Abstract
We evaluated antimicrobial resistance in Neisseria gonorrhoeae isolated from men enrolled in a randomized trial of male circumcision to prevent HIV. Urethral specimens from men with discharge were cultured for N. gonorrhoeae. MICs were determined by agar dilution. Clinical and Laboratory Standards Institute (CLSI) criteria defined resistance: penicillin, tetracycline, and azithromycin MICs of ≥2.0 μg/ml; a ciprofloxacin MIC of ≥1.0 μg/ml; and a spectinomycin MIC of ≥128.0 μg/ml. Susceptibility to ceftriaxone and cefixime was shown by an MIC of ≤0.25 μg/ml. Additionally, PCR amplification identified mutations in parC and gyrA genes in selected isolates. From 2002 to 2009, 168 N. gonorrhoeae isolates were obtained from 142 men. Plasmid-mediated penicillin resistance was found in 65%, plasmid-mediated tetracycline resistance in 97%, and 11% were ciprofloxacin resistant (quinolone-resistant N. gonorrhoeae [QRNG]). QRNG appeared in November 2007, increasing from 9.5% in 2007 to 50% in 2009. Resistance was not detected for spectinomycin, cefixime, ceftriaxone, or azithromycin, but MICs of cefixime (P = 0.018), ceftriaxone (P < 0.001), and azithromycin (P = 0.097) increased over time. In a random sample of 51 men, gentamicin MICs were as follows: 4 μg/ml (n = 1), 8 μg/ml (n = 49), and 16 μg/ml (n = 1). QRNG increased rapidly and alternative regimens are required for N. gonorrhoeae treatment in this area. Amid emerging multidrug-resistant N. gonorrhoeae, antimicrobial resistance surveillance is essential for effective drug choice. High levels of plasmid-mediated resistance and increasing MICs for cephalosporins suggest that selective pressure from antibiotic use is a strong driver of resistance emergence.
INTRODUCTION
In sub-Saharan Africa, gonorrhea prevalence in the adult population is estimated at 2 to 3%, with incidence rates estimated at 58 cases per 1,000 males and 65 per 1,000 females, the highest among developing countries (37).
Antimicrobial resistance in Neisseria gonorrhoeae has been an ongoing issue since the discovery of plasmid-mediated penicillin resistance (PPNG) in 1976; clinically important chromosomally mediated penicillin and tetracycline resistance emerged in the early 1980s, and plasmid-mediated tetracycline resistance (TRNG) emerged in 1985. Oral fluoroquinolones (quinolones) became the first-line recommended therapy for gonococcal infection in 1993 (6). However, quinolone resistance rapidly developed, initially in the western Pacific (42), and by 2007 became so widely prevalent that the CDC rescinded this recommendation (9). Resistance to previously recommended ciprofloxacin and ofloxacin regimens currently exceeds 40% in some Asian countries (42). Substantial quinolone resistance has been identified only in South Africa, but ongoing systematic data collection and analysis are limited, and there are no surveillance data from Africa similar to the gonococcal surveillance programs in the United States, the western Pacific, Europe, or Australia (37).
We conducted a randomized trial of male circumcision to prevent HIV acquisition in men aged 18 to 24 years old in Kisumu, Kenya (3). The trial took place from 2002 through 2006, with continued follow-up of participants through 2010. Men were routinely tested and treated for ulcerative and nonulcerative sexually transmitted infections (STIs) at 6-month intervals as well as whenever symptomatic episodes occurred. This provided a unique opportunity to study the prevalence and types of antimicrobial resistance for urogenital N. gonorrhoeae isolates in a systematic sample over this time period and to study the emergence of quinolone resistance.
MATERIALS AND METHODS
Study design and participants.
This is an analysis of data collected between February 2002 and July 2009 from men aged 18 to 24 enrolled in a randomized controlled trial of male circumcision to reduce HIV acquisition in Kisumu, Kenya. Details of recruitment and enrollment have been previously described (3). Briefly, for inclusion in the study men had to be uncircumcised, HIV negative, sexually active in the last 12 months, aged 18 to 24 years, have a hemoglobin level of >9.0 mmol/liter, and be resident in Kisumu District with no plans to move away for the duration of follow-up (2 years). Men were excluded if their foreskin covered less than half the glans or if they had hemophilia or another bleeding disorder, a medical condition contraindicating surgery, or an absolute indication for circumcision. This study was approved by the Institutional Review Boards of the University of Illinois at Chicago, the Kenyatta National Hospital, RTI International, and the University of Manitoba.
Data collection.
All consenting participants underwent a standardized medical examination and history and a personal interview in the participant's language of choice (English, Dholuo, or Kiswahili) to obtain sociodemographic and behavioral risk factor data. All exposure variables except age and residence at baseline were taken from the preceding visit closest in time to the culture-positive isolate (see below).
Detection of N. gonorrhoeae.
Participants were asked to provide first-void urine specimens which were tested for N. gonorrhoeae and Chlamydia trachomatis by PCR assay (Amplicor CT/NG test; Roche Diagnostics, Montreal, Canada). Men with urethral discharge on physical examination had a urethral swab taken for PCR testing for N. gonorrhoeae and C. trachomatis and for culturing of N. gonorrhoeae. Urine and urethral swabs were sent to the University of Nairobi Department of Medical Microbiology for PCR testing. All tests were conducted according to the manufacturer's instructions. Culturing was performed in the study lab in Kisumu. Urethral swabs were inoculated directly onto Thayer-Martin chocolate agar and incubated at 35°C in a moist atmosphere containing 5% CO2 for 24 to 48 h. N. gonorrhoeae isolates were identified by colony morphology, Gram stain, and oxidase test and then subcultured on plain chocolate agar for 24 h and frozen at −80°C.
Treatment.
Men presenting with urethritis were presumptively treated with doxycycline at 100 mg twice daily for 7 days and 400 mg of oral norfloxacin as a single dose, which was accordant with the Kenyan national guidelines for treatment of STIs (31). Asymptomatic men testing positive for N. gonorrhoeae or C. trachomatis by PCR were traced and treated with the same regimen. Trichomonas infection was treated with oral metronidazole at 2 g as a single dose. Beginning in February 2009, the presumptive treatment for urethritis changed to doxycycline at 100 mg twice daily for 7 days and 400 mg of cefixime as a single dose, with cefixime also used for the treatment of laboratory-detected N. gonorrhoeae infection. Infected men were given coupons to give to their sex partners for free treatment. However, there was no follow-up of these coupons, and it is unknown how many partners were treated.
Antimicrobial susceptibility testing.
Antimicrobial susceptibilities of N. gonorrhoeae to azithromycin (compliments of Pfizer, Pointe-Claire/Dorval, Québec, Canada), cefixime (compliments of Wyeth-Ayerst Laboratories, Mason, MI), ciprofloxacin (compliments of Bayer, Etobicoke, Ontario, Canada), spectinomycin (compliments of Pharmacia & Upjohn, Kalamazoo, MI), ceftriaxone, erythromycin, penicillin, gentamicin, and tetracycline (Sigma-Aldrich Canada Ltd., Oakville, Ontario, Canada) were determined by the National Microbiology Laboratory, Public Health Agency of Canada (Winnipeg, Canada) using the agar dilution method with a GC medium base (Difco Laboratories, Detroit, MI) containing 1% Kellogg's defined supplement and 2-fold dilutions of antibiotic. N. gonorrhoeae ATCC 49226 and World Health Organization (WHO) strains B, C, and D were used as controls. Breakpoints were in accordance with the Clinical and Laboratory Standards Institute (CLSI): MICs of ≥2 μg/ml (penicillin), ≥2 μg/ml (tetracycline), ≥1 μg/ml (ciprofloxacin), and ≥128 μg/ml (spectinomycin) were considered resistant, and MICs of ≤0.25 μg/ml for cefixime and ceftriaxone were considered susceptible (11). The breakpoint for resistance for erythromycin and azithromycin was ≥2 μg/ml (14, 38). A random sample of 51 specimens underwent susceptibility testing for gentamicin. Isolates were subcultured on GC medium base containing 0.2% BioX and incubated for 24 h at 35°C in a 5% CO2 atmosphere with or without antibiotics and maintained in brain heart infusion (BHI) medium containing 20% glycerol and stored at −80°C. Auxotyping was performed for nutritional requirements. Plasmids were isolated using the Promega Wizard Plus SV Minipreps DNA purification system (Fisher Scientific, Ottawa, Ontario, Canada) according to the manufacturer's instructions, and plasmid profiles were determined by agarose gel electrophoresis. The presence of the tet(M)-containing plasmid was confirmed using PCR (44).
When agar dilution testing was conducted in 2009, it was discovered that 63 isolates (1 from 2003, 1 from 2004, 23 from 2005, 24 from 2006, 9 from 2007, and 5 from 2008) were nonrecoverable. Therefore, to assess quinolone resistance, these specimens underwent PCR amplification at The Johns Hopkins Bayview Infectious Diseases Division to detect mutations in the parC and gyrA genes of the quinolone resistance-determining region, as previously described (17). Previous studies have shown these mutations in these genes to have a high correlation with increased fluoroquinolone MICs (27, 45–46).
Data analysis.
We examined the prevalence of N. gonorrhoeae (detected by culture and PCR) and quinolone and other antimicrobial resistance by year (2002 to 2009) as well as changes in MICs for each antimicrobial assessed. The statistical significance of trends over time was assessed by a nonparametric trend test. To better understand the epidemiology of resistant infection and how it may have spread within the community, we compared characteristics of men with quinolone-resistant infection to men with quinolone-susceptible infection and made similar comparisons for other antibiotics showing increased MICs. Increased MICs of cefixime (≥0.016 μg/ml), ceftriaxone (≥0.016 μg/ml), azithromycin (≥0.25 μg/ml), and gentamicin (≥16 μg/ml) were selected based on previous publications (5, 12, 20, 36, 39). Differences between categorical variables were assessed by chi-square test and with Fisher's exact test when n was <5. Descriptive analyses include frequencies and MIC 50th and 90th percentiles for each antimicrobial, presented by time period (2002 to 2006 versus 2007 to 2009), to examine the putative changes in MICs at the time that quinolone resistance emerged. Data were analyzed using STATA/SE 10.0 for Windows (Stata Corp., College Station, TX).
RESULTS
Between February 2002 and September 2005, 2,784 men were enrolled and randomized as to circumcision (n = 1,391) or delayed circumcision (n = 1,390). Follow-up visits, baseline sociodemographic characteristics, sexual risk behaviors, and STI infection were well balanced between the two arms (3). From February 2002 through July 2009, the prevalence of N. gonorrhoeae infection decreased from 3.8% in 2002 to 2.7% in 2009 (Fig. 1), representing 331 N. gonorrhoeae infections detected by PCR and/or culture. Of these, there were 168 culture-positive N. gonorrhoeae results occurring in 142 men. Melt curves for the parC and gyrA genes failed for 2 of the 63 nonviable isolates, and PCR testing was not performed. Results then are restricted to 166 isolates, of which 26 (16%) were second infections. All 140 initial infections were treated; having more than one N. gonorrhoeae culture-positive visit was not correlated with quinolone or penicillin resistance or demographic or behavioral characteristics, and the median time from initial to subsequent infection was 301 days. Therefore, these 26 repeat infections were treated as independent observations (i.e., new infections) for statistical analysis of MIC and resistance. Demographic and behavioral characteristics, year of recovery, and auxotype are shown in Table 1.
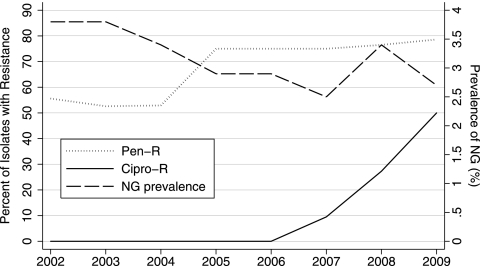
Fig. 1.
Prevalence of N. gonorrhoeae (NG) over time with proportions of ciprofloxacin and penicillin resistance. Penicillin resistance (Pen-R) was defined by MIC of ≥2 μg/ml, and ciprofloxacin resistance (Cipro-R) was defined by MIC of ≥1 μg/ml or gyrA or parC mutation.
Table 1.
Demographic and behavioral characteristics of culture-positive N. gonorrhoeae episodes
| Characteristic | n (%) (N = 140)a |
|---|---|
| Age group (yr) at baseline | |
| 18–20 | 67 (48) |
| 21–24 | 73 (52) |
| Residence at baseline | |
| Kisumu | 60 (43) |
| Other | 80 (57) |
| Circumcision status at time of infection | |
| Circumcised | 71 (51) |
| Uncircumcised | 69 (49) |
| Condom use at last sexual intercourse | |
| No | 68 (51) |
| Yes | 66 (49) |
| Gave gifts or money to a woman for sex in | |
| past 6 months | |
| No | 110 (87) |
| Yes | 17 (13) |
| Two or more sex partners in past 30 days | |
| No | 106 (79) |
| Yes | 28 (21) |
| Any antibiotic use in past 6 months | |
| No | 130 (93) |
| Yes | 10 (7) |
| Year of culture-positive isolate | |
| identification (n = 166) | |
| 2002 | 18 (11) |
| 2003 | 19 (11) |
| 2004 | 17 (10) |
| 2005 | 27 (16) |
| 2006 | 28 (17) |
| 2007 | 21 (13) |
| 2008 | 22 (13) |
| 2009 | 14 (8) |
| Auxotypeb (n = 105) | |
| Nonrequiring | 48 (46) |
| Proline | 47 (45) |
| Mixed | 7 (7) |
| Other | 2 (2) |
| Untypeable | 1 (1) |
Not all cells sum to N (total number of cases) due to missing data.
Auxotyping was performed for nutritional requirements for leucine, ornithine, citrulline, proline, arginine, hypoxanthine, uracil, and methionine. Strains that did not have requirements for these amino acid supplements were designated nonrequiring. Mixed is proline (n = 6) in combination with ornithine (n = 2) or hypoxanthine (n = 4); one isolate was methionine/hypoxanthine.
Quinolone resistance.
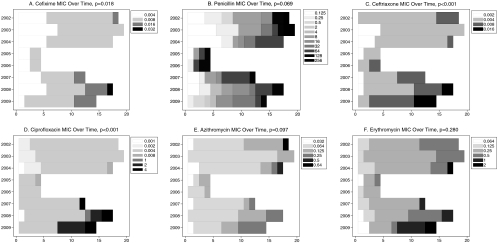
Over the period from 2002 to 2009, the MIC50 and MIC90 for ciprofloxacin were 0.004 μg/ml and 2 μg/ml, respectively (range, 0.001 to 4) (Table 2), but the MIC90 increased from 0.004 μg/ml to 4.0 μg/ml from the period 2002 to 2006 to the period 2007 to 2009. Resistance to ciprofloxacin (MIC ≥ 1 μg/ml) or the presence of mutant parC or gyrA was first detected in November 2007. Overall, 15/166 (9.0%; 95% confidence interval [CI]: 4.6 to 13.4%) specimens were quinolone resistant: 13/105 (12.4%) by agar dilution testing and 2/61 (3.3%) by PCR (one gyrA mutant/parC mutant; one gyrA mutant/parC wild type). The trend of increasing proportions of resistant specimens (Fig. 1) in 2007 (9.5%; 95% CI: 1.2 to 30%), 2008 (27.3%; 95% CI: 10.7 to 50.2%), and 2009 (50%; 95% CI: 23 to 77%) is statistically significant (P < 0.001). The increase in MIC over time was also statistically significant (Fig. 2D) (P < 0.001).
Table 2.
Results of agar dilution testing: distribution of MICs by antibiotic and time
| Antibiotic | MIC %a | MIC (μg/ml) at indicated time of isolate recovery |
|
|---|---|---|---|
| 2002–2006 (n = 62) | 2007–2009 (n = 43) | ||
| Penicillin | 50 | 8.0 | 16.0 |
| 90 | 128.0 | 128.0 | |
| Ciprofloxacin | 50 | 0.004 | 0.004 |
| 90 | 0.004 | 4.0 | |
| Azithromycin | 50 | 0.064 | 0.064 |
| 90 | 0.125 | 0.25 | |
| Cefixime | 50 | 0.008 | 0.008 |
| 90 | 0.008 | 0.016 | |
| Ceftriaxone | 50 | 0.004 | 0.008 |
| 90 | 0.008 | 0.016 | |
| Tetracycline | 50 | 32.0 | 32.0 |
| 90 | 64.0 | 32.0 | |
| Erythromycin | 50 | 0.25 | 0.25 |
| 90 | 0.5 | 1.0 | |
| Spectinomycin | 50 | 16.0 | 16.0 |
| 90 | 32.0 | 32.0 | |
For MIC %, 50 and 90 represent MIC50 and MIC90, respectively.
Fig. 2.
(A to F) MICs (μg/ml) by year: cefixime (A), penicillin (B), ceftriaxone (C), ciprofloxacin (D), azithromycin (E), and erythromycin (F). The x axis indicates the number of specimens and the legend indicates the MIC. The statistical significance (P value) of the nonparametric test for a trend of increasing MIC over time is presented after each caption.
MIC profiles of other antimicrobials.
Overall, 68 (65%) isolates exhibited penicillin resistance (MIC ≥ 2 μg/ml). All of these were plasmid related (66 were 3.2 MDa and 2 were 4.5 MDa). All isolates exhibited the 2.6-MDa cryptic plasmid. The auxotype was primarily nonrequiring (46%) or proline (45%) and did not vary over time. The increase in MIC over time was statistically significant for cefixime (P = 0.018) and ceftriaxone (P < 0.001) (Fig. 2A and 2C, respectively). There were 12 (11%) isolates with cefixime MICs of ≥0.016 μg/ml and 6 (6%) isolates with ceftriaxone MICs of ≥0.016 μg/ml. The increases in MIC over time for penicillin (P = 0.069) and azithromycin (P = 0.097) were marginally statistically significant (Fig. 2B and 2E, respectively). There were 2 (2%) isolates with increased azithromycin MICs (0.5 μg/ml, 0.64 μg/ml). There was no change over time in the erythromycin (P = 0.280), spectinomycin, and tetracycline MICs (results not shown for spectinomycin or tetracycline). All isolates were susceptible to spectinomycin. Three (3%) exhibited erythromycin resistance (MIC ≥ 2 μg/ml), and 97% (n = 102) exhibited the 25.2-MDa plasmid and were resistant to tetracycline (MIC ≥ 2 μg/ml). Of the 51 specimens with gentamicin susceptibility testing results, 1 (2%) had an MIC of 4 μg/ml, 49 (96%) had an MIC of 8 μg/ml, and 1 (2%) had an MIC of 16 μg/ml.
Comparison of participant characteristics by MIC.
A greater proportion of isolates with ciprofloxacin resistance (MIC ≥ 1 μg/ml), increased azithromycin MIC (≥0.25 μg/ml), and increased ceftriaxone MIC (≥0.016 μg/ml) occurred among residents of Kisumu District versus residents of neighboring districts: 92% (12/13) versus 48% (44/92) for ciprofloxacin (P = 0.003); 83% (10/12) versus 50% (44/88) for azithromycin (P = 0.034); and 11% (6/56) versus 0% (0/49) (P = 0.029) for ceftriaxone. Compared to patients producing isolates with cefixime MICs of <0.016 μg/ml, having two or more sex partners was more common among patients producing isolates with cefixime MICs of ≥0.016 μg/ml (42% [5/12] versus 20% [18/90], P = 0.092), and condom use was less common (17% [2/12] versus 49% [44/90], P = 0.061). When data examined were restricted to those obtained upon initial visits, only the association between Kisumu District residence and ciprofloxacin resistance remained significant at the P level of <0.05.
Age at baseline, circumcision status, exchange of gifts or money for sex, and antibiotic use during the previous 6 months were not associated with MIC as a continuous or categorical variable for any antibiotic. Antibiotic prescription or dispensation in 32,039 medical visits was as follows: penicillins at 4,225 (13.2%, including augmentin [n = 469]) visits, doxycycline at 950 (3.0%) visits, cephalosporins (cefixime or ceftriaxone) at 108 (0.3%) visits, quinolones (norfloxacin and ciprofloxacin) at 1,043 (3.3%) visits, spectinomycin at 65 (0.2%) visits, erythromycin at 311 (1.0%) visits, and azithromycin at 34 (0.1%) visits. Antibiotic prescribing at the cohort level (frequency of prescription of penicillins, tetracyclines, macrolides, quinolones, cephalosporins, and spectinomycin; examined individually) did not change significantly over time and was not associated with MIC levels for any antibiotics.
Increased MIC, by either trend or cutoff, was not associated with having multiple N. gonorrhoeae culture-positive visits for any of the antibiotics assessed. Quinolone resistance did not differ by single infection or reinfection (13.7% single/initial visit versus 9.4% at reinfection, P = 0.750), nor did penicillin resistance (81% at single/initial versus 79% at reinfection, P > 0.99). By nonparametric trend test, an increasing MIC was not associated with reinfection for any antimicrobial.
DISCUSSION
The levels of Neisseria gonorrhoeae antimicrobial resistance measured in our sample of young men in Kisumu, Kenya, from 2002 to 2009 are some of the highest reported. Of note, the resistance observed was entirely plasmid related, making it among the highest prevalences of plasmid-mediated penicillin and tetracycline resistance observed (2, 12, 41). The differences in mechanisms giving rise to plasmid-mediated and chromosomal resistance indicate that this is in response to intense selective pressure.
Our findings are not surprising in light of the high prevalence of quinolone resistance worldwide (37) and increasing reports of quinolone resistance in South Africa. In response to clinical failures in patients treated with ciprofloxacin in Durban, South Africa, Moodley et al. measured a 22% prevalence of quinolone resistance in 139 N. gonorrhoeae isolates by using molecular methods (32). Among men attending health clinics in Cape Town and Johannesburg, the prevalence of ciprofloxacin resistance increased from 7% and 11%, respectively, in 2004 to 27% and 32%, respectively, in 2007 (25), while a 2004-2005 study in the Pretoria region found 7% quinolone resistance (13). Additionally, similar prevalences of quinolone resistance observed in North America and Europe are not accompanied by high levels of plasmid-mediated resistance (12).
We observed statistically significant increases in cephalosporin MICs over time, which is in keeping with increased MICs for cefixime and ceftriaxone in N. gonorrhoeae isolates from Southeast Asia, Europe, and Australia (10, 18, 41). The shift in MIC over time for cefixime and ceftriaxone appears to have been preceded by an increase in penicillin resistance (Fig. 1), which is consistent with what is known about mechanisms of beta-lactamase resistance. A correlation between chromosomally mediated penicillin resistance and increased MIC for cephalosporins has been observed, and they appear to be linked by mosaic and nonmosaic penA and penB alleles (21, 22, 33). Conversely, studies by Whiley et al. (40) and Takahata et al. (35) support a nonclonal link between resistance emergence in cephalosporins and penicillin. Other genetic loci may be involved (4), and the mechanism by which the mutations are acquired is not completely understood, though genetic material is possibly acquired from other Neisseria spp. (1, 48). Dispensation of cephalosporins in our cohort was rare. While this is not a measure of community-level use of these drugs, the men in the cohort were unlikely to seek care outside of the study, as medical care was provided for free to study participants. The much higher cost of cephalosporins relative to quinolones (28) is likely a barrier to generalized use in the community. Increases in cephalosporin MICs in N. gonorrhoeae isolates from men in our cohort in this time period may not be the result of generalized overuse of cephalosporins. Sequencing of additional genetic loci may further our understanding of origins of cephalosporin and other antimicrobial resistance emergence. Epidemiologic study of factors associated with quinolone resistance and with increased MICs of other antimicrobials can augment surveillance to allow early anticipation of emergence of resistance.
The increased MICs for cephalosporins we observed contribute to the growing concern regarding multidrug-resistant N. gonorrhoeae. In areas of Japan and Korea, up to 40% of isolates show tetracycline, penicillin, fluoroquinolone, and multiple oral cephalosporin resistance (29). A decreased ability to treat N. gonorrhoeae is a serious public health concern because of the high burden of disease, the important sequelae, the risk for continued transmission through increased duration of infectiousness, the contribution to HIV transmission and acquisition (26, 37), and the potential for shared resistance with other Neisseria spp. Acquisition of extended-spectrum beta-lactamase resistance is even more alarming, as the resistance plasmids can be transferred to other bacterial species. Suggested strategies to stem antimicrobial resistance in N. gonorrhoeae include multiple dosing, increased dosing, and drug cycling (10). However, the extremely high levels of plasmid-mediated resistance we observed suggest that selective pressure coming from community antibiotic use is a stronger driver of resistance emergence than suboptimal N. gonorrhoeae treatment. Strategies from the WHO for containing antimicrobial resistance more broadly aim at the root of resistance emergence, primarily the appropriate use of antimicrobials, improved infection control practices, and the reduced use of antimicrobials in food animal production (43). The Antimicrobial Resistance Containment and Surveillance approach recommends that interventions simultaneously address procurement and management at the regulatory level, as well as demand and use at the individual level (34). With high levels of quinolone resistance, if cephalosporins become ineffective, the future of N. gonorrhoeae control will be in a dire state, especially in limited-resource settings.
Results of por sequencing suggest that recombination and diversity may be increased within high-risk groups as individuals are exposed to multiple gonococcal strains (16, 30). The association between fluoroquinolone resistance and high-risk groups is echoed by findings of increased quinolone resistance among men who have sex with men (7, 15). While resistance was not more common among men with repeat N. gonorrhoeae infections in our cohort, increased cefixime MIC was associated with multiple recent sex partners and lack of condom use, providing suggestive epidemiologic data that core transmitter sexual behavior may accelerate the emergence of resistance. The association between residence in Kisumu District and increased MICs for ciprofloxacin, azithromycin, and ceftriaxone may reflect different circulating gonococcal strains, as other explanatory variables examined in this analysis did not vary by residence.
Spectinomycin is an effective alternative drug choice for the treatment of uncomplicated N. gonorrhoeae (8), and we observed no resistance in this study. However, delivery by injection makes spectinomycin unfavorable for resource-poor settings and for administration by lower-skilled health care workers, and spectinomycin is not recommended for pharyngeal infection (9). While MIC values that define resistance in documented treatment failure have not been determined for aminoglycosides, only one (2%) of our isolates had a gentamicin MIC that might be considered raised. Results of studies from the 1970s through the 1990s demonstrate a curative efficacy of 92 to 98% for gentamicin against N. gonorrhoeae (5, 20, 36). While several studies from the 1990s found N. gonorrhoeae sensitivity to gentamicin ranging from 94 to 100% by using an MIC breakpoint of 16 (19, 23–24), a study of 47 men with N. gonorrhoeae-positive urethral discharge in Malawi in 2000 and 2001 found 7 (15%) had gentamicin MICs of >16 (47). Gentamicin may prove a relevant potential alternative therapy if cephalosporin resistance emerges, and it should be included in routine antimicrobial resistance surveillance.
Fluoroquinolones are no longer recommended as the first-line therapy when resistance exceeds 5% (37, 42). However, the average wholesale prices of 250 mg of intramuscular (i.m.) ceftriaxone (the lowest unit dose commercially available, which is commonly used) and a 400-mg tablet of cefixime are several times higher than those of the equivalent doses of norfloxacin or ciprofloxacin (28). In developing countries, where resources are limited, the higher cost of newer antimicrobials can be a significant financial burden. The geographic variability in the prevalence and type of gonococcal antibiotic resistance (37, 42) presents a challenge for adequate drug choice and dosing. Currently, there is no regular gonococcal antimicrobial susceptibility testing in Kenya. Selectively applying antimicrobial regimens in different geographic locales in an effort to minimize costs would require local and timely susceptibility testing. At a minimum, sentinel or periodic surveillance programs in select geographic locations should be implemented. In addition to identifying the most effective drug class and member, surveillance can be used to evaluate the effects on antimicrobial susceptibility of altered pharmacological regimens, and should incorporate measures of community-level antibiotic prescribing and bacterial burden. The rising rates of quinolone resistance and associated treatment failure will lead to gonococcal disease progression and continued transmission (37), making alternative regimens necessary. Unfortunately, the replacement of quinolones with cephalosporins may be only a temporary solution to improved N. gonorrhoeae management, in light of the growing potential for multidrug-resistant N. gonorrhoeae.
Limitations.
Our ability to identify factors associated with antimicrobial resistance and MIC shift over time was limited by sample size. Nevertheless, the 7.5-year observation period provides insight into the occurrence of quinolone resistance over time, as well as a suggestion of the possibility of decreased susceptibility of Neisseria gonorrhoeae to cephalosporins and macrolides. The interpretation of trends in MIC over time is impeded by the lack of data from 2005 and 2006. Thus, our power to detect changes is decreased, and the increase in MICs may have occurred earlier than observed. However, we were able to assess quinolone resistance in all isolates by augmenting detection with PCR analysis. Finally, while this was not a probability sample of the Kisumu District population, our cohort was sampled from the community and sexual partners are located locally. Therefore, we believe the high prevalence of quinolone resistance we observed is strongly suggestive of levels of quinolone resistance in the community.
Conclusions.
The level of quinolone resistance we detected (26% in 2007 to 2009) in Kisumu, Kenya, is greater than the level (5%) (37) at which an alternative antibiotic regimen is recommended, and this indicates that clinicians should not be using ciprofloxacin or norfloxacin to treat gonorrhea. A nonquinolone antibiotic such as cefixime or ceftriaxone would be appropriate to treat diagnosed or suspected gonorrhea in this area, as indicated by the low MIC90 values we observed. The coexistence of multiple other types of resistance and increased MICs for cephalosporins contributes to a growing concern about multidrug-resistant N. gonorrhoeae. Ongoing surveillance for antimicrobial resistance in Kenya is lacking and is an essential public health capacity to ensure timely response to emerging cephalosporin and multidrug-resistant N. gonorrhoeae and the selection of pharmacological regimens offering effective cures. The prevention of antimicrobial resistance emergence will require a population-level approach rather than a disease-specific approach.
ACKNOWLEDGMENTS
We have no financial or other conflicts of interest to report. Robert C. Bailey receives funds for research and implementation of male circumcision interventions from the U.S. Government and the Bill and Melinda Gates Foundation through Family Health International. This research was supported by grant AI50440 from the Division of AIDS, National Institute of Allergies and Infectious Disease of the U.S. National Institutes of Health, by AI50440-S (American Recovery and Reinvestment Act), and by grant HCT 44180 from the Canadian Institutes of Health Research (CIHR). Stephen Moses was supported by a CIHR Investigator Award. Jonathan M. Zenilman was supported by grant R01AI076859 from the National Institute of Allergies and Infectious Disease of the U.S. National Institutes of Health. The Kisumu trial has been registered in http://www.clinicaltrials.gov under the number NCT00059371.
Footnotes
Published ahead of print on 23 May 2011.
REFERENCES
- 1. Ameyama S., et al. 2002. Mosaic-like structure of penicillin-binding protein 2 gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob. Agents Chemother. 46:3744–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Australian Gonococcal Surveillance Programme 2010. Annual report of the Australian Gonococcal Surveillance Programme, 2009. Commun. Dis. Intell. 34:89–95 http://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf Accessed 17 November 17 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bailey R. C., et al. 2007. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369:643–656 [DOI] [PubMed] [Google Scholar]
- 4. Bala M., Sood S. 2010. Cephalosporin resistance in Neisseria gonorrhoeae. J. Glob. Infect. Dis. 2:284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowie W., Ronald A. R., Krywulak W., Cates C. Y., Boutros P. 1974. Gentamicin in the treatment of gonorrhoea in females. Br. J. Vener. Dis. 50:208–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 1993. 1993 Sexually transmitted diseases treatment guidelines. MMWR Recommend. Rep. 42(RR-14):1–102 [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2004. Increases in fluoroquinolone-resistant Neisseria gonorrhoeae among men who have sex with men–United States, 2003, and revised recommendations for gonorrhea treatment, 2004. MMWR Morb. Mortal. Wkly. Rep. 53:335–338 [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention 2006. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recommend. Rep. 55(RR-11):44. [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention 2007. Update to CDC's Sexually Transmitted Diseases Treatment Guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb. Mortal. Wkly. Rep. 56:332–336 [PubMed] [Google Scholar]
- 10. Chisholm S. A., et al. 2010. Expanded cephalosporin MIC creep among gonococci: time for a pharmacodynamic rethink? J. Antimicrob. Chemother. 65:2141–2148 [DOI] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing: nineteenth informational supplement. M100–S20, vol. 30, no. 1 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 12. Cole M. J., et al. 2010. European surveillance of antimicrobial resistance in Neisseria gonorrhoeae. Sex. Transm. Infect. 86:427–432 [DOI] [PubMed] [Google Scholar]
- 13. De Jongh M., Dangor Y., Adam A., Hoosen A. A. 2007. Gonococcal resistance: evolving from penicillin, tetracycline to the quinolones in South Africa—implications for treatment guidelines. Int. J. STD AIDS 18:697–699 [DOI] [PubMed] [Google Scholar]
- 14. Ehret J. M., Nims L. J., Judson F. N. 1996. A clinical isolate of Neisseria gonorrhoeae with in vitro resistance to erythromycin and decreased susceptibility to azithromycin. Sex. Transm. Dis. 23:270–272 [DOI] [PubMed] [Google Scholar]
- 15. Farhi D., et al. 2007. Increasing rates of quinolone-resistant Neisseria gonorrhoeae in Paris, France. J. Eur. Acad. Dermatol. Venereol. 21:818–821 [DOI] [PubMed] [Google Scholar]
- 16. Fudyk T. C., et al. 1999. Genetic diversity and mosaicism at the por locus of Neisseria gonorrhoeae. J. Bacteriol. 181:5591–5599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giles J. A., et al. 2004. Quinolone resistance-determining region mutations and por type of Neisseria gonorrhoeae isolates: resistance surveillance and typing by molecular methodologies. J. Infect. Dis. 189:2085–2093 [DOI] [PubMed] [Google Scholar]
- 18. Golparian D., Hellmark B., Fredlund H., Unemo M. 2010. Emergence, spread and characteristics of Neisseria gonorrhoeae isolates with in vitro decreased susceptibility and resistance to extended-spectrum cephalosporins in Sweden. Sex. Transm. Infect. 86:454–460 [DOI] [PubMed] [Google Scholar]
- 19. Guyot A., Jarrett B., Sanvee L., Dore D. 1998. Antimicrobial resistance of Neisseria gonorrhoeae in Liberia. Trans. R. Soc. Trop. Med. Hyg. 92:670–674 [DOI] [PubMed] [Google Scholar]
- 20. Hira S. K., et al. 1985. Efficacy of gentamicin and kanamycin in the treatment of uncomplicated gonococcal urethritis in Zambia. Sex. Transm. Dis. 12:52–54 [DOI] [PubMed] [Google Scholar]
- 21. Huang C. T., et al. 2010. Characteristics and dissemination of mosaic penicillin-binding protein 2-harboring multidrug-resistant Neisseria gonorrhoeae isolates with reduced cephalosporin susceptibility in northern Taiwan. Antimicrob. Agents Chemother. 54:4893–4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ison C. A., Bindayna K. M., Woodford N., Gill M. J., Easmon C. S. 1990. Penicillin and cephalosporin resistance in gonococci. Genitourin. Med. 66:351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain S. K., Kulkarni M. G., Banker D. D. 1994. Antibiotic susceptibility pattern of gonococcal isolates. Indian J. Med. Sci. 48:233–236 [PubMed] [Google Scholar]
- 24. Joesoef M. R., et al. 1994. Antimicrobial susceptibilities of Neisseria gonorrhoeae strains isolated in Surabaya, Indonesia. Antimicrob. Agents Chemother. 38:2530–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis D. A., et al. 2008. Escalation in the relative prevalence of ciprofloxacin-resistant gonorrhoeae among men with urethral discharge in two South African cities: association with HIV seropositivity. Sex. Transm. Infect. 84:352–355 [DOI] [PubMed] [Google Scholar]
- 26. Lewis D. A. 2010. The Gonococcus fights back: is this time a knock out? Sex. Transm. Infect. 86:415–421 [DOI] [PubMed] [Google Scholar]
- 27. Lindbäck E., Rahman M., Jalal S., Wretlind B. 2002. Mutations in gyrA, gyrB, parC, and parE in quinolone-resistant strains of Neisseria gonorrhoeae. APMIS 110:651–657 [DOI] [PubMed] [Google Scholar]
- 28. Management Sciences for Health in collaboration with World Health Organization 2009. International drug price indicator guide, 2009 ed. Frye J. (ed.) http://erc.msh.org/dmpguide/pdf/DrugPriceGuide_2009_en.pdf Accessed 1 February 2011
- 29. Matsumoto T. 2008. Trends of sexually transmitted diseases and antimicrobial resistance in Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 31(Suppl. 1):S35–S39 [DOI] [PubMed] [Google Scholar]
- 30. McKnew D. L., Lynn F., Zenilman J. M., Bash M. C. 2003. Porin variation among clinical isolates of Neisseria gonorrhoeae over a 10-year period, as determined by Por variable region typing. J. Infect. Dis. 187:1213–1222 [DOI] [PubMed] [Google Scholar]
- 31. Ministry of Health, Government of Kenya 2002. Clinical guidelines for diagnosis and treatment of common conditions in Kenya—2002, 2nd ed http://collections.infocollections.org/whocountry/en/d/Jh4329e/ Accessed 11 November 2010
- 32. Moodley P., Martin I. M., Pillay K., Ison C. A., Sturm A. W. 2006. Molecular epidemiology of recently emergent ciprofloxacin-resistant Neisseria gonorrhoeae in South Africa. Sex. Transm. Dis. 33:357–360 [DOI] [PubMed] [Google Scholar]
- 33. Pandori M., et al. 2009. Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob. Agents Chemother. 53:4032–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simonsen G. S., Tapsall J. W., Allegranzi B., Talbot E. A., Lazzari S. 2004. The antimicrobial resistance containment and surveillance approach–a public health tool. Bull. World Health Organ. 82:928–934 [PMC free article] [PubMed] [Google Scholar]
- 35. Takahata S., Senju N., Osaki Y., Yoshida T., Ida T. 2006. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 50:3638–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tan N. J., Rajan V. S., Pang R., Sng E. H. 1980. Gentamicin in the treatment of infections due to penicillinase-producing gonococci. Br. J. Vener. Dis. 56:394–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tapsall J. 2001. Antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization. http://www.who.int/drugresistance/Antimicrobial_resistance_in_Neisseria_gonorrhoeae.pdf Accessed 15 December 2008
- 38. Tapsall J. W., et al. 1998. Failure of azithromycin therapy in gonorrhea and discorrelation with laboratory test parameters. Sex. Transm. Dis. 25:505–508 [DOI] [PubMed] [Google Scholar]
- 39. Whiley D. M., et al. 2010. Alterations of the pilQ gene in Neisseria gonorrhoeae are unlikely contributors to decreased susceptibility to ceftriaxone and cefixime in clinical gonococcal strains. J. Antimicrob. Chemother. 65:2543–2547 [DOI] [PubMed] [Google Scholar]
- 40. Whiley D. M., Limnios E. A., Ray S., Sloots T. P., Tapsall J. W. 2007. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob. Agents Chemother. 51:3111–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. World Health Organization Western Pacific Programme et al 2010. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian regions, 2007-2008. Commun. Dis. Intell. 34:1–7 [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization 2001. Western Pacific Region Gonococcal Antimicrobial Susceptibility Programme (GASP) report—2000. Commun. Dis. Intell. 25:274–27711806663 [Google Scholar]
- 43. World Health Organization 2001. WHO global strategy for containment of antimicrobial resistance. WHO document WHO/CDS/CSR/DRS/2001.2. World Health Organization, Geneva, Switzerland [Google Scholar]
- 44. Xia M., Pang Y., Roberts M. C. 1995. Detection of two groups of 25.2 mDa Tet M plasmids by polymerase chain reaction of the downstream region. Mol. Cell. Probes 9:327–332 [DOI] [PubMed] [Google Scholar]
- 45. Yang Y., et al. 2006. Antimicrobial susceptibility and molecular determinants of quinolone resistance in Neisseria gonorrhoeae isolates from Shanghai. J. Antimicrob. Chemother. 58:868–872 [DOI] [PubMed] [Google Scholar]
- 46. Yong D., et al. 2004. Epidemiological characteristics and molecular basis of fluoroquinolone-resistant Neisseria gonorrhoeae strains isolated in Korea and nearby countries. J. Antimicrob. Chemother. 54:451–455 [DOI] [PubMed] [Google Scholar]
- 47. Zachariah R., et al. 2002. Behavioural characteristics, prevalence of Chlamydia trachomatis and antibiotic susceptibility of Neisseria gonorrhoeae in men with urethral discharge in Thyolo, Malawi. Trans. R. Soc. Trop. Med. Hyg. 96:232–235 [DOI] [PubMed] [Google Scholar]
- 48. Zhao S., et al. 2009. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob. Agents. Chemother. 53:3744–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]