.7 h in mice versus 1.8 h for daptomycin and ∼0.5 h for vancomycin) (22, 26, 30). The long half-life would be expected to result in a longer time above MICs and, when combined with the long PAE, would result in continued pressure on pathogens.
As with other lipopeptides targeting Gram-positive bacteria, such as daptomycin (11) and amphomycin (18), MX-2401 activity was Ca2+ dependent. Thus, MICs of MX-2401 against Gram-positive organisms decreased in a dose-dependent manner with increasing Ca2+ concentrations, with the lowest MICs observed at physiological Ca2+ levels. For amphomycin and its analogs, this Ca2+-dependent effect has been speculated to be related to the ability of Ca2+ to stabilize the interaction of the lipid tail of amphomycin with the lipid moiety of undecaprenylphosphate (3, 18). Daptomycin, however, exhibits a different mechanism of action, as Ca2+ helps daptomycin to interact with and aggregate in the membrane (2, 15, 19, 24), leading to membrane depolarization and eventual bacterial cell death (28). Nevertheless, the presence of Ca2+ had less of an effect on the activity and secondary structure/membrane interaction of MX-2401 than on those of daptomycin, consistent with its different mechanism of action, inhibiting the biosynthesis of the cell wall and teichoic acid precursors (25).
Another distinctive characteristic of MX-2401 is that, in contrast to daptomycin, it was able to retain its microbiological activity in the presence of lung surfactant. Daptomycin is approved for treatment of complicated skin and skin-structure infections; however, the drug failed to achieve statistical noninferiority criteria for the treatment of severe community-acquired pneumonia (29). This is despite the fact that the drug exhibits potent bactericidal activity against S. pneumoniae. Further research suggested that daptomycin antimicrobial activity was dramatically inhibited by pulmonary surfactant (29). As a result, the drug failed to show activity in a mouse model of bronchial-alveolar pneumonia, even at a dose of 100 mg/kg (29). Therefore, it was critical to investigate if MX-2401 activity was affected by pulmonary surfactant. In vitro studies showed that activity of MX-2401 against S. pneumoniae and S. aureus was not significantly affected by the presence of lung surfactant. Moreover, MX-2401 was efficacious in a model of bronchial-alveolar pneumonia, where up to a 5 log10 reduction in bacterial counts was observed. It was suggested that inhibition of daptomycin by pulmonary surfactant is directly related to its mechanism of action. Daptomycin demonstrates Ca2+-dependent insertion into and disruption of the bacterial membrane (28, 29). In contrast, MX-2401 bactericidal activity is not associated with membrane depolarization, even though the drug is able to induce a very modest degree of membrane depolarization that requires concentrations significantly above MICs (25). The observed delay between bactericidal activity and the start of membrane depolarization indicates that the limited depolarization may be a consequence, rather than the cause, of cell death. This is further supported by the data generated here that showed that addition of lung surfactant resulted in a dose-dependent inhibition of membrane depolarization by MX-2401 (and amphomycin) but did not affect the bactericidal effect of this drug.
Overall the results presented here suggest that MX-2401 is an expanded-spectrum lipopeptide that exhibits several improved features, including a novel mechanism of action, lack of inhibition by lung surfactant, and long half-life compared to the narrow-spectrum lipopeptide daptomycin. The properties of MX-2401 described here, as well as the documented microbiological, physiological, and mechanistic properties described elsewhere (9, 10, 13, 22, 25), indicate that this drug may fulfill a serious medical need for the treatment of life-threatening infections with Gram-positive bacteria.
ACKNOWLEDGMENTS
We thank Laurel Workman for her technical help with the postantibiotic effect work and Jeremy Fenn for technical support during kill-kinetic experiments.
S.K.S. acknowledges funding from NSERC (Discovery grant) and the Michael Smith Foundation for Health Research (Career Investigator Scholar). R.E.W.H. holds a Canada Research Chair and acknowledges additional support from the Canadian Institutes for Health Research.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Angeletti R. H., Ali G., Shen N., Gee P., Nieves E. 1992. Effects of calcium on recombinant bovine chromogranin A. Protein Sci. 1:1604–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ball L. J., Goult C. M., Donarski J. A., Micklefield J., Ramesh V. 2004. NMR structure determination and calcium binding effects of lipopeptide antibiotic daptomycin. Org. Biomol. Chem. 2:1872–1878 [DOI] [PubMed] [Google Scholar]
- 3. Banerjee D. K. 1989. Amphomycin inhibits mannosylphosphoryldolichol synthesis by forming a complex with dolichylmonophosphate. J. Biol. Chem. 264:2024–2028 [PubMed] [Google Scholar]
- 4. Cheng J. T. J., Hale J. D., Elliott M., Hancock R. E., Straus S. K. 2011. The importance of bacterial membrane composition in the structure and function of aurein 2.2 and selected variants. Biochim. Biophys. Acta 1803:622–633 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed Document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. Approved standard MS100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 7. Cooper M. A., Jin Y. F., Ashby J. P., Andrews J. M., Wise R. 1990. In-vitro comparison of the post-antibiotic effect of vancomycin and teicoplanin. J. Antimicrob. Chemother. 26:203–207 [DOI] [PubMed] [Google Scholar]
- 8. Craig W., Gudmundsson S. 1996. Postantibiotic effect, p. 296–329 In Lorian V. (ed.), Antibiotics in laboratory medicine, 4th ed Williams & Wilkins, Baltimore, MD [Google Scholar]
- 9. Craig W. A., Andes D., Satmstad T. 2010. In vivo pharmacodynamics of a new lipopeptide MX-2401. Antimicrob. Agents Chemother. 54:5092–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dugourd D., et al. 2005. Novel lipopeptide amphomycin derivatives have activity against resistant and sensitive Gram-positive bacteria, abstr. A-086. Abstr. 105th Gen. Meet. Am. Soc. Microbiol [Google Scholar]
- 11. Hanberger H., Nilsson L. E., Maller R., Isaksson B. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ho S. W., et al. 2008. Effect of divalent cations on the structure of the antibiotic daptomycin. Eur. Biophys. J. 37:421–433 [DOI] [PubMed] [Google Scholar]
- 13. Hoban D. J., Weshnoweski B., Vashisht R., Zhanel G. G., Dugourd D. 2008. In vitro activity of MX-2401, a novel lipopeptide against multi-drug resistant (MDR) Staphylococcus aureus (SA), abstr. F1-363. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 14. Hunter T. H. 1950. Speculations on the mechanism of cure of bacterial endocarditis. JAMA 144:524–527 [DOI] [PubMed] [Google Scholar]
- 15. Jung D., Rozek A., Okon M., Hancock R. E. W. 2004. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem. Biol. 11:949–957 [DOI] [PubMed] [Google Scholar]
- 16. Jung D., Powers J. P., Straus S. K., Hancock R. E. W. 2008. Lipid-specific binding of the calcium-dependent antibiotic daptomycin leads to changes in lipid polymorphism of model membranes. Chem. Phys. Lipids 154:120–128 [DOI] [PubMed] [Google Scholar]
- 17. Klevens R. M., et al. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771 [DOI] [PubMed] [Google Scholar]
- 18. Lakey J. H., Maget-Dana R., Ptak M. 1988. Conformational change on calcium binding by the lipopeptide antibiotic amphomycin. A C.D. and monolayer study. Biochem. Biophys. Res. Commun. 150:384–390 [DOI] [PubMed] [Google Scholar]
- 19. Muraih J. K., Pearson A., Silverman J., Palmer M. 2011. Oligomerization of daptomycin on membranes. Biochim. Biophys. Acta 1808:1154–1160 [DOI] [PubMed] [Google Scholar]
- 20. Omoni A. O., Aluko R. E. 2006. Effect of cationic flaxseed protein hydrolysate fractions on the in vitro structure and activity of calmodulin-dependent endothelial nitric oxide synthase. Mol. Nutr. Food Res. 50:958–966 [DOI] [PubMed] [Google Scholar]
- 21. Pankuch G. A., Jacobs M. R., Appelbaum P. C. 2003. Postantibiotic effects of daptomycin against 14 staphylococcal and pneumococcal clinical isolates. Antimicrob. Agents Chemother. 47:3012–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pasetka C. J., Erfle D. J., Cameron D. R., Clement J. J., Rubinchik E. 2010. Novel antimicrobial lipopeptides with long in vivo half-lives. Int. J. Antimicrob. Agents 35:182–185 [DOI] [PubMed] [Google Scholar]
- 23. Reder-Christ K., et al. 2011. Model membrane approaches to determine the role of calcium for the antimicrobial activity of friulimicin. Int. J. Antimicrob. Agents 37:256–260 [DOI] [PubMed] [Google Scholar]
- 24. Rotondi K. S., Gierasch L. M. 2005. A well-defined amphipathic conformation for the calcium-free cyclic lipopeptide antibiotic, daptomycin, in aqueous solution. Biopolymers 80:374–385 [DOI] [PubMed] [Google Scholar]
- 25. Rubinchik E., et al. 2011. Mechanism of action and limited cross resistance of a new lipopeptide MX-2401. Antimicrob. Agents Chemother. 55:2743–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Safdar N., Andes D., Craig W. A. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sculier J. P., Klastersky J. 1984. Significance of serum bactericidal activity in gram-negative bacillary bacteremia in patients with and without granulocytopenia. Am. J. Med. 76:429–435 [DOI] [PubMed] [Google Scholar]
- 28. Silverman J. A., Perlmutter N. G., Shapiro H. M. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silverman J. A., Mortin L. I., VanPraagh A. D. G., Li T., Alder J. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J. Infect. Dis. 191:2149–2152 [DOI] [PubMed] [Google Scholar]
- 30. Vogelman B., Gudmundsson S., Turnidge J., Leggett J., Craig W. A. J. 1988. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J. Infect. Dis. 157:287–298 [DOI] [PubMed] [Google Scholar]
- 31. Weinstein M. P., Stratton C. W., Hawley H. B., Ackley A., Reller L. B. 1987. Multicenter collaborative evaluation of a standardized serum bactericidal test as a predictor of therapeutic efficacy in acute and chronic osteomyelitis. Am. J. Med. 83:218–222 [DOI] [PubMed] [Google Scholar]
- 32. Wunderink R. G., Rello J., Cammarata S. K., Croos-Dabrera R. V., Kollef M. H. 2003. Linezolid vs. vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789–1797 [PubMed] [Google Scholar]