Abstract
The Bacillus subtilis acyl lipid desaturase (Δ5-Des) is an iron-dependent integral membrane protein able to selectively introduce double bonds into long-chain fatty acids. In the last decade since its discovery, the molecular mechanism of Δ5-Des expression has been studied extensively. However, the mechanism of desaturation, which must rely on unknown bacterial proteins for electron transfer, has not yet been explored. The B. subtilis genome encodes three proteins that can act as potential electron donors of Δ5-Des, ferredoxin (Fer) and two flavodoxins (Flds) (YkuN and YkuP), which are encoded by the ykuNOP operon. Here we report that the disruption of either the fer gene or the ykuNOP operon decreases the desaturation of palmitic acid by ∼30%. Nevertheless, a fer ykuNOP mutant abolished the desaturation reaction almost completely. Our results establish Fer and the two Flds as redox partners for Δ5-Des and suggest that the Fer and Fld proteins could function physiologically in the biosynthesis of unsaturated fatty acids in B. subtilis. Although Flds have extensively been described as partners in a number of redox processes, this is the first report describing their role as electron donors in the fatty acid desaturation reaction.
INTRODUCTION
Desaturase enzymes introduce cis double bonds in fatty acyl aliphatic chains (23), producing unsaturated fatty acids (UFAs). They play critical roles in adjusting the physiological properties of membrane lipids and in several essential cellular processes (3). Fatty acid desaturases are derived from two evolutionary lineages, the soluble acyl-acyl carrier protein (ACP) desaturases, found primarily in the plastids of higher plants, e.g., the castor Δ9-18:0-ACP desaturase (22), and a larger group of membrane-bound desaturases found in a wide range of taxa, typified by the Saccharomyces cerevisiae Ole1 Δ9-desaturase (Ole1p) (27) and the Bacillus subtilis Δ5-desaturase (Δ5-Des) (1, 2, 4). The complex process of introducing a double bond into fatty acids requires iron cofactors, molecular oxygen, and two reducing equivalents for catalysis (24, 25). The latter are supplied from NAD(P)H by two different functionally equivalent electron transport systems that are specific to the subcellular compartment rather than to the class of the desaturase. In the case of the desaturase in the plant endoplasmic reticulum and the acyl coenzyme A (CoA) desaturases of animals and fungi, the donor is cytochrome b5, either in the form of a cytochrome b5-fused domain or in the free form. For the soluble acyl-ACP desaturase and the integral membrane acyl lipid desaturases from plastids and cyanobacteria, electrons are delivered by ferredoxins (Fers), which are ubiquitous soluble iron-sulfur proteins involved in a variety of redox reactions (25). It should be noted, however, that a number of desaturases in heterologous hosts have been characterized (11, 18). Nevertheless, this approach precludes the study of the in vivo activities of these enzymes in the presence of their natural electron donors.
B. subtilis contains a membrane-bound desaturase, encoded by the des gene (1), which introduces a cis double bond at the Δ5 position of the acyl chains of membrane phospholipids (4). This enzyme, like many membrane proteins, has proven difficult to overexpress and purify; however, a topological analysis based on experimental grounds has provided important information regarding the structure. The topology of Δ5-Des, determined in Escherichia coli, revealed that this desaturase consists of six transmembrane segments and one membrane-associated domain (6). These results, as well as results from the systematic mutagenesis of conserved His residues, defined that the putative binuclear iron active site (defined by the conserved His ligands) is located within several intervening membrane-associated domains (6). The des gene, coding for Δ5-Des, was isolated by the functional complementation of Escherichia coli UFA auxotrophs (1), indicating that the desaturase works by using the electron donors of the E. coli heterologous host. However, the identity and intrinsic details concerning the electron transport system required for acyl desaturation reactions in B. subtilis are unknown. As a first step to study this important question, we undertook a genetic approach to identify the electron donors required to provide the 2 electrons required for Δ5-Des catalysis. Since database searching revealed that B. subtilis does not contain cytochrome b5 homologues, we assumed that, like other membrane-bound desaturases, Fer would be the electron donor required for fatty acid desaturation.
In this work, we demonstrate that the fer gene, encoding a 4Fe-4S ferredoxin (Fer) (9), as well as the ykuN and ykuP genes, coding for two flavodoxins (Flds) (13), which are mobile electron carriers containing flavin mononucleotide (FMN) as the prosthetic group, are able to transfer electrons to the B. subtilis Δ5-Des to catalyze the O2-dependent desaturation of the acyl chain of membrane phospholipids. Thus, Flds are isofunctional with Fer, mediating essentially the same redox process required for UFA biosynthesis in B. subtilis.
MATERIALS AND METHODS
Bacterial strains, media, and reagents.
Bacterial strains used in the present study are listed in Table 1. Escherichia coli and Bacillus subtilis strains were routinely grown in Luria-Bertani (LB) broth at 37°C (18). Spizizen salts (26) supplemented with 0.5% glucose, 0.01% each tryptophan and phenylalanine, and trace elements (10) were used as the minimal medium (MM) for B. subtilis. In nutritional screening procedures, amino acids were added at a final concentration of 0.01%. Minimal medium supplemented with methionine, isoleucine, and valine (MIV) was designated MM-MIV. Antibiotics were added to media at the following concentrations: the macrolides erythromycin (Erm) at 0.5 μg ml−1 and lincomycin (Lm) at 12.5 μg ml−1, ampicillin (Amp) at 100 μg ml−1, chloramphenicol (Cm) at 5 μg ml−1, and kanamycin (Km) at 5 μg ml−1. Restriction enzymes and DNA ligase were obtained from Promega Life Science. Oligonucleotides were purchased from Invitrogen.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| Bacillus subtilis | ||
| JH642 | trpC2 pheA1 | Laboratory stock |
| L43 | JH642 fer::Kmr | This study |
| L43C | JH642 fer::KmramyE::Plac-fer Cmr | This study |
| LSC9 | JH642 ykuNOP::Pspac-ykuNOP PykuNOP-lacZ Ermr Lmr | This study |
| LSC28 | L43 ykuNOP::Pspac-ykuNOP PykuNOP-lacZ Kmr Ermr Lmr | This study |
| LSC29 | LSC28 thrC::Pxyl-ykuN Kmr Ermr Lmr Spr | This study |
| LSC30 | LSC28 thrC::Pxyl-ykuP Kmr Ermr Lmr Spr | This study |
| Escherichia coli DH5α | supE44 thi-1 ΔlacU169(φ80lacZΔM15) endA1 recA1 hsdR17gyrA96relA1 trp6 cysT329::lacinmλpI(209) | Laboratory stock |
| Plasmids | ||
| pCR-Blunt II-TOPO | E. coli cloning vector; Kmr | Invitrogen |
| pGEMT-Easy | E. coli cloning vector; Ampr | Promega |
| pJM114 | Integrative vector used for gene disruption; Kmr | 17 |
| pJM114F1 | pJM114 with fragment F1 cloned into the SacI/BamHI site | This study |
| pJM114F1F2 | pJM114F1 with fragment F2 cloned into the XhoI/KpnI site | This study |
| pLM1 | pGEMT Easy containing a 609-bp fragment corresponding to the 5′ end of the fer gene | This study |
| pLM2 | pGEMT Easy containing a 555-bp fragment corresponding to the 3′ end of the fer gene | This study |
| pLM3 | pGEM-T Easy containing a 308-bp fer gene | This study |
| pDR67 | amyE integration plasmid with the IPTG-inducible Pspac promoter; Cmr | 17 |
| pDR67Cfd | Contains the fer gene cloned into HindIII/BglII sites in pDR67 under the control of the Pspac promoter | This study |
| pJM116 | Integrative vector to construct transcriptional fusions to lacZ; integrates at the amyE locus of B. subtilis; Cmr | 17 |
| pMutin | Integrates at a specific locus of B. subtilis; Ermr Lmr | 28 |
| pLSC1 | pCR-Blunt II-TOPO containing a 549-bp fragment corresponding to the 5′ end of the ykuNOP operon | This study |
| pLSC9 | Pspac::ykuNOP-lacZ cloned into pMutin; Ermr Lmr | This study |
| pDG1731 | Integrates at the thrC locus of B. subtilis; Spr | Laboratory stock |
| pGS247 | Xylose promoter cloned into pDG1731; Spr | Laboratory stock |
| pLSC10 | ykuN cloned under the control of Pxyl in pGS247; Kmr Ermr Lmr Spr | This study |
| pLSC11 | ykuP cloned under the control of Pxyl in pGS247; Kmr Ermr Lmr Spr | This study |
Cmr, Tetr, Kmr, Ermr, Lmr, and Ampr denote resistance to chloramphenicol, tetracycline, kanamycin, 0.5 μg erythromycin ml−1, 12.5 μg lincomycin ml−1, and ampicillin, respectively.
General molecular techniques.
Chromosomal DNA was isolated according to standard techniques (19). Plasmids were constructed by using standard methods and were amplified in E. coli DH5α cells. Plasmid DNA was prepared by using the Wizard DNA purification system (Promega Life Science). E. coli competent cells were transformed with supercoiled plasmid DNA by using the calcium chloride procedure (19). The transformation of B. subtilis was carried out according to a method described previously by Dubnau and Davidoff-Abelson (7). The amy phenotype was assayed with colonies grown during 48 h in LB starch plates by flooding the plates with a 1% I2-KI solution (21). amy+ colonies produced a clear halo, while amy-deficient colonies gave no halo. All plasmids and primers used in this study are listed in Tables 1 and 2, respectively.
Table 2.
Oligonucleotide primers
| Primer | Sequence (5′–3′)a |
|---|---|
| yKunEcoRI-up | TGAGAATTCTTATCATTTAAAGTGATA |
| yKunBamHI-low | CCTTTATGGATCCTGGATTTTTTC |
| ferSacI556 | CAAGGGAGCTCGCTAAGGGCATGTCTC |
| ferBamHI1137 | TACATGGATCCTTGTCTACGATTGTGTAC |
| ferXhoI | ATTCTCTCGAGGTGGCGGATGAGCCATTTG |
| ferKpnI | GCGATGGTACCGCAATTATTGCCATTCTG |
| CfdHindIII-up | GTTCCACCAAGCTTAATCTGGGAG |
| CfdBglII-low | CGTTCGAGATCTTTTTTTAGTTAC |
| CykuNHindIII-up | GAAAAGCTTTATCAACTAATGGGGTGATAAC |
| CykuNBamHI-low | CCGGATCCTTTATGAAACATGGATTTTTTCC |
| CykuPHindIII-up | AAAGCTTCATTAGAGGAGGAACAAGGAA |
| CykuPBamHI-low | CCGGATCCTGATTTTCTACCTCATTACTGT |
Restrictions sites are underlined.
Plasmid and strain construction. (i) Insertional mutagenesis of the fer gene and construction of a fer mutant strain.
Plasmid pJM114F1F2 (Table 1), used to insertionally disrupt the fer gene, was constructed as follows. Two sets of primers were designed to amplify the predicted fer coding sequence plus additional flanking regions from the B. subtilis genome. The 609-bp upstream fragment containing the 5′ region of the fer gene was amplified by PCR with primers ferSacI556 and ferBamHI1137 (Table 2). The 555-bp downstream fragment containing the 3′ region of the fer gene was amplified by PCR with primers ferXhoI and ferKpnI. The PCR products were ligated into pGEMT Easy (Promega Life Science) to generate the intermediate vectors pLM1 and pLM2, respectively, and sequenced. Plasmid pLM1 was subsequently digested with SacI and BamHI (Promega) and cloned upstream of the Km resistance cassette carried on plasmid pJM114 (17), yielding plasmid pJM114F1. The insert of pLM2 was then cloned into XhoI and KpnI sites downstream of the Km resistance cassette from pJM114F1 to generate plasmid pJM114F1F2 (Table 1). This construct was transformed into B. subtilis JH642, and transformants were selected on LB agar supplemented with Km at 5 μg ml−1. As a result of the expected double crossover, the Km resistance cassette replaced part of the chromosomal copy of the target gene, thereby creating a fer gene knockout. Gene replacement in the mutant clones was confirmed by PCR.
To construct a plasmid bearing the fer gene, a 308-bp fragment of B. subtilis DNA was amplified by PCR with primers CferHindIII-up and CferBglII-low (Table 2). The PCR product was then cloned into pGEMT Easy, yielding plasmid pLM3, which was then transformed into E. coli cells and sequenced. The fragment was cloned into the HindIII and BglII sites of vector pDR67 to generate a plasmid, pDR67CFer, with the fer gene under the transcriptional control of the Pspac promoter (Table 1). This plasmid was integrated into the amyE locus of strain L43, and transformants were selected on LB agar supplemented with 5 μg ml−1 Km and 5 μg ml−1 Cm. As a result of the expected double crossover, the construction replaced the amyE gene, yielding an amyE-deficient phenotype. The resulting strain was named L43C (Table 1). For the expression of fer, 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added.
(ii) Construction of conditional ykuNOP mutant strains.
To construct the ykuNOP conditional mutant, integrative plasmid pMUTIN4 (28) containing the IPTG-inducible Pspac promoter was used. A 549-bp DNA fragment was amplified by PCR using primers yKunEcoRI-up and yKunBamHI-low (Table 2), cloned into PCR-Blunt II-TOPO, and sequenced, rendering plasmid pLSC1. The cloned PCR fragment was excised with EcoRI and BamHI and ligated into vector pMUTIN4 cleaved with the same enzymes to yield plasmid pLSC9. This plasmid was introduced into B. subtilis strains JH642 and L43, and transformants were selected by plating cultures onto LB agar supplemented with macrolides. The resulting plasmid was integrated into the ykuNOP operon by a single-crossover event, generating B. subtilis strains LSC9 and LSC28 (Table 1). This approach resulted in the conditional inactivation of the target gene whose expression can be controlled by the IPTG-induced Pspac promoter.
For complementation experiments, the ykuN and ykuP genes of B. subtilis were amplified by PCR with the primers listed in Table 2. The PCR products were then cloned into the pGEMT Easy vector (Promega), transformed into E. coli cells, and sequenced. The fragments were cloned into the HindIII and BamHI sites of vector pGS247, yielding plasmids pLSC10 and pLSC11, with the ykuN and ykuP genes under the transcriptional control of the Pxyl promoter, respectively (Table 1). These plasmids were introduced into B. subtilis strain LSC28 by transformation, and transformants were selected on LB agar supplemented with 100 μg ml−1 spectinomycin, macrolides, and 5 μg ml−1 Km. As a result of the expected double crossover, the construction replaced the thrC gene, yielding a thrC-deficient phenotype. The resulting strains were named LSC29 and LSC30, respectively (Table 1). These strains were routinely grown in medium supplemented with 1% xylose.
Growth and metabolic labeling of mutant strains.
Strain L43 was grown overnight in LB medium, and on the following day cells were washed twice and diluted 1:10 in MM or MM-MIV, as indicated. Strain L43C was grown overnight in MM with 0.5 mM IPTG, while LSC29 and LSC30 cultures were grown in MM-MIV with 1% xylose. B. subtilis LSC9 was grown in MM-MIV without and inducer in all cases. For the measurement of unsaturated fatty acid (UFA) biosynthesis, cells were grown overnight at 37°C. The following day, fresh cultures were started by the dilution of the cultures grown overnight (1:10) in MM or MM-MIV, as indicated. Cells were grown to the mid-exponential phase and labeled during 2 h with 0.2 μCi of [14C]palmitate (specific activity, 58 mCi/mM) at 25°C. Strain LSC28 was grown overnight in MM-MIV supplemented with of 0.5 mM IPTG. On the following day, fresh cultures were started by washing twice and diluting the cultures grown overnight at a 1:10 dilution in the same medium without inducer; when indicated, 2-ml samples were taken and labeled with 0.2 μCi [14C]palmitate for 2 h at 25°C. After a 4-h period of arrested growth, the inducer was added at a final concentration of 0.5 mM. Cells were incubated during 2 h, and a 2-ml sample was processed as described above. Following incubation, cells were collected and lipids were prepared according to the method of Bligh and Dyer (5) and were converted into methyl esters as described previously (4). Labeled methyl esters were applied onto 10% silver nitrate-impregnated Silica Gel G plates (0.5-mm thickness; Analtech). Chromatographic separation was achieved with a toluene solvent system at −20°C and detected by using a phosphorimager screen. The spots of the different fatty acids were quantified by use of ImageQuant 5.2.
RESULTS
Lipid desaturation in a fer null mutant. B. subtilis contains a single gene, fer, encoding a 4Fe-4S ferredoxin (9). To evaluate whether this protein is required for fatty acid desaturation, we constructed strain L43, in which the fer gene was disrupted with a kanamycin resistance cassette. Physiological and biochemical characterizations of this strain revealed that this mutant grew normally in rich LB medium but showed a lag phase of 20 h in minimal medium (MM), which is about 6-fold longer than that observed for the parent wild-type strain, B. subtilis JH642 (Fig. 1A). Nevertheless, after this prolonged lag phase in MM, the fer mutant reassumed exponential growth, with a generation time similar to that of the parental strain (Fig. 1A). When tested on agar plates, strain L43 formed colonies after 4 to 6 days in MM but grew normally when supplemented with casein hydrolysate, indicating that this growth defect was due to an amino acid metabolism deficiency (data not shown). To identify the amino acid(s) required by strain L43, we used a nutritional screening procedure described previously by Miller (16). We found that the fer mutant strain grew as well as wild-type B. subtilis in MM supplemented with methionine, valine, and isoleucine (MM-MIV) (Fig. 1A), thus indicating that Fer is required for the synthesis of these amino acids. These results also suggest that after the 20-h lag period, the cells might have accumulated enough of an alternative electron donor to support Fer critical functions, restoring cells growth and probably acting as an electron donor for the Δ5-desaturation.
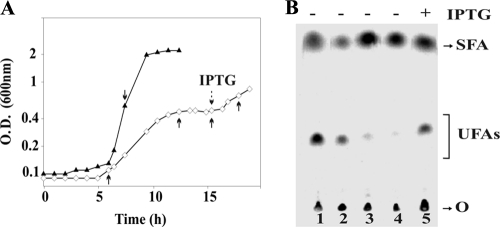
Fig. 1.
Growth phenotype and autoradiogram of the products of [14C]palmitate labeling of strains L43 and LSC9. (A) Strains JH642, L43, and LSC9 were grown as described in Materials and Methods. Cells were harvested, washed, and resuspended in fresh MM. Strain L43 was supplemented (○) or not supplemented (□) with 0.01% MIV, while strains JH642 (▿) and LSC9 (▴) were grown in MM-MIV. Strain LSC9 was grown without IPTG. (B) UFAs synthesized by strains JH642, L43, and LSC9 at 25°C. Cultures were grown to the mid-exponential phase at 37°C in minimal medium. Two milliliters of the cultures was challenged with 0.2 μCi [14C]palmitate and further shifted to 25°C for 2 h. The lipids were then extracted and transesterified, and the resulting methyl esters were separated by thin-layer chromatography silver-nitrate-impregnated silica plates. Lanes 1 and 3, strain JH642; lane 2, strain L43; lane 4, strain LSC9.
To test if the fer gene product was required for UFA biosynthesis, we labeled fer+ strain JH642 and fer-deficient strain L43 growing in MM and MM-MIV with radioactive palmitate and assayed the conversion of this fatty acid to cis-hexadecenoic acid. As shown in Fig. 1B the formation of cis-hexadecenoic acid from palmitic acid was detected in both strains JH642 and L43; however, the desaturation of palmitic acid in the Fer-deficient strain was reproducibly found to be about 80% of the lipid desaturation activity observed for the parental strain (Table 3). The fact that there are no differences in the amounts of UFAs in both media indicates that even when growth was restored by the addition the MIV amino acids, where no other electron sources are allowed to accumulate, the activity of Δ5-Des is influenced slightly by the depletion of Fer.
Table 3.
Effect of Fer depletion on Δ5-desaturase activity in B. subtilis strainsa
| Fatty acid type | Mean % fatty acids ± SD |
|||||
|---|---|---|---|---|---|---|
| JH642 |
L43 |
L43C |
||||
| MM | MM-MIV | MM | MM-MIV | MM | MM-MIV | |
| SFAs | 60 ± 2 | 58 ± 3 | 68.3 ± 0.6 | 67 ± 3 | 61 ± 2 | 60 ± 4 |
| UFAs | 40 ± 2 | 42 ± 3 | 31.7 ± 0.6 | 33 ± 3 | 39 ± 2 | 40 ± 4 |
Cells were grown to log phase at 37°C in MM or MM-MIV. Two-milliliter samples were labeled with 0.2 μCi [14C]palmitate at 25°C for 2 h. Labeled fatty acids were extracted and methyl esters were prepared as described in Materials and Methods. Each sample was fractionated by argentation–thin-layer chromatography. The spots of the different fatty acids were quantified by use of ImageQuant 5.2. Values are the means of data from five independent experiments and are expressed as percentages of total fatty acids. SFAs, saturated fatty acids; UFA, unsaturated fatty acids.
To confirm that the growth defect in MM as well as the decrease in the desaturase activity observed for L43 were due to the absence of fer, complementation analyses were carried out. A plasmid in which fer was placed under the control of the IPTG-inducible Pspac promoter was introduced into strain L43. The induction of fer in the resulting strain (L43C) allowed growth in MM (Fig. 1A) and restored the desaturation of palmitic acid to levels comparable to those of the wild-type strain (Table 3), thereby confirming that the absence of a functional copy of fer caused the growth phenotype and decreased the desaturase activity of strain L43 (Fig. 1A). This result demonstrates that although the desaturase activity in strain L43 is reproducibly diminished, it still proceeds in the absence of Fer, suggesting that an alternative electron donor accounts for the remainder of the activity.
Lipid desaturation in a flavodoxin-depleted strain.
B. subtilis has two flavodoxins encoded in the operon ykuNOP (13). It was demonstrated that the products of these genes, YkuN and YkuP, are FMN binding flavodoxins able to transport electrons to cytochrome P450 BioI (13), while the ykuO gene encodes a protein of unknown function. Since Flds shuttle electrons between different acceptors in the redox-based metabolisms of a number of bacteria (20), we decided to test if these flavoproteins could mediate electron transfer to Δ5-Des. To this end, we constructed a conditional mutant, LSC9, that expressed the ykuNOP operon under the control of the Pspac promoter (Table 1). This mutant grew as well as the wild-type strain in MM (either on agar plates or in liquid medium) not supplemented with IPTG, showing that the depletion of the gene products encoded by the ykunNOP operon does not affect the synthesis of any essential nutrient (Fig. 2A). However, the Fld deficiency affected the desaturation reaction, similarly to that in the absence of Fer, since in the absence of IPTG the desaturation of palmitic acid was about 70% of the lipid desaturation activity found in the presence of the inducer (Table 4 and Fig. 1B).
Fig. 2.
Growth phenotypes and autoradiogram of the products of [14C]palmitate labeling of LSC28. (A) Strains JH642 (▴) and LSC28 (⋄) were grown as described in Materials and Methods. Cells were harvested, washed, and resuspended in fresh medium supplemented with 0.01% MIV. After ∼15 h of growth, 0.5 mM IPTG was added to a culture of strain LSC28 (dashed arrow). (B) Argentation-thin-layer chromatography analysis of fatty acid methyl esters. Cells were grown at 37°C as described as in A. At the growth stages indicated by the solid arrows in A, 2 ml of the cultures was challenged with 0.2 μCi [14C]palmitate and further shifted to 25°C for 2 h. The lipids were then extracted and transesterified, and the resulting methyl esters were separated by thin-layer chromatography silver-nitrate-impregnated silica plates. Lane 1, JH642; lanes 2 to 5, LC28.
Table 4.
Desaturase activities in B. subtilis strains depleted of Fldsa
| Fatty acid type | Mean % fatty acids ± SD |
|||||
|---|---|---|---|---|---|---|
| JH642 | LSC9 −IPTG | LSC28 |
||||
| −IPTG | −IPTG | −IPTG | +IPTG | |||
| SFAs | 51 ± 7 | 66 ± 6 | 62 ± 4 | 97.5 ± 0.5 | 99 ± 1 | 70 ± 4 |
| UFAs | 49 ± 7 | 34 ± 6 | 38 ± 4 | 2.5 ± 0.5 | 1 ± 1 | 30 ± 4 |
Cells were grown to log phase at 37°C in MM-MIV, and LSC28 was grown with or without IPTG. Two-milliliter samples were labeled with 0.2 μCi [14C]palmitate at 25°C for 2 h. Labeled fatty acids were extracted, fractionated, and quantified as described in Table 3. Values are the means of data from five independent experiments and are expressed as percentages of total fatty acids. SFAs, saturated fatty acids; UFA, unsaturated fatty acids.
Lipid desaturation is blocked in the absence of Fer and Fld.
The above-described results indicate that both Fer and Flds act as electron donors for Δ5-Des. Nevertheless, it was still possible that another not-yet-identified protein, in addition to Fer and Flds, could also act as an electron donor for fatty acid desaturation in B. subtilis. To test this possibility, we constructed strain LSC28, a derivative of B. subtilis strain L43 which lacks Fer, and conditionally expresses the ykuNOP operon under the control of the Pspac inducible promoter. This strain was able to grow in rich LB medium or MM-MIV supplemented with IPTG (data not shown). However, the removal of the inducer resulted in no growth in either rich medium or MM-MIV, indicating that the absence of Fer and Flds is lethal for B. subtilis (data not shown). It should be noted that the removal of IPTG did not result in the immediate cessation of growth, but rather, cell proliferation continued until the preexisting proteins were diluted out by subsequent cell division, as illustrated in Fig. 2A. The addition of IPTG when the cell density reached a plateau reestablished the growth of strain LSC28, indicating that the cessation of proliferation was due to the depletion of the ykuNOP gene products and the absence of Fer. Lipid desaturation in strain LSC28 was assessed by the labeling of the cells with [14C]palmitate during time periods when the gene products were becoming limiting to growth, as indicated in Fig. 2A. In the absence of the inducer the fatty acid desaturation decreased progressively, reaching values that were about 2% of the total radioactivity incorporated into fatty acids. When IPTG was added, UFA synthesis was reestablished (Table 4 and Fig. 2B). These results indicate that lipid desaturation in B. subtilis is almost completely abolished in the absence of Fer and the two Flds.
The ability of each B. subtilis Fld to support lipid desaturation was assessed by complementation analysis. Constructs in which either ykuN or ykuP was placed under the control of a xylose-inducible promoter (Pxyl) were introduced into fer ykuNOP strain LSC28, giving strains LSC29 and LSC30, respectively. The xylose induction of ykuN or ykuP in these strains allowed growth in MM-MIV and the desaturation of labeled palmitic acid (Table 5). These experiments demonstrated that both flavodoxins are competent redox partners for the lipid desaturation reaction in B. subtilis, although YkuP was particularly good at supporting electron transfer and catalysis by Δ5-Des (Table 5).
Table 5.
Desaturase activities in B. subtilis strainsa
| Fatty acid type | Mean % fatty acids ± SD |
||||||
|---|---|---|---|---|---|---|---|
| JH642 | LSC29 |
LSC30 |
|||||
| No supplement | +Xyl | +IPTG | No supplement | +Xyl | +IPTG | ||
| SFAs | 50 ± 3 | 94 ± 4 | 85 ± 3 | 71 ± 3 | 93 ± 4 | 66 ± 4 | 69 ± 2 |
| UFAs | 50 ± 3 | 6 ± 4 | 15 ± 3 | 29 ± 3 | 7 ± 4 | 34 ± 4 | 31 ± 2 |
Cells were grown to log phase at 37°C in MM-MIV with 1% xylose (Xyl) or 0.5 mM IPTG as indicated. Two-milliliter samples were labeled with 0.2 μCi [14C]palmitate at 25°C for 2 h. Labeled fatty acids were extracted, fractionated, and quantified as described in Table 3. Values are the means of data from five independent experiments and are expressed as percentages of total fatty acids. SFAs, saturated fatty acids; UFA, unsaturated fatty acids.
DISCUSSION
The B. subtilis Δ5-Des is an attractive enzyme for the study of the structure-function relationship of membrane-bound acyl-lipid desaturases. In contrast to the soluble desaturases, integral membrane desaturases are notoriously recalcitrant to overexpression in heterologous systems and, with notable exceptions, have been very difficult to purify. Moreover, the electron donors of most members of this important family of proteins have not yet been identified because of the difficulties associated with assays of their in vitro activities. We therefore employed a series of in vivo approaches to establish the electron donors of Δ5-Des. In this report we describe that Fer or Fld is a catalytically competent electron donor for Δ5-Des during the conversion of palmitic acid to palmitoleic acid. Thus, this is the first report describing the electron donors of a bacterial desaturase other than those of cyanobacteria (11, 29) and the first to identify a flavoprotein that can transfer electrons to a lipid desaturase and, in conjunction with Fer, support the desaturation of B. subtilis lipids.
The fer gene has not been considered essential based on the results of the systematic genome-wide inactivation of B. subtilis genes in a study performed by using a standard laboratory rich LB medium (12). During this work aimed at identifying the electron donors of Δ5-Des, we found that a null mutant in fer is associated with an amino acid metabolism deficiency for isoleucine, valine, and methionine only in MM. Why does the deficiency in Fer lead to this phenotype in B. subtilis? Two members of the Adx/Pdx subfamily of [2Fe-2S] cluster proteins, ferredoxin (Fdx) in E. coli and Yah1 in yeast mitochondria, have attracted much attention in recent years (for recent reviews, see references 8 and 14). Genetic studies have shown that the disruption of E. coli fdx or the depletion of yeast Yah1p results in a marked decrease in the activities of various Fe-S proteins. Thus, the deficiency of Fer in B. subtilis could affect the activity of the ilvB gene product, dihydroxy acid dehydratase, which is an Fe-S protein that is essential for the biosynthesis of branched-chain amino acids, such as isoleucine and valine. On the other hand, it was reported that E. coli cells subjected to transient oxidative stress during growth in MM develop methionine auxotrophy, due to the inactivation of the enzyme that catalyzes the last step of methionine biosynthesis, cobalamine-independent methionine synthase (MetE). It has been well established that the absence of ferredoxin increases susceptibility to oxidative stress in plants (31). Thus, it is likely that B. subtilis fer mutants are also more sensitive to oxidative stress under nonideal growth conditions, such as nutrient limitation, leading to the inactivation of MetE.
Fld enzymes are found in a wide range of bacteria and eukaryotic algae and act as electron carriers between other redox proteins. The two Flds in B. subtilis have recently been characterized and were shown to support lipid hydroxylation by B. subtilis cytochrome P450 BioI and nitric acid production by nitric oxide synthase (13, 30), respectively. In the present study we found that the two Fld enzymes from B. subtilis were isofunctional with Fer, supporting electron transfer and catalysis by Δ5-Des. Nevertheless, the function of Fer in the biosynthesis of branched-chain amino acids and methionine seems to be poorly replaced by the two Flds. This assumption is derived from the observation that a fer strain reassumes exponential growth in MM after a lag phase of 20 h, suggesting that this prolonged lag phase is required for the accumulation of Flds to fulfill the critical role of Fer in amino acid biosynthesis. Therefore, our results indicate that in B. subtilis, Fld and Fer cannot interact with the same redox partners with equivalent efficiencies. More importantly, our findings demonstrate that both Fer and Fld are the physiological electron donor system for UFA biosynthesis in B. subtilis.
The expression of Δ5-Des is a highly regulated environmental process dependent on a two component-regulatory system (15). It remains to be determined whether the binding of Fer or Fld to Δ5-Des is a dynamic process that could potentially contribute to the regulation of the catalytic complex and mechanism of activity of the enzyme under different environmental conditions.
ACKNOWLEDGMENTS
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Agencia Nacional de Promoción Científica y Tecnológica (FONCYT). L.C.C. is a fellow of CONICET, and S.G.A. and D.M. are career investigators from the same institution. D.M. is an international research scholar of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 10 June 2011.
REFERENCES
- 1. Aguilar P. S., Lopez P., de Mendoza D. 1998. A Bacillus subtilis gene induced by cold shock encodes a membrane phospholipid desaturase. J. Bacteriol. 180:2194–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguilar P. S., Lopez P., de Mendoza D. 1999. Transcriptional control of the low-temperature-inducible des gene, encoding the Δ5 desaturase of Bacillus subtilis. J. Bacteriol. 181:7028–7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aguilar P. S., de Mendoza D. 2006. Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Mol. Microbiol. 62:1507–1514 [DOI] [PubMed] [Google Scholar]
- 4. Altabe S. G., Aguilar P., Caballero G. M., de Mendoza D. 2003. The Bacillus subtilis acyl lipid desaturase is a Δ5 desaturase. J. Bacteriol. 185:3228–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917 [DOI] [PubMed] [Google Scholar]
- 6. Diaz A. R., Mansilla M. C., Vila A. J., de Mendoza D. 2002. Membrane topology of the acyl-lipid desaturase from Bacillus subtilis. J. Biol. Chem. 277:48099–48106 [DOI] [PubMed] [Google Scholar]
- 7. Dubnau D., Davidoff-Abelson R. 1971. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor recipient complex. J. Mol. Biol. 56:209–221 [DOI] [PubMed] [Google Scholar]
- 8. Ewen K. M., Kleser M., Bernhardt R. 2011. Adrenodoxin: the archetype of vertebrate-type [2Fe-2S] cluster ferredoxins. Biochim. Biophys. Acta 1814:111–125 [DOI] [PubMed] [Google Scholar]
- 9. Green A. J., et al. 2003. Expression, purification and characterization of a Bacillus subtilis ferredoxin: potential electron transfer donor to cytochrome P450 BioI. J. Inorg. Biochem. 93:92–99 [DOI] [PubMed] [Google Scholar]
- 10. Harwood C. R., Cuttings S. M. (ed.). 1990. Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England [Google Scholar]
- 11. Hongsthong A., et al. 2006. Revealing the complementation of ferredoxin by cytochrome b5 in the Spirulina-Δ6-desaturation reaction by N-terminal fusion and coexpression of the fungal-cytochrome b5 domain and Spirulina-Δ6-acyl-lipid desaturase. Appl. Microbiol. Biotechnol. 72:1192–1201 [DOI] [PubMed] [Google Scholar]
- 12. Kobayashi K., et al. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. U. S. A. 100:4678–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawson R. J., von Wachenfeldt C., Haq I., Perkins J., Munro A. W. 2004. Expression and characterization of the two flavodoxin proteins of Bacillus subtilis, YkuN and YkuP: biophysical properties and interactions with cytochrome P450 BioI. Biochemistry 43:12390–12409 [DOI] [PubMed] [Google Scholar]
- 14. Lill R. 2009. Function and biogenesis of iron-sulphur proteins. Nature 460:831–838 [DOI] [PubMed] [Google Scholar]
- 15. Mansilla M. C., de Mendoza D. 2005. The Bacillus subtilis desaturase: a model to understand phospholipid modification and temperature sensing. Arch. Microbiol. 183:229–235 [DOI] [PubMed] [Google Scholar]
- 16. Miller J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 17. Perego M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615–624 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, DC [Google Scholar]
- 18. Petrini G. A., Altabe S. G., Uttaro A. D. 2004. Trypanosoma brucei oleate desaturase may use a cytochrome b5-like domain in another desaturase as an electron donor. Eur. J. Biochem. 271:1079–1086 [DOI] [PubMed] [Google Scholar]
- 19. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 20. Sancho J. 2006. Flavodoxins: sequence, folding, binding, function and beyond. Cell. Mol. Life Sci. 63:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sekiguchi J., Takada N., Okada H. 1975. Genes affecting the productivity of α-amylase in Bacillus subtilis Marburg. J. Bacteriol. 121:688–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shanklin J., Somerville C. 1991. Stearoyl-acyl-carrier-protein desaturase from higher plants is structurally unrelated to the animal and fungal homologs. Proc. Natl. Acad. Sci. U. S. A. 88:2510–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shanklin J., Whittle E., Fox B. G. 1994. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787–12794 [DOI] [PubMed] [Google Scholar]
- 24. Shanklin J. E., Cahoon E. B. 1998. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49:611–641 [DOI] [PubMed] [Google Scholar]
- 25. Sperling P., Ternes P., Zank T. K., Heinz E. 2003. The evolution of desaturases. Prostaglandins Leukot. Essent. Fatty Acids 68:73–95 [DOI] [PubMed] [Google Scholar]
- 26. Spizizen J. 1958. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. Natl. Acad. Sci. U. S. A. 44:1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stukey J. E., McDonough V. M., Martin C. E. 1990. The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 265:20144–20149 [PubMed] [Google Scholar]
- 28. Vagner V., Dervyn E., Ehrlich S. D. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097–3104 [DOI] [PubMed] [Google Scholar]
- 29. Wada H., Schmidt H., Heinz E., Murata N. 1993. In vitro ferredoxin-dependent desaturation of fatty acids in cyanobacterial thylakoid membranes. J. Bacteriol. 175:544–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Z. Q., et al. 2007. Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase. J. Biol. Chem. 282:2196–2202 [DOI] [PubMed] [Google Scholar]
- 31. Zurbriggen M. D., Tognetti V. B., Carrillo N. 2007. Stress-inducible flavodoxin from photosynthetic microorganisms. The mystery of flavodoxin loss from the plant genome. IUBMB Life 59:355–360 [DOI] [PubMed] [Google Scholar]