Abstract
Moraxella catarrhalis is a common pathogen found in children with upper respiratory tract infections and in patients with chronic obstructive pulmonary disease during exacerbations. The bacterial species is often isolated together with Streptococcus pneumoniae and Haemophilus influenzae. Outer membrane vesicles (OMVs) are released by M. catarrhalis and contain phospholipids, adhesins, and immunomodulatory compounds such as lipooligosaccharide. We have recently shown that M. catarrhalis OMVs exist in patients upon nasopharyngeal colonization. As virtually all M. catarrhalis isolates are β-lactamase positive, the goal of this study was to investigate whether M. catarrhalis OMVs carry β-lactamase and to analyze if OMV consequently can prevent amoxicillin-induced killing. Recombinant β-lactamase was produced and antibodies were raised in rabbits. Transmission electron microscopy, flow cytometry, and Western blotting verified that OMVs carried β-lactamase. Moreover, enzyme assays revealed that M. catarrhalis OMVs contained active β-lactamase. OMVs (25 μg/ml) incubated with amoxicillin for 1 h completely hydrolyzed amoxicillin at concentrations up to 2.5 μg/ml. In functional experiments, preincubation of amoxicillin (10× MIC) with M. catarrhalis OMVs fully rescued amoxicillin-susceptible M. catarrhalis, S. pneumoniae, and type b or nontypeable H. influenzae from β-lactam-induced killing. Our results suggest that the presence of amoxicillin-resistant M. catarrhalis originating from β-lactamase-containing OMVs may pave the way for respiratory pathogens that by definition are susceptible to β-lactam antibiotics.
INTRODUCTION
After Streptococcus pneumoniae and nontypeable Haemophilus influenzae (NTHi), Moraxella catarrhalis is the most common cause of bacterial respiratory infections in humans. M. catarrhalis causes acute otitis media in children and exacerbations in adults with chronic obstructive pulmonary disease (COPD), but it can also be found in patients diagnosed with sinusitis and laryngitis. M. catarrhalis resides in the palatine tonsils and invades epithelial cells in the respiratory tract (11, 14, 25, 27).
One important characteristic of M. catarrhalis is that the bacterium, like most other Gram-negative species, releases outer membrane vesicles (OMVs). Over recent years, OMVs have been shown to contain several virulence factors allowing M. catarrhalis to evade the immune system and thus effectively colonize the host (31, 34, 37). Vesicles are formed when part of the bacterial outer membrane bulges out and pinches off, creating vesicles with sizes ranging from 50 to 250 nm (7, 19, 34, 36). OMVs are composed of proteins and phospholipids found in the outer cell membrane but can also contain certain periplasmic proteins closely associated with the membrane. Interestingly, OMVs also contain immunomodulatory compounds, which enable bacteria to interact with the host immune system without requiring close contact (16). When we analyzed M. catarrhalis OMVs in detail using a proteomics approach combining 2-dimensional SDS-PAGE and matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry, 57 different periplasmic or outer membrane proteins were identified (31).
While most M. catarrhalis clinical strains recovered before 1975 were susceptible to β-lactam antibiotics, strains isolated in the mid-1980s showed a rapid increase in resistance against β-lactams. It was found that these resistant isolates produced one of two variants of a defined β-lactamase encoded by the gene bro-1 or bro-2 (38). However, since these alleles are considered to be >99% identical (2), it was not surprising that no functional differences could be found between strains. More than 97% of all M. catarrhalis strains are today considered to be β-lactamase positive, and a majority of these (>90%) have the bro-1 allele, whereas the bro-2 allele occurs less frequently (2, 13, 17).
M. catarrhalis is often found in mixed infections, and in up to 50% of all M. catarrhalis clinical cultures either S. pneumoniae or NTHi has also been identified (15). In contrast to M. catarrhalis, most S. pneumoniae and H. influenzae isolates are susceptible to β-lactam antibiotics; that is, on a worldwide basis, ≈14% of S. pneumoniae and ≈21% of NTHi clinical isolates are resistant to β-lactams (5). One possible advantage of the coinfection of M. catarrhalis with the two other bacterial species was convincingly shown in a mouse model, where β-lactamase-producing M. catarrhalis conferred protection for S. pneumoniae against β-lactam antibiotics (12).
Since β-lactamase is found in the periplasm, we hypothesized that OMVs might harbor β-lactamase and function as a long-distance delivery system to confer antimicrobial resistance for M. catarrhalis, but also for the other two bacterial species dwelling in the respiratory tract. We show the presence of β-lactamase in OMVs isolated from M. catarrhalis and that OMVs hydrolyze amoxicillin and consequently rescue β-lactamase-negative H. influenzae and S. pneumoniae isolates from amoxicillin-induced killing.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and MICs.
Reference strains and clinical isolates from the Department of Laboratory Medicine Malmö are shown in Table 1. S. pneumoniae ATCC 6303 (American Type Culture Collection) was grown in Todd-Hewitt broth supplemented with 0.5% yeast extract and cultured on sheep blood agar plates. All other species were cultured on chocolate agar plates. M. catarrhalis, NTHi 772, and H. influenzae capsule type b (Hib) Eagan were grown in brain heart infusion (BHI) broth (Difco/Becton, Lawrence, KS) at 37°C in 5% CO2. H. influenzae was grown with NAD and hemin (10 μg/ml for each). Bacteria were tested for β-lactamase activity using nitrocefin disks (bioMérieux, Marcy l'Étoile, France). H. influenzae strains were additionally tested for β-lactamase status against penicillin G and cefaclor according to the manufacturer's instructions (Biodisk, Solna, Sweden). Escherichia coli strains DH5α and BL21 were cultured in Luria-Bertani (LB) broth at 37°C in a humid atmosphere containing 5% CO2.
Table 1.
Clinical isolates, reference strains, and presence of bro-1 or bro-2 genes encoding M. catarrhalis β-lactamase
| Clinical isolate/strain | Site of isolation | Age | Gender | Clinical manifestation | MIC (μg/ml)a | Amoxicillin susceptibility | β-Lactamase status | bro genotype | Reference or source |
|---|---|---|---|---|---|---|---|---|---|
| M. catarrhalis | |||||||||
| Bc5 | Nasopharynx (reference strain) | 0.032 | Sensitive | Negative | 20 | ||||
| RH4 | Blood (reference strain) | 2.0 | Resistant | Positive | bro-1 | 6 | |||
| KR395 | Tympanic cavity | 66 yr | Female | Cough | 0.064 | Sensitive | Negative | 20 | |
| KR492 | Nasopharynx | 9 mo | Male | Otitis media | 0.50 | Resistant | Positive | bro-1 | This study |
| KR493 | Nasopharynx | 4 yr | Female | Recurring fever | 16.0 | Resistant | Positive | bro-1 | This study |
| KR522 | Nasopharynx | 35 yr | Male | Unknown | 8.0 | Resistant | Positive | bro-1 | This study |
| KR523 | Nasopharynx | 79 yr | Male | Unknown | 8.0 | Resistant | Positive | bro-2 | This study |
| KR526 | Nasopharynx | 33 yr | Male | Cough, sore throat | 1.0 | Resistant | Positive | bro-1 | This study |
| KR542 | Nasopharynx | 2 mo | Male | Unknown | 6.0 | Resistant | Positive | bro-1 | This study |
| KR923 | Nasopharynx | 4 yr | Male | Otitis media | 3.0 | Resistant | Positive | bro-1 | This study |
| H. influenzae | |||||||||
| NTHi 722 | Nasopharynx (reference strain) | 0.19 | Sensitive | Negative | 34 | ||||
| Hib Eagan | Reference strain | 0.50 | Sensitive | Negative | 10 | ||||
| S. pneumoniae | |||||||||
| ATCC 6303 | Reference strain | 0.094 | Sensitive | Negative | 29 |
MICs were determined by Etest.
To determine MICs for amoxicillin (Table 1), both Etests (Biodisk) and conventional colony counting (CFU) after incubation of bacteria in BHI broth (3) were used. A starting concentration of 107 CFU was used for determination of the MIC in solution. M. catarrhalis isolates with MICs of ≤0.125 μg/ml amoxicillin were susceptible and those with MICs of >0.125 μg/ml were resistant (21). NTHi and S. pneumoniae isolates with MICs of ≤1 μg/ml and MICs of ≤0.5 μg/ml amoxicillin, respectively, were considered susceptible.
Identification of M. catarrhalis bro-1 and bro-2 genes.
The β-lactamase genes bro-1 and bro-2 were identified using PCR with primers 5′-TGTGCGAAGCTACCATAACACTGAGT-3′ and 5′-GGGGGCTTGTTGGGTCATAAATTTTTC-3′, followed by DNA sequencing. The bro-1 and bro-2 phenotypes are distinguished by a single amino acid change (aspartic acid to glycine) at position 294 that is caused by substitution of a single base pair (2).
Cloning of bro, recombinant protein expression, and antibody production.
In order to produce recombinant Bro for immunization purposes, the bro-1 gene was isolated from genomic DNA obtained from M. catarrhalis strain RH4 (Table 1) using PCR primers 5′-AGGAGATAATGATGGATCCCCGTCA-3′ and 5′-GGGATTTACCAAGCTTGGGCTGGGTGA-3′ containing BamHI and HindIII restriction enzyme cleavage sites (underlined), respectively. The resulting PCR product (878 bp) was subsequently cloned into the vector pET26b(+) (Novagen, Darmstadt, Germany). To avoid presumptive toxicity, the vector was first transformed into E. coli strain DH5α, and positive clones were selected using LB broth supplemented with 50 μg/ml kanamycin. Plasmids were further transformed into the expression host E. coli BL21(DE3), and protein production was performed essentially as previously described by Singh et al. (32). Briefly, protein expression was induced by addition of 1 mM isopropyl-1-thio-β-d-galactoside (IPTG) to mid-log-phase cultures (optical density at 600 nm [OD600], 0.6 to 0.8) for 3 h at 37°C. Subsequently, bacteria were sonicated, and proteins were purified using affinity chromatography (Histrap FF Crude; GE Healthcare Biosciences, Pittsburgh, PA) using His tag elution buffer (50 mM Tris HCl, 500 mM NaCl, and 250 mM imidazole, pH 7.5). After purification and protein concentration, a rabbit anti-β-lactamase (RH4) antiserum was prepared using an immunization protocol as described previously (23). Briefly, rabbits were immunized intramuscularly with 200 μg purified recombinant RH4 β-lactamase, which had been emulsified in complete Freund's adjuvant (Difco, Becton Dickinson, Franklin Lanes, NJ) and boosted on days 14 and 28 with the same doses of protein in incomplete Freund's adjuvant. Blood was drawn 2 weeks later, and antibodies were purified against recombinant RH4 β-lactamase coupled to a CnBr-Sepharose column (VWR International, Leicestershire, United Kingdom). Antibodies were used in flow cytometry analysis and Western blotting as described below.
Isolation of M. catarrhalis OMVs.
OMVs were isolated using the method of Rosen et al. (30). Bacteria were grown in BHI broth overnight, and after centrifugation, the supernatants were filtered through 0.2-μm-pore-size filters (Sartorius, Goettingen, Germany). Thereafter, the flowthrough was concentrated using 100,000-kDa Vivaspin centrifugal concentrators (Vivascience, Hannover, Germany). The precipitate containing the extracellular vesicles was collected by centrifugation at 100,000 × g for 1 h and was washed with phosphate-buffered saline (PBS). Protein concentrations were determined by spectrophotometry using a NanoDrop Technologies spectrophotometer (Wilmington, DE), and the resulting OMV suspensions were checked on BHI agar to confirm that preparations were free of bacteria.
SDS-PAGE and Western blot analysis.
To analyze whether OMVs carry β-lactamase, OMV content was analyzed by 12% SDS-PAGE. Either the gels were stained with Bio-Rad Coomassie brilliant blue R-250 (Munich, Germany) or the proteins were transferred from the gel to an Immobilon-P membrane at 20 V overnight (Millipore, Bedford, MA). Following transfer, membranes were blocked with PBS containing 0.1% Tween (PBS-Tween) and 5% milk powder for 1 h. After several washes with PBS-Tween, the membrane was incubated with rabbit anti-β-lactamase polyclonal antibody (pAb) diluted 1:200 in PBS-Tween for 1 h as described previously (23). After repeated washing steps, horseradish peroxidase (HRP)-conjugated goat anti-rabbit pAbs (Dako, Glostrup, Denmark) diluted 1:1,000 were added for 1 h. The membranes were then washed and developed using enhanced chemiluminescence Western blot detection reagents (Amersham Pharmacia Biotech, Uppsala, Sweden).
Flow cytometry analysis.
To analyze for the presence of β-lactamase in OMVs, 3 μg OMVs was fixed with 3.5% formaldehyde for 15 min and then permeabilized with saponin (0.2%) for 5 min at room temperature (RT). OMVs were further incubated with recombinant RH4 rabbit anti-β-lactamase pAb diluted 1:5 in PBS-bovine serum albumin (2.5%), followed by addition of fluorescein isothiocyanate (FITC)-conjugated swine anti-rabbit pAb (Dako). Between each labeling step, OMVs were washed by ultracentrifugation at 100,000 × g for 30 min. Samples were analyzed in an EPICS XL-MCL flow cytometer (Beckman Coulter, Hialeah, FL). A gate excluding signals of ≤2.0% was set.
Transmission electron microscopy (TEM).
After fixation of the specimens, ultrathin sections were mounted on gold grids and subjected to antigen retrieval through the use of metaperiodate. Grids were floated on top of drops of immune reagents displayed on a sheet of Parafilm. Free aldehyde groups were blocked with 50 mM glycine. Grids were thereafter blocked with 5% (vol/vol) goat serum diluted in incubation buffer consisting of 0.2% bovine serum albumin-C in PBS, pH 7.6 (Aurion, Wageningen, Netherlands) for 15 min. OMVs were then incubated overnight with primary antibodies (dilution, 1:50 to 1:100) at +4°C. Grids were washed in 200 μl incubation buffer and thereafter floated on drops containing the gold conjugate reagents with sizes of 10 and 5 nm (diluted 1:10 to 1:20 in incubation buffer) for 1 h at RT. After further washes in incubation buffer, sections were postfixed in 2% glutaraldehyde and sections were washed in distilled water. They were then poststained with uranyl acetate and lead citrate and examined under an electron microscope (JEM 1230; Joel, Tokyo, Japan) operated at a 60-kV accelerating voltage. Images were recorded with a Gatan Multiscan 791 charge-coupled-device camera (Gatan, Pleasanton, CA).
Quantification of M. catarrhalis OMV β-lactamase activity.
β-Lactamase activity was determined using the chromogenic cephalosporin nitrocefin as previously described (3). Briefly, OMV preparations (0.3 μg/ml) were incubated with nitrocefin (0.5 mg/ml; Oxoid, Thermo Scientific, Cambridge, United Kingdom) for 30 min at 37°C in the dark. Following incubation, samples were spun down at 13,000 × g for 3 min in order to remove larger proteins aggregated in the preparations. The chromogen hydrolysis and subsequent color change of supernatants were determined immediately with the NanoDrop spectrophotometer at OD485. The enzymatic activity was estimated using a standard curve, where OD485 was related to the number of moles nitrocefin hydrolyzed. This was quantified using recombinant β-lactamase (VWR). Readings were thereafter converted to the number of mol nitrocefin hydrolyzed per min per mg protein. In order to compare β-lactamase activity in OMVs compared to that in the parent strain, whole parent cells were heated to 95°C for 7 min in order to lyse the bacteria. The nitrocefin hydrolysis capacity of OMVs versus that of the parent strain lysate was then compared on a weight basis. Furthermore, in order to determine the localization of the β-lactamase in OMVs, they were treated with 100 μg/ml proteinase K (Sigma Aldrich, St. Louis, MO) for 1 h at 50°C. After deactivation with 10 mM phenylmethylsulfonyl fluoride (PMSF; Sigma Aldrich) and 4-(2-aminoethyl)benzenesulfonyl fluoride hydrocholoride (AEBSF; USB, Cleveland, OH), samples were incubated with 0.02% saponin and enzyme activity was measured as described above.
Measurement of β-lactamase-induced amoxicillin hydrolysis.
Amoxicillin concentrations were estimated using an agar diffusion method (18). Sarcina lutea, a highly β-lactam-susceptible Gram-positive bacterium from the family Clostridiaceae, was plated on agar and allowed to dry. Perforations were made in the agar, and samples containing OMVs, which had been preincubated with amoxicillin for 1 h at 37°C, were added in duplicate. To allow diffusion of antibiotics into the agar as well as subsequent growth of bacteria, plates were left overnight at 37°C. The inhibitory zones (where no bacterial growth was observed) were measured, and a standard curve was compiled.
Inactivation of amoxicillin by β-lactamase transferred by OMVs.
Bacterial cultures were grown in a starting culture to a concentration of 106 to 107 CFU/ml, followed by incubation with various concentrations of OMVs that had been preincubated at 37°C for 1 h with amoxicillin at concentrations of 10× MIC (Table 1). Cultures were grown in microtiter plates (Nunclon Surface; Thermo Fisher Scientific, Waltham, MA) at 37°C with 5% CO2. Bacterial growth was measured at OD600, and at each time point triplicates of each culture were plated on chocolate agar or sheep blood plates and incubated overnight.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism, version 5, software (San Diego, CA). Student's t test was used to determine statistical differences for unpaired comparisons. All data are expressed as means ± standard errors of the means (SEMs), where n corresponds to the number of experiments performed. Significant values were defined as P values of ≤0.05.
RESULTS
M. catarrhalis OMVs carry β-lactamase.
Moraxella-dependent resistance against amoxicillin was evaluated in a set of different strains (Table 1). Etests were used to define MICs which were also confirmed using conventional counting of CFU. To confirm that amoxicillin resistance in M. catarrhalis strains was due to the presence of β-lactamase genes, chromosomal analysis was included. Since the two alleles bro-1 and bro-2 have previously been found to encode a β-lactamase in M. catarrhalis, clinical M. catarrhalis isolates (n = 8) and two well-defined reference strains were screened by PCR and sequenced. As can be seen in Fig. 1, eight strains carried the bro gene, and of these, only one was positive for bro-2, which concurs with the assumed frequency of about 10% in a defined population (3). The amoxicillin-resistant strains M. catarrhalis KR526 and RH4, with MICs of ≥1 μg/ml, were chosen for detailed analysis. For comparison, amoxicillin-susceptible M. catarrhalis KR395 and Bc5, with MICs of 0.064 and 0.032 μg/ml, respectively, were also included.
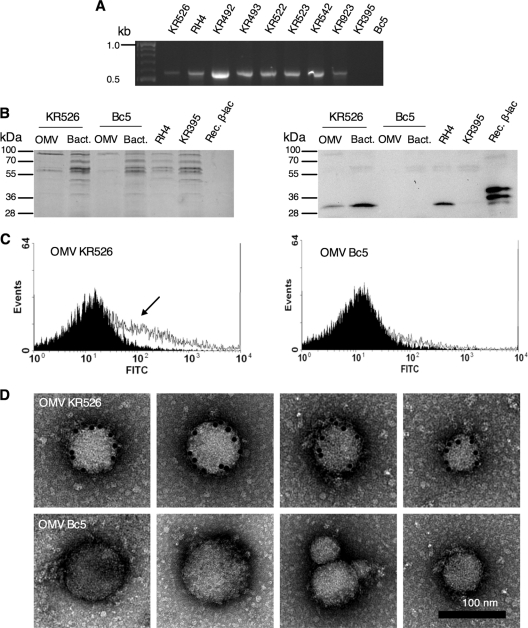
Fig. 1.
Amoxicillin-resistant M. catarrhalis strains produce OMV containing β-lactamase. (A) Eight M. catarrhalis strains out of 10 were positive for the bro gene (522 bp), as revealed by PCR analysis. bro alleles were not found in strains KR395 and Bc5. (B) M. catarrhalis OMVs and total bacterial lysates (10 μg each) were subjected to SDS-PAGE (left), followed by detection of β-lactamase (35 kDa) by Western blotting (right). Lysates of whole M. catarrhalis RH4 and KR395 bacteria were used as positive and negative controls, respectively. Recombinant RH4 β-lactamase (0.6 μg) was also included. The upper band represents the recombinant protein at a size of 37.7 kDa. The lower band most likely results from N-terminal degradation. The His tag located at the C-terminal end was not affected by degradation, since it was possible to purify the recombinant β-lactamase using affinity chromatography. (C) Flow cytometry using antiserum raised against recombinant RH4 β-lactamase. The arrow shows a positive shift with β-lactamase containing OMV KR526, whereas OMVs from Bc5 were negative. OMVs without the β-lactamase antiserum (black) were compared to OMVs incubated with β-lactamase antiserum (white). (D) Gold-labeled anti-β-lactamase antibodies confirmed the presence of β-lactamase by TEM.
To verify that M. catarrhalis strains expressed β-lactamase, Western blot analyses with total bacterial cell lysates and their corresponding released OMVs were done using a rabbit anti-β-lactamase antiserum (Fig. 1B). Interestingly, OMVs originating from the β-lactamase-positive strain M. catarrhalis KR526 contained β-lactamase. This was in contrast to OMVs isolated from the β-lactamase-negative strain M. catarrhalis Bc5, which did not contain any β-lactamase. Total cell lysates of M. catarrhalis strains RH4 and KR395 were used as additional positive and negative controls, respectively. Moreover, our recombinant RH4 β-lactamase produced in E. coli was included as a positive control.
The presence of β-lactamase in OMVs was also verified by flow cytometry after permeabilization with saponin (Fig. 1C). M. catarrhalis KR526 vesicles contained more β-lactamase than Bc5 vesicles when they were analyzed with the anti-β-lactamase antiserum, followed by incubation with FITC-conjugated secondary antibodies. A clear shift (increased fluorescence intensity) was observed when OMVs were analyzed by flow cytometry. However, β-lactamase was not detected in OMVs analyzed in the absence of saponin (data not shown), suggesting that β-lactamase was mainly located inside the vesicles. TEM with gold-labeled anti-β-lactamase pAb further showed the presence and absence of β-lactamase in KR526 and Bc5 OMVs, respectively (Fig. 1D). Taken together, these experiments demonstrated that OMVs derived from amoxicillin-resistant and β-lactamase-positive M. catarrhalis also contained β-lactamase.
β-Lactamase-positive M. catarrhalis OMVs hydrolyze amoxicillin.
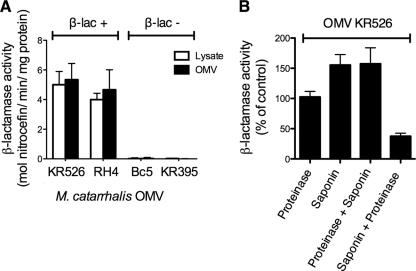
To determine the β-lactamase activity in our OMV preparations, the chromogenic substrate nitrocefin was used. OMVs from four clinical M. catarrhalis strains as well as lysates from their parent bacteria were analyzed. The β-lactamase activity was expressed as the number of moles nitrocefin hydrolyzed per minute per mg protein (Fig. 2A). The M. catarrhalis strains KR526 and RH4 and their respective OMVs were confirmed to be β-lactamase positive and Bc5 and KR395 negative. Interestingly, although a higher MIC of amoxicillin was required for M. catarrhalis RH4, OMVs from KR526 were shown to have the highest β-lactamase enzyme content on a weight basis. M. catarrhalis KR526 was thus selected for further experiments. However, it was also determined that there was no significant difference between the enzyme content of OMVs and those of their parent bacteria. This suggested that β-lactamase was not enriched in vesicles.
Fig. 2.
M. catarrhalis OMVs contain enzymatically active β-lactamase. (A) The β-lactamase contents of whole-cell lysates and OMVs from four different M. catarrhalis strains were analyzed. OMVs contained approximately the same β-lactamase content as whole-cell lysates. (B) The β-lactamase enzyme was found on the inside of the OMVs. The β-lactamase activity was quantified by the ability of the enzyme to hydrolyze the β-lactam nitrocefin, leading to a change in absorbance from OD380 to OD485, as determined by spectrophotometry. β-Lactamase enzyme or OMVs were treated with proteinase K (100 μg/ml) and/or saponin (0.2%), and enzyme content was subsequently determined. As a negative control, OMVs were first treated with saponin, followed by proteinase K. In panel A, the β-lactamase content was expressed as the number of moles nitrocefin hydrolyzed per minute per mg OMVs. In panel B, β-lactamase activity was defined as the percentage of enzyme activity of OMV in the absence of amoxicillin that was set to 100%. Data shown are means and SEM of at least three independent experiments.
After the β-lactamase content of OMVs was quantified, the precise localization of β-lactamase was determined. OMV preparations were consequently pretreated with proteinase K to digest β-lactamase associated with the outer membrane of the vesicles. Preparations were thereafter treated with saponin to permeabilize OMVs. Proteinase K-treated OMVs were found to have approximately the same enzymatic activity as nontreated ones. When OMVs were additionally treated with saponin, the β-lactamase activity increased. As a control, OMVs were first treated with saponin and subsequently proteinase K in order to verify that proteinase K digested free β-lactamase. These results suggest that only a minor portion of β-lactamase was associated with the membrane of the vesicles and that most of the β-lactamase was found inside the vesicles.
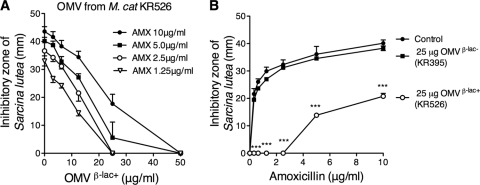
The biological β-lactamase activity in the OMV preparations was determined by an antibiotic bioassay. β-Lactamase-positive and -negative OMVs were preincubated for 1 h with amoxicillin, and thereafter, antibiotic concentrations were quantified by the ability to hydrolyze the highly amoxicillin-susceptible indicator bacterium Sarcina lutea. Various concentrations of β-lactamase-positive OMVs from M. catarrhalis KR526 were incubated with amoxicillin (range, 1.25 to 10 μg/ml) (Fig. 3A). OMVs at 25 μg/ml were found to completely hydrolyze amoxicillin at concentrations up to 2.5 μg/ml, whereas a partial hydrolysis was observed at ≥5 μg/ml amoxicillin and 25 μg/ml OMVs. In contrast to β-lactamase-positive M. catarrhalis KR526 OMVs, the β-lactamase-negative OMVs from KR395 did not hydrolyze amoxicillin; that is, no differences were found in the control with amoxicillin compared to samples with amoxicillin preincubated with OMVs deficient in β-lactamase (Fig. 3B). For comparison, samples preincubated with β-lactamase-positive OMVs (25 μg/ml) from KR526 completely hydrolyzed amoxicillin concentrations up to 2.5 μg/ml. To summarize, our results indicated that OMVs from M. catarrhalis carrying β-lactamase were able to hydrolyze and thus deactivate amoxicillin in a dose-dependent manner.
Fig. 3.
β-Lactamase-carrying M. catarrhalis OMVs hydrolyze amoxicillin. (A) Amoxicillin (AMX)-induced killing at 1.25 to 10 μg/ml amoxicillin was gradually reduced with increasing concentrations (0 to 50 μg/ml) of β-lactamase-containing OMVs. Amoxicillin concentrations were determined by measuring inhibitory growth zones of the β-lactam-susceptible bacterium Sarcina lutea. (B) β-Lactamase-positive and -negative M. catarrhalis OMVs at 25 μg/ml were incubated with increasing amoxicillin concentrations. The data are presented as means and SEMs of at least three independent experiments. β-lac+ and β-lac−, β-lactamase positive and negative, respectively. ***, P ≤ 0.001.
Amoxicillin-susceptible M. catarrhalis is protected against amoxicillin by β-lactamase-carrying OMVs derived from another M. catarrhalis strain.
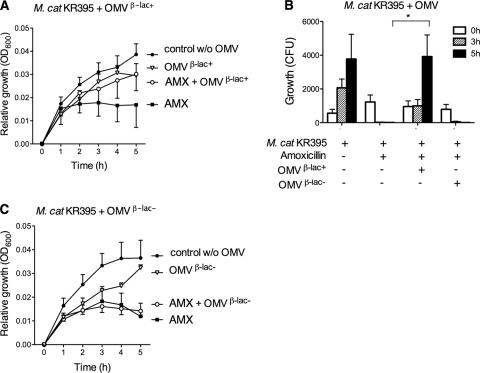
Since several strains of M. catarrhalis have been shown to reside in one individual (2), β-lactamase-positive M. catarrhalis OMVs may play an important role in coinfections with β-lactamase-negative M. catarrhalis. To investigate this phenomenon, amoxicillin with or without preincubation with M. catarrhalis OMVs was analyzed against β-lactamase-negative and, thus, amoxicillin-susceptible M. catarrhalis KR395. Preincubation of amoxicillin (1 μg/ml) with OMVs derived from KR526 carrying β-lactamase rescued bacteria (107 CFU/ml) from amoxicillin-induced killing and resulted in bacterial growth comparable to that of the control without amoxicillin, as revealed by optical density measurements (Fig. 4A).
Fig. 4.
β-Lactamase-positive M. catarrhalis OMVs protect amoxicillin-susceptible M. catarrhalis strains from being killed by amoxicillin. β-Lactamase-susceptible M. catarrhalis KR935 (107 CFU/ml) was grown with amoxicillin (1 μg/ml) that had been preincubated in the presence of 25 μg/ml β-lactamase-positive OMVs (A and B) or β-lactamase-negative OMVs (B and C). β-Lactamase-positive and -negative OMVs were isolated from M. catarrhalis KR526 (β-lac+) and Bc5 (β-lac−), respectively. Growth was expressed either as relative growth compared to starting concentrations measured as absorbance (OD600) (A and C) or as numbers of CFU (B). Mean values and SEMs of at least three independent experiments are shown. *, P ≤ 0.05.
To determine the number of viable bacteria upon incubation with amoxicillin with or without OMVs, the numbers of CFU were also counted (Fig. 4B). The results confirmed the optical density measurements, and in these experiments, it was even more evident that amoxicillin-susceptible M. catarrhalis KR395 was rescued by KR526 OMVs that were loaded with β-lactamase. In contrast, the protective effect was not seen with M. catarrhalis isolates that were incubated with amoxicillin and pretreated with β-lactamase-negative OMVs from M. catarrhalis Bc5, as demonstrated by changes in absorbance over time (Fig. 4C). Vesicles without β-lactamase neither inhibited nor promoted bacterial growth in the absence of amoxicillin. Thus, OMVs containing β-lactamase hydrolyzed amoxicillin and promoted growth of non-β-lactamase-producing and, hence, amoxicillin-susceptible M. catarrhalis.
M. catarrhalis OMVs containing β-lactamase rescue amoxicillin-susceptible NTHi and S. pneumoniae.
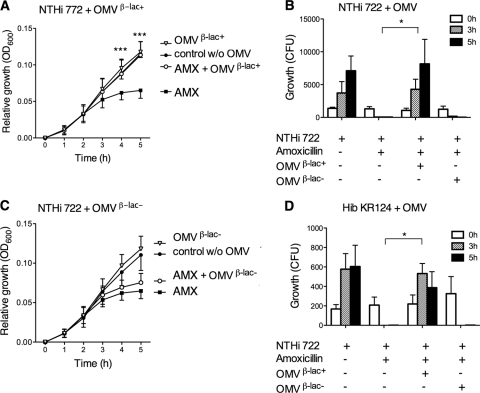
To reveal whether OMVs isolated from β-lactamase-producing M. catarrhalis also protect other respiratory pathogens from amoxicillin, the susceptible strain NTHi 722 (107 CFU/ml) was exposed to amoxicillin (2 μg/ml) that had been pretreated with KR526 OMVs containing β-lactamase. A significant difference in growth rate could be seen between cultures that were exposed to amoxicillin and preincubated with β-lactamase-carrying OMVs and the control exposed to amoxicillin only (Fig. 5A). This was also confirmed when the numbers of CFU were determined (Fig. 5B). Finally, when NTHi 722 was incubated with amoxicillin exposed to β-lactamase-negative Bc5 OMVs, no difference was found compared to the results for amoxicillin-treated NTHi 722 in the absence of OMVs (Fig. 5C). The addition of OMVs in the absence of amoxicillin did not interfere with bacterial growth.
Fig. 5.
Vesicles from β-lactamase-positive M. catarrhalis protect NTHi against amoxicillin. Amoxicillin-susceptible NTHi 772 (A to C) or Hib KR124 (D) (107 CFU/ml) was grown with amoxicillin (2 μg/ml) that had been preincubated with either 25 μg/ml β-lactamase-positive OMVs (A and B) or β-lactamase-negative OMVs (B and C). OMVs were isolated from M. catarrhalis KR526 and Bc5, which were β-lactamase positive and negative, respectively. Growth was expressed either as relative growth compared to starting concentrations measured as absorbance at OD600 (A and C) or as numbers of CFU (B and D). The results are shown as means and SEMs of at least three independent experiments. *, P ≤ 0.05; ***, P ≤ 0.001.
The incidence of encapsulated Hib has decreased in the Western Hemisphere due to successful vaccine campaigns, but Hib is still a significant problem in certain developing countries. We therefore also included Hib in our study. In parallel with NTHi, β-lactamase-containing M. catarrhalis OMVs rescued Hib to the same extent as NTHi in the presence of amoxicillin (Fig. 5D).
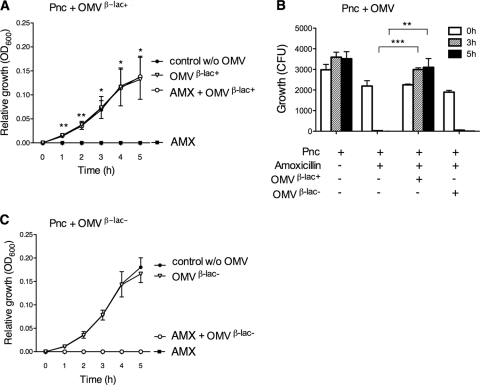
It is a well-known fact that S. pneumoniae is significantly more susceptible to amoxicillin than M. catarrhalis and NTHi (9). In parallel with M. catarrhalis (Fig. 4) and NTHi (Fig. 5), S. pneumoniae ATCC 6303 (106 CFU/ml) was rescued from amoxicillin-induced killing when amoxicillin (1 μg/ml) was preincubated with β-lactamase-carrying M. catarrhalis OMVs (Fig. 6A). This protective effect was also verified by determination of the numbers of CFU (Fig. 6B) but was not observed with amoxicillin preparations preincubated in the presence of β-lactamase-negative OMVs (Fig. 6C). S. pneumoniae incubated with β-lactamase-positive or -negative OMVs in the absence of amoxicillin did not interfere with bacterial growth. Taken together, OMVs derived from β-lactamase-producing M. catarrhalis hydrolyzed amoxicillin, resulting in significantly increased survival of NTHi and Hib, and, finally, pneumococci that were all susceptible to amoxicillin.
Fig. 6.
M. catarrhalis β-lactamase-containing OMVs protect S. pneumoniae (pneumococci [Pnc]) from being killed by amoxicillin. Amoxicillin-susceptible S. pneumoniae ATCC 6303 (106 CFU/ml) was grown with amoxicillin (1 μg/ml) preincubated with either 25 μg/ml β-lactamase-positive OMVs (A and B) or β-lactamase-negative OMVs (B and C). OMVs were isolated from M. catarrhalis KR526 (β-lac+) and Bc5 (β-lac−). Growth was expressed either as relative growth compared to starting concentrations measured as absorbance (OD600) (A and C) or as numbers of CFU (B). The data are presented as means and the standard errors of at least three independent experiments. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
DISCUSSION
In this study we have shown that OMVs from M. catarrhalis carry β-lactamase at high concentrations and that these vesicles are able to protect amoxicillin-susceptible M. catarrhalis, as well as H. influenzae and pneumococci, against amoxicillin-induced killing.
It has previously been demonstrated that β-lactamase, along with other proteins as well as DNA, can be transferred between different strains of Pseudomonas aeruginosa (1, 4, 26), a pathogen found in, for example, patients with cystic fibrosis (CF) (33). Thereby, β-lactamase can be shared between strains and thus obliterate the need for each strain to carry its own resistance gene. Using electron microscopy and enzyme studies, Ciofu et al. showed that β-lactamase was packaged inside the secreted P. aeruginosa OMVs (4). In the present study, we identified functional β-lactamase inside M. catarrhalis OMVs, as judged by flow cytometry and permeabilization with saponin. TEM supported our observations with flow cytometry. Thus, antibiotic resistance could indeed be conferred to other M. catarrhalis strains by the aid of OMVs hydrolyzing amoxicillin. We also determined that β-lactamase was localized inside the OMVs and thus derived from the periplasm of the parent bacteria. This is in accordance with the findings of a study by Bootsma et al. (2a), where it was suggested that the β-lactamase activity was found in the inner leaflet of the outer membrane facing the periplasmic compartment of M. catarrhalis. Our results further support the notion that amoxicillin can traverse the outer membrane and enter the lumen of OMVs and that this compartment may be the localization of amoxicillin hydrolysis.
Acute otitis media has previously been associated with a complex polymicrobial state. The explanation for this common phenomenon of mixed infections, however, largely remains unclear. S. pneumoniae and H. influenzae are often found to be copathogens in infections with M. catarrhalis, and the reason for this has been speculated over (15). Budhani and Struthers found that S. pneumoniae cells growing in a biofilm in the presence of β-lactamase-positive M. catarrhalis were protected against killing when they were treated with amoxicillin (3). Additionally, in vivo experiments showed that mice infected intranasally with pneumococci and treated with amoxicillin or penicillin died from pneumococcal pneumonia if they were coinfected with β-lactamase-producing M. catarrhalis (12). In contrast, this effect was not found when mice were coinoculated with β-lactamase-negative M. catarrhalis. The transfer of β-lactam resistance is thus thought to be an important advantage for bacteria coinhabiting with β-lactamase-positive M. catarrhalis. Interestingly, we found that β-lactamase was transferred from M. catarrhalis by means of OMVs protecting S. pneumoniae and H. influenzae against amoxicillin, suggesting this to be a novel mechanism for conveying antimicrobial resistance.
Several secretion systems are used by bacterial species in order to invade their hosts and cause infection. Both type III and IV secretion systems allow bacteria to deliver proteins directly into the cytoplasm of the host cell. OMVs have been identified to be a novel secretion system, where no contact is required between the invading bacteria and its host. OMVs adhere and fuse with host cells at lipid rafts in cell membranes, thus allowing them to deliver various bacterial factors (36, 37). In this way, OMVs can secrete virulence factors from a distance to host cells, still causing infection but staying clear of the host immune response. Several virulence factors of the common pathogen M. catarrhalis are packaged into OMVs, such as the Moraxella IgD-binding protein (MID), UspA1/UspA2, CopB, outer membrane protein (OMP) CD, OMP E, and lipooligosaccharides (LOSs) (31, 35, 37). We recently showed that OMVs secreted from M. catarrhalis interact with human tonsillar B cells (37). Through induction of B-cell-receptor clustering and Toll-like receptor signaling, OMVs were found to bind and activate B cells. The superantigen MID (8, 24, 35a) and DNA associated with the OMV membrane were found to be essential for maximal B-cell activation in a nonimmunogenic fashion.
We have previously shown that the M. catarrhalis OMVs also protect H. influenzae against complement-mediated attacks (34). The M. catarrhalis proteins UspA1/UspA2 bind and deplete the third component of the complement system (C3), an essential protein of the complement cascade in serum (22, 39). Furthermore, it was established that OMVs containing UspA1 and UspA2 interfered with the complement cascade and thereby increased the survival of H. influenzae. Such a symbiotic relationship between two common pathogens might also be of benefit to M. catarrhalis, since the promotion of the copathogen H. influenzae may cause increased inflammation, leading to upregulation of epithelial cell surface receptors. This may also facilitate the adherence of M. catarrhalis and thus potentiate infection (34).
Conferment of β-lactamase in OMVs is a novel mechanism by which M. catarrhalis not only enhances survival of its own species but also promotes infection of coinhabiting pathogens such as H. influenzae and S. pneumoniae. Considering the problem with the current global spread of antibiotic resistance, it is of highest importance to elucidate all possible mechanisms by which bacteria can cause infection through avoidance of antimicrobial agents. OMVs are an interesting novel target for innovative therapies in combination with conventional antibiotics during treatment of chronic and acute bacterial infections.
ACKNOWLEDGMENTS
We are grateful to Holger von Fircks (Meda/Recip, Solna, Sweden), who provided us with amoxicillin, and Marta Brant and Anki Striby for technical assistance.
This work was supported by grants from the Alfred Ósterlund, the Anna and Edwin Berger, the Marianne and Marcus Wallenberg, the Greta and Johan Kock, the Janne Elgqvist, and the Gyllenstiernska Krapperup Foundations; the Swedish Medical Research Council; the Cancer Foundation at the University Hospital in Malmö; and the Skåne County Council's Research and Development Foundation.
Footnotes
Published ahead of print on 16 May 2011.
REFERENCES
- 1. Bomberger J. M., et al. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bootsma H. J., van Dijk H., Vauterin P., Verhoef J., Mooi F. R. 2000. Genesis of BRO beta-lactamase-producing Moraxella catarrhalis: evidence for transformation-mediated horizontal transfer. Mol. Microbiol. 36:93–104 [DOI] [PubMed] [Google Scholar]
- 2a. Bootsma H. J., et al. 1999. Moraxella (Branhamella) catarrhalis BRO beta-lactamase: a lipoprotein of gram-positive origin? J. Bacteriol. 181:5090–5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Budhani R. K., Struthers J. K. 1998. Interaction of Streptococcus pneumoniae and Moraxella catarrhalis: investigation of the indirect pathogenic role of beta-lactamase-producing moraxellae by use of a continuous-culture biofilm system. Antimicrob. Agents Chemother. 42:2521–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ciofu O., Beveridge T. J., Kadurugamuwa J., Walther-Rasmussen J., Hoiby N. 2000. Chromosomal beta-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 45:9–13 [DOI] [PubMed] [Google Scholar]
- 5. Darabi A., Hocquet D., Dowzicky M. J. 2010. Antimicrobial activity against Streptococcus pneumoniae and Haemophilus influenzae collected globally between 2004 and 2008 as part of the Tigecycline Evaluation and Surveillance Trial. Diagn. Microbiol. Infect. Dis. 67:78–86 [DOI] [PubMed] [Google Scholar]
- 6. de Vries S. P., et al. 2010. Genome analysis of Moraxella catarrhalis strain RH4, a human respiratory tract pathogen. J. Bacteriol. 192:3574–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellis T. N., Kuehn M. J. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74:81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forsgren A., Brant M., Karamehmedovic M., Riesbeck K. 2003. The immunoglobulin D-binding protein MID from Moraxella catarrhalis is also an adhesin. Infect. Immun. 71:3302–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forsgren A., Walder M. 1984. Activity of common antibiotics against Branhamella catarrhalis, Haemophilus influenzae, pneumococci, group A streptococci and Staphylococcus aureus in 1983. Acta Otolaryngol. Suppl. 407:43–49 [DOI] [PubMed] [Google Scholar]
- 10. Hallström T., Trajkovska E., Forsgren A., Riesbeck K. 2006. Haemophilus influenzae surface fibrils contribute to serum resistance by interacting with vitronectin. J. Immunol. 177:430–436 [DOI] [PubMed] [Google Scholar]
- 11. Heiniger N., Spaniol V., Troller R., Vischer M., Aebi C. 2007. A reservoir of Moraxella catarrhalis in human pharyngeal lymphoid tissue. J. Infect. Dis. 196:1080–1087 [DOI] [PubMed] [Google Scholar]
- 12. Hol C., Van Dijke E. E., Verduin C. M., Verhoef J., van Dijk H. 1994. Experimental evidence for Moraxella-induced penicillin neutralization in pneumococcal pneumonia. J. Infect. Dis. 170:1613–1616 [DOI] [PubMed] [Google Scholar]
- 13. Jetter M., Spaniol V., Troller R., Aebi C. 2010. Down-regulation of porin M35 in Moraxella catarrhalis by aminopenicillins and environmental factors and its potential contribution to the mechanism of resistance to aminopenicillins. J. Antimicrob. Chemother. 65:2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan M. A., et al. 2010. bro {beta}-lactamase and antibiotic resistances in a global cross-sectional study of Moraxella catarrhalis from children and adults. J. Antimicrob. Chemother. 65:91–97 [DOI] [PubMed] [Google Scholar]
- 15. Krishnamurthy A., McGrath J., Cripps A. W., Kyd J. M. 2009. The incidence of Streptococcus pneumoniae otitis media is affected by the polymicrobial environment particularly Moraxella catarrhalis in a mouse nasal colonisation model. Microbes Infect. 11:545–553 [DOI] [PubMed] [Google Scholar]
- 16. Kuehn M. J., Kesty N. C. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655 [DOI] [PubMed] [Google Scholar]
- 17. Levy F., Walker E. S. 2004. BRO beta-lactamase alleles, antibiotic resistance and a test of the BRO-1 selective replacement hypothesis in Moraxella catarrhalis. J. Antimicrob. Chemother. 53:371–374 [DOI] [PubMed] [Google Scholar]
- 18. Lourenco F. R., Kaneko T. M., Pinto Tde J. 2007. Evaluating measurement uncertainty in the microbiological assay of vancomycin from methodology validation data. J. AOAC Int. 90:1383–1386 [PubMed] [Google Scholar]
- 19. Mashburn-Warren L., McLean R. J., Whiteley M. 2008. Gram-negative outer membrane vesicles: beyond the cell surface. Geobiology 6:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mollenkvist A., et al. 2003. The Moraxella catarrhalis immunoglobulin D-binding protein MID has conserved sequences and is regulated by a mechanism corresponding to phase variation. J. Bacteriol. 185:2285–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nash D. R., Flanagan C., Steele L. C., Wallace R. J., Jr 1991. Comparison of the activity of cefixime and activities of other oral antibiotics against adult clinical isolates of Moraxella (Branhamella) catarrhalis containing BRO-1 and BRO-2 and Haemophilus influenzae. Antimicrob. Agents Chemother. 35:192–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nordstrom T., Blom A. M., Tan T. T., Forsgren A., Riesbeck K. 2005. Ionic binding of C3 to the human pathogen Moraxella catarrhalis is a unique mechanism for combating innate immunity. J. Immunol. 174:3628–3636 [DOI] [PubMed] [Google Scholar]
- 23. Nordstrom T., Forsgren A., Riesbeck K. 2002. The immunoglobulin D-binding part of the outer membrane protein MID from Moraxella catarrhalis comprises 238 amino acids and a tetrameric structure. J. Biol. Chem. 277:34692–34699 [DOI] [PubMed] [Google Scholar]
- 24. Nordstrom T., Jendholm J., Samuelsson M., Forsgren A., Riesbeck K. 2006. The IgD-binding domain of the Moraxella IgD-binding protein MID (MID962-1200) activates human B cells in the presence of T cell cytokines. J. Leukoc. Biol. 79:319–329 [DOI] [PubMed] [Google Scholar]
- 25. Perez Vidakovics M. L., Riesbeck K. 2009. Virulence mechanisms of Moraxella in the pathogenesis of infection. Curr. Opin. Infect. Dis. 22:279–285 [DOI] [PubMed] [Google Scholar]
- 26. Renelli M., Matias V., Lo R. Y., Beveridge T. J. 2004. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology 150:2161–2169 [DOI] [PubMed] [Google Scholar]
- 27. Riesbeck K., Nordstrom T. 2006. Structure and immunological action of the human pathogen Moraxella catarrhalis IgD-binding protein. Crit. Rev. Immunol. 26:353–376 [DOI] [PubMed] [Google Scholar]
- 28. Reference deleted.
- 29. Rodgers G. L., Arguedas A., Cohen R., Dagan R. 2009. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine 27:3802–3810 [DOI] [PubMed] [Google Scholar]
- 30. Rosen G., Naor R., Rahamim E., Yishai R., Sela M. N. 1995. Proteases of Treponema denticola outer sheath and extracellular vesicles. Infect. Immun. 63:3973–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schaar V., et al. 2011. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cell. Microbiol. 13:432–449 [DOI] [PubMed] [Google Scholar]
- 32. Singh B., et al. 2010. Vitronectin binds to the head region of Moraxella catarrhalis ubiquitous surface protein A2 and confers complement-inhibitory activity. Mol. Microbiol. 75:1426–1444 [DOI] [PubMed] [Google Scholar]
- 33. Speert D. P., et al. 2002. Epidemiology of Pseudomonas aeruginosa in cystic fibrosis in British Columbia, Canada. Am. J. Respir. Crit. Care Med. 166:988–993 [DOI] [PubMed] [Google Scholar]
- 34. Tan T. T., Mörgelin M., Forsgren A., Riesbeck K. 2007. Haemophilus influenzae survival during complement-mediated attacks is promoted by Moraxella catarrhalis outer membrane vesicles. J. Infect. Dis. 195:1661–1670 [DOI] [PubMed] [Google Scholar]
- 35. Tan T., Nordstrom T., Forsgren A., Riesbeck K. 2005. The respiratory pathogen Moraxella catarrhalis adheres to epithelial cells by interacting with fibronectin through ubiquitous surface proteins A1 and A2. J. Infect. Dis. 192:1029–1038 [DOI] [PubMed] [Google Scholar]
- 35a. Tan T. T., Riesbeck K. 2007. Current progress of adhesins as vaccine candidates for Moraxella catarrhalis. Expert Rev. Vaccines 6:949–956 [DOI] [PubMed] [Google Scholar]
- 36. Ünal C., Schaar V., Riesbeck K. 2010. Bacterial outer membrane vesicles in disease and preventive medicine. Semin. Immunopathol. [Epub ahead of print.] doi:10.1007/s00281-010-0231-y [DOI] [PubMed] [Google Scholar]
- 37. Vidakovics M. L., et al. 2010. B cell activation by outer membrane vesicles—a novel virulence mechanism. PLoS Pathog. 6:e1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wallace R. J., Jr., et al. 1989. BRO beta-lactamases of Branhamella catarrhalis and Moraxella subgenus Moraxella, including evidence for chromosomal beta-lactamase transfer by conjugation in B. catarrhalis, M. nonliquefaciens, and M. lacunata. Antimicrob. Agents Chemother. 33:1845–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zipfel P. F., Sherka C. 2009. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 10:729–740 [DOI] [PubMed] [Google Scholar]