Abstract
Most surveys for class 1 integrons are at least partly predicated on PCR screening that targets integron conserved regions. However, class 1 integrons are structurally diverse, so dependence on conserved regions may lead to missing clinically relevant examples of class 1 integrons. Here, we surveyed a commensal population of bacteria from patients in an intensive care unit to identify class 1 integrons irrespective of their structure or genetic context. We identified several examples of class 1 integrons linked to complete Tn402-like or Tn402 hybrid transposition modules and diverse insertion points with respect to the inverted repeat IRi boundary. The diversity and abundance of class 1 integrons identified are such that many novel elements seen here would not have been identified by commonly used methods, and they revealed an additional level of complexity.
TEXT
Class 1 integrons contribute substantially to the spread of antibiotic resistance determinants in Gram-negative pathogens (22, 24). The use of PCR methodologies that allow the rapid recovery of cassette arrays even in the absence of information about actual resistance gene content has led to a great number of reports on the diversity of class 1 integrons based on the gene cassette(s) they carry (14). Approaches of this type have helped in identifying the resistance genes carried by class 1 integrons and the phylogenetic and geographical distribution of such genes once captured. Consequently, these approaches represent a useful collective tool for the identification of new resistance genes as they are recruited into the class 1 integron gene pool (22). However, fewer studies have investigated the genetic context in which class 1 integrons are found. Since class 1 integrons are not self-mobilizable, they are dependent on other elements (e.g., transposons and plasmids) to be disseminated. Thus, as well as recovering cassette arrays, investigating their genetic context is important in understanding how resistance is spread into and between pathogenic bacteria.
Approaches that reveal class 1 integron contexts include studies that investigate whole genomes (1) and whole mobile DNA regions, such as plasmids (30) and pathogenicity islands (6, 7). Other studies may be more focused and target class 1 integrons and their context directly in subsets of isolates selected based on criteria such as location (18), presence of specific resistance genes (8), and source species. These studies collectively reveal remarkable gene context complexity, with rearrangements being driven by a variety of processes, including transposition, homologous recombination, and site-specific recombination (18, 21). These studies emphasize that interplay between mobile genetic elements such as conjugative plasmids, transposons, and integrons has collectively spread resistance genes through disparate bacteria. This cooperative approach often leads to rearrangements of resistance genes in new hosts and creates difficulties in therapeutic management.
The structural elements that make up class 1 integrons include the inverted repeats IRi and IRt along with sequences between them, including the 5′-conserved segment (5′-CS) (intI1 and attI1), gene cassette(s), and the 3′-conserved segment (3′-CS) that includes qacEΔ1 and sul1 (9, 28). This structure represents the most common found in clinical isolates and is presumed to have evolved from an ancestor that had a complete Tn402-like transposition module (2, 12). It was subsequent evolution that led to the acquisition of the 3′-CS and the loss of some tni genes. Most clinical class 1 integrons, therefore, are defective transposons, although transposition may be possible if these functions are provided in trans (Fig. 1) (2, 9). Within this basic structure, other rearrangements have also occurred. For example, it is becoming clear that IS26 mediates other changes to the class 1 integron/transposon structure that includes loss of parts of the 3′-CS (7) and 5′-CS (19). Further, class 1 integrons with a complete tni module and no 3′-CS are being found with increasing frequency (11, 13, 17). Consequently, it is likely that the common approaches to studying class 1 integron structure and context driven by PCR targeting of the 3′-CS (14) will underestimate the structural diversity of class 1 integrons and bias the recovery of class 1 types. Here, we used a combination of genomic library construction and PCR methods to survey the genetic context of class 1 integrons recovered from commensal bacteria. Also, atypical class 1 integron structure was detected by testing for the presence or absence of common features shared by clinical class 1 integrons. Considerable diversity and multiple examples of class 1 integrons, including those with functional Tn402-like transposition modules, were revealed.
Fig. 1.
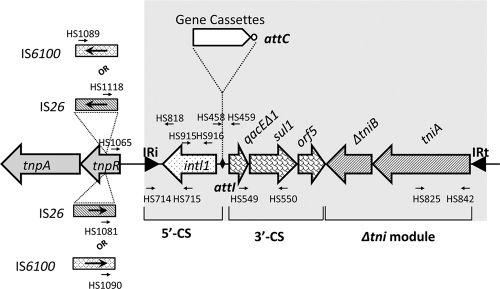
Schematic representation of a Tn402-like integron inserted into a Tn21 subgroup transposon backbone. The IRi junction PCR assay using primer pair HS1065/HS818 amplifies the region existing in this kind of recombined structure. The shaded box shows the class 1 integron bounded within two inverted repeats, IRi and IRt, and its basic structural features (5′-CS, 3′-CS, potential gene cassette[s], and tni module), together with positions of specific primer pairs used to detect each feature. To detect the presence of the IS26 element, which could be inserted in either orientation as shown, two separate PCRs were carried out per isolate, with primer pairs HS1081/HS916 and HS1118/HS916. Similarly, two specific primer pairs were used to detect the presence of IS6100.
Bacterial strains were obtained from random patients admitted to a hospital intensive care unit (ICU) in Sydney, New South Wales, Australia, in the period from April 2004 to July 2005. These mixed cultures were enriched after recovery from perineal swabs and endotracheal aspirates. A 0.1-ml volume of stock was subcultured in 5 ml of Lennox broth overnight at 37°C in the absence of antibiotic selection. From 183 mixed population enrichments, 38 were positive for class 1 integrons in the community sample by PCR with intI1-specific primers. Individual clones were isolated from these by plating on both Luria-Bertani agar and MacConkey agar and growing at 37°C in the absence of antibiotic selection. Diverse morphologies were periodically picked from plates over 120 h and screened by PCR for intl1 by using the primers HS915 and HS916 (see Table S1 in the supplemental material). From the 588 colonies screened, 34 independent intI1 isolates were identified for further study.
The ability of the Tn402-like transposition system to target res sites (12) has led to the spread of class 1 integrons to a range of different transposons and plasmids. Most common among these in clinical isolates is the coassociation between class 1 integrons and members of the Tn21 family of transposons (16, 21). For this reason, all intI1-positive isolates were further screened by PCR using a degenerate primer designed to bind within the tnpR genes of different Tn21-like transposons (HS1065) coupled with one that targets the 5′-CS of the integron (HS818) (Fig. 1). The region surrounding and including the insertion point of an integron to a Tn21-like transposon can be retrieved in this way. Similar strategies have been used before (3, 8), but HS1065 is likely to detect more members of the Tn21 subgroup. Results of these PCRs are summarized in the supplemental material (see Table S2).
Nine of the 34 intI1-positive isolates failed to generate any amplicons with HS1065 and HS818. However, a total of 36 amplicons were obtained from the remaining 25 intI1-positive isolates. Twenty isolates generated a single amplicon, and five generated multiple products, implying multiple class 1 integrons in different genetic contexts. The cohort of five isolates collectively yielded 16 amplicons, with two isolates, WM77a05 and WM82a10, generating four amplicons (see Table S2 in the supplemental material). At least five different-sized PCR products were observed, and subsequent sequencing of all 36 amplicons revealed a total of eight distinct genetic contexts (Table 1). Seven corresponded to insertions in Tn21-like transposons at exactly the same positions as seen before, including in Tn21 itself or a hybrid of Tn21 and another transposon, reinforcing the important role of this transposon in disseminating class 1 integrons (16). One of these, the Tn1721-like context (Table 1), identified in four isolates, corresponded to insertion in the Tn21-like transposon Tn1721 at a novel position, representing a third lineage of this Tn1721-class 1 integron association (see Fig. S1 in the supplemental material). The eighth genetic context, detected in two isolates, was a nontransposon type and could be traced back to a plasmid res region (32). Together with the nine isolates from which genetic contexts were not retrieved by the Tn21-specific primer HS1065, about one-third of integrons in this population are positioned in genetic elements additional to Tn21 and other previously identified relatives.
Table 1.
Genetic contexts detected at the IRi boundary of the 36 amplicons obtained from PCR by using the primer pair HS1065/HS818
| Genetic contexta | No. of occurrences | Amplicon size (bp) | Accession no. | Position (nt)b |
|---|---|---|---|---|
| Tn1696 | 4 | 351 | U12338.3 | 3506–3856 |
| Tn1721-likec | 4 | 398 | HQ730118 | 3504–3901 |
| Tn1403 | 3 | 420 | AF313472 | 3504–3923 |
| Tn21 | 7 | 724 | AF071413.3 | 3503–4226 |
| Tn5051-Tn21 hybrid | 4 | 730 | CP000603 | 156570–155841 |
| Tn1721-Tn21 hybrid | 7 | 774 | AB207867.1 | 3504–4277 |
| Tn6005 | 5 | 345 | EU591509 | 9022–9366 |
| Nontransposon | 2 | 1,054 | EF219134.2 | 10281–11330 |
Named transposons comprise distinct but related transposition modules and therefore distinct transposon/integron lineages.
The nucleotide numbers specify the equivalent regions in the cited GenBank entry.
The integron is inserted in a Tn1721-like transposon backbone, but the insertion point is different from that of Tn1721. See the text and also Fig. S1 in the supplemental material.
The insertion sequences (ISs) IS26 and IS6100 can be inserted in or near class 1 integrons (3, 5, 15, 29), and this can prevent amplification of the IRi boundary by using a primer analogous to HS1065 (18). Therefore, all 34 isolates were tested for the presence of these two ISs beyond IRi by using HS916 and different IS-specific primers (Fig. 1; see Table S1 in the supplemental material). Eight isolates with IS26 beyond IRi and one with IS6100 (see Table S2) were identified. Five of these nine represented isolates that had other class 1 integrons present, and two, WM21a40 and WM146a05, were from the cohort of nine that had formerly not generated an IRi boundary PCR product. In these two isolates, IS26 was inserted 497 bases beyond the end of IRi in a Tn21 backbone, and the sequences were identical from the IS26 element through to the end of the sul1 gene in the 3′-CS. These two isolates were collected from two different patients nearly 6 months apart. The remaining six IS26-containing structures were relatively varied in their architecture. WM178a02 comprised a Tn21 backbone with IS26 inserted 644 bases beyond IRi, while in WM31a01, IS26 was inserted 656 bases beyond IRi in a Tn21/Tn1721 hybrid backbone, with the hybrid junction being found in the res site when the sequence is compared to the two presumed parents.
In three isolates, WM77a05, WM128a13, and WM48a45, IS26 truncated the intI1 gene at a point 654 bases beyond the start of the intI1 gene, as seen previously (31). Two isolates were Escherichia coli isolates, and the third, WM48a45, was a Klebsiella sp. This IS26 analysis brings the total number of integrons detected in WM77a05 to five. In WM98a10, IS6100 had deleted part of the 5′-CS, 348 bases beyond the start of intI1, and additional PCR mapping identified a second copy of IS6100 beyond the dfrA14 cassette, as seen elsewhere (4, 20). Of the 34 intI1-positive isolates, seven failed to generate an IRi boundary amplicon by using primers that target Tn21-like tnpR genes, and two of these, WM5a20 and WM7a34, were devoid of any identifiable clinical class 1 integron feature beyond the presence of the intI1 gene itself (see Table S2 in the supplemental material). It is possible that these may represent class 1 integrons more typical of those in nonclinical bacteria that are not linked to Tn402 or relatives at all.
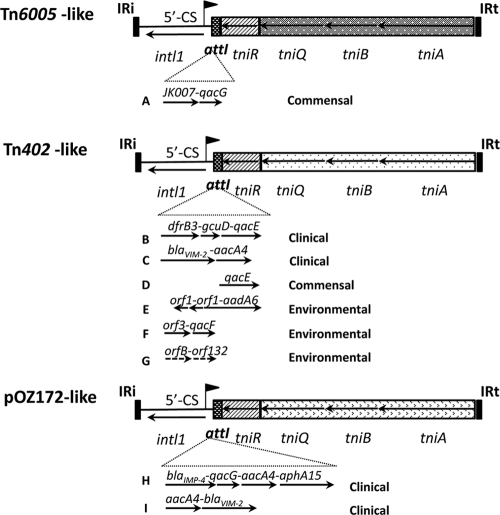
To further assess the diversity of class 1 integron types in this sampled population, all 34 intI1-positive isolates were screened by PCR for the 3′-CS by using the primers HS549 and HS550 (Fig. 1; see Table S1 in the supplemental material), for a Tn402-like tniA gene (HS825 and HS842) and for gene cassette arrays (HS458 and HS459). Nine isolates generated a product with all three primer sets; however, the majority, 25 isolates, failed to generate a product with at least one of the three (see Table S1). Nine isolates failed to generate a product with all three primer sets. To investigate the structure of some of these atypical class 1 integrons, two that gave a single amplicon in the HS1065/HS818 PCR, WM88a32 and WM2b02, were selected for construction of fosmid libraries (18). WM88a32 was one of five isolates that had an IRi boundary identical to that of Tn6005 (13) and failed to generate either sul1 or tniA amplicons. These PCR results were consistent with data for Tn6005, since this transposon carries a functional Tn402-like hybrid tni module, but its tniA gene differs from that of Tn402, and neither HS825 nor HS842 binds to the Tn6005 tniA variant. Extensive sequencing of a fosmid clone revealed that WM88a32 contains an integron identical to the originally identified Tn6005 transposon (Fig. 2). Thus, WM88a32 is now the second example of this variant class 1 integron/transposon for which the Tn402-like tni module is a hybrid compared to the tni module seen in most class integrons, including Tn402 itself (13). The integron/transposon inserted into the Tn5036 mercury resistance transposon, Tn6006 (13), to create Tn6005 is 13,731 bp in length, and the element in WM88a32 was identical across this entire region (13). Tn6005 is mobilizable between species, since JKB7 is an Enterobacter cloacae isolate, whereas WM88a32 is an Escherichia coli isolate. Four other isolates with the same Tn5036/IRi boundary as in Tn6005 were investigated by targeted PCR using primers specific for the Tn6005 tniA gene and for the JK007 cassette (Fig. 2). WM98a10 and WM82a10 each generated a band the same size as WM88a32, whereas WM174a01 and WM77a05 failed to generate a product. We conclude, therefore, that a Tn6005-like element is present in at least three isolates, but there is also diversity of structure in integrons that possess this Tn6005 IRi boundary.
Fig. 2.
Comparison of class 1 integrons with complete tni modules. Each of the three known tni module types is indicated. The flag symbol indicates the promoter, designated Pc, that transcribes inserted resistance genes. Different cassette arrays that have been identified inserted at attI1 are shown under each type of tni module. The types of sources of the isolates carrying each array are indicated on the right. For all elements recovered in this study, the indicated regions were completely sequenced. A, JKB7 (Enterobacter cloacae) (13) and WM88a32 (Escherichia coli) (this study); B, Tn402 (Enterobacter aerogenes) (23, 27); C, LD209 (Pseudomonas putida) (17); D, WM2b02 (Klebsiella pneumonia) (this study); E, LMCB014 (Comomonas testosteroni) (25); F, DCB015 (Pseudomonas alcaligenes) (25); G, 11BF10 (Pseudomonas aeruginosa) (26); H, pOZ172 (Citrobacter youngae) (10); I, PPV2-2 (Pseudomonas putida) (11).
The Klebsiella pneumoniae isolate WM2b02 represented one of four isolates with the novel IRi/Tn1721-like boundary and gave an amplicon for a Tn402-like tniA but was negative for a 3′-CS (see Table S2 in the supplemental material). Sequencing of an intI1-positive fosmid clone confirmed the presence of a complete tni module that was identical to that of Tn402 and a single qacE gene cassette (Fig. 2). Two of the three additional isolates, WM82a10 and WM98a10, generated a PCR product with primers linking the tniA and qacE genes (HS842/HS549; see Table S1 in the supplemental material) of a length consistent with a complete tni module but no 3′-CS. The third, WM77a05, failed to generate a product. We infer that the integron in WM77a05 is not identical to the others that have this IRi/Tn1721-like boundary. With the Tn1721 and Tn6005 integron data taken together, it is clear that functional integrons/transposons are well established in the commensal population. Collectively, they were recovered from at least four different patients and are mobile, since the four isolates with functional transposition modules (WM82a10, WM98a10, WM88a32, and WM2b02) include examples of E. coli, E. cloacae, and K. pneumoniae. Apart from the examples reported here in a commensal population, other recent examples with class 1 integrons linked to functional Tn402 modules include bacteria from sediment and freshwater (25) as well as marine environments (26). In addition, examples in a clinical context exist. These include the Tn402 exemplar and the integron/transposon in the Pseudomonas putida strain LD209 (17), both of which possess a Tn402-like transposition module, and those with a pOZ172-like hybrid transposition, pOZ172, and the integron in PPV2-2 (11). In the last two cases, the hybrid module is different from that seen in Tn6005 (Fig. 2). Collectively, these functional integrons/transposons demonstrate dynamic exchange of cassettes, given the diverse combinations of cassettes observed (Fig. 2).
There are at least type two types of tni variants, the Tn6005 type (89% identity with Tn402 in the tniQBA genes) and the type seen in plasmid pOZ172 (10) and the plasmid from PPV2-2 (11) (84% identity with Tn402 in the tniQBA genes). In pOZ172 and the PPV2-2 plasmid, the tniQBA genes are identical to the equivalent genes in the transposon Tn5053 (12). In both hybrid types, tniR is identical to the tniR of Tn402, with divergence occurring at the res site, implying that the hybrids are derived by site-specific resolution of two modules. We have two independent examples (WM82a10 and WM98a10) in our sampled population of strains carrying two functional transposon module types (i.e., Tn402-like and Tn6005-like). Thus, these resolution events may be more common than realized.
This study reinforces that the recovery of class 1 cassette arrays that involves the targeting of conserved segments greatly underestimates integron abundance and structural diversity. Also, these elements with complete transposition modules may represent an important link in moving new and existing resistance genes from the general environment into pathogens, given that they are common and coincident in strains with the type of class integrons that commonly carry multiple resistance cassettes. Thus, more detailed analysis of the mobile DNA content of resistance pathogens and the commensals they interact with may assist in identifying new important resistance genes before they add to the burden of clinical resistance.
Nucleotide sequence accession numbers.
The sequences of fosmid clones WM2b02, WM98a10, and WM31a01 are lodged in GenBank under accession numbers HQ730118, HQ730119, and HQ730120, respectively.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia (grant number 543402).
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 31 May 2011.
REFERENCES
- 1. Adams M. D., et al. 2008. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J. Bacteriol. 190:8053–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown H., Stokes H., Hall R. 1996. The integrons In0, In2, and In5 are defective transposon derivatives. J. Bacteriol. 178:4429–4437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cain A. K., Liu X., Djordjevic S. P., Hall R. M. 2010. Transposons related to Tn1696 in IncHI2 plasmids in multiply antibiotic resistant Salmonella enterica serovar Typhimurium from Australian animals. Microb. Drug Resist. 16:197–202 [DOI] [PubMed] [Google Scholar]
- 4. Carattoli A., et al. 2010. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the U. S. A. J. Antimicrob. Chemother. 65:2070–2075 [DOI] [PubMed] [Google Scholar]
- 5. Dawes F. E., et al. 2010. Distribution of class 1 integrons with IS26-mediated deletions in their 3′-conserved segments in Escherichia coli of human and animal origin. PLoS One 5:e12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Djordjevic S. P., et al. 2009. Emergence and evolution of multiply antibiotic-resistant Salmonella enterica serovar Paratyphi B D-tartrate-utilizing strains containing SGI1. Antimicrob. Agents Chemother. 53:2319–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doublet B., Praud K., Weill F.-X., Cloeckaert A. 2009. Association of IS26-composite transposons and complex In4-type integrons generates novel multidrug resistance loci in Salmonella genomic island 1. J. Antimicrob. Chemother. 63:282–289 [DOI] [PubMed] [Google Scholar]
- 8. Espedido B. A., Partridge S. R., Iredell J. R. 2008. blaIMP-4 in different genetic contexts in Enterobacteriaceae isolates from Australia. Antimicrob. Agents Chemother. 52:2984–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall R. M., Brown H. J., Brookes D. E., Stokes H. W. 1994. Integrons found in different locations have identical 5′ ends but variable 3′ ends. J. Bacteriol. 176:6286–6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawkey P. M., Xiong J., Ye H., Li H., M'Zali F. H. 2001. Occurrence of a new metallo-β-lactamase IMP-4 carried on a conjugative plasmid in Citrobacter youngae from the People's Republic of China. FEMS Microbiol. Lett. 194:53–57 [DOI] [PubMed] [Google Scholar]
- 11. Juan C., et al. 2010. Metallo-beta-lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas aeruginosa clones. J. Antimicrob. Chemother. 65:474–478 [DOI] [PubMed] [Google Scholar]
- 12. Kholodii G. Y., et al. 1995. Four genes, two ends, and a res region are involved in transposition of Tn5053: a paradigm for a novel family of transposons carrying either a mer operon or an integron. Mol. Microbiol. 17:1189–1200 [DOI] [PubMed] [Google Scholar]
- 13. Labbate M., Chowdhury P. R., Stokes H. W. 2008. A class 1 integron present in a human commensal has a hybrid transposition module compared to Tn402: evidence of interaction with mobile DNA from natural environments. J. Bacteriol. 190:5318–5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lévesque C., Piche L., Larose C., Roy P. H. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levings R. S., Partridge S. R., Djordjevic S. P., Hall R. M. 2007. SGI1-K, a variant of the SGI1 genomic island carrying a mercury resistance region, in Salmonella enterica serovar Kentucky. Antimicrob. Agents Chemother. 51:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liebert C., Hall R., Summers A. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marchiaro P., et al. 2010. First report of a Tn402-like class 1 integron carrying blaVIM-2 in Pseudomonas putida from Argentina. J. Infect. Devel. Count. 4:412–416 [PubMed] [Google Scholar]
- 18. Márquez C., et al. 2008. Urinary tract infections in a South American population: dynamic spread of class 1 integrons and multidrug resistance by homologous and site-specific recombination. J. Clin. Microbiol. 46:3417–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miriagou V., Carattoli A., Tzelepi E., Villa L., Tzouvelekis L. S. 2005. IS26-associated In4-type integrons forming multiresistance loci in enterobacterial plasmids. Antimicrob. Agents Chemother. 49:3541–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parkhill J., et al. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848–852 [DOI] [PubMed] [Google Scholar]
- 21. Partridge S. R., Hall R. M. 2004. Complex multiple antibiotic and mercury resistance region derived from the r-det of NR1 (R100). Antimicrob. Agents Chemother. 48:4250–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Partridge S. R., Tsafnat G., Coiera E., Iredell J. R. 2009. Gene cassettes and cassette arrays in mobile resistance integrons. FEMS Microbiol. Rev. 33:757–784 [DOI] [PubMed] [Google Scholar]
- 23. Rådström P., et al. 1994. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J. Bacteriol. 176:3257–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Recchia G. D., Hall R. M. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015–3027 [DOI] [PubMed] [Google Scholar]
- 25. Rosewarne C. P., Pettigrove V., Stokes H. W., Parsons Y. M. 2010. Class 1 integrons in benthic bacterial communities: abundance, association with Tn402-like transposition modules and evidence for coselection with heavy-metal resistance. FEMS Microbiol. Ecol. 72:35–46 [DOI] [PubMed] [Google Scholar]
- 26. Sajjad A., Holley M. P., Labbate M., Stokes H. W., Gillings M. R. 2011. A preclinical class 1 integron with a complete Tn402-like transposition module. Appl. Environ. Microbiol. 77:335–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shapiro J. A., Sporn P. 1977. Tn402: a new transposable element determining trimethoprim resistance that inserts in bacteriophage lambda. J. Bacteriol. 129:1632–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stokes H., Hall R. 1989. A novel family of potentially mobile elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3:1669–1683 [DOI] [PubMed] [Google Scholar]
- 29. Targant H., Doublet B., Aarestrup F. M., Cloeckaert A., Madec J. Y. 2010. IS6100-mediated genetic rearrangement within the complex class 1 integron In104 of the Salmonella genomic island 1. J. Antimicrob. Chemother. 65:1543–1545 [DOI] [PubMed] [Google Scholar]
- 30. Venturini C., Beatson S. A., Djordjevic S. P., Walker M. J. 2010. Multiple antibiotic resistance gene recruitment onto the enterohemorrhagic Escherichia coli virulence plasmid. FASEB J. 24:1160–1166 [DOI] [PubMed] [Google Scholar]
- 31. Yamane K., et al. 2007. New plasmid-mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 51:3354–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zong Z., Partridge S. R., Iredell J. R. 2010. ISEcp1-mediated transposition and homologous recombination can explain the context of blaCTX-M-62 linked to qnrB2. Antimicrob. Agents Chemother. 54:3039–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.