Abstract
Drug resistance in Mycobacterium tuberculosis has become a serious global health threat, which is now complicated by the emergence of extensively drug-resistant strains. New drugs that are active against drug-resistant tuberculosis (TB) are needed. We chose to search for new inhibitors of the enoyl-acyl carrier protein (ACP) reductase InhA, the target of the first-line TB drug isoniazid (also known as isonicotinoic acid hydrazide [INH]). A subset of a chemical library, composed of 300 compounds inhibiting Plasmodium falciparum enoyl reductase, was tested against M. tuberculosis. Four compounds were found to inhibit M. tuberculosis growth with MICs ranging from 1 μM to 10 μM. Testing of these compounds against M. tuberculosis in vitro revealed that only two compounds (CD39 and CD117) were bactericidal against drug-susceptible and drug-resistant M. tuberculosis. These two compounds were also bactericidal against M. tuberculosis incubated under anaerobic conditions. Furthermore, CD39 and CD117 exhibited increased bactericidal activity when used in combination with INH or rifampin, but CD39 was shown to be toxic to eukaryotic cells. The compounds inhibit InhA as well the fatty acid synthase type I, and CD117 was found to also inhibit tuberculostearic acid synthesis. This study provides the TB drug development community with two chemical scaffolds that are suitable for structure-activity relationship study to improve on their cytotoxicities and bactericidal activities in vitro and in vivo.
INTRODUCTION
The fight against tuberculosis (TB), a disease caused by the bacillus Mycobacterium tuberculosis, is facing new challenges with the surge of multidrug-resistant (MDR) TB and the recent emergence of extensively drug-resistant (XDR) TB (8). TB strains resistant to the first-line anti-TB drugs (FLDs) isoniazid (also known as isonicotinoic acid hydrazide [INH]) and rifampin (RIF) are classified as MDR-TB, while XDR-TB is defined as MDR-TB resistant to any fluoroquinolone and one or more of the three injectable drugs (6). The need for novel TB drugs has spurred a new interest in TB drug development, and several new TB drugs are currently being tested in clinical trials (18). Nevertheless, expanding the pharmacopeia of active TB drugs remains an important goal, as M. tuberculosis has proven to be more than adept at acquiring drug resistance. One strategy to develop new drugs effective against MDR- and XDR-TB is to target essential functions that are not aims of the current anti-TB drug regimen. An alternative is to develop new drugs with novel requirements for inhibition of a bona fide target, with the goal of circumventing extant drug resistance.
TB is resistant to most commonly used antibacterial agents due in part to its unusual cell wall structure. The mycobacterial cell wall contains unique long-chain (C70 to C90), α-alkyl, β-hydroxy fatty acids called mycolic acids. The synthesis of these fatty acids requires the coenzyme A (CoA)-dependent fatty acid synthase type I (FASI) and the acyl carrier protein (ACP)-dependent multienzyme fatty acid synthase type II (FASII) systems. FASI produces fatty acids of up to C16 and C26 chain lengths, and FASII elongates these fatty acids to meromycolates. Condensation of meromycolates with the end product of FASI produces mycolic acids (4, 37). The FASII enzymes are viable targets for drug development, since eukaryotic cells use only a FASI-type enzyme to synthesize fatty acids (10). Two enzymes of the mycobacterial FASII system are already targets of antimycobacterial drugs: the β-keto-acyl-ACP reductase KasA, targeted by thiolactomycin (34), and the enoyl-ACP reductase InhA, which is inhibited by INH (40). INH is a prodrug that is activated by the catalase-peroxidase KatG (13) to form an isonicotinoyl radical which reacts with NAD to produce an INH-NAD adduct (17, 31, 42), which inhibits InhA (17, 23, 29, 40, 42). A large majority of INH-resistant (INHR) M. tuberculosis clinical isolates have mutations in the activator of INH, KatG, not the target of INH, InhA (38). The inhA gene is essential in mycobacteria, and inhibition of InhA enzymatic activity leads to mycobacterial cell death (39). InhA, as a drug target, has been validated by several studies (5, 9, 20, 24, 33, 36). GlaxoSmithKline and the TB Alliance have conducted an InhA target-based screen of a million compounds and are in the lead optimization phase (http://www.tballiance.org/new/portfolio/html-portfolio-item.php?id=5). Series of analogs of triclosan, another InhA inhibitor (25), that showed good activities against the enoyl reductase InhA of M. tuberculosis (7), as well as the enoyl reductase PfENR of Plasmodium falciparum (15), have also been synthesized. Our goal was to identify new InhA inhibitors that, unlike INH, do not require activation by KatG and could therefore be active against katG-deficient INHR M. tuberculosis strains. Therefore, our screening activities commenced with the extension of a previously conducted campaign against PfENR (14). Our experience with numerous chemotypes, including diaryl ether phenols, indoles, and pyrazoles, supported the notion that PfENR inhibitors may also show significant efficacy against InhA. Thus, we examined a subset of 300 hits (ChemDiv; San Diego, CA) against PfENR, characterized by >40% enzyme inhibition at 10 μM compound and drug-like properties (less than two violations of Lipinski's Rule of Five [19]). Herein, we describe the screening and testing of these compounds against whole cells of M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains and media.
The M. tuberculosis strains were obtained from laboratory stocks. The clinical isolates were selected from an anonymized collection of clinical M. tuberculosis isolates. The isolates were derived from clinical tuberculosis patients in Mexico. 5071 and 5483 are single nucleotide polymorphism (SNP) cluster group 3b, while 12081 is SNP cluster group 5, both consistent with the predominant strain types in the Americas. The strains were grown in Sauton medium (16) supplemented with 0.05% (vol/vol) tyloxapol unless otherwise indicated. The solid medium used was Middlebrook 7H10 medium (Difco) supplemented with 10% (vol/vol) ADS enrichment (50 g albumin, 20 g dextrose, 8.5 g sodium chloride, in 1 liter of water) and 0.2% (vol/vol) glycerol. The chemical library was obtained from ChemDiv (San Diego, CA). All other chemicals used in this study were obtained from Sigma (St. Louis, MO) and ChemBridge (San Diego, CA) or were synthesized utilizing established methods (3, 11, 30).
MIC determination.
Each compound from the chemical library was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/liter. The MICs were determined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (21). In this assay, the MIC was determined by visual inspection as the lowest concentration of compound that prevented the conversion of MTT (yellow solution) to formazan (blue/violet solution). If a compound reacted with MTT, the MIC determination was then performed in Falcon tubes containing 2-fold serial dilution of the compound in Sauton medium (2 ml), to which was added the M. tuberculosis strain (≈104 CFU in 0.1 ml of Sauton). The tubes were incubated at 37°C for 4 weeks. Compounds were tested at concentrations ranging from 0.15 mg/liter to 40 mg/liter. In broth dilution assays, the MIC was determined as the lowest concentration of compound that prevented visible growth.
Determination of anaerobic bactericidal activity of ChemDiv compounds.
Select ChemDiv compounds were tested for bactericidal activity on nongrowing populations of M. tuberculosis. M. tuberculosis strain mc27000 (H37Rv ΔRD1 ΔpanCD) was grown under a normal aerobic atmosphere in 7H9 medium supplemented with ADS, 0.5% glycerol, 0.05% tyloxapol, and 50 mg/liter pantothenic acid to an optical density at 600 nm (OD600) of 0.5. Actively growing cultures were then shifted to a hypoxic glove box (Coy Laboratory Products, Grass Lake, MI) containing an anaerobic atmosphere (<0.0001% O2, 5% CO2, 10% H2, 85% N2), which rapidly arrested growth but did not have a measurable effect on culture viability. After 48 h of anaerobic incubation, bacilli were inoculated 1:50 to anaerobic 7H9 (supplemented as described above) containing test compounds at the indicated concentrations. Viability was assessed over time by determining CFU per milliliter of culture on 7H10 medium supplemented with ADS, 0.2% glycerol, and 50 mg/liter pantothenic acid.
Analysis of FAMEs and MAMEs by TLC.
M. tuberculosis H37Rv cultures (10 ml), grown in Sauton medium to an OD600 of ∼0.35, were treated with selected ChemDiv compounds for 20 h and then labeled with [1-14C]acetate for 6 h. The cultures were spun, and the cell pellets were washed once with distilled water, resuspended in distilled water (1 ml), and added to a 40% solution of tetrabutylammonium hydroxide (1 ml). The suspension was heated at 100°C for 16 h. After cooling, methyl iodide (0.1 ml) and methylene chloride (2 ml) were added, and the samples were mixed for 1 h at 20°C. The organic phase was washed with a 3N aqueous hydrochloric acid solution followed by a wash with distilled water, drying, and resuspension in methylene chloride (0.2 ml). The fatty acid methyl esters (FAMEs) and mycolic acid methyl esters (MAMEs) were analyzed by spotting 5 μl of the extract onto a silica gel 60 F254 thin-layer chromatography (TLC) plate. The TLC plate was eluted three times with hexane-ethyl acetate (95/5, vol/vol) and the FAMEs and MAMEs were detected by autoradiography after exposure for 24 to 48 h at −80°C.
Analysis of fatty acids by HPLC.
M. tuberculosis H37Rv cultures (10 ml), grown in Sauton medium to an OD600 of ≈0.35, were treated with different concentrations of CD117 (0, 1, 5, and 10 mg/liter) for 20 h, followed by labeling with [1-14C]acetate for 6 h. After centrifugation, the cell pellets were washed once with distilled water (10 ml) and then saponified with a 20% aqueous solution of tetrabutylammonium hydroxide (1 ml) overnight at 100°C. The fatty acids were extracted, derivatized to their UV-absorbing p-bromophenacyl esters, and analyzed on a Hewlett-Packard model HP1100 high-performance liquid chromatograph (HPLC) coupled to an IN/US β-RAM flowthrough radioisotope beta-gamma radiation detector as described previously (39). Fatty acid standards were derivatized and analyzed by HPLC similarly, and the resulting chromatograms were used to identify the peaks from the experimental chromatograms.
Cytotoxicity assay.
African green monkey kidney cells (Vero cells, ATCC, Manassas VA) were seeded into 96-well plates and cultured with Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum in the presence of varying amounts of CD39 (0.39 to 50 mg/liter), CD117 (0.39 to 50 mg/liter), INH (2.5 to 5000 mg/liter), and RIF (0.25 to 500 mg/liter). After incubation at 37°C for 2 days, cell viability was measured using the MTT assay (22). Plates were read at 595 nm using a DTX880 plate reader.
Macrophage experiment.
Bone marrow-derived macrophages (BMDM) were prepared from C57BL/6 mice by flushing isolated femurs with Dulbecco modified Eagle medium (DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, and 1× nonessential amino acids (complete DMEM). The cells were cultured for 7 days, on petri dishes, in complete DMEM also supplemented with recombinant murine macrophage colony-stimulating factor (M-CSF; Peprotech) at 20 ng/ml; the cytokine was replenished once during this 7-day culture period. After 7 days, the cells were detached using cold phosphate-buffered saline (PBS) with 5 mM EDTA, washed in complete DMEM, and resuspended in DMEM with M-CSF at 10 ng/ml. The cells were seeded into 24-well plates (2 × 105 to 3 × 105 cells/well) and permitted to adhere overnight prior to infection. The macrophages were infected with M. tuberculosis H37Rv that was grown to an OD600 of ∼1 in 7H9 medium supplemented with oleic acid-albumin-dextrose-catalase (OADC), 0.2% glycerol, and 0.05% Tween 80, washed and resuspended in complete DMEM and diluted to the appropriate titer in complete DMEM. The bacteria were added to the wells at an approximate multiplicity of infection (MOI) of 5. Following 4 h of incubation at 37°C to permit bacterial uptake, macrophage monolayers were washed twice with PBS to remove extracellular bacteria, following which wells were replenished with complete DMEM containing M-CSF at 10 ng/ml. At this point, the test compounds were added to the wells. These included CD117, INH, or RIF, alone or in combination, as well as DMSO as the vehicle control. At various times after infection, the medium was removed to a tube containing sufficient SDS to give a final concentration of 0.025%; the cell monolayers were lysed with 0.025% SDS and combined with the medium. The lysates were diluted in PBS with 0.05% Tween 80 and plated onto 7H10 agar (with 10% OADC, 0.5% glycerol) for determination of bacterial numbers.
RESULTS
Screening against whole-cell M. tuberculosis.
The 300 compounds previously identified as PfENR inhibitors (14) were tested by spotting 5, 10, 20, and 40 μg of each compound on an M. tuberculosis H37Rv lawn. After incubation of the plates for 3 weeks at 37°C, clearing of the mycobacterial lawn was observed for 64 compounds, which were then tested for inhibition of M. tuberculosis growth in liquid cultures at a concentration of 20 mg/liter. Only 11 compounds retarded or inhibited the growth of M. tuberculosis at this concentration. Their MICs were determined, and they ranged between 1 μM and >100 μM against wild-type (wt) M. tuberculosis (Table 1). The four compounds with MICs below 10 μM (CD13, CD39, CD59, and CD117) were then investigated further for bactericidal activity. M. tuberculosis H37Rv was first treated with each compound at the concentration of 20 mg/liter, and kill curves were performed by measuring CFU for a period of 3 weeks (Fig. 1). Treatment of M. tuberculosis with CD13 and CD59 resulted in no more than a 2-log decrease in CFU titers after 3 weeks. By contrast, treatment of M. tuberculosis with CD117 and CD39 was a bactericidal event with up to a 4-log decrease in CFU titers. To examine if the activity of the compounds was strain specific, we treated M. tuberculosis Erdman, M. tuberculosis CDC1551, and M. tuberculosis Beijing with CD39 or CD117 at 20 mg/liter and observed the same reduction in CFU as with M. tuberculosis H37Rv.
Table 1.
MIC and chemical structure of active ChemDiv compounds against M. tuberculosis strains
ND, not done.
Fig. 1.
Growth of M. tuberculosis H37Rv treated with CD13, CD39, CD59, and CD117 at 20 mg/liter. Cultures, grown in Sauton medium, were incubated at 37°C, and CFU were determined by plating 10-fold dilutions of each cultures onto Middlebrook 7H10 agar plates. Each growth curve was done in triplicate, and the averages with standard deviations are plotted.
Activity of CD39 and CD117 against drug-resistant M. tuberculosis strains.
Since our goal was to identify InhA inhibitors that would be active against INHR clinical isolates, where the main mechanism of INH resistance is mutations in katG (38), we tested CD39 and CD117 against mc24977, an M. tuberculosis H37Rv strain that has a base pair deletion (del g371) in katG resulting in a frameshift mutation and high resistance to INH (MIC > 100 mg/liter). Surprisingly, both compounds were more active against this INHR M. tuberculosis strain than they were against wt M. tuberculosis (Fig. 2). Treatment of mc24977 with either CD39 or CD117 resulted in a 6-log decrease in CFU titers after 14 days. To assess if this increased activity against the INHR M. tuberculosis strain was a general phenomenon or existed only in a katG mutant, we tested three clinical isolates with different drug resistance patterns: CI5071, resistant to INH and streptomycin; CI5483, which is ethambutol and streptomycin resistant; and CI12081, resistant to INH, RIF, and streptomycin. Again, we observed a better inhibition of the drug-resistant (DR) TB strains than of wt M. tuberculosis (Fig. 2). This increase in bactericidal activity was not observed for CD13 and CD59. These two compounds showed the same level of killing for wt M. tuberculosis and for DR-TB strains.
Fig. 2.
Treatment of drug-resistant M. tuberculosis strains with INH (1 mg/liter), CD39 (20 mg/liter), and CD117 (20 mg/liter) in Sauton medium. mc24977 is an INH-resistant laboratory strain containing a single base pair deletion in katG. The three clinical isolates CI5483, CI5071, and CI12081 are resistant to ethambutol and streptomycin, INH and streptomycin, and INH, RIF, and streptomycin, respectively. The cultures were incubated with shaking at 37°C, and CFU were determined by plating 10-fold serial dilutions onto Middlebrook 7H10 plates. Each experiment was done in duplicate, and the averages are plotted with standard deviations.
Activity of CD39 and CD117 in combination with FLDs.
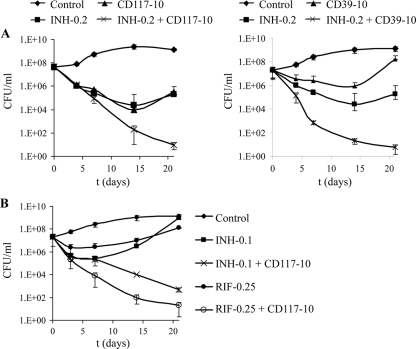
Since monotherapy is not an accepted protocol for treatment of TB, the interactions of the compounds CD39 or CD117 with current FLDs were tested to determine if the compounds could be used in combination with FLDs. We tested a combination of CD39 or CD117 (10 mg/liter) with INH at a concentration of 0.2 mg/liter (4× MIC). After 3 weeks of treatment, a reduction of more than 6 logs of CFU/ml was observed when M. tuberculosis H37Rv was treated with INH and CD39 or CD117 (Fig. 3A). By contrast, the culture of M. tuberculosis treated with CD39 alone had almost the same titer as the untreated culture, while treatment of M. tuberculosis with INH or CD117 resulted in a 2-log decrease in CFU after 3 weeks (Fig. 3A). Similar killing was also obtained when the concentration of CD39 or CD117 was decreased to 1 mg/liter in combination with 0.2 mg/liter of INH (data not shown). The addition of INH to the compounds was essential to the increased activity, since decreasing the amount of INH by half resulted in reduced killing (Fig. 3B). When used in combination with a low concentration of RIF (0.25 mg/liter), a limited effect was observed with CD39 (1-log increase in killing), while the combination of CD117 and RIF resulted in a 4-log increase in killing compared to CD117 alone (Fig. 3B).
Fig. 3.
Combination of CD39 and CD117 with FLDs. M. tuberculosis H37Rv, grown in Sauton, was treated with compounds alone or in combination with INH (A and B) or RIF (B). The cultures were incubated with shaking at 37°C. CFU were determined by plating 10-fold serial dilutions onto Middlebrook 7H10 plates. Concentrations are given in mg/liter. Each experiment was done in triplicate, and the averages with standard deviations are plotted.
Activity of CD39 and CD117 under anaerobic conditions.
Another challenge faced by TB eradication programs is the ability of M. tuberculosis to enter latency and persist in an infected person for many years or decades. To fully eradicate TB, new drugs will have to be able to kill these persistent populations of bacilli. As the hypoxic lumen of the granuloma is predicted to be a site of residence for dormant persistent bacilli, a common in vitro model for M. tuberculosis dormancy is the Wayne model (41), where mycobacterial cultures are subjected to a gradual loss of oxygen that likely mimics the hypoxic conditions inside granulomas. To test if CD39 and CD117 were active against nongrowing hypoxic bacilli, M. tuberculosis was grown under aerobic conditions to early log phase and then shifted to anaerobic conditions inside an anaerobic chamber. Once the bacilli were adapted to this condition, they were subcultured to anaerobic medium containing different concentrations of CD39 or CD117, and CFU titers were determined over time (Fig. 4). The effects of CD39 and CD117 were found to be concentration dependent. In contrast to what was observed for aerobic treatment, CD117 showed limited activity for the first 2 weeks of anaerobic treatment (Fig. 4A). However, this period of anaerobic tolerance was followed by a rapid concentration-dependent decrease in culture viability. Conversely, treatment of hypoxic bacilli with high concentrations of CD39 (10 and 20 mg/liter) resulted in a rapid acute decrease in CFU titers. Yet, after 6 days of treatment, continued bactericidal activity was observed only at the highest concentration (20 mg/liter) that was tested. In comparison, INH at 1 mg/liter has no activity against M. tuberculosis incubated under hypoxic conditions, while RIF at 1 mg/liter maintained bactericidal activity, resulting in a 3-log killing of M. tuberculosis in 21 days (data not shown).
Fig. 4.

Bactericidal activity of CD39 and CD117 on hypoxically dormant M. tuberculosis. M. tuberculosis mc27000 was grown aerobically in 7H9 medium supplemented with ADS, 0.5% glycerol, 0.05% tyloxapol, and 50 mg/liter pantothenic acid. Bacilli were shifted to an anaerobic atmosphere containing <0.0001% O2, 5% CO2, 10% H2, and 85% N2. After 48 h, cells were subcultured to anaerobic 7H9 (supplemented as above) containing the indicated concentrations (in mg/liter) of CD117 (A) and CD39 (B), and colony-forming-units (CFU) were determined by plating 10-fold dilutions of each culture onto Middlebrook 7H10 agar plates.
Activity of CD39 and CD117 on eukaryotic cells.
M. tuberculosis is an intracellular pathogen. In the early stages of infection, M. tuberculosis is primarily found in alveolar macrophages. Therefore, it was necessary to determine the level of toxicity of the compounds to eukaryotic cells and then assess if the compounds could kill M. tuberculosis inside a macrophage. Toxicity data were obtained using African green monkey kidney cells (Vero cells). The highest tolerated dose (HTD) in Vero cells was 3 μM for CD39 (0.8 mg/liter). CD117 at the highest concentration tested (132 μM, 50 mg/liter) had a limited effect (20% cell viability reduction) on the Vero cells. For comparison, the HTDs for INH and RIF in this assay were 14 mM (1.9 g/liter) and 0.6 mM (0.5 g/liter), respectively. The ratio between the maximum concentration of drug tolerated by the mammalian cells and the MIC was 0.3 for CD39 and 132 for CD117, leading us to conclude that CD39 was toxic and would require chemical modifications for further study. Thus, only CD117 was tested for killing of M. tuberculosis in a macrophage. Bone marrow-derived macrophages (BMDM) from C57BL/6 mice were infected with M. tuberculosis H37Rv for 4 h and then washed to remove extracellular bacteria prior to the addition of CD117 (10 and 20 mg/liter) (Fig. 5A). The effect of CD117 was dose dependent and resulted in a 1-log decrease in CFU after 24 h, followed by a continuous small decrease in CFU at 20 mg/liter. At 10 mg/liter, a small decrease in CFU was observed for the first 2 days (70%), followed by stabilization (Fig. 5A). Since the in vitro bactericidal activity increased when INH or RIF was added to CD117, the combination of CD117 with low concentrations of INH (0.2 mg/liter) or RIF (0.25 mg/liter) was also tested in BMDM infected with H37Rv. Surprisingly, no improvement in the killing of H37Rv was observed when combining INH and CD117 (Fig. 5B). In contrast, the combination of RIF and CD117 resulted in >3-log killing of H37Rv (Fig. 5C). The combined effect of CD117 and RIF was more important than the additional effect of RIF alone, which had limited killing activity (60% reduction in CFU), and of CD117 alone (1.5-log killing) after 6 days. A similar pattern was observed with lower concentrations of CD117 (5 and 10 mg/liter). Adding INH to CD117 at 5 or 10 mg/liter did not improve killing of M. tuberculosis, while the addition of RIF (0.25 mg/liter) to CD117 (5 and 10 mg/liter) resulted in an additional 1-log killing compared to CD117 alone (data not shown).
Fig. 5.
Growth of M. tuberculosis H37Rv, treated with CD117 at 10 or 20 mg/liter (A), INH ± CD117 (B), or RIF ± CD117 (C), in bone marrow-derived macrophages (BMDM). Drug treatment was initiated after a 4-h infection period. At each time point, macrophages were lysed, and 10-fold serial dilutions were plated on Middlebrook 7H10 plates to determine CFU counts. Concentrations are given in mg/liter. Each experiment was done in triplicate, and the averages with standard deviations are plotted.
Mode of action of CD39 and CD117.
InhA is the NADH-dependent enoyl-ACP reductase of the FASII system, involved in mycolic acid biosynthesis. It was previously shown that inhibition of InhA resulted in inhibition of mycolic acid biosynthesis and accumulation of long-chain fatty acids (39). To determine if CD39 and CD117 did in fact target InhA, mycolic acid biosynthesis in M. tuberculosis treated with CD39 or CD117 was analyzed by thin-layer chromatography. At 10 mg/liter, only CD117 had an effect on the biosynthesis of mycolic acids (MAMEs), while treatment of M. tuberculosis with CD39 resulted in a fatty acid (FAME) and mycolic acid profile similar to that of the control (Fig. 6A). When the concentration of compounds was doubled, inhibition of mycolic acid biosynthesis was observed for M. tuberculosis treated with CD39 and CD117 (Fig. 6B). Furthermore, inhibition of fatty acid biosynthesis was almost complete for M. tuberculosis treated with CD117, and a decrease in fatty acid production was also observed in the CD39 treatment.
Fig. 6.

Analysis of fatty acid (FAMEs) and mycolic acid (MAMEs) biosynthesis by thin-layer chromatography (TLC). M. tuberculosis H37Rv, grown in Sauton medium, was treated with CD39 or CD117 at either 10 mg/liter (A) or 20 mg/liter (B) for 20 h and then labeled with [1-14C]acetate for 6 h. Fatty acids were saponified, extracted, and derivatized (see Materials and Methods). Equal amounts of extracted fatty acids were spotted on TLC. The TLC was run three times in hexane-ethyl acetate (95/5, vol/vol), and fatty acids were detected by autoradiography. The control is untreated M. tuberculosis.
To further determine whether InhA was the target of CD39 and CD117, the compounds were tested against mc24914, an M. tuberculosis strain overexpressing InhA (40). Although mc24914 was 3 to 4 times more resistant to the compounds than wt M. tuberculosis, the level of resistance was weak compared to the resistance of mc24914 to INH (Table 1). For comparison, mc24914 is 15 times more resistant to INH than is the wt. The low-level resistance to CD39 and CD117 observed in mc24914 suggested that, while the compounds do inhibit InhA, they must also target other enzymes.
During the testing of the compounds against wt M. tuberculosis, we examined if the inhibition observed was medium dependent. All the in vitro experiments described above had been done in Sauton medium supplemented with tyloxapol. Surprisingly, the compounds showed no activity against M. tuberculosis grown in Middlebrook 7H9 supplemented with oleic acid-albumin-dextrose-catalase (OADC) but were still as active in Middlebrook 7H9 supplemented with albumin-dextrose-saline (ADS). Antimycobacterial drugs, such as isoxyl, are known to inhibit oleic acid biosynthesis. To examine if there was a correlation between the mode of action of the compounds and oleic acid biosynthesis, fatty acids produced by M. tuberculosis when treated with increasing concentrations of CD117 were analyzed by high-performance liquid chromatography (HPLC). No decrease in oleic acid biosynthesis was observed when M. tuberculosis was treated with 1, 5, or 10 mg/liter of CD117, but a reduction in tuberculostearic acid (TA) was found (Fig. 7A). Analysis of the fatty acid distribution showed that the loss of tuberculostearic acid was dependent on the concentrations of CD117 used (Fig. 7B). Interestingly, an accumulation of long-chain fatty acids was also observed with increased concentrations of CD117, an event known to occur when InhA and subsequently the FASII system are inhibited (39).
Fig. 7.
Analysis of fatty acid production by high-performance liquid chromatography (HPLC). M. tuberculosis H37Rv was treated with 0, 1, 5, or 10 mg/liter of CD117 for 20 h and then labeled with [1-14C]acetate for 6 h. Fatty acids were saponified, extracted, and derivatized (see Materials and Methods) prior to HPLC analysis. (A) HPLC chromatograms. The peaks were identified by comparison with known standards. Labels are as follows: 16:0, palmitic acid; 18:1, oleic acid; 18:0, stearic acid; TA, tuberculostearic acid; 20:0, eicosanoic acid; 24:0, tetracosanoic acid; 26:0, hexacosanoic acid. (B) Percentage of fatty acid (FA) in each experiment as determined by the chromatograms' peak intensity reports.
To find out which other gene or genes were targeted by CD117, the isolation of CD117-resistant mutants was attempted in two independent experiments. M. tuberculosis H37Rv (109 cells) was plated on Middlebrook 7H10 plates supplemented with ADS and containing CD117 at 10 and 20 times the MIC. The plates were incubated for 5 to 6 weeks at 37°C. No CD117-resistant mutants could be obtained.
Chemical structure versus antimycobacterial activity.
To assess which part of the CD117 molecule was important for the antimycobacterial activity, analogs of CD117 were tested against M. tuberculosis H37Rv. CD117 has two major structural components: the tetrahydrobenzothienopyrimidine and the ethyl 2-thioacetate chain (Table 2). The thioacetate group was modified by replacing the ethyl ester with a methyl ester (analog CD117a), an amide (CD117e), or an alcohol (CD117f) or removing the ethyl ester (CD117d). At the other end of the molecule, the t-butyl group was excised (analog CD117b). Regarding the tricyclic heterocycle (comprised of pyrimidine, thiophene, and cyclohexene elements), the pyrimidine ring was opened (analog CD117c). Only CD117a retained antimycobacterial activity (Table 2). From this limited set of CD117 analogs, we can conclude that one essential chemical group in CD117 is the thioacetate, since replacing the carboxylic group by an alcohol or removing it resulted in total loss of the antimycobacterial activity. Furthermore, changing the ester into an amide group led to a severe reduction in activity, lending support to the idea that the ester moiety may serve as a prodrug for the carboxylic acid. Another important group is the t-butyl group attached to the cyclohexene ring, since its removal resulted in a decrease of the antimycobacterial activity. The fact that such small changes in the chemical structure of CD117 affected its antimycobacterial activity argues against the possibility that the compound's activity is nonspecific.
Table 2.
CD117 chemical modifications' effect on antimycobacterial activity
DISCUSSION
Drug resistance in M. tuberculosis has become a major health problem, and the WHO estimates that worldwide up to 28% of the TB cases are INHR and that, in previously treated TB cases, the level of INH resistance can reach 60%. The main target of INH is the enoyl reductase InhA, yet the main mechanism of INH resistance in TB clinical isolates is mutations in the activator of INH, KatG. In order to identify new inhibitors of InhA that did not require KatG activation, we tested a subset (300 compounds) of a chemical library, composed of inhibitors of the P. falciparum enoyl-reductase PfENR, against whole-cell M. tuberculosis. Although the enoyl reductases of M. tuberculosis and P. falciparum have limited similarities at the protein level (28% identical, 44% similar), two compounds, CD39 and CD117, were found to have bactericidal activity against drug-susceptible M. tuberculosis under both aerobic and anaerobic conditions. More importantly, both compounds were also bactericidal against DR- and MDR-TB. Treatment of MDR-TB with one dose of CD39 and CD117 resulted in the near sterilization of the cultures. This sterilization effect might reflect the lack of fitness of MDR-TB strains, since the compounds were in fact much more active against MDR-TB than against wt M. tuberculosis.
The novel TB drugs currently in clinical trials were not discovered through target-based cell screening but through either whole-cell phenotypic screening (TMC207 [1]) or chemical modification of previous hits (PA-824 [35], SQ109 [12], and OPC-67683 [28]). The advantage of target-based chemical library screening is that the target of the identified inhibitors is known, which means that the mode of action and mode of resistance can be easily identified. In this study, although the initial enzymatic screen was done to identify inhibitors of PfENR, the whole-cell screening of M. tuberculosis led to the identification of CD39 and CD117 that, indeed, did target the M. tuberculosis enoyl-reductase InhA, as shown by biochemistry (inhibition of mycolic acid biosynthesis) and genetics (MICs increased 4-fold for an M. tuberculosis strain overexpressing inhA). Nonetheless, their inhibitory activity was not limited to this enzyme. Treatment of M. tuberculosis with these compounds resulted in the inhibition of mycolic acid and long-chain fatty acid biosyntheses, indicating that the compounds inhibit both fatty acid synthases types I and II. Furthermore, CD117 has also been shown to inhibit tuberculostearic acid biosynthesis. This combination of phenotypes is reminiscent of isoxyl, a drug used in the 1960s for the treatment of TB. Isoxyl (1,3-bis[4-(3-methylbutoxy)phenyl]thiourea) inhibits the biosynthesis of mycolic acids (43), short-chain fatty acids (26), oleic acid, and subsequently tuberculostearic acid (27). Isoxyl targets the Δ9-desaturase DesA3, resulting in the inhibition of oleic acid biosynthesis (27), but the targets involved in mycolic and fatty acid biosyntheses are still unknown. One of the advantages of antibacterials having multiple targets is the reduced development of drug resistance (32). For drugs having a single target, like RIF, a single point mutation in one gene (rpoB) renders the strain resistant and the drug useless. The same is true for INH, although here it is the gene involved in the activation of the drug that leads to resistance. The frequency of mutation with CD117 was extremely low (<10−9), which illustrates the fact that CD117 targets multiple enzymes. The benefits of antibiotic polypharmacology have been well described in the past, and compounds like CD117 and certainly isoxyl should certainly be developed or rediscovered in order to fight the rise of drug resistance in M. tuberculosis clinical strains.
A search in PubChem (pubchem.ncbi.nlm.nih.gov) revealed that CD117 had been previously tested in one high-throughput screen (HTS), but no bioassay result was reported for CD39. CD117 was found inactive in an HTS for protein interaction modulators targeting the tyrosine 3-mono-oxygenase/tryptophan 5-mono-oxygenase activation protein in Homo sapiens. Interestingly, 2 analogs of CD117 (CD117e and the methyl ester of CD117b) were found active in an HTS for new inhibitors of Mycobacterium tuberculosis H37Rv performed by the Southern Research Institute in Birmingham, Alabama. In general, a search for all tetrahydrobenzothienopyrimidines in this data set and all other publicly accessible TB data available from Collaborative Drug Discovery, Inc. demonstrated that, indeed, this compound family displays activity against M. tuberculosis where most notably substitution on the 2 and 4 positions of the pyrimidine ring drives the structure-activity relationships (SARs). This observation coupled with our preliminary investigations into the SARs of CD117 argues against the possibility that the small molecule's whole-cell activity is nonspecific. Both CD39 and CD117 are candidates for structure-activity relationship study to improve activity, decrease toxicity, and increase solubility (CD39 and CD117 have partition coefficient [log P] values of 3.97 and 1.33, respectively). CD39 features an ortho-nitrofuran linked to an iminothiazolidinone via a three-carbon unsaturated spacer. Modifying the nitro substituent, the 2-furyl moiety, or the connecting three-carbon spacer (chain length and degree of unsaturation) could lead to a less toxic compound with reasonable antimycobacterial activity. PA-824 and OPC-67683 were actually discovered by chemical modification of an initial hit, CGI-17341, a bicyclic 5-nitroimidazole derivative with decent activity against M. tuberculosis in vitro and in vivo but high mutagenic properties (2). The sterilizing activities of CD117 and CD39 against MDR-TB illustrate the potential of the chemical skeletons of these two molecules for further drug development, especially against MDR-TB.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI26170, AI43268, AI46669, and CFAR AI051519. A.D.B. was a Merck Fellow of the Helen Hay Whitney Foundation. James C. Sacchettini acknowledges Robert A. Welch Foundation grant A-0015.
Footnotes
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Andries K., et al. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227 [DOI] [PubMed] [Google Scholar]
- 2. Ashtekar D. R., et al. 1993. In vitro and in vivo activities of the nitroimidazole CGI 17341 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 37:183–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azab M. E. 2008. Utility of the enaminonitrile moiety in the synthesis of some biologically active thienopyrimidine derivatives. Phosphorus Sulfur Silicon Relat. Elem. 183:1766–1782 [Google Scholar]
- 4. Bhatt A., Molle V., Besra G. S., Jacobs W. R., Jr., Kremer L. 2007. The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Mol. Microbiol. 64:1442–1454 [DOI] [PubMed] [Google Scholar]
- 5. Boyne M. E., et al. 2007. Targeting fatty acid biosynthesis for the development of novel chemotherapeutics against Mycobacterium tuberculosis: evaluation of A-ring-modified diphenyl ethers as high-affinity InhA inhibitors. Antimicrob. Agents Chemother. 51:3562–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2006. Revised definition of extensively drug-resistant tuberculosis. MMWR Morb. Mortal. Wkly. Rep. 55:1176 [Google Scholar]
- 7. Freundlich J. S., et al. 2009. Triclosan derivatives: towards potent inhibitors of drug-sensitive and drug-resistant Mycobacterium tuberculosis. ChemMedChem 4:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gandhi N. R., et al. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580 [DOI] [PubMed] [Google Scholar]
- 9. He X., Alian A., Ortiz de Montellano P. R. 2007. Inhibition of the Mycobacterium tuberculosis enoyl acyl carrier protein reductase InhA by arylamides. Bioorg. Med. Chem. 15:6649–6658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heath R. J., Rock C. O. 2004. Fatty acid biosynthesis as a target for novel antibacterials. Curr. Opin. Invest. Drugs 5:146–153 [PMC free article] [PubMed] [Google Scholar]
- 11. Hegab M. I., Hassan N. A., Rashad A. E., Fahmy A. A., Abdel-Megeid F. M. E. 2007. Synthesis, reactions, and antimicrobial activity of some fused thieno[2,3-d]pyrimidine derivatives. Phosphorus Sulfur Silicon Relat. Elem. 182:1535–1556 [Google Scholar]
- 12. Jia L., et al. 2005. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br. J. Pharmacol. 144:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnsson K., Schultz P. G. 1994. Mechanistic studies of the oxidation of isoniazid by the catalase peroxidase from Mycobacterium tuberculosis. J. Am. Chem. Soc. 116:7425–7426 [Google Scholar]
- 14. Kuo M. R. 2006. Ph.D. thesis Structure, function, and inhibition of enoyl reductases. Texas A&M University, College Station, TX [Google Scholar]
- 15. Kuo M. R., et al. 2003. Targeting tuberculosis and malaria through inhibition of enoyl reductase: compound activity and structural data. J. Biol. Chem. 278:20851–20859 [DOI] [PubMed] [Google Scholar]
- 16. Larsen M. H., Biermann K., Jacobs W. R., Jr 2007. Laboratory maintenance of Mycobacterium tuberculosis. Chapter 10, Unit 10A 11. Current protocols in microbiology. http://www.currentprotocols.com/protocol/mc10a01 [DOI] [PubMed]
- 17. Lei B., Wei C. J., Tu S. C. 2000. Action mechanism of antitubercular isoniazid. Activation by Mycobacterium tuberculosis KatG, isolation, and characterization of inhA inhibitor. J. Biol. Chem. 275:2520–2526 [DOI] [PubMed] [Google Scholar]
- 18. Lienhardt C., Vernon A., Raviglione M. C. 2010. New drugs and new regimens for the treatment of tuberculosis: review of the drug development pipeline and implications for national programmes. Curr. Opin. Pulm. Med. 16:186–193 [DOI] [PubMed] [Google Scholar]
- 19. Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3–26 [DOI] [PubMed] [Google Scholar]
- 20. Luckner S. R., Liu N., am Ende C. W., Tonge P. J., Kisker C. 2010. A slow, tight binding inhibitor of InhA, the enoyl-acyl carrier protein reductase from Mycobacterium tuberculosis. J. Biol. Chem. 285:14330–14337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin A., et al. 2005. Multicenter study of MTT and resazurin assays for testing susceptibility to first-line anti-tuberculosis drugs. Int. J. Tuberc. Lung Dis. 9:901–906 [PubMed] [Google Scholar]
- 22. Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen M., Quemard A., Broussy S., Bernadou J., Meunier B. 2002. Mn(III) pyrophosphate as an efficient tool for studying the mode of action of isoniazid on the InhA protein of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2137–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliveira J. S., Vasconcelos I. B., Moreira I. S., Santos D. S., Basso L. A. 2007. Enoyl reductases as targets for the development of anti-tubercular and anti-malarial agents. Curr. Drug Targets 8:399–411 [DOI] [PubMed] [Google Scholar]
- 25. Parikh S. L., Xiao G., Tonge P. J. 2000. Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry 39:7645–7650 [DOI] [PubMed] [Google Scholar]
- 26. Phetsuksiri B., et al. 1999. Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis. Antimicrob. Agents Chemother. 43:1042–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Phetsuksiri B., et al. 2003. Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis. J. Biol. Chem. 278:53123–53130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Protopopova M., et al. 2007. In search of new cures for tuberculosis. Med. Chem. 3:301–316 [DOI] [PubMed] [Google Scholar]
- 29. Rawat R., Whitty A., Tonge P. J. 2003. The isoniazid-NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. Proc. Natl. Acad. Sci. U. S. A. 100:13881–13886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosowsky A., Chaykovsky M., Chen K. K., Lin M., Modest E. J. 1973. 2,4-Diaminothieno(2,3-d)pyrimidines as antifolates and antimalarials. 1. Synthesis of 2,4-diamino-5,6,7,8-tetrahydrothianaphthena(2,3-d)pyrimidines and related compounds. J. Med. Chem. 16:185–188 [DOI] [PubMed] [Google Scholar]
- 31. Rozwarski D. A., Grant G. A., Barton D. H., Jacobs W. R., Jr., Sacchettini J. C. 1998. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science 279:98–102 [DOI] [PubMed] [Google Scholar]
- 32. Silver L. L. 2007. Multi-targeting by monotherapeutic antibacterials. Nat. Rev. Drug Discov. 6:41–55 [DOI] [PubMed] [Google Scholar]
- 33. Sivaraman S., Zwahlen J., Bell A. F., Hedstrom L., Tonge P. J. 2003. Structure-activity studies of the inhibition of FabI, the enoyl reductase from Escherichia coli, by triclosan: kinetic analysis of mutant FabIs. Biochemistry 42:4406–4413 [DOI] [PubMed] [Google Scholar]
- 34. Slayden R. A., et al. 1996. Antimycobacterial action of thiolactomycin: an inhibitor of fatty acid and mycolic acid synthesis. Antimicrob. Agents Chemother. 40:2813–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stover C. K., et al. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962–966 [DOI] [PubMed] [Google Scholar]
- 36. Sullivan T. J., et al. 2006. High affinity InhA inhibitors with activity against drug-resistant strains of Mycobacterium tuberculosis. ACS Chem. Biol. 1:43–53 [DOI] [PubMed] [Google Scholar]
- 37. Takayama K., Wang C., Besra G. S. 2005. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 18:81–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vilcheze C., Jacobs W. R., Jr 2007. The mechanism of isoniazid killing: clarity through the scope of genetics. Annu. Rev. Microbiol. 61:35–50 [DOI] [PubMed] [Google Scholar]
- 39. Vilcheze C., et al. 2000. Inactivation of the inhA-encoded fatty acid synthase II (FASII) enoyl-acyl carrier protein reductase induces accumulation of the FASI end products and cell lysis of Mycobacterium smegmatis. J. Bacteriol. 182:4059–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vilcheze C., et al. 2006. Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat. Med. 12:1027–1029 [DOI] [PubMed] [Google Scholar]
- 41. Wayne L. G., Hayes L. G. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilming M., Johnsson K. 1999. Spontaneous formation of the bioactive form of the tuberculosis drug isoniazid. Angew Chem. Int. Ed. 38:2588–2590 [DOI] [PubMed] [Google Scholar]
- 43. Winder F. G., Collins P. B., Whelan D. 1971. Effects of ethionamide and isoxyl on mycolic acid synthesis in Mycobacterium tuberculosis BCG. J. Gen. Microbiol. 66:379–380 [DOI] [PubMed] [Google Scholar]