Abstract
We have compared the efficacy of terbinafine (TRB) and itraconazole (ITZ), the recommended drugs for the treatment of chromoblastomycosis, with that of posaconazole (PSC) and voriconazole (VRC) in athymic mice infected with the fungus Fonsecaea pedrosoi. Three weeks after challenge, mice were treated for 4 months with PSC at 10 or 20 mg/kg of body weight/day, with VRC at 10 or 20 mg/kg/day, with ITZ at 25 or 50 mg/kg/day, or with TRB at 150 or 250 mg/kg/day. The progress of the infection was evaluated by measuring the size of the lesions, by histopathological studies, and by cultures of the excised tissue. The two doses of PSC followed by the highest doses of ITZ and TRB showed the best results while VRC did not show efficacy. PSC could be an alternative in the treatment of chromoblastomycosis.
INTRODUCTION
Chromoblastomycosis is a chronic fungal infection common to tropical and subtropical areas; it affects cutaneous and subcutaneous tissues and is acquired by direct inoculation of the fungus through the skin (27). The disease is caused by several species but mainly by Fonsecaea pedrosoi and Cladophialophora carrionii and less frequently by Fonsecaea monophora, Phialophora verrucosa, Rhinocladiella aquaspersa, Exophiala dermatitidis, Exophiala jeanselmei, and Exophiala spinifera (21, 27). The clinical characteristics of the disease include the development of nodular lesions at the site of the inoculation, which can progress slowly over the years to irregular verrucose hyperkeratotic forms, scaly plaques, or scarring atrophic skin lesions. Histopathologically, this disease is characterized by the presence of sclerotic bodies (muriform cells) although occasionally pigmented hyphae can be also present (27).
Depending on the stage of the disease, different therapeutic options have been recommended to treat chromoblastomycosis; these include surgery, thermotherapy, and chemotherapy (27). Itraconazole (ITZ) and terbinafine (TRB) are the most commonly used drugs in the treatment of chromoblastomycosis (27), and combinations of them are especially indicated in refractory infections (11). Although new azoles like posaconazole (PSC) and voriconazole (VRC) have shown good efficacy against cerebral and disseminated phaeohyphomycosis (2, 16, 18, 22, 31), little is known about their role in the treatment of chromoblastomycosis.
We have compared the efficacies of VRC and PSC with those of traditionally used drugs in a murine model of chromoblastomycosis.
MATERIALS AND METHODS
Establishing the model.
Three clinical isolates, two of F. pedrosoi (FMR 6630 and FMR 5211) and one of P. verrucosa (FMR 5210), were tested for the development of a reproducible murine chromoblastomycosis. On the day of infection, 2-week cultures on potato dextrose agar (PDA) of each strain were suspended in sterile saline and filtered through sterile gauze to remove clumps of cells or hyphae. The resulting suspensions were adjusted to the desired inoculum based on hemocytometer counts. Dilutions of the original suspensions were cultured on PDA plates to test viability. Three different inocula for each fungal isolated were tested, i.e., 1 × 105 CFU, 7 × 105 CFU, and 3 × 106 CFU. To try to reproduce previous studies of other authors who developed murine models of chromoblastomycosis (1, 6), two different mouse strains and two routes of inoculation were tested. Athymic CD-1 and OF-1 male mice (Charles River, Criffa S.A., Barcelona, Spain) were used to develop such models. Animals were anesthetized by isoflurane inhalation. One day before challenge and every 9 days, half of the OF-1 mice were immunosuppressed with 200 mg/kg of body weight of cyclophosphamide and 150 mg/kg of 5-fluorouracil (24) while the remaining half remained immunocompetent. Athymic CD-1 mice were challenged subcutaneously over the left thigh with 0.1 ml of each inoculum (1). Immunocompetent or immunosuppressed OF-1 mice were similarly challenged but also infected intraperitoneally with 1 ml of the same inocula in different abdominal sites (6). Groups of 5 mice were randomly established and housed in standard boxes with corncob bedding and had free access to food and water.
All animal care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare and Ethics Committee.
Therapy study.
Once the fungal strain and the most suitable testing conditions to produce a reproducible chromoblastomycosis model were chosen, groups of eight CD-1 athymic mice were randomly established for each treatment, and one group served as a control. Mice were subcutaneously infected with 3 × 106 CFU of the strain F. pedrosoi FMR 6630, which was the only inoculum tested able to produce chronic infections, in 0.1 ml of sterile saline solution over the left thigh. The in vitro susceptibilities of the strain F. pedrosoi FMR 6630 to PSC, VRC, TRB, and ITZ were tested using a broth microdilution method according to the CLSI guidelines for filamentous fungi (7).
The drugs tested were PSC (Noxafil; Schering-Plough, Ltd., Hertfordshire, United Kingdom), ITZ (Canadiol; Laboratorios Dr. Esteve S. A., Barcelona, Spain), TRB (Novartis Farmacéutica S. A., Barcelona, Spain), and VRC (Vfend; Pfizer S. A., Madrid, Spain). All drugs were administered orally once daily, with testing the following doses: PSC at 10 or 20 mg/kg, ITZ at 25 or 50 mg/kg, TRB at 150 or 250 mg/kg, and VRC at 10 or 20 mg/kg. These doses were selected on the basis of previous experimental studies that demonstrated that these doses delivered plasma levels in mice similar to those in humans (13, 15, 17, 19, 30, 32, 34). Drug treatment did not begin until 3 weeks after challenge to ensure that all animals developed the infection, and the treatment lasted for 4 months. Control groups received no treatment. Mice treated with VRC received 0.25 ml of grapefruit juice by gavage twice daily, 30 min before and approximately 6 h after the VRC administration (29, 30, 33). In order to prevent bacterial infections, all mice received 5 mg/day of ceftazidime subcutaneously from days 1 to 7 after challenge.
The efficacies of the different drugs were evaluated by measuring the skin lesions in 3-week intervals using a caliper. The areas of the lesions were assessed using the mathematical formula of the ellipse (28). At the end of the experiment, histopathological studies and qualitative skin cultures were also performed. The compiled data of the lesion areas were analyzed by a Kruskal-Wallis test. When this test was significant, we used a Mann-Whitney U test to compare treatments with the control group. Statistical significance of the tissue cultures studied was estimated by Fisher's exact test. When P was <0.05, the differences were considered statistically significant.
For the histopathological study, approximately half of the tissue of each lesion was excised and fixed with 10% buffered formalin. Samples were dehydrated, paraffin embedded, and sliced into 2-μm sections, which were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), Grocott methenamine silver, or Giemsa and examined blind by light microscopy. Fine-needle aspiration cytologies of the nodules were also performed. For the skin culture study, the remaining half of the excised tissue was cultured on PDA supplemented with chloramphenicol, incubated at 30°C, and examined daily for 7 days.
RESULTS
Establishing the model.
We did not observe signs of disease after 4 months of the intraperitoneal inoculation of the fungus in immunocompetent mice. The subcutaneous inoculation of the fungus in immunocompetent mice produced lesions of chromoblastomycosis; however, they cured spontaneously after a few weeks. Similarly, subcutaneous inoculation of the fungus in immunocompromised OF-1 mice showed disappointing results, too. Finally, the chosen model was the subcutaneous inoculation in CD-1 athymic mice of the strain FMR 6630 since this method was able to produce a chronic infection in the animals, with the presence of typical lesions of chromoblastomycosis in the area of inoculation. Such lesions persisted at least 1 year after challenge.
Therapy study.
For strain FMR 6630, the MIC of TRB, PSC, and VRC was 0.12 μg/ml, and the MIC of ITZ was 0.5 μg/ml.
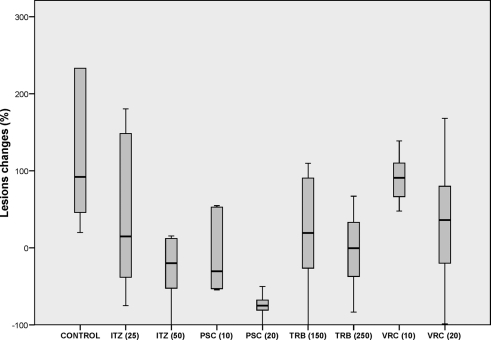
The lesions of the control animals were macroscopically characterized by the presence of dark subcutaneous soft nodules that in some cases ulcerated and disseminated throughout the thigh (Fig. 1). With the exception of animals treated with VRC at 10 mg/kg, the sizes of the lesions tended to decrease during the treatment, with a substantial reduction in the degree of ulceration and dissemination. Table 1 and Fig. 2 show the changes in the sizes of the lesions and the percentage of positive cultures at the end of the therapy. After 4 months of therapy, mice treated with TRB at 250 mg/kg, ITZ at 50 mg/kg, or PSC at 20 mg/kg showed lesions significantly smaller than those of the control group. PSC at 20 mg/kg was the most effective treatment, being able to reduce the sizes of the lesions by 75% in comparison to the start of therapy. PSC at 10 mg/kg achieved an important reduction (30.4%) in the lesion area although this was not significant with respect to the control group (P value of 0.076) due to the high dispersion of the values. ITZ at 50 mg/kg and TRB at 250 mg/kg reduced the areas of the lesions by 20% and 1%, respectively. The other therapies were not able to reduce the sizes of the lesions.
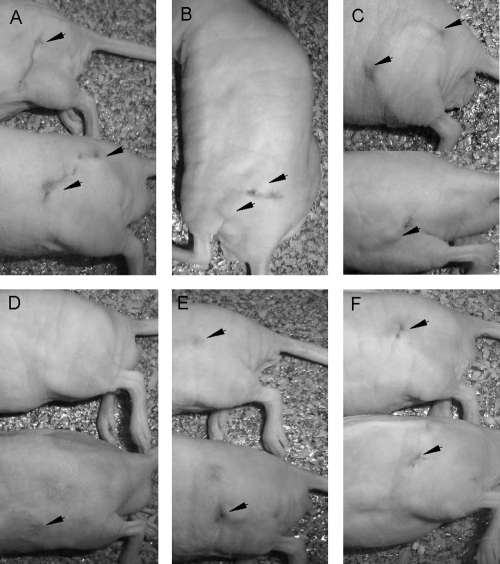
Fig. 1.
Macroscopic lesions in athymic mice 4 months after challenge with 3 × 106 CFU of the strain F. pedrosoi FMR 6630. Control mice (A and B) and mice treated with VRC at 20 mg/kg (C) show nodular disseminated lesions in the left thigh. (D) Lesions of mice treated with PSC at 20 mg/kg are almost unapparent. Mice treated with ITZ at 50 mg/kg (E) and mice treated with TRB at 250 mg/kg (F) show small nodules.
Table 1.
Changes in the sizes of the lesions of murine chromoblastomycosis by F. pedrosoi and percentage of positive cultures after 16 weeks of therapy
| Drug and dose (mg/kg of body wt) | % Change in lesion sizea | % of positive cultures |
|---|---|---|
| Control | 92.0 (368.3) | 100 |
| ITZ | ||
| 25 | 14.7 (220.9) | 100 |
| 50 | −20.0 (89.9) **b | 75 |
| PSC | ||
| 10 | −30.4 (107.6) | 75 |
| 20 | −75.0 (31.4) ** | 50** |
| TRB | ||
| 150 | 19.3 (163.3) | 100 |
| 250 | −0.6 (110.3) ** | 100 |
| VRC | ||
| 10 | 90.8 (67.3) | 100 |
| 20 | 35.9 (138.3) | 100 |
Lesions changes are expressed as median values (interquartile range).
**, Mann-Whitney test P value of <0.05 in comparison with the control group.
Fig. 2.
Box plot of changes in the sizes of the lesions of murine chromoblastomycosis caused by F. pedrosoi. Horizontal lines indicate median values.
The histological study of control mice showed well-delimited subcutaneous nodules with a central zone of necrosis and an important inflammatory response with presence of polymorphonuclear neutrophils, histiocytes, and a few multinucleated giant cells. The sizes of the nodules and the inflammatory responses were higher in control animals and in those treated with VRC than in mice treated with the other therapies (Fig. 3). Nodule sections showed fungal pigmented hyphae and abundant brown pigmented globose sclerotic bodies, mainly around the necrosis areas (Fig. 4).
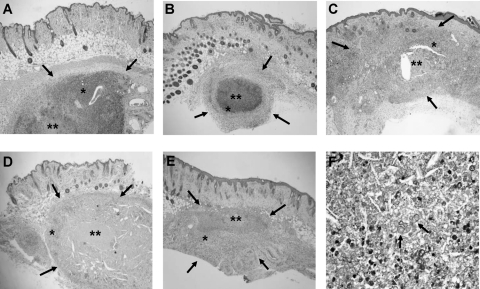
Fig. 3.
Histological sections of the nodular lesions in athymic mice 4 months after challenge with 3 × 106 CFU of F. pedrosoi FMR 6630. Control mice (A) and mice treated with VRC at 20 mg/kg (B) show subcutaneous nodules (delimited by arrows) with important inflammatory responses (*) and central zones of necrosis (**). Mice treated with TRB at 250 mg/kg (C), mice treated with ITZ at 50 mg/kg (D), and mice treated with PSC at 20 mg/kg (E) show smaller subcutaneous nodules with central necrosis but with a less intense inflammatory response (H&E staining; magnification, ×25). (F) Histological nodule section showing fungal elements with sclerotic bodies (PAS stain; magnification, ×200).
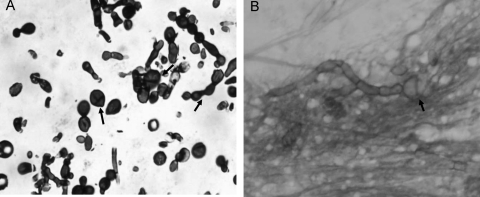
Fig. 4.
Fine-needle aspiration cytology of a nodule of an athymic mouse 4 months after challenge with 3 × 106 CFU of the strain F. pedrosoi FMR 6630 showing septate hyphae and sclerotic bodies (arrows). (A) Grocott stain (magnification, ×400). (B) Giemsa stain (magnification, ×400).
DISCUSSION
The aim of the current study was to evaluate the use of newer azoles as potential antifungal alternatives for the treatment of chromoblastomycosis in a murine model. First, we tried to reproduce the murine chromoblastomycoses described in the literature, and although we repeated the same experimental conditions reported by other authors, we were not successful in developing a chronic infection. This could be explained by the different levels of virulence of the strains of a given species, as has been demonstrated in our preliminary study, and by the low rate of cutaneous signs of infection in the intraperitoneal infection model (6). We were able to obtain mice with chronic chromoblastomycosis only after subcutaneous inoculation in athymic mice. The T cell depletion of these mice facilitated the establishment of the infection since these cells appear to have a critical role in the host mechanism of defense against F. pedrosoi (1). In addition, the lack of hair on these mice allowed a better visualization of the lesions. This model proved to be reproducible and was chosen for the present study.
In our study, PSC was more effective than the other therapies in reducing the size of the lesions and the percentages of positive cultures from affected tissue. Previous experimental studies demonstrated that this drug was active in infections caused by dematiaceous fungi (5, 10, 20), including chromoblastomycosis (8). In the clinical setting, PSC has been successfully used in cases of cerebral and disseminated phaeohyphomycosis (2, 22). Such good efficacy against dematiaceous fungi, together with the drug's good tolerance in long-term therapies, make PSC a promising drug for the treatment of chromoblastomycosis (14, 23).
ITZ is currently the most commonly used drug in the treatment of chromoblastomycoses (4); nevertheless, infections by F. pedrosoi strains resistant to that drug have also been reported (3, 25, 26). In our study ITZ, especially at high doses, also showed efficacy in the treatment of the experimental infection.
VRC has shown good in vitro activity against dematiaceous fungi (9) and has been effective in cases of cerebral phaeohyphomycosis (16, 18, 31). However, its efficacy in chromoblastomycosis has been poorly studied (12), and further studies are necessary to evaluate the potential use of VRC in such infections. Under our experimental conditions, the increase in the size of the lesions despite the use of VRC, together with a high inflammatory response and null reduction of positive cultures, showed the lack of efficacy of this drug.
Due to its high degree of effectiveness and tolerability, TRB is considered one of the best options in the treatment of chromoblastomycosis (27). In our study, while TRB, especially at the highest dose, was able to reduce the inflammatory response to the infection at similar levels to PSC and ITZ, it showed less efficacy in reducing the sizes of the lesions and the number of positive cultures than PSC at both doses and ITZ at 50 mg/kg.
In conclusion, our study suggests that PSC could be a potential alternative to ITZ and TRB in the treatment of chromoblastomycosis.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Ahrens J., Graybill J. R., Abishawl A., Tio F. O., Rinaldi M. G. 1989. Experimental murine chromomycosis mimicking chronic progressive human disease. Am. J. Trop. Med. Hyg. 40:651–658 [DOI] [PubMed] [Google Scholar]
- 2. Al-Abdely H. M., et al. 2005. Successful therapy of cerebral phaeohyphomycosis due to Ramichloridium mackenziei with the new triazole posaconazole. Med. Mycol. 43:91–95 [DOI] [PubMed] [Google Scholar]
- 3. Andrade T. S., Castro L. G., Nunes R. S., Gimenes V. M., Cury A. E. 2004. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses 47:216–221 [DOI] [PubMed] [Google Scholar]
- 4. Bonifaz A., Carrasco-Gerard E., Saúl A. 2001. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses 44:1–7 [DOI] [PubMed] [Google Scholar]
- 5. Calvo E., et al. 2010. Murine model of a disseminated infection by the novel fungus Fonsecaea monophora and successful treatment with posaconazole. Antimicrob. Agents Chemother. 54:919–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cardona-Castro N., Agudelo-Flórez P. 1999. Development of a chronic chromoblastomycosis model in immunocompetent mice. Med. Mycol. 37:81–83 [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed Approved standard M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Defaveri J., Graybill J. R. 1990. Treatment of chronic murine chromoblastomycosis with the triazole SCH39304. Am. J. Trop. Med. Hyg. 42:601–606 [DOI] [PubMed] [Google Scholar]
- 9. Espinel-Ingroff A., Boyle K., Sheehan D. J. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101–105 [DOI] [PubMed] [Google Scholar]
- 10. Graybill J. R., Najvar L. K., Johnson E., Bocanegra R., Loebenberg D. 2004. Posaconazole therapy of disseminated phaeohyphomycosis in a murine model. Antimicrob. Agents Chemother. 48:2288–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta A. K., Taborka P. R., Sanzovo A. D. 2002. Alternate week and combination itraconazole and terbinafine therapy for chromoblastomycosis caused by Fonsecaea pedrosoi in Brazil. Med. Mycol. 40:529–534 [DOI] [PubMed] [Google Scholar]
- 12. Hamza S. H., Mercado P. J., Skelton H. G., Smith K. J. 2003. An unusual dematiaceous fungal infection of the skin caused by Fonsecaea pedrosoi: a case report and review of the literature. J. Cutan. Pathol. 30:340–343 [DOI] [PubMed] [Google Scholar]
- 13. Kan V. L., Bennett J. E. 1988. Efficacies of four antifungal agents in experimental sporotrichosis. Antimicrob. Agents Chemother. 32:1619–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keating G. M., Posaconazole 2005. Drugs 65:1553–1567 [DOI] [PubMed] [Google Scholar]
- 15. Krishna G., Sansone-Parsons A., Martinho M., Kantesaria B., Pedicone L. 2007. Posaconazole plasma concentrations in juvenile patients with invasive fungal infection. Antimicrob. Agents Chemother. 51:812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koo S., Klompas M., Marty F. M. 2010. Fonsecaea monophora cerebral phaeohyphomycosis: case report of successful surgical excision and voriconazole treatment and review. Med. Mycol. 48:769–774 [DOI] [PubMed] [Google Scholar]
- 17. Leyden J. 1988. Pharmacokinetics and pharmacology of terbinafine and itraconazole. J. Am. Acad. Dermatol. 38:S42–47 [DOI] [PubMed] [Google Scholar]
- 18. Lyons M. K., Blair J. E., Leslie K. O. 2005. Successful treatment with voriconazole of fungal cerebral abscess due to Cladophialophora bantiana. Clin. Neurol. Neurosurg. 107:532–534 [DOI] [PubMed] [Google Scholar]
- 19. MacCallum D. M., Odds F. C. 2002. Influence of grapefruit juice on itraconazole plasma levels in mice and guinea pigs. J. Antimicrob. Chemother. 50:219–224 [DOI] [PubMed] [Google Scholar]
- 20. Mariné M., Pastor F. J., Guarro J. 2009. Combined antifungal therapy in a murine model of disseminated infection by Cladophialophora bantiana. Med. Mycol. 47:45–49 [DOI] [PubMed] [Google Scholar]
- 21. Najafzadeh M. J., et al. 2009. Genetic diversity and species delimitation in the opportunistic genus Fonsecaea. Med. Mycol. 47:17–25 [DOI] [PubMed] [Google Scholar]
- 22. Negroni R., et al. 2004. Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clin. Infect. Dis. 38:e15–e20 [DOI] [PubMed] [Google Scholar]
- 23. Negroni R., et al. 2005. Posaconazole treatment of refractory eumycetoma and chromoblastomycosis. Rev. Inst. Med. Trop. Sao Paulo 47:339–346 [DOI] [PubMed] [Google Scholar]
- 24. Ortoneda M., Capilla J., Pastor F. J., Serena C., Guarro J. 2004. Interaction of granulocyte colony-stimulating factor and high doses of liposomal amphotericin B in the treatment of systemic murine scedosporiosis. Diagn. Microbiol. Infect. Dis. 50:247–251 [DOI] [PubMed] [Google Scholar]
- 25. Poirriez J., et al. 2000. A case of chromoblastomycosis treated by a combination of cryotherapy, shaving, oral 5-fluorocytosine and oral amphotericin B. Am. J. Trop. Med. Hyg. 63:61–63 [DOI] [PubMed] [Google Scholar]
- 26. Pradinaud R., Bolzinger T. 1991. Treatment of chromoblastomycosis. J. Am. Acad. Dermatol. 25:869–870 [DOI] [PubMed] [Google Scholar]
- 27. Queiroz-Telles F., et al. 2009. Chromoblastomycosis: an overview of clinical manifestations, diagnosis and treatment. Med. Mycol. 47:3–15 [DOI] [PubMed] [Google Scholar]
- 28. Siebenhaar F., et al. 2007. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am. J. Pathol. 170:1910–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sugar A. M., Liu X. P. 2000. Effect of grapefruit juice on serum voriconazole concentrations in the mouse. Med. Mycol. 38:209–212 [DOI] [PubMed] [Google Scholar]
- 30. Sugar A. M., Liu X. P. 2001. Efficacy of voriconazole in treatment of murine pulmonary blastomycosis. Antimicrob. Agents Chemother. 45:601–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takei H., Goodman J. C., Powell S. Z. 2007. Cerebral phaeohyphomycosis caused by Cladophialophora bantiana and Fonsecaea pedrosoi: report of three cases. Clin. Neuropathol. 26:21–27 [DOI] [PubMed] [Google Scholar]
- 32. Trifilio S., et al. 2005. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 35:509–513 [DOI] [PubMed] [Google Scholar]
- 33. Warn P. A., et al. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J. Antimicrob. Chemother. 58:1198–1207 [DOI] [PubMed] [Google Scholar]
- 34. Wiederhold N. P., Najvar L. K., Bocanegra R., Graybill J. R., Patterson T. F. 2009. Efficacy of posaconazole as treatment and prophylaxis against Fusarium solani. Antimicrob. Agents Chemother. 54:1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]