Abstract
Ciprofloxacin, the first fluoroquinolone to be used to treat lower respiratory tract infections (LRTI), demonstrates poor potency against Streptococcus pneumoniae, and its use has been associated with the emergence of resistance. During the last decade, fluoroquinolones with enhanced in vitro activity against S. pneumoniae have replaced ciprofloxacin for the treatment of LRTI. Here, we analyzed the impact of more active fluoroquinolone usage on pneumococci by examining the fluoroquinolone usage, prevalence of fluoroquinolone resistance, and mutations in the genes that encode the major target sites for the fluoroquinolones (gyrA and parC) in pneumococcal isolates collected in Canada-wide surveillance. A total of 26,081 isolates were collected between 1998 and 2009. During this time period, total per capita outpatient use of fluoroquinolones increased from 64 to 96 prescriptions per 1,000 persons per year. The proportion of prescriptions for respiratory tract infection that were for fluoroquinolones increased from 5.9% to 10.7%, but the distribution changed: the proportion of prescriptions for ciprofloxacin decreased from 5.3% to 0.5%, and those for levofloxacin or moxifloxacin increased from 1.5% in 1999 to 5.9% in 2009. The prevalence of ciprofloxacin resistance (MIC ≥ 4 μg/ml), levofloxacin resistance, and moxifloxacin resistance remained unchanged at <2%. Multivariable analyses showed that prevalence of mutations known to be associated with reduced susceptibility to fluoroquinolones did not change during the surveillance period. If fluoroquinolone therapy is required, the preferential use of fluoroquinolones with enhanced pneumococcal activity to treat pneumococcal infections may slow the emergence of resistance in S. pneumoniae.
INTRODUCTION
Streptococcus pneumoniae is the most common bacterial cause of respiratory tract infections (RTIs). Ciprofloxacin, the first fluoroquinolone to be used to treat lower respiratory tract infections (LRTIs) (29), demonstrates poor potency against S. pneumoniae, and its introduction has been associated with the rapid emergence of resistance in pneumococci (5, 6, 9, 16, 26, 29).
Since the late 1990s, newer fluoroquinolones with enhanced pneumococcal activity, including levofloxacin, gatifloxacin, and moxifloxacin, have replaced these earlier antimicrobials as the fluoroquinolones of choice for the treatment of LRTIs (1, 4). There is concern that this change may enhance treatment success in the short term but promote the spread of highly resistant strains in the long term (28).
In order to determine the impact of the use of these newer agents on fluoroquinolone resistance in pneumococci, we characterized isolates collected as part of a Canada-wide surveillance program from 1998 to 2009 and related this to fluoroquinolone use during this time period.
MATERIALS AND METHODS
The Canadian Bacterial Surveillance Network is comprised of private and hospital-affiliated laboratories from across Canada and provides service to community and tertiary care hospitals, community clinics, physician offices, and long-term care facilities (25). All 10 Canadian provinces and two of three territories are represented. For surveillance of resistance in S. pneumoniae from 1998 to 2009, participating laboratories, based on their size and catchment area, were asked each year to collect either the first 20 or 100 consecutive clinical isolates, followed by all sterile-site isolates of S. pneumoniae. All laboratories were asked to provide the date of collection, source of specimen, and patient age and gender. In metropolitan Toronto and Peel Region (population, 3.5 million), all laboratories participated in surveillance and submitted all sterile-site and respiratory isolates of S. pneumoniae, providing population-based surveillance of disease in this region.
Isolates were transported on chocolate agar slants or swabs to Mount Sinai Hospital in Toronto, where they were confirmed to be S. pneumoniae by standard methodology, screened to ensure that duplicate isolates from the same patient were not included, and stored at −70°C. In vitro susceptibility testing was performed by broth microdilution, and results were interpreted according to Clinical and Laboratory Standards Institute guidelines using nonmeningeal parenteral breakpoints unless otherwise specified (7, 8). For the purposes of this study, isolates were defined as nonsusceptible if their MICs were in the intermediate or resistant category and as resistant to ciprofloxacin if their ciprofloxacin MICs were ≥4 μg/ml (6, 11).
IMS Health Canada (IMS) provided the annual per capita rate of prescriptions for fluoroquinolones and the percentage of each drug in this class recommended to treat RTIs from 1998 to 2009. IMS collects data on dispensed prescriptions from a representative sample of retail pharmacies in Canada. These pharmacies are stratified by outlet type, location, and size and are used to estimate the total Canadian retail prescription market. The indications for use of antimicrobials were determined by a quarterly IMS audit that collects treatment data from a sample of over 650 office-based Canadian physicians and identifies usage and treatment patterns by drug and by physician specialty.
The quinolone resistance-determining regions (QRDRs) of the parC and gyrA genes were amplified and sequenced (23). Multiple nucleotide sequences were performed with the Clustal W2 program (http://www.ebi.ac.uk/Tools/clustalw2/index.html). We sequenced the QRDRs of all isolates with a ciprofloxacin MIC of ≥4 μg/ml, moxifloxacin MIC of ≥0.25 μg/ml, or levofloxacin MIC of ≥4 μg/ml. In order to test the hypothesis that this selection would identify all isolates with at least one mutation in the QRDRs of gyrA, we also sequenced a 15% sample of other isolates, stratified by fluoroquinolone MIC combinations.
The serotypes of all fluoroquinolone-resistant isolates, of all isolates from population-based surveillance in the metropolitan Toronto and Peel region, and of a representative sample of other susceptible isolates were determined on the basis of the Quellung reaction, using commercial antisera obtained from the Statens Serum institute (Copenhagen, Denmark) (18).
Statistical analysis.
Statistical analysis was performed using SPSS, version 15 (SPSS, Inc., Chicago, IL), and SAS, version 9.1 (SAS, Cary, NC). A chi-square or Fisher's exact test was used to assess the difference in proportions between two or more groups, and Wilcoxon rank sum tests were used to assess differences in continuous variables. P values of less than 0.05 were considered statistically significant. Logistic regression models were used to adjust the estimated effect of time on fluoroquinolone resistance for covariates that might be potential confounders, mediators, or effect modifiers, based both on relationships within the surveillance data and information from other publications. Secondary analysis was performed with data from population-based surveillance in Toronto/Peel. Logistic regression models were also used to identify factors associated with increased prevalence of and gyrA and parC mutations.
RESULTS
From January 1998 to December 2009, a total of 119 laboratories, including 12 community-based laboratories and 107 laboratories serving hospitals ranging in size from 76 to 1,211 beds, submitted 26,081 pneumococcal isolates for testing (Table 1). The median number of isolates submitted per center was 71 (range, 1 to 2,952). A total of 46 laboratories submitted isolates each year for the entire surveillance period.
Table 1.
Factors associated with reduced susceptibility to ciprofloxacin in 26,081 isolates of S. pneumoniae collected between 1998 and 2009 by the Canadian Bacterial Surveillance Networka
| Factorb | No. (%) of isolates with a ciprofloxacin MIC ≥ 4 μg/ml | P valuec |
|---|---|---|
| Geographic aread | 0.002 | |
| Central Canada | 415/18,981 (2.2) | |
| Eastern Canada | 64/3,265 (2.0) | |
| Western Canada | 50/3,725 (1.3) | |
| Patient age | <0.001 | |
| <15 yrs | 17/6,524 (0.26) | |
| 15–64 yrs | 173/10,253 (1.7) | |
| >64 yrs | 336/8,720 (3.9) | |
| Source of isolate | <0.001 | |
| Blood or other sterile fluid | 106/10,080 (1.0) | |
| Respiratory tract, nonsterile | 389/10,450 (3.7) | |
| Other | 32/5,410 (0.59) | |
| Type of laboratorye | <0.001 | |
| Community | 53/4,149 (1.3) | |
| Hospital, <200 beds | 12/922 (1.3) | |
| Hospital, 200–499 beds | 172/9,013 (1.9) | |
| Hospital, 500–699 beds | 93/4,125 (2.3%) | |
| Hospital, ≥700 beds | 193/6,936 (2.8%) | |
| Penicillin MIC of isolate | <0.001 | |
| <0.125 μg/ml | 396/21,876 (1.8) | |
| 0.125–1 μg/ml | 64/2,391 (2.7) | |
| >1 μg/ml | 85/1,787 (5.3) |
Data shown are for reduced susceptibility to ciprofloxacin (MIC ≥ 4 μg/ml); analysis of factors associated with levofloxacin and moxifloxacin yielded indistinguishable results.
The submitting center (geographic area) was missing for 2 (<.1%) isolates, the patient's age was unknown for 302 (1.1%) specimens, and the type of specimen (source) was unknown for 32 (0.12%) isolates.
Likelihood ratio chi-square test.
Central Canada includes the provinces of Manitoba, Ontario, and Quebec; eastern Canada includes the Atlantic provinces; western Canada includes Saskatchewan, Alberta, British Columbia, the Yukon, and the Northwest Territories.
A total of 936 isolates were submitted from laboratories that service hospitals of various sizes.
Of the 26,081 isolates, 10,127 (38.9%) were recovered from blood or other sterile sites (9,368 from blood, 301 from cerebrospinal fluid, 212 from pleural fluid, and 246 from other sterile sites), 10,500 (41.1%) were recovered from nonsterile respiratory sites (9,096 from sputum/endotracheal aspirates, 1,136 from bronchoscopy specimens, 268 from other respiratory tract sites), and 5,422 (20.8%) were from other sites (3,511 from eye swabs, 1,641 from ear swabs, and 270 from other sites). The source of 32 specimens was unknown. The patient's age was specified for 25,779 (98.8%) isolates: among these, 6,537 (25.4%) specimens were from children under the age of 15 years, 10,485 (40.7%) were from adults 16 to 64 years of age, and 8,757 (34.0%) were from adults 65 years of age or older.
The prevalence of isolates resistant to oral penicillin increased from 5.7% (82 of 1,428) in 1998 to 8.3% (123 of 1,491) in 2009. Over the same time period, the prevalence of resistance to intravenous penicillin (nonmeningeal breakpoints) increased from 0.07% (1 of 1,428) to 0.54% (8 of 1,491), resistance to amoxicillin increased from 0.07% (1 of 1,428) to 3.5% (50 of 1,491), resistance to ceftriaxone (meningeal breakpoints) increased from 2.5% (35 of 1,428) to 5.1% (76 of 1,491), resistance to erythromycin increased from 10.5% (150 of 1,428) to 24.4% (363 of 1,491), resistance to trimethoprim-sulfamethoxazole increased from 12.3% (175 of 1,428) to 13.7% (204 of 1,491), and resistance to tetracycline increased from 9.1% (113 of 1,248) to 12.5% (187 of 1,491).
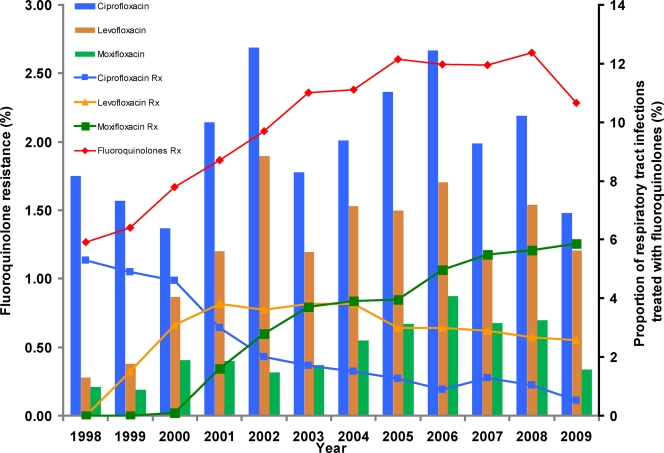
Out-patient use of fluoroquinolone antibiotics in Canada increased from 64 prescriptions per 1,000 persons in 1998 to 96 prescriptions/1,000 persons in 2009. The proportion of Canadian antibiotic prescriptions for RTIs that were for fluoroquinolones increased from 5.9% (in 1998) to 10.7% (in 2009) (Fig. 1).Over the same time period, the proportion of prescriptions for RTIs that were for ciprofloxacin decreased from 5.3% to 0.5%. The proportion of prescriptions for levofloxacin increased from 1.5% in 1999 to 3.8% in 2001 and then remained stable, and the proportion that was for moxifloxacin increased from 0.1% in 2000 to 5.9% in 2009.
Fig. 1.
Prevalence of fluoroquinolone-resistant isolates (bars) and proportion of fluoroquinolone usage (lines) for the treatment of respiratory tract infections in Canada. The percentages of total fluoroquinolones and individual fluoroquinolones used for treatment of respiratory tract infection are shown. Resistance was defined as a MIC of ≥4 μg/ml for ciprofloxacin and levofloxacin and of ≥2 μg/ml for moxifloxacin. The left y axis depicts prevalence of fluoroquinolone resistance found among pneumococcal isolates collected during the surveillance. The right y axis shows the proportion of respiratory tract infections treated with total fluoroquinolones and individual fluoroquinolones.
The prevalence of ciprofloxacin resistance in pneumococcal isolates increased from 1.7% (136 of 7,968) in 1998 to 2001 to 2.2% (225 of 10,157) in 2002 to 2005 and 2.4% (184 of 7,761) in 2006 to 2009 (P = 0.004) (Fig. 1). Levofloxacin resistance increased from 0.73% (58 of 7,968) in 1998 to 2001 to 1.5% (156 of 10,157) in 2002 to 2005 and 1.4% (112 of 7,761) in 2006 to 2009 (P = 0.001), and moxifloxacin resistance increased from 0.31% (25 out of 7,968) in 1998 to 2001 to 0.48% (49 out of 10,157) in 2002 to 2005 and 0.68% (53 of 7,761) in 2006 to 2009 (P < 0.001). The 375 pneumococcal isolates which were resistant to at least one fluoroquinolone were submitted by 59 different centers from all 10 provinces of Canada. Forty-one different serotypes were identified; the most frequent were serotypes 19F (55 isolates), 23F (46 isolates), 11A (29 isolates), 14 (30 isolates), 9V (22 isolates), 6A (21 isolates), 6B (20 isolates), and 3 (17 isolates). In analysis of population-based data, isolates of serotypes 35A, 17F, 11A, and 19F were statistically significantly more likely to be fluoroquinolone resistant than isolates of other serotypes. There was no evidence of temporal or geographic clustering of fluoroquinolone-resistant isolates of any serotype (data not shown).
In univariable analysis, pneumococci resistant to fluoroquinolones were significantly more likely to be isolated from older patients, from respiratory tract specimens, from patients from central Canada, and from specimens submitted from patients in larger hospitals (>200 beds) (Table 1). In multivariable analysis, pneumococci resistant to fluoroquinolones were significantly more likely to be isolated from older patients (odds ratio [OR], 1.2 per decade of age; 95% confidence intervals [CI], 1.15 to 1.30) and from respiratory tract specimens (OR, 3.3; 95% CI, 2.7 to 3.9). There was no association between date of isolation of the specimen and resistance to ciprofloxacin, levofloxacin, or moxifloxacin. There was no significant difference in results in a secondary analysis based on isolates from Toronto/Peel only; date of isolation of the specimen was not associated with resistance to ciprofloxacin, levofloxacin, or moxifloxacin.
In temporal association with the licensure of the 7-valent conjugate vaccine in Canada in June 2001 and its subsequent incorporation into publicly funded infant immunization programs, the proportion of submitted pneumococcal isolates from specimens obtained from children decreased from 34% (2,656 of 7,758 isolates) in 1998 to 2001 to 16.8% (1,312 of 7,815) in 2006 to 2009 (P < 0.001), and the proportion of isolates with serotypes included in the 7-valent conjugate vaccine decreased from 65% (1,759 of 2,715) in 1998 to 2001 to 27% (1,189 of 4,393) in 2006 to 2009. In isolates from population-based surveillance in the metropolitan Toronto/Peel region, resistance to ciprofloxacin was 2.2% in isolates with serotypes included in the 7-valent conjugate vaccine and 2.8% in other isolates (P = 0.13). In comparison, resistance to levofloxacin was 1.5% in isolates with serotypes included in the vaccine and 2.3% in other isolates (P = 0.01), and resistance to moxifloxacin was 0.5% in isolates of serotypes included in the vaccine and 1.0% in other isolates (P = 0.009).
In multivariable analysis, pneumococci resistant to ciprofloxacin were significantly more likely to be isolated from older patients (OR, 1.4 per decade; 95% CI, 1.3 to 1.5), from respiratory tract specimens (OR, 3.8; 95% CI, 2.7 to 5.4), from isolates whose serotypes were included in the 7-valent conjugate vaccine (OR, 1.5; 95% CI, 1.1 to 2.1), and from specimens obtained was early during the surveillance period (OR per year, 1.08; 95% CI, 1.0 to 1.15). There was no association between date of isolation of the specimen and resistance to levofloxacin or moxifloxacin.
Of the 4,558 isolates selected for molecular characterization of the QRDRs of parC and gyrA, 22 isolates (0.48%) could not be retrieved for sequencing, and 3 isolates could not be sequenced due to technical problems. There were 963 isolates with one or more mutations in the QRDR of the parC gene but none in the gyrA gene. Among these isolates, the most common mutations were at positions 137 (Lys-137) (761 isolates), 79 (Ser-79) (179 isolates), and 83 (Asn-83) (47 isolates). Forty-two isolates had mutations at 20 other positions in parC (data not shown). We found 29 isolates with mutations in the QRDR of only gyrA: 18 with a mutation at position 81 (Ser-81), 1 with a mutation at position 85 (Glu-85), 1 with mutations at both positions 81 and 85, and 9 with mutations at other sites. Of the 268 isolates containing mutations in both parC and gyrA, the most common substitutions were at positions 79 (Ser-79) of parC and at 81 (Ser-81) of gyrA (191 isolates), followed by substitutions at positions 79 (Ser-79) of parC and 85 (Glu-85) of gyrA (39 isolates) and substitutions at positions 83 (Asn-83) of parC and 81 (Ser-81) of gyrA (16 isolates).
Previous studies and our prior analysis of mutations in these isolates have shown that substitutions at positions 78, 79, 83, 91, 115, and 129 of parC and substitutions at positions 81 and 85 of gyrA are associated with reduced fluoroquinolone susceptibility while other mutations in these QRDRs do not affect fluoroquinolone susceptibility (13, 21, 23). Therefore, we considered only those isolates with mutations affecting fluoroquinolone susceptibility in our analysis of mutations potentially selected for by the use of fluoroquinolone antibiotics. We identified mutations affecting fluoroquinolone susceptibility in gyrA alone in none of 3,343 isolates with a moxifloxacin MIC of <0.25 μg/ml, 8 of 883 (0.9%) isolates with a moxifloxacin MIC of 0.25 μg/ml, and 18 of 49 (37%) isolates with a moxifloxacin MIC of ≥0.5 μg/ml (P < 0.0001). Similarly, isolates with mutations affecting fluoroquinolone susceptibility in both gyrA and parC were identified in none of 4,067 isolates with a ciprofloxacin MIC of <4 μg/ml, 2 of 143 (1.4%) isolates with a ciprofloxacin MIC of 4 μg/ml, 18 of 60 (30%) of isolates with a ciprofloxacin MIC of 8 μg/ml, and 234 of 255 (92%) isolates with a ciprofloxacin MIC of ≥8 μg/ml (P < 0.0001). The prevalence of isolates with mutations in only gyrA increased from 0.05% (4 of 7,968) in 1998 to 2001 to 0.08% (8 of 10,157) in 2002 to 2005 to 0.32% (14 of 4,348) in 2006 to 2007 (P < 0.0001). Similarly, the prevalence of isolates with mutations in both gyrA and parC increased from 0.68% (54 of 7,968) in 1998 to 2001 to 1.5% (147 of 10,157) in 2002 to 2006 and then remained stable in 2006 to 2007 (53 of 4,348, or 1.2%) (P < 0.0001). However, in multivariable analysis adjusted for the age of the patient, the source of the specimen, and whether the isolate was a serotype included in the 7-valent conjugate vaccine, there was no increase in the prevalence of mutations over the decade of surveillance.
The first pneumococcal isolate with a mutation in the QRDR of gyrA only was identified in 2000. The 26 isolates were recovered from specimens from adults (median age, 62 years; range, 29 to 89 years) from 19 different laboratories in five provinces. Eighteen specimens were sputum, four were blood cultures, two were bronchoscopy specimens, and one each was from an ear swab and pleural fluid. Fourteen different serotypes were identified among the 26 isolates.
DISCUSSION
Antibiotics continue to be an important weapon in the health care armamentarium. Their major weaknesses are serious adverse events—such as Clostridium difficile-associated disease and tendon rupture for fluoroquinolones—and the fact that their use selects for antibiotic resistance in both the microorganisms they are intended to treat and other microorganisms that are incidentally exposed. Developing new antibiotics and minimizing the use of currently available antibiotics are the two most important strategies for combating the threat of antibiotic resistance. However, both of these strategies have proven difficult to implement. Thus, it is also important to understand whether differences in how antibiotics are used can increase or decrease selective pressure. The introduction of ciprofloxacin was associated with the rapid emergence of ciprofloxacin resistance in pneumococci in Canada and around the world (2, 6, 19, 27), and the volume of fluoroquinolone use has been shown to be strongly associated with resistance. When newer fluoroquinolones more active against pneumococci than ciprofloxacin were introduced in the late 1990s, it was hypothesized that their use would reduce selective pressure for resistance in pneumococci. Our surveillance demonstrates that, despite overall increases in the total use of ciprofloxacin and the added use of newer fluoroquinolones for the treatment of RTIs, fluoroquinolone resistance in pneumococci has not increased.
Two reasons were postulated to explain why using newer fluoroquinolones might reduce selective pressure for resistance in pneumococci. The parameter that best predicts the efficacy of the fluoroquinolones in eradicating pneumococci is the ratio of the area under the concentration-time curve (AUC) to the MIC. In vitro and in vivo studies and clinical trials have determined that maintenance of an unbound AUC/MIC ratio of 30 to 40 maximizes the bactericidal efficacy of fluoroquinolones against S. pneumoniae and minimizes the selection of resistant isolates during therapy (2). Ciprofloxacin at doses used for therapy never achieves this target, levofloxacin at a dose of 500 mg once a day will just achieve it, and moxifloxacin and gatifloxacin used at recommended daily doses will achieve AUC/MIC ratios well above it (19, 27, 28). The inherent activity of levofloxacin, gatifloxacin, and moxifloxacin against pneumococci may thus minimize the emergence of resistance during therapy and, thus, the broader selection of resistance in a population (14).
Another putative benefit of the newer fluoroquinolones gatifloxacin and moxifloxacin in reducing selective pressure compared to ciprofloxacin is their affinity for both the GyrA and ParC enzymes such that wild-type S. pneumoniae needs to acquire resistance mutations in both genes to survive at drug concentrations achieved during therapy. Since independent mutations generally arise once per 107 cell divisions or less, the likelihood that relevant mutations in both parC and gyrA genes will occur in the same strain is about once in 1014 cell divisions (14), which is unlikely to occur during therapy infection. Using gatifloxacin or moxifloxacin to treat a pneumococcal infection may thus be analogous to using combined antimicrobial therapy to reduce the likelihood of the emergence of resistance in the treatment of Mycobacterium tuberculosis and HIV infections (22).
Our surveillance for mutations associated with fluoroquinolone resistance mutations over time supports the hypothesis that, over time, the selective pressure has changed from ciprofloxacin to more active fluoroquinolones. Ciprofloxacin and levofloxacin preferentially target ParC, whereas moxifloxacin and gatifloxacin target both ParC and GyrA (15). Prior to the introduction of gatifloxacin and moxifloxacin, all isolates with mutations observed in the QRDRs of S. pneumoniae had mutations in the parC gene (3, 10). Since the introduction of gatifloxacin and moxifloxacin, the number of isolates with parC mutations has remained stable, but there has been an increase in the proportion of isolates with gyrA-only mutations (12, 13, 24).
Introduction of the 7-valent conjugate pneumococcal vaccine, which includes serotypes that are often associated with nonsusceptibility to penicillin and other antimicrobial drugs, has resulted in changes in the epidemiology of pneumococcal disease including the proportion of antimicrobial-resistant strains causing invasive pneumococcal disease (17, 20). Although strains of serotypes included in the 7-valent vaccine were somewhat more likely to be resistant to fluoroquinolones than other strains, we found that the introduction of the 7-valent conjugate pneumococcal vaccine in Canada did not have an impact on the prevalence of fluoroquinolone resistance in pneumococci.
In summary, our data suggest that the introduction of more active fluoroquinolones with approximately equal affinity to two different binding sites has resulted in a significant reduction in selective pressure for fluoroquinolone resistance in S. pneumoniae. Although volume of antibiotic use is the most important determinant of antimicrobial resistance, there are some circumstances in which the selection of particular antimicrobials within a class may also be important, and recommendations for antimicrobial use should take these effects into account. In the case of pneumococcal resistance to fluoroquinolones, the change to new antimicrobial agents may have helped to control the emergence fluoroquinolone-resistant S. pneumoniae.
ACKNOWLEDGMENTS
We are indebted to all of the hospitals and laboratories participating in the Canadian Bacterial Surveillance Network.
This study was funded through the National Centre of Excellence in Bacterial Diseases and by an unrestricted educational grant from Bayer Healthcare AG.
S.N.P., R.M., D.R.P., and K.G. have no conflicts. A.M. has received speaker fees from and served on advisory boards for Bayer, Inc., and Wyeth Pharmaceuticals (Wyeth Pharmaceuticals is now a fully owned subsidiary of Pfizer Inc.). She has also received unrestricted investigator initiated grants from Bayer Healthcare AG and Wyeth Pharmaceuticals. G.J.T. has served on the speaker's bureau and advisory board of Wyeth Pharmaceuticals and GlaxoSmithKline, Inc., and has received an unrestricted investigator originated grant from Wyeth Pharmaceuticals. D.E.L. has been a member of Advanced Life Sciences, Boehringer Ingelheim USA Corporation, Pfizer, Bayer, Abbott, and Wyeth advisory board committees. He has also consulted with MPM Asset management LLC and has received research support from Advanced Life Sciences, Cerexa, Inc., GlaxoSmithKline, Inc., Wyeth Pharmaceuticals, and Bayer Healthcare AG. D.E.L. has received speaker fees from Pfizer, Inc., Bayer Healthcare, Wyeth Pharmaceuticals, Schering Plough, Abbott Laboratories, and Novartis.
The other members of the Canadian Bacterial Surveillance Network and their participating laboratories are as follows: S. Porter-Pong, B. Willey and A. Plevneshi, Mount Sinai Hospital, Toronto, Ontario; K. Weiss, Hopital Maisonneuve-Rosemont, Montreal, Quebec; G. Zhanel and D. Hoban, Health Sciences Center, Winnipeg, Manitoba; M. Kuhn, Southeast Healthcare Corporation-Moncton Site, Moncton, New Brunswick; D. Church, Calgary Laboratory Services, Calgary, Alberta; R. Davidson and K. Forward, QEII Elizabeth Health Sciences Center, Halifax, Nova Scotia; A. Simor and M. Vearnecombe, Sunnybrook Health Sciences Center and Women's College Hospital, Toronto, Ontario; H. R. Devlin and M. Muller, St. Michael's Hospital, Toronto, Ontario; Y. Hussein, Cape Breton Regional Health Care Complex, Sydney, Nova Scotia; P. C. Kibsey, Victoria General Hospital, Victoria, British Columbia; J. Blondeau, Royal University Hospital, Saskatoon, Saskatchewan; G. K. Harding, St. Boniface General Hospital, Winnipeg, Manitoba; L. Thibault, Dr. Georges L. Dumont Hopital, Moncton, New Brunswick; F. Smaill and M. Loeb, Hamilton Health Sciences Corporation, Chedoke-McMaster, Hamilton, Ontario; M. Gourdeau, Hopital de l'Enfant-Jesus, Quebec City, Quebec; S. Richardson, Hospital for Sick Children, Toronto, Ontario; G. J. Hardy, Saint John Regional Hospital, Saint John, New Brunswick; P. R. Laberge, Centre Hospitalier Regional de Sept-Iles, Sept-Iles, Quebec; L. Ang, Queen Elizabeth Hospital, Charlottetown, Prince Edward Island; M. Yorke, Westman Regional Laboratory, Brandon, Manitoba; D. Richardson, William Osler Health Center, Brampton, Ontario; J. Downey and P. Da Camara, Toronto East General and Orthopedic Hospital, Inc., Toronto, Ontario; M. Bergeron, Centre Hospitalier de l'Université Laval, Sainte-Foy, Québec; J. Hutchinson, Health Care Corp., St. John's, Newfoundland; S. Krajden, St. Joseph's Health Care Center, Toronto, Ontario; R. Price, Royal Victoria Hospital, Barrie, Ontario; J. F. Paradis, Hopital de Chicoutimi, Chicoutimi, Quebec; L. Bocci, Regional Hospital Centre, Bathurst, New Brunswick; M. Savard, David Thompson Regional Laboratories, Red Deer, Alberta; K. Ostrowska, Trillium Health Centre, Mississauga, Ontario; K. Katz and B. Mederski, North York General Hospital, Toronto, Ontario; C. Tremblay, Hotel Dieu de Quebec, Quebec City, Quebec; L. Abbott, Dr. Everett Chalmers Hospital, Fredericton, New Brunswick; D. Yamamura, Lifelabs, Toronto, Ontario, and D. Noria, A. Gelbloom, and D. Rose, The Scarborough Hospital, Scarborough, Ontario; D. Chen, York Central Hospital, Richmond Hill, and Markham Stouffville Hospital, Markham, Ontario; B. Boaretto, Southlake Regional Health Center, Newmarket, Ontario; I. Davis and I. Kitai, Rouge Valley Health System, Scarborough and Ajax, Ontario; P. Garrod and N. Rau, Halton Health Services, Hamilton, Ontario; M. Silverman, Lakeridge Health, Oshawa, Ontario; A. Sarabia, Credit Valley Hospital, Missisauga, Ontario; K. S. Lee and M. Baqi, Humber River Regional Hospital, Toronto, Ontario; P. Shokry, Markham Stouffville Hospital, Markham, Ontario; M. Lovgren, National Center for Streptococcus, Edmonton, Alberta; A. Haworth, Joseph Brant Memorial Hospital, Burlington, Ontario; and S. Walmsley, University Health Network, Toronto, Ontario, Canada.
Footnotes
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Adam H. J., Hoban D. J., Gin A. S., Zhanel G. G. 2009. Association between fluoroquinolone usage and a dramatic rise in ciprofloxacin-resistant Streptococcus pneumoniae in Canada, 1997–2006. Int. J. Antimicrob. Agents 34:82–85 [DOI] [PubMed] [Google Scholar]
- 2. Ambrose P. G., et al. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bast D. J., et al. 2000. Fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae: contributions of type II topoisomerase mutations and efflux to levels of resistance. Antimicrob. Agents Chemother. 44:3049–3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhavnani S. M., Hammel J. P., Jones R. N., Ambrose P. G. 2005. Relationship between increased levofloxacin use and decreased susceptibility of Streptococcus pneumoniae in the United States. Diagn. Microbiol. Infect. Dis. 51:31–37 [DOI] [PubMed] [Google Scholar]
- 5. Canton R., Morosini M., Enright M. C., Morrissey I. 2003. Worldwide incidence, molecular epidemiology and mutations implicated in fluoroquinolone-resistant Streptococcus pneumoniae: data from the global PROTEKT surveillance programme. J. Antimicrob. Chemother. 52:944–952 [DOI] [PubMed] [Google Scholar]
- 6. Chen D. K., McGeer A., de Azavedo J. C., Low D. E. 1999. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N. Engl. J. Med. 341:233–239 [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A, 7th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2007. Performance standards for antimicrobial susceptibility testing. Supplement M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Critchley I. A., et al. 2002. Phenotypic and genotypic analysis of levofloxacin-resistant clinical isolates of Streptococcus pneumoniae collected in 13 countries during 1999–2000. Int. J. Antimicrob. Agents 20:100–107 [DOI] [PubMed] [Google Scholar]
- 10. Davies T. A., et al. 2002. Prevalence of single mutations in topoisomerase type II genes among levofloxacin-susceptible clinical strains of Streptococcus pneumoniae isolated in the United States in 1992 to 1996 and 1999 to 2000. Antimicrob. Agents Chemother. 46:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davies T. A., et al. 2006. Infrequent occurrence of single mutations in topoisomerase IV and DNA gyrase genes among US levofloxacin-susceptible clinical isolates of Streptococcus pneumoniae from nine institutions (1999–2003). J. Antimicrob. Chemother. 57:437–442 [DOI] [PubMed] [Google Scholar]
- 12. de la Campa A. G., et al. 2009. Changes in fluoroquinolone-resistant Streptococcus pneumoniae after 7-valent conjugate vaccination, Spain. Emerg. Infect. Dis. 15:905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de la Campa A. G., et al. 2004. Fluoroquinolone resistance in penicillin-resistant Streptococcus pneumoniae clones, Spain. Emerg. Infect. Dis. 10:1751–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Vecchi E., Nicola L., Ossola F., Drago L. 2009. In vitro selection of resistance in Streptococcus pneumoniae at in vivo fluoroquinolone concentrations. J. Antimicrob. Chemother. 63:721–727 [DOI] [PubMed] [Google Scholar]
- 15. Ferrara A. M. 2005. New fluoroquinolones in lower respiratory tract infections and emerging patterns of pneumococcal resistance. Infection 33:106–114 [DOI] [PubMed] [Google Scholar]
- 16. Ho P. L., et al. 2001. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J. Antimicrob. Chemother. 48:659–665 [DOI] [PubMed] [Google Scholar]
- 17. Karnezis T. T., Smith A., Whittier S., Haddad J., Saiman L. 2009. Antimicrobial resistance among isolates causing invasive pneumococcal disease before and after licensure of heptavalent conjugate pneumococcal vaccine. PLoS One 4:e5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lovgren M., Spika J. S., Talbot J. A. 1998. Invasive Streptococcus pneumoniae infections: serotype distribution and antimicrobial resistance in Canada, 1992–1995. CMAJ 158:327–331 [PMC free article] [PubMed] [Google Scholar]
- 19. Low D. E. 2005. Fluoroquinolone-resistant pneumococci: maybe resistance isn't futile? Clin. Infect. Dis. 40:236–238 [DOI] [PubMed] [Google Scholar]
- 20. Messina A. F., et al. 2007. Impact of the pneumococcal conjugate vaccine on serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Dallas, TX, children from 1999 through 2005. Pediatr. Infect. Dis. J. 26:461–467 [DOI] [PubMed] [Google Scholar]
- 21. Munoz R., De La Campa A. G. 1996. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob. Agents Chemother. 40:2252–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palumbi S. R. 2001. Humans as the world's greatest evolutionary force. Science 293:1786–1790 [DOI] [PubMed] [Google Scholar]
- 23. Patel S. N., Melano R., McGeer A., Green K., Low D. E. 2010. Characterization of the quinolone resistant determining regions in clinical isolates of pneumococci collected in Canada. Ann. Clin. Microbiol. Antimicrob. 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pletz M. W., et al. 2006. Fluoroquinolone resistance in invasive Streptococcus pyogenes isolates due to spontaneous mutation and horizontal gene transfer. Antimicrob. Agents Chemother. 50:943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Powis J., et al. 2004. In vitro antimicrobial susceptibilities of Streptococcus pneumoniae clinical isolates obtained in Canada in 2002. Antimicrob. Agents Chemother. 48:3305–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richter S. S., et al. 2002. The molecular epidemiology of penicillin-resistant Streptococcus pneumoniae in the United States, 1994–2000. Clin. Infect. Dis. 34:330–339 [DOI] [PubMed] [Google Scholar]
- 27. Shams W. E., Evans M. E. 2005. Guide to selection of fluoroquinolones in patients with lower respiratory tract infections. Drugs 65:949–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y. C., Lipsitch M. 2006. Upgrading antibiotic use within a class: tradeoff between resistance and treatment success. Proc. Natl. Acad. Sci. U. S. A. 103:9655–9660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhanel G. G., et al. 2002. A critical review of the fluoroquinolones: focus on respiratory infections. Drugs 62:13–59 [DOI] [PubMed] [Google Scholar]