Abstract
The quorum regulatory cascade is poorly characterized in Vibrio parahaemolyticus, in part because swarming and virulence factors—the hallmarks of the organism—are repressed by this scheme of gene control, and quorum sensing seems to be silenced in many isolates. In these studies, we examine a swarming-proficient, virulent strain and identify an altered-function allele of the quorum regulator luxO that is demonstrated to produce a constitutively active mimic of LuxO∼P. We find that LuxO* affects the expression of three small regulatory RNAs (Qrrs) and the activity of a translational fusion in opaR, the output regulator. Tests for epistasis showed that luxO* is dominant over luxO and that opaR is dominant over luxO. Thus, information flow through the central elements of the V. parahaemolyticus quorum pathway is proven for the first time. Quorum-sensing output was explored using microarray profiling: the OpaR regulon encompasses ∼5.2% of the genome. OpaR represses the surface-sensing and type III secretion system 1 (T3SS1) regulons. One novel discovery is that OpaR strongly and oppositely regulates two type VI secretion systems (T6SS). New functional consequences of OpaR control were demonstrated: OpaR increases the cellular cyclic di-GMP (c-di-GMP) level, positively controls chitin-induced DNA competency, and profoundly blocks cytotoxicity toward host cells. In expanding the previously known quorum effects beyond the induction of the capsule and the repression of swarming to elucidate the global scope of genes in the OpaR regulon, this study yields many clues to distinguishing traits of this Vibrio species; it underscores the profoundly divergent survival strategies of the quorum On/Off phase variants.
INTRODUCTION
Many members of the Vibrionaceae are well known for their capacities to communicate and to control group activities such as biofilm formation, virulence, and luminescence via cell-to-cell signaling (reviewed in reference 48). However, quorum sensing has not been intensively investigated in Vibrio parahaemolyticus. This ubiquitous marine organism is the leading worldwide cause of seafood-borne gastroenteritis (43, 47, 60). Although its pathogenic strategies are not well understood, V. parahaemolyticus possesses a powerful arsenal of potential virulence factors, including proteases, hemolysins, two type VI secretion systems (T6SS1 and T6SS2), and two type III secretion systems (T3SS1 and TSS2) (37). The two T3SS, which are specially designed to inject effector virulence factors into eukaryotic host cells, have garnered much attention recently and have been shown to play distinct and critical roles in the pathogenicity of the organism (9, 27). Another hallmark of the organism is a marked proficiency at surface colonization, which is determined by its vigorous capacity to swarm and form robust biofilms (reviewed in reference 39). Our lack of knowledge about quorum sensing in V. parahaemolyticus stems in part from the fact that the archetypal V. parahaemolyticus strains appear defective in cell density-dependent regulation. Specifically, evidence suggests that the quorum pathway represses the two most characteristic traits of the species, swarming and virulence, and that phase variation in the quorum pathway selects for the surface-mobile and pathogenic cell type (26, 28, 40).
Nevertheless, in all genomes that have been sequenced, Vibrio species share similarity in the generally conserved central components of the quorum-sensing pathway. Moreover, for the species that have been examined, a paradigm of information flow through this pathway seems to be preserved (reviewed in reference 63). At a low cell density, when the concentrations of autoinducer molecules are also low, sensor histidine kinases phosphorylate the σ54-dependent LuxO regulator via the small histidine phosphorelay protein LuxU. LuxO∼P induces transcription of small quorum-regulatory RNAs (Qrrs), and the Qrrs destabilize the mRNA for the central output regulator of the system. At a high cell density in the presence of autoinducers, the histidine kinases become LuxO phosphatases, resulting in an inactive form of LuxO. The Qrrs are no longer transcribed, and the mRNA for the central output regulator is then translated. Although the backbone of the quorum-sensing system seems to work similarly in the vibrios that have been studied, the organisms differ with respect to the number and kinds of autoinducers and cognate sensory receptor kinases, the number of Qrr genes, and the kinds of genes in the output regulon (reviewed in reference 46).
The feature that is most distinguishing among the Vibrio species is the composition of the output regulon. This is reflected in the diverse names that have been assigned to the central terminal output regulator. The best known is V. harveyi LuxR, and its hallmark target is luminescence (55). Other characterized orthologs include V. cholerae HapR (hemagglutinin [HA]/protease), V. fischeri LitR (light and symbioses), V. anguillarum VanT (protease, pigment, and biofilm), V. vulnificus SmcR (starvation metalloprotease), V. tubiashii VtpR (multiple metalloproteases), and V. parahaemolyticus OpaR (colony opacity) (10, 16, 24, 29, 40, 42). Many of these quorum-controlled genes are pertinent to social activities such as biofilm formation and virulence (reviewed in reference 48); however, even the direction of output regulation can differ among the Vibrio spp. For example, HapR represses the expression of the extracellular polysaccharide locus (vps) in V. cholerae, whereas LitR and OpaR induce the expression of extracellular polysaccharide in V. fischeri and V. parahaemolyticus (reviewed in reference 74).
V. parahaemolyticus undergoes reversible phase variation, resulting in different colony morphologies: opaque (OP) and translucent (TR) (39). OP strains form mounded, opaque colonies, do not swarm, and possess a thick capsule. This capsular polysaccharide (CPS) determines colony opacity and “stickiness,” which is apparent, for example, when a colony is touched with a toothpick. Producing less CPS, the TR cell type is not sticky and forms flat, translucent colonies. TR strains are swarming proficient. Although both OP and TR strains form robust biofilms, their architecture and structural integrity are different because of differences in the amounts of CPS and other cell surface molecules (13, 14).
In strain BB22, the OP/TR phenotypic variation has been shown to be a consequence of alteration of the quorum pathway. Some TR strains have been found to contain genetic alterations in the opaR locus, specifically, small insertions and deletions (13, 40). These TR strains can be complemented with a functional copy of opaR, and the phenotype then converts to OP; conversely, targeted introduction of a mutation in opaR is sufficient to convert the OP phenotype to TR (40). OpaR represses the expression of the lateral flagellar genes and induces cps expression (21, 28). There is some evidence that OpaR regulates T3SS1 (26) and that regulation of swarming and T3SS gene expression are linked (19, 20). In Vibrio harveyi, LuxR has been demonstrated to repress the T3SS; it does so by directly binding the regulatory region controlling the expression of exsA, which encodes the master transcriptional regulator of the T3SS (26, 66). In V. parahaemolyticus BB22, OpaR is inferred to work similarly, because a T3SS1 effector, VopD, could be detected in concentrated cell-free culture fluids prepared from a TR strain bearing an insertion in opaR, but could not be detected in the OP opaR+ strain, by using an antibody directed against V. harveyi VopD (26).
In this work we examine the quorum pathway in V. parahaemolyticus by probing the function of the upstream elements that regulate opaR and the output targets regulated by OpaR. Until now, we have been stymied in dissecting this pathway, due in part to the nature of its central wiring: loss of function of luxO caused no discernible phenotype with respect to swarming, biofilm formation, or lateral flagellar or cps gene expression (L. McCarter and S. Jaques, unpublished data). Though consistent with the predictions of the paradigm, because the high-cell-density phenotype is in fact a functionally LuxO− phenotype due to lack of phosphorylation, these were negative results. Our findings in this report originate in the characterization of a TR variant that was found by sequencing to contain an intact opaR locus. This swarming-proficient, highly cytotoxic strain is demonstrated to produce an altered form of LuxO (LuxO*) that behaves in a constitutively active manner. By using various combinations of mutations, we tested and confirmed elements of the paradigmatic vibrio quorum-sensing circuit. Transcriptional profiling illuminated the scope of the OpaR regulon, and new quorum-controlled activities were examined. Thus, this work provides the first evidence of information flow through the quorum-sensing pathway in V. parahaemolyticus and offers insight into the scope of activities controlled by this signaling cascade.
MATERIALS AND METHODS
Bacterial strains, media, and nomenclature.
The bacterial strains and plasmids used in this work are described in Table 1. The V. parahaemolyticus strains are derivatives of BB22 (5). V. harveyi BB7 was obtained from Michael Silverman (4). The Vibrio strains were routinely grown at 30°C on plates unless otherwise indicated. Heart infusion (HI) broth contained 25 g/liter heart infusion (Difco) and 15 g/liter NaCl; heart infusion plates contained HI broth with 20 g/liter granulated agar (Difco) for isolating colonies and examining OP/TR colony morphology and 15 g/liter Bacto agar (Difco) for promoting swarming motility. Congo red medium contained 25 g/liter heart infusion, 15 g/liter granulated agar, 2.5 mM CaCl2, and 250 mg/liter Congo red. The calcium and dye (solubilized in ethanol) were added after the medium was autoclaved. Supplements were used at the following concentrations: 75 μg/ml kanamycin, 25 μg/ml gentamicin, and 10 μg/ml tetracycline.
Table 1.
Bacterial strains, plasmids, and primers
| Strain, plasmid, or primer | Genotype, description,a or sequenceb | Parent, reference, or construction |
|---|---|---|
| Strains | ||
| BB7 | Vibrio harveyi | 55 |
| BB22 | V. parahaemolyticus | 5 |
| LM1017 | luxO1017 flgB313L::lux (luxO* constitutive allele) (TR) | 41 |
| LM4476 | luxO1017 (TR) | LM1017 × pLM1776 (allelic replacement) |
| LM5312 | “Wild type” (OP) | BB22 |
| LM5431 | opaR2 (TR) | 59 |
| LM5392 | flaM1P::EzKan opaR14 (TR) | LM5314 |
| LM5674 | ΔopaR1 (TR) | BB22c |
| LM5738 | flgB1L::Tn5lux(Kanr) opaR2 (TR) | LM5431d |
| LM5949 | ΔopaR1flgCL3061::lacZ (Camr) (TR) | LM5674e |
| LM6633 | “Wild type” (OP) | LM5674 × pLM1950 (allelic replacement) |
| LM6693 | flgB1L::lux(Kanr) (OP) | LM5738 × pLM1950 (allelic replacement) |
| LM6820 | ΔopaR1 ΔswrT2::lacZ (Camr) (TR) | LM5674 |
| LM7595 | LM6693/pLM3286 | |
| LM7919 | BB7/pLM1877 | |
| LM7920 | BB7/pLM3286 | |
| LM7921 | BB7/pLM3942 | |
| LM7956 | LM6693/pLM1877 | |
| LM8602 | ΔopaR1flgCL3061::lacZ (Camr) scrC292::Tn5lux(Kanr) (TR) | 15 |
| LM9513 | LM5674/pLM3286 | |
| LM9514 | LM5674/pLM1877 | |
| LM9515 | LM4476/pLM3286 | |
| LM9516 | LM4476/pLM1877 | |
| LM9651 | LM6633/pLM3942 | |
| LM9652 | LM6633/pLM3286 | |
| LM9653 | LM6633/pLM1877 | |
| LM9655 | LM6693/pLM3942 | |
| LM9656 | LM5674/pLM3942 | |
| LM9688 | ΔluxO::Genr (OP) | LM4476 × pLM3294 (allelic replacement) |
| LM9736 | flgB313L::lux luxO1017opaR3282::TnlacZ/in (Camr) (TR) | LM1017 × pLM3282 (allelic replacement) |
| LM9738 | opaR3282::TnlacZ/in (TR) | LM5312 × pLM3282 (allelic replacement) |
| LM9752 | luxO1017 opaR3282::TnlacZ/in (TR) | LM4476 × pLM3282 (allelic replacement) |
| LM9757 | flgB313L::lux ΔluxO::Genr (OP) | LM1017 × pLM3294 (allelic replacement) |
| LM9847 | ΔluxO::Genr (OP) | LM5312 × pLM3294 (allelic replacement) |
| LM9849 | ΔopaR1 ΔluxO::Genr (TR) | LM5674 × pLM3294 (allelic replacement) |
| LM9903 | ΔluxO::GenropaR3282::TnlacZ/in (TR) | LM9688 × pLM3282 (allelic replacement) |
| LM9946 | flgCL3061::lacZ (Camr) scrC292::Tn5lux(Kanr) (OP) | LM8602 × pLM1950 (allelic replacement) |
| LM10036 | ΔopaR1 VPA1435::Tn5lux(Kanr) ΔswrT2::lacZ (Camr) (TR) | LM6820f |
| LM10037 | VPA1435::Tn5lux(Kanr) ΔswrT2::lacZ (Camr) (OP) | LM10036 × pLM1950 (allelic replacement) |
| Plasmids | ||
| pLM1776 | TetrflgBL+ cosmid | 59 |
| pLM1877 | Genr Apr IPTG-inducible expression vector | 8 |
| pLM1950 | Tetr cosmid containing opaR locus from LM5312 (opaR+) | 40 |
| pLM3230 | Tetr cosmid containing 4-kb luxO locus from LM5674 (luxO+) | pLAFR3 |
| pLM3282 | opaR3282::TnlacZ/in (Camr) | pLM1950 |
| pLM3286 | Genr Apr IPTG-inducible luxOU operon from LM5674 (luxO+) | pLM1877 |
| pLM3294 | ΔluxO::Genr | pLM3230 |
| pLM3942 | Genr Apr IPTG-inducible luxOU operon from LM1017 (luxO*) | pLM1877 |
| Primers | ||
| Jkluxofbam | CTTGGATCCGCAAAGCGTAATGCGATTATTG | |
| Jkluxorpst | CTGCTGCAGCACTAAGTAGCCACTTGAGACTGC | |
| Δluxogen1 | GATCGACATTAATATTGTCGGTACAGGTAGAGATGCCATTGAAAGTCTCAATCATCGTGTAGGCTGGAGCTGCTTC | |
| Δluxogen2 | CATTCCATGCTTGTAGTTTGCGGTAAATCGTTGACGGACTGACATCAAGATACCCAGCGCATATGAATATCCTCCTTAG | |
| VP1386for | CCAGTCAATGGTGCAAAAATTGAGG | |
| VP1386rev | CGAAACTGTACATCAGCTAAATGC | |
| VPA1026for | GGCATTGTCTCTGCTGCAAAGCTCG | |
| VPA1026rev | GCCAAGACATATTCTTTTCCTACTCGC | |
| VP2232for | CTGTGTTGGTTAAGCGTATTGCTCAC | |
| VP2232rev | CGTTGCATCGCTTGGCTTAAGTATTTG |
Gene deletions were constructed by using the phage lambda Red recombination system on plasmids carrying V. parahaemolyticus DNA in Escherichia coli (12). The ΔluxO mutation was created by deleting 1,094 nucleotides of the coding region and inserting a gentamicin resistance cassette that was amplified from pUCGM (54). The gentamicin cassette and luxO are transcribed in the same direction. The translational fusion in opaR was obtained by mutagenizing an opaR-containing plasmid in E. coli with TnlacZ/in (38). The site of insertion, identified by sequencing, occurred at nucleotide 322. Mutations were transferred to the V. parahaemolyticus chromosome after conjugation to introduce the plasmid bearing the mutant allele via allelic replacement. Introduction of the opaR::Tn mutation into OP strain LM5312 to make strain LM9738 produced the TR, swarming-proficient phenotype (data not shown). With a similar allelic exchange strategy, chromosomal mutations were repaired by transfer of a cosmid bearing the wild-type locus and serial passage to allow recombinational repair (and subsequent loss of the cosmid). In this manner, the ΔopaR1 allele in strain LM5674 was repaired using cosmid pLM1950 to make strain LM6633, and the laf::lux reporter in strain LM1017 was repaired using cosmid pLM1776 to make strain LM4476. All strain constructions were verified by PCR.
The luxOU operons were cloned using Phusion high-fidelity DNA polymerase (New England Biolabs) and the pLM1877 vector, which contains an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter (8). There is some expression from this promoter in the absence of IPTG, so IPTG was not used for the expression experiments described here. The expression plasmids were sequenced and compared to sequences derived directly from PCRs performed on chromosomal DNA. The luxOU operon was cloned prior to our sequencing of the BB22 locus, and the cloning primers were designed according to the annotated sequence; upon analysis of the region after sequencing, we noted the potential for an earlier start site for the coding region: 14 amino acids (aa) upstream in our strain BB22 (as well as in the sequenced RIMD strain).
Microarray growth conditions.
A single colony was streaked as a lawn onto an HI plate. The following day, 5 ml of HI broth was used to suspend the cells on the plate, and the cells were then diluted to an optical density at 600 nm (OD600) of 0.05. Fifty microliters of the diluted cells was spread onto an HI plate. After 6 h of growth, cells were harvested from the plate and diluted to an OD600 of 1.0 by using RNAprotect (Qiagen) that had been diluted 2-fold in 1× Dulbecco's phosphate-buffered saline (DPBS), pH 7.1 (Gibco). Samples were checked for phase variation by plating for single colonies to visualize OP and TR colony morphology, and the frequency of phase shifting was less than 1%. Protein samples were analyzed by immunoblotting for TR-specific lateral and constitutive polar flagellin production. RNA samples were analyzed for polar and lateral flagellin gene expression using primers specific for polar and lateral fliA genes (20).
Microarray analyses.
RNA isolation, cDNA synthesis, labeling, and hybridization have been described elsewhere, as has the custom Affymetrix GeneChip (20). Samples (∼4.5 μg) were hybridized to the chips in the DNA Core Facility at the University of Iowa according to the standard Affymetrix protocols for E. coli. The GeneChip (rhofispaa52026F; feature size, 11 μm) was designed using the V. parahaemolyticus RIMD2210633 genome. Chip annotation generally conforms to original genome annotation, and tag numbers were assigned VP numbers for chromosome 1 and VPA numbers for chromosome 2. Additional probe sets were designed for some intergenic regions, including the 5 quorum-regulating RNAs and some other potential coding regions not assigned in the original annotation. V. parahaemolyticus strain BB22, with which these studies were performed, has not been sequenced; however, probing of the Affymetrix GeneChip with genomic BB22 DNA yielded present calls for ∼98% of the predicted open reading frames (ORFs). It seems reasonable to conclude that these transcriptome studies provide a good reflection of genome-wide expression patterns in BB22, although some fraction of gene activity could not be assessed.
Each condition used to query the chips was repeated at least 2 times using independently isolated RNA samples. Analysis was done using the Affymetrix GeneChip operating software (version 1.2.1) and Affymetrix GeneChip genotyping analysis software (GTYPE, version 4.0). Using the raw signal output generated by GTYPE, processing was carried out, and comparisons were made, by using R, version 2.8.1, GUI 1.27 Tiger build 32-bit (5301) (http://www.r-project.org/) within Bioconductor software for bioinformatics (http://www.bioconductor.org) (18). The complete data set was preprocessed using the guanine cytosine robust multiarray analysis (GCRMA) method to perform optical adjustment, background adjustment, normalization, and summarization functions (71). Tests for differential expression were performed using an analysis of variance (ANOVA) model with a false discovery rate (FDR) of 0.03. The P and Q values for each differentially expressed gene are supplied in Table S1 in the supplemental material. A Q value of 0.01 for a gene means that if the requirements for statistical significance were lowered just enough to allow this gene to meet them, the overall FDR of the study would be 1% (6). Hierarchical clustering was performed using the normalized expression values for each gene and the Heatmap.2 package for R with Bioconductor.
RT-PCR.
Reverse transcriptase PCR (RT-PCR) was carried out using the Promega (Madison, WI) Access RT-PCR system according to the manufacturer's instructions except that 25-μl reaction mixtures were used. The annealing temperature was 56°C. Quality control reactions were performed to check RNA for DNA contamination using primers specific for the polar and lateral fliA genes. PCR was run for 30 cycles with a 1-min extension time. Each reaction mixture contained 50 ng of RNA and 4 primers, the gene-specific pair and a control pair of primers designed to amplify the constitutively expressed polar flagellar gene fliA. RNA samples were prepared from cells grown identically to, but independently of, those for the microarray experiments.
Coculture cytotoxicity assays.
Chinese hamster ovary (CHO) cells (ATCC CCL-61) were used in coculture experiments as previously described (19). Briefly, the bacteria were grown overnight on HI plates at 30°C. The bacterial cells were harvested from plates and were suspended in prewarmed Ham's F-12 medium (Gibco) at 1.5 × 106/ml to yield an approximate multiplicity of infection (MOI) of 15. The cocultures were incubated at 37°C under 5% CO2 for 5 ho. The bacterial inoculum was also serially diluted and plated in order to calculate the actual MOI and to monitor colony morphology. Cytotoxicity was assayed by measuring the release of lactate dehydrogenase (LDH) using the CytoTox 96 nonradioactive cytotoxicity kit (Promega Corp., Madison, WI). Five replicate wells were measured per data point. The percentage of lysis was calculated by comparison to total lysis obtained in control reactions using 0.9% Triton X-100; control wells without bacteria were used to calculate the background level of lysis. Maximal cytotoxicity for strain LM5674 was usually ∼70% of detergent-induced lysis.
β-Galactosidase and luminescence assays.
For plate-grown cells, strains were grown overnight on plates, suspended, and diluted to an OD600 of 0.05 in HI broth, and 50 μl was spread on plates with supplements as indicated in the figure legends. Cells were periodically harvested from these plates by suspension in 5 ml of medium. Activity in broth cultures was measured by periodically removing 0.5-ml aliquots from 25-ml cultures grown in 250-ml flasks. β-Galactosidase measurements were performed according to the method of Miller (45), except that Koch's lysis solution was used to permeabilize the cells (51). Bioluminescence was measured by using a TD20/22 luminometer (Turner Designs) and is reported as specific light units (SLU), which are relative total light units per minute per milliliter per OD600 unit. Assays were performed in triplicate, and each experiment was performed at least three times with similar results. P values were calculated by using Student's t test (2-tailed distribution with 2-sample, equal-variance calculations).
c-di-GMP measurements.
Cells were grown as described under “Microarray growth conditions” above. Sixteen OD600 units were extracted in 375 μl and were analyzed as described previously (20) by the Mass Spectrometry Facility at Michigan State University using liquid chromatography-tandem mass spectrometry (LC-MS-MS) on a Quattro Premier XE mass spectrometer (Waters) coupled with an Acquity Ultra Performance LC system (Waters). Cyclic di-GMP (c-di-GMP) was detected by electrospray ionization using multiple-reaction monitoring in negative-ion mode at m/z 689.16 → 344.31. In order to normalize OD600 to milligrams of protein extracted, the protein concentration was determined by using the Bradford method (Bio-Rad protein assay). A culture of known optical density grown as described under “Microarray growth conditions” above in liquid or on a surface was centrifuged, and the cell pellet was dissolved in 0.1 N NaOH by heating for 15 min at 95°C as described previously (57). The protein standard was bovine serum albumin (BSA). The conversion factors (± standard errors of the means) were 193 (±14.2) and 220 (±27.1) μg protein per OD600 unit for LM5674 and LM6633, respectively. The conversion factors were derived from 4 independent growth experiments and quadruplicate assays per experiment.
Chitin transformation of LM5312 and LM5674 with chromosomal DNA.
Chromosomal DNA was prepared from a 1-ml overnight culture of HI-grown strain LM5392 (flaM::Kanr) in 300 μl (68). Recipient strains (LM5674 or LM5312) were grown overnight in minimal marine medium (MMM) supplemented with 0.1% Casamino Acids, 5 mM MgSO4, and 0.5% chitin (final concentrations). MMM contained 1 g/liter K2SO4, 1.1 g/liter NH4Cl, 4.7 g/liter KH2PO4, 13.5 g/liter K2HPO4, and 20 g/liter NaCl. Chitin was prepared by a modification of the colloidal chitin procedure of Berger and Reynolds (7): instead of being ground during hydrolysis and filtered through glass wool, the chitin was serially decanted. Strains were subcultured by 1:100 dilution in 2.5 ml and were grown for 4 h with shaking at 30°C. DNA (20 μl at ∼100 to 200 ng/μl) was added to 0.5-ml aliquots of culture, and the tubes were incubated statically for 2 h at 30°C. Fresh medium (0.5 ml) without chitin was added, and cultures were incubated overnight with shaking at 30°C and were then plated on selective medium. Transformants were selected on HI plates with 75 μg/ml kanamycin, and introduction of the disrupted polar flagellar regulatory gene was verified by examination of the swimming motility phenotype (Swim−) and PCR. Chitin transformation experiments were repeated at least 3 times.
Statistical significance.
Statistical significance was tested by using Student's t test (2-tailed distribution with 2-sample, equal-variance calculations).
Accession numbers.
The microarray data have been submitted to the NCBI and have been assigned GEO accession number GSE28216. The GenBank accession numbers for the DNA sequences of the luxO genes from LM5312 and LM1017 are JF317958 and JF317959, respectively.
RESULTS
Not all TR strains have defects in opaR.
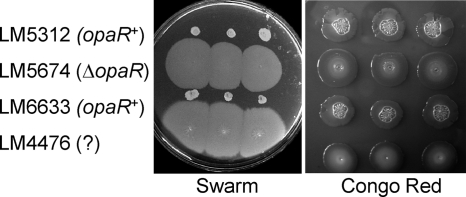
Strain BB22, which was isolated in Bangladesh prior to 1983, switches reversibly between the OP and TR colony types. Prior work demonstrated that mutations in the opaR locus of strain BB22 cause the TR phenotype. Specifically, spontaneous phase variants arising as translucent flares from an OP colony can have insertion or deletion mutations in opaR, such as the 5-bp insertion in LM5431 (opaR2), the 10-bp deletion in LM5093 (ΔopaR6), and the 83-bp deletion in LM5674 (ΔopaR1) (13). Most aged TR colonies (though not LM5674, which seems fixed due to its large deletion) will show occasional conversion of cells to OP types upon restreaking. LM5312 is a representative BB22 OP strain. It is highly swarm defective and produces a mounded colony that is crinkly on a Congo red plate (Fig. 1, top row). In contrast, the TR strain LM5674 is swarming proficient and produces a flat colony on Congo red medium (Fig. 1, second row). Recombinational repair of LM5674 by using allelic exchange to introduce the opaR+ allele and make strain LM6633 (Fig. 1, third row) produced a strain phenotypically indistinguishable from LM5312.
Fig. 1.
Swarming motility and colony morphology of V. parahaemolyticus phase variants. Three single colonies from each strain—LM5312 (opaR+), LM5674 (ΔopaR), LM6633 (opaR+), and LM4476 (?)—were inoculated with a toothpick onto an HI swarm agar and Congo red medium. The swarm plates were photographed after overnight growth at 30°C. Congo red plates were incubated overnight at 30°C and were allowed to develop for 1 week at room temperature before photography. LM5312, LM5674, and LM4476 are spontaneous phase variants of strain BB22; strain LM6633 was constructed by allelic exchange to repair the opaR locus of strain LM5674. The genetic basis for the translucent phenotype of strain LM4476 was unknown; hence the “?” for its genotype. In this work, we demonstrate that strain LM4476 carries a missense mutation, designated luxO*.
However, we were intrigued by the fact that not all TR strains derived from OP strains have a defect that could be mapped to opaR. One such strain is the laf::lux reporter strain LM1017, which has been used by our laboratory for a number of years to study the regulation of swarming (41). LM4476 (Fig. 1, bottom row) is a congenic derivative of LM1017 that has been repaired by allelic exchange of the flgBL locus to swarming proficiency. Like opaR mutants, the strain is swarming competent and forms flat, translucent colonies on Congo red plates. Sequence analysis of the opaR locus of LM4476, obtained by using oligonucleotide primers specific for the flanking genes (hpt and lpd), revealed that this opaR sequence was identical to that of the OP strain LM5312 (AF035967.1).
The expression profile of LM4476 suggests that OpaR has been silenced.
To gain an understanding of the genome-wide differences between OP and TR strains, microarray profiling was performed. Strains were grown on plates and were harvested for RNA sampling at a time corresponding to the time of induction of the swarming regulon (20). Hierarchical clustering created the dendrogram in Fig. 2A, showing the similarity of the expression profiles. Independently isolated RNA samples were used for each hybridization query of a custom Affymetrix GeneChip. The analyses included 4 samples (a to d) of LM5674 (ΔopaR), 2 samples (a and b) of LM4476, and 2 samples (a and b) of LM6633 (opaR+). A single microarray hybridization (c) was also performed with RNA prepared from OP strain LM5312 (opaR+). LM4476 clustered with LM5674 (ΔopaR), whereas the two opaR+ strains, LM6633 and LM5312, clustered with each other, and their expression profiles were distant from that of either TR strain.
Fig. 2.
The transcriptome of LM4476 clusters with that of the ΔopaR strain and appears to silence OpaR. (A) Hierarchical clustering analysis. The microarray expression profiles were compared in the statistical computing program R using the Heatmap.2 package. Lowercase letters indicate that three biological replicates were carried out for opaR+ strains LM6633 (a and b) and LM5312 (c), two replicates for LM4476 (a and b), and four replicates for ΔopaR strain LM5674 (a to d). (B) Quorum-regulatory RNA expression. Each bar represents the averaged normalized log2 expression value for the indicated qrr gene. Of the 5 predicted small quorum-regulatory RNAs (Qrrs), 3 showed average expression levels for two biological replicate microarrays of LM4476 that were statistically significantly higher than the averages for four replicates of LM5674 (ΔopaR) or the averages for the three microarrays of LM6633 and LM5312 (opaR+). All P values were <0.002. Error bars indicate standard errors of the means. Data for Qrr1 and Qrr5 are not shown, because no significant changes in gene expression were detected. (C) The level of translation of opaR is decreased in LM4476. A translational fusion of lacZ to opaR was introduced into the chromosomes of LM5312 and LM4476, generating strains LM9738 and LM9752, respectively. β-Galactosidase activity was measured over a time course of growth on HI plates and is reported as Miller units at 6 h; the difference between the strains was statistically significant (P < 0.0001). Error bars represent standard deviations for triplicate samples in a single representative experiment. The experiment was repeated three times, and the results of a representative experiment are shown.
The phenotype and global gene expression profile of LM4476 were most like those of the ΔopaR strain; therefore, a likely hypothesis for its TR phenotype was that opaR was being silenced in some way other than by its mutation. The microarray data provided a key by revealing the altered expression of some of the small regulatory RNAs, the Qrrs. The V. parahaemolyticus genome contains 5 predicted Qrrs (36). The average log2 expression levels for three of these, Qrr2, Qrr3, and Qrr4, were elevated in LM4476 and were significantly different from those in LM5674 (ΔopaR) and the opaR+ strains LM5312 and LM6633 (Fig. 2B). (The values for the LM5312 and LM6633 arrays were averaged together.) Because the sequences of the qrr loci are quite similar and small (∼100 nucleotides), we note that it was not possible to generate specific probes for Qrr4. As a consequence, the Qrr4 probe set is likely to reflect some cumulative level of multiple Qrr transcripts.
The microarray data supported the notion that OpaR was being silenced in LM4476 due to an alteration in the upstream elements of the quorum-sensing pathway. To test this notion directly, a translational fusion of LacZ to amino acid 107 of OpaR (which is 202 aa long) was employed. The opaR::′lacZ fusion was recombined into the chromosomes of LM4476 and LM5312. The introduction of this allele had no effect on the TR phenotype of LMM4476 but converted the OP strain LM5312 to the TR phenotype (data not shown). β-Galactosidase levels, measured after 6 h of growth on plates, were 30-fold higher in the LM5312 background (strain LM9738) than in the LM4476 background (strain LM9752) (Fig. 2C).
The luxO allele of LM4476 is different from those of other strains.
In the paradigmatic vibrio model, the expression of the Qrrs is controlled by the σ54-dependent regulator LuxO. When LuxO is phosphorylated at a low cell density, the Qrrs are expressed, leading to destabilization of target mRNA encoding the terminal output regulator. Only at a high cell density, when LuxO is believed not to be phosphorylated and hence to be inactive, is there significant production of the output regulator. For V. harveyi, the output regulator is LuxR, and the consequence is luminescence at a high cell density. The elevated levels of Qrr expression observed in LM4476 and the correspondingly low level of the opaR::′lacZ fusion suggested that the LuxO protein might be altered in this strain. The luxO gene occurs in an operon with luxU, which encodes the small quorum phosphorelay protein. This operon was sequenced from LM5312 (opaR+), LM5674 (ΔopaR), and LM4476 (opaR+). The sequences of luxO from LM5312 and LM5674 were identical, and the LM4476 sequence contained one nucleotide change in luxO (A662C), resulting in a D221A substitution in the amino acid sequence. We refer to this allele as luxO*. The luxU alleles in the three strains were identical.
LuxO from LM4476 is constitutively active.
Taken together, the data suggest that the altered form of LuxO in LM4476 is a constitutively active variant of LuxO. To examine this possibility, the luxOU operons from LM4476 and LM5674 were cloned into an expression vector. The clones were transferred to Vibrio harveyi. Conservation of the central components of quorum sensing is high enough that heterologous complementation has been a useful tool for investigation of the Vibrio species. This, for example, is how opaR and hapR were identified (29, 40) and the functioning of the quorum pathway in V. cholerae explored (22, 36). Luminescence was measured over a time course of growth in liquid culture (Fig. 3A). The growth rates of the strains were similar. As the density of the V. harveyi culture increased, cultures with the vector control and the luxOU clone derived from LM5674 showed increasing luminescence, achieving maximal light outputs of ∼5,000,000 and 8,600,000 SLU (light units normalized to OD), respectively. These values were not significantly different (P > 0.01). In contrast, the strain overexpressing luxO*U derived from LM4476 reached a maximal light output of only 298 SLU. This observed degree of repression is consistent with the performance of site-directed mutations introduced into V. harveyi luxO that produce a mimic of LuxO∼P: strains bearing these mutations [luxOvh(D47E) and luxOvh(F94W)] produced 100,000- to 10,000-fold less light than wild-type or loss-of-function luxO strains (17).
Fig. 3.
The cloned luxO* operon elicits a low-cell-density response in V. harveyi and V. parahaemolyticus. (A) The luxO operon was cloned from LM5674 (luxO+) and LM4476 (luxO*) into the pLM1877 vector. The luxO expression plasmids were introduced into the wild-type V. harveyi strain BB7. Luminescence was measured periodically during growth in liquid culture. The luminescence normalized to the optical density (SLU) is plotted against the optical density of the liquid culture. (B) The luxO clones were also expressed in V. parahaemolyticus strain LM6693, an opaR+ strain with a luminescence reporter in the lateral flagellar gene flgBL. Luminescence was measured periodically during growth on plates. The luminescence normalized to the optical density (SLU) is plotted against the time of growth on plates. The growth of these strains was similar. Error bars represent standard deviations for triplicate samples in a representative experiment. The experiments were repeated three times with similar results. In both panels, the values for the luxO* clone were significantly different from those for the vector (P < 0.005 at all time points).
A similar experiment was conducted with V. parahaemolyticus. The expression clones were introduced into an OP strain carrying a lateral flagellar reporter (opaR+ laf::lux). In this case, strains were grown on plates and were harvested periodically to measure luminescence. The growth rates of the strains were similar. The OP strain was unable to express lateral flagellar genes: the vector control strain produced a maximal luminescence level of ∼4 SLU. Ectopic expression of luxOU elicited no effect: the luminescence profile of the strain carrying luxOU was similar to that of the strain with the vector. In contrast, luxO*U expression increased lateral flagellar gene expression to a maximal light value of ∼23,600 SLU (Fig. 3B). Ectopic expression of luxO*U cloned from LM4476, and not that of the clone derived from LM5674, was sufficient to induce a TR, swarming-proficient morphotype in the OP strain LM5312 (data not shown). Thus, as one would predict, because wild-type LuxO should be inactive at a high cell density due to lack of phosphorylation, the altered-function luxO* allele was dominant over the wild-type allele. In preventing luminescence in V. harveyi and inducing swarming gene expression in V. parahaemolyticus, V. parahaemolyticus LuxO* appeared to effectively silence the output regulators, the positively acting V. harveyi LuxR and the negatively acting V. parahaemolyticus OpaR.
LuxO-mediated regulation within the quorum-sensing pathway.
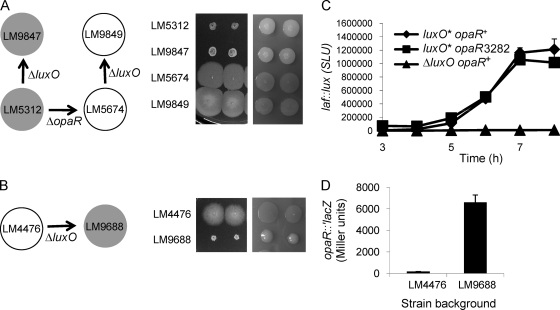
The experiments described above provide the first evidence of roles for the elements of the quorum-sensing pathway upstream of OpaR in V. parahaemolyticus, and they generally conform to the paradigmatic model. To test the model and further substantiate the V. parahaemolyticus pathway, a deletion mutation in luxO was introduced into various strains via allelic recombination. LM9847 (ΔluxO) was derived from the opaR+ strain LM5312, and its phenotype with respect to swarming ability and a mounded, opaque colony type is indistinguishable from that of the parental strain (Fig. 4A). Such a result was predicted and is consistent with the quorum-sensing model. At a high cell density, the σ54-dependent LuxO regulator is not phosphorylated and hence is inactive; therefore, the high-cell-density phenotype should be effectively equivalent to the deletion phenotype. Similarly, the introduction of the ΔluxO allele into the ΔopaR strain LM5674 to produce strain LM9849 had no effect on swarming or the flat, translucent colony phenotype (Fig. 4A). Therefore, opaR is epistatic to luxO.
Fig. 4.
Epistasis tests with luxO and opaR alleles. (A and B) (Left) Schemes of strain construction. Arrows indicate strain lineages; filled circles, strains with an OP colony type; open circles, strains with a TR colony type. (Right) Strains were grown on HI media permissive or restrictive for swarming (with low or high agar concentrations) (left and right panels, respectively). Shown are the swarming motilities and colony morphologies of opaR+ and ΔopaR strains (A) and of LM4476 (B) with and without the ΔluxO allele. (C) laf::lux gene expression in the LM1017 (luxO*) background. Strains LM1017 (luxO* opaR+ laf::lux), LM9736 (luxO* opaR3282 laf::lux), and LM9757 (ΔluxO opaR+ laf::lux) were grown on plates and were harvested periodically in order to measure luminescence, reported as SLU. The introduction of an opaR mutation into strain LM1017 to produce LM9736 had no significant effect on luminescence, whereas the luminescence profile of LM9757, which was derived from LM1017 by introducing the ΔluxO allele, was significantly different from those of the other strains (P < 0.001 at all time points). Error bars represent standard deviations of triplicate measurements in one representative experiment of three. (D) The introduction of the ΔluxO allele into LM4476 increases the level of OpaR translation. A translational fusion of lacZ to opaR was introduced into the chromosomes of LM4476 and LM9688, generating strains LM9752 and LM9903, respectively. β-Galactosidase activity was measured over a time course of growth on HI plates and is reported as Miller units at 6 h. The difference between strains was statistically significant (P < 0.0001). Error bars represent standard deviations of triplicate samples in a single representative experiment. The experiment was repeated three times, and the results of a representative experiment are shown.
In contrast, but as predicted, the swarming proficiency and translucent colony morphology of strain LM4476 (luxO*) was greatly affected by the introduction of the ΔluxO allele to produce strain LM9688 (Fig. 4B). LM9688 (ΔluxO) was severely defective in swarming and produced mounded, opaque colonies on swarm-restrictive medium. Mutations were also recombined into the chromosome of the laf::lux reporter strain LM1017, allowing the quantification of the effects on swarming gene expression (Fig. 4C). Luminescence in LM9757 (ΔluxO opaR+ laf::lux) was severely repressed compared to that in LM1017 (luxO* opaR+ laf::lux) After 7 h of growth, LM1017 produced ∼1,200,000 SLU, while LM9757 emitted ∼8,000 SLU. In contrast, the introduction of a mutation in opaR into LM1017 had little effect on laf::lux expression. The reporter strains LM1017 and LM9736 (luxO* opaR3282 laf::lux) displayed similar profiles of laf::lux induction during growth on surfaces. The introduction of an opaR mutation into the luxO* strain was superfluous, since OpaR was already being silenced in LM1017.
Consistent with the prediction from these phenotypes, the introduction of the ΔluxO allele into LM4476 relieved the repression of an opaR::′lacZ fusion that was recombined onto the chromosome (Fig. 4D). β-Galactosidase activity, measured after 6 h of growth on plates, was ∼35-fold higher in LM9903 (in which the luxO* allele was replaced with the ΔluxO allele) than in LM9752 (luxO*); the activity in LM9903 was similar to the degree of expression observed in LM9738 (LM5312 luxO+) (Fig. 2C). Thus, these results support the paradigmatic vibrio model for the role of LuxO and its relation to the other core elements of the quorum-signaling pathway.
Quorum-sensing output control: the OpaR regulon.
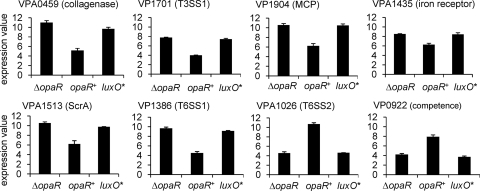
The microarray analyses provided insight into the conserved elements of the quorum-sensing pathway upstream of and controlling OpaR. It was also designed to begin to establish the output regulon, which is unique to each Vibrio species. By use of a 4-fold discriminator, 210 genes were expressed at higher levels and 113 genes were expressed at lower levels in the ΔopaR strain (LM5674) than in the opaR+ strains (LM6633 and LM5312). The complete list of differentially regulated genes (4-fold or greater change; FDR, 0.03) with P and Q values is provided in Table S1 in the supplemental material. A graphical representation of the expression profiles for selected genes is shown in Fig. 5. The profiles for the luxO* strain LM4476 are provided for comparison: they generally resemble those of the ΔopaR strain. The data are plotted as the normalized expression values (log2) for each strain and give an indication of significance for some genes, i.e., the magnitude of expression as well as the differences between strains. Genes expressed at high levels, such as those encoding lateral flagellin or ribosomal proteins, have expression values of ∼10 to 12, and genes that are not transcribed or are very poorly transcribed have values of ∼3 or lower. We note that many of the genes regulated by OpaR are expressed at high levels.
Fig. 5.
Microarray expression profiles of select OpaR-regulated genes. Bars indicate the average log2 normalized expression level of each gene in the ΔopaR (LM5674), opaR+ (LM6633 and LM5312), or luxO* (LM4476) strains. The array data for the two opaR+ strains examined were highly similar (see Fig. 2A), so expression values were averaged together (2 microarray experiments for LM6633 and 1 for LM5312). Error bars represent standard errors of the means.
The microarray data replicated gene expression patterns for gene sets that were known to be OpaR controlled, providing validation of the robustness of the transcriptome comparison. For example, genes in the capsular polysaccharide operon (VPA1403 to VPA1412) showed ∼5- to 8-fold induction by OpaR. This is consistent with the sticky, mounded colony phenotype of the OP cell type (Fig. 1) and with prior studies demonstrating that capsule and cps reporter gene levels are higher in OP (opaR+) than in TR (opaR-defective) strains (14, 21). Similarly, lateral flagellar genes were derepressed 14- to 229-fold in the ΔopaR strain. This degree of regulation is in keeping with the ability of TR and not OP strains to swarm over surfaces (Fig. 1) and with laf reporter expression data (28).
Recently, by examining a swarming-proficient strain (ΔopaR), we defined the surface-sensing regulon, a set of ∼70 genes that are specifically regulated by the polar flagellum-mediated surface-sensing mechanism (20). These genes are not expressed during growth in liquid culture; they include the lateral flagellar gene system and diverse nonflagellar genes, some of which encode potential sensory and virulence factors. These genes were OpaR controlled (see Table S1 in the supplemental material). For example, VPA0459, encoding a collagenase, showed ∼60-fold higher expression in the ΔopaR strain than in the opaR+ strain (Fig. 5). Thus, the comparison of the opaR+ to the ΔopaR transcriptome reveals an expanded role for OpaR: not only acting as the master negative regulator of the lateral flagellar gene system, but also controlling the entire surface-sensing regulon.
New members of the OpaR regulon include genes pertinent to c-di-GMP, T6SS, and competence.
Some of the newly identified members of the OpaR regulon were examined further (Fig. 6). One differentially regulated operon, VPA1435 to VPA1438, encodes a predicted TonB-dependent iron transport system, which was derepressed ∼5-fold in the ΔopaR cell type compared to the opaR+ strain (Fig. 5). OpaR control of this operon was confirmed by using a luminescence reporter fusion to VPA1435 (Fig. 6A). The ΔopaR strain containing VPA1435::lux produced ∼26-fold more light than the congenic opaR+ strain.
Fig. 6.
Examination of select OpaR-regulated genes. (A) Luminescence reporter in VPA1435, an iron acquisition gene, measured in strains LM10036 (ΔopaR) and LM10037 (opaR+) after 6 h of growth on plates. (B) Luminescence reporter in scrC, measured in strains LM8602 (ΔopaR) and LM9946 (opaR+) after 6 h of growth on plates. For both reporter gene analyses, the error bars represent standard deviations for triplicate samples in one representative experiment of three. Light emission was significantly different for the two strains (P < 0.0001 for each reporter). (C) Cyclic di-GMP levels measured in strains LM5674 (ΔopaR) and LM6633 (opaR+) after 6 h of growth on plates. The average concentrations of two replicates were calculated as picomoles per milligram of bacterial protein by using an experimentally derived conversion factor from optical density to milligrams of protein for each strain. Error bars represent standard deviations of the replicates. The differences in c-di-GMP levels between the two strains are statistically significant (P < 0.0001). (D) Chitin-induced competence. LM5674 (ΔopaR) and LM5312 (opaR+) were grown in medium with chitin and were transformed with chromosomal DNA (∼1 μg) prepared from the polar flagellar mutant strain LM539 (flaMP::Kanr). The y axis shows the average number of kanamycin-resistant colonies obtained from three independent transformations. Error bar, standard error. No Kanr transformants were obtained for LM5674. (E) Reverse transcriptase PCR of a T6SS1 gene (VP1386) and a T6SS2 gene (VPA1026) using RNA prepared from LM6633 (opaR+) and LM5674 (ΔopaR). Primers for the constitutively expressed polar flagellar sigma factor gene fliA were used to generate the loading control band in each RT-PCR mixture.
The genes in the scrABC operon (VPA1513 to VPA1511) were also derepressed in the ΔopaR strain. For example, the difference in expression for scrA (VPA1513), the first gene in the operon, was ∼20-fold between the ΔopaR and opaR+ strains. (Fig. 5). OpaR-dependent repression of this operon was confirmed by using a scrC::lux reporter; luminescence was 25-fold higher in the ΔopaR strain after 6 h of growth (Fig. 6B). This finding seems consistent, because scrABC is known to be part of the surface-sensing regulon. The scrABC operon positively regulates swarming motility by modulating cellular c-di-GMP levels (15). In fact, many genes pertinent to c-di-GMP modulation display differential regulation between the opaR+ and ΔopaR cell types. Eighteen genes predicted to have the capacity to influence c-di-GMP signaling by virtue of containing highly conserved EAL, GGDEF, or HD-GYP domains were observed to be >2-fold differentially expressed. We reasoned that opaR+ and ΔopaR strains might have measurable differences in their cellular nucleotide pools. LM6633 (opaR+) and LM5674 (ΔopaR) were grown as for the microarray experiments. The harvested cells were extracted with formic acid for analysis by high-performance liquid chromatography-coupled mass spectrometry. The mean observed c-di-GMP concentration for LM6633 (20.7 pmol mg of total bacterial protein−1) was significantly higher (P < 0.0001) than that for LM5674 (7.64 pmol mg of total bacterial protein−1) (Fig. 6C).
VP0922 was induced more than 12-fold in the opaR+ strain (Fig. 5). Its V. cholerae homolog (VC1917) is required for natural, chitin-induced competency (44); moreover, some other genes demonstrated to play roles in competency were also induced in the opaR+ strain, though not as strongly. For example, the expression of VP1241, encoding a TfoX homolog (VC1153), was 3.1-fold higher in the opaR+ strain than in the ΔopaR strain. Since HapR regulates the competence of V. cholerae, we wondered if this was true of V. parahaemolyticus. Chromosomal DNA was prepared from a swimming-defective strain carrying the flaMP::Kanr mutation. DNA was added to cultures of LM5312 (opaR+) and LM5674 (ΔopaR) that were growing in medium with chitin. After time was allowed for phenotypic expression, cells were plated on selective medium with kanamycin. Colonies were obtained only for the opaR+ strain, never for the ΔopaR strain (Fig. 6D). The kanamycin-resistant transformants were unable to swim. This experiment and permutations using donor DNA with differently marked mutations (and with different drug resistance cassettes), as well as with recipient strains with other opaR alleles, were repeated with the same results. The opaR+ strain consistently exhibited the capacity to acquire DNA, whereas the ΔopaR strain was deficient.
Two additional large gene sets were regulated strongly and oppositely. Each encodes components of predicted T6SS. These sets were located on separate chromosomes. Twenty-nine linked genes forming 7 predicted operons encoding T6SS1 were expressed in the ΔopaR strain but not the opaR+ cell type. The expression of VP1386, a representative T6SS1 gene, was 36-fold increased in the ΔopaR strain (Fig. 5). Other genes in this region were expressed 4- to 41-fold more strongly in the ΔopaR strain (see Table S1 in the supplemental material). On chromosome 2, 22 linked genes in 3 predicted operons were expressed most highly in the opaR+ strain; for example, VPA1026, a representative T6SS2 gene, displayed ∼70-fold induction (Fig. 5). The other predicted (on the basis of homology or operon structure) T6SS2 genes were induced 4- to 94-fold by OpaR (see Table S1 in the supplemental material). To confirm this regulation observed in the microarray analysis, reverse transcriptase PCR (RT-PCR) was performed using RNA prepared independently of the microarray RNA samples with primers specific for VPA1026 and VP1386. A control set of primers was included in each reaction mixture to amplify the constitutively expressed polar flagellar mRNA for fliA. The results of the RT-PCR corroborate the reciprocal control of these genes by OpaR: a product for the T6SS2 gene was observed only in the opaR+ strain, and a strong product for T6SS1 was observed in the ΔopaR strain (Fig. 6E).
OpaR-silenced strains are more cytotoxic than OpaR+ strains.
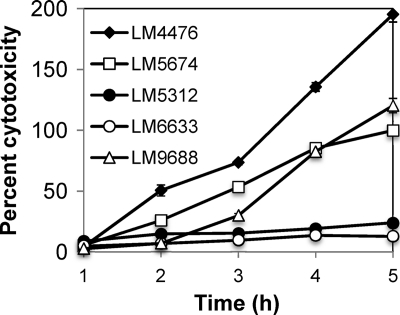
V. parahaemolyticus OpaR and its V. harveyi ortholog LuxR have been found to negatively affect the secretion of the T3SS substrate VopD by immunoblotting of concentrated cell-free supernatants (26), and LuxR has been demonstrated to repress directly the exsBA operon of V. harveyi (66); however, neither the effects of OpaR on the entire T3SS1 regulon nor the biological significance of OpaR regulation with respect to virulence has been assessed. Genes encoding type III secretion components on chromosome 1 (T3SS1) were derepressed 4.7- to 13.6-fold in the ΔopaR strain LM5674 compared to the opaR+ strains in the microarray analysis (see Table S1 in the supplemental material). One example is VP1701 (encoding the regulator ExsC), expression of which was 13.6-fold higher in the ΔopaR strain; it was similarly derepressed in the luxO* strain LM4476 (Fig. 5). To test the functional significance of the repression by OpaR, cytotoxicity toward host cells in coculture was examined (Fig. 7). Host cell lysis in this assay is dependent on T3SS1 (19, 49). The OpaR+ strains LM5312 and LM6633 (LM5674 repaired to opaR+) displayed little toxicity toward the host Chinese hamster ovary (CHO) cells as measured by release of the host enzyme lactate dehydrogenase. Both OpaR-silenced strains (LM5674 and LM4476) were significantly more cytotoxic than the OpaR+ strains. LM4476 appeared more cytotoxic than LM5674. Introduction of the ΔluxO allele to replace luxO* in strain LM4476 to make LM9688 resulted in a significant reduction in cytotoxicity. The differences in virulence between LM4476 and LM9688 were largest at early times of coculture; for example, after 2 h of coculture with CHO cells, the normalized level of lysis caused by LM4476 was 51%, compared to 7% lysis induced by LM9688. At the same time point, the OpaR+ strains LM5312 and LM6633 elicited 15% and 7% killing, respectively. At later times of coincubation, the cytotoxicity of LM9688 increased, a result consistent with the accumulation of some basal level of toxicity; nevertheless, throughout the coculture, LM9688 displayed significantly less host cell lysis than its parent, LM4476 (P < 0.0001). Thus, strains that produce OpaR displayed reduced virulence compared to strains in which OpaR production is silenced, either by opaR mutation or by expression of the luxO* allele.
Fig. 7.
OpaR-silenced strains are more cytotoxic than OpaR+ strains. V. parahaemolyticus strains LM4476 (OpaR− luxO*), LM5674 (ΔopaR), LM5312 (OpaR+), LM6633 (OpaR+), and LM9688 (LM4476 OpaR+ ΔluxO) were grown overnight on HI plates and were used to infect Chinese hamster ovary cells at an MOI of 15. Cytotoxicity was measured periodically by lactate dehydrogenase release and is shown as a normalized percentage of maximal lysis for LM5674, which was equivalent to 80% of the lysis achieved using 0.9% Triton X-100. Error bars are obscured by the symbols and represent standard errors of the means for 5 replicate coculture samples in one representative experiment; the experiment was repeated 3 times with similar results. Differences in cytotoxicity were statistically significant when LM5674 was compared to LM5312 or LM6633 (P < 0.0001 at 3, 4, and 5 h and <0.05 at 2 h). The cytotoxicity of LM4476 was significantly different from that of LM9688 (P < 0.0001 at 2, 3, 4, and 5 h); it was also different from that of LM5674 (P < 0.0001 at 3, 4, and 5 h and <0.01 at 2 h). The cytotoxicities of LM5312 and LM6633 were not significantly different from each other.
DISCUSSION
The gammaproteobacterium V. parahaemolyticus is found in diverse aquatic habitats, freely swimming or attached to a variety of abiotic and biotic surfaces, including plankton and shellfish, or within a host organism (32). Effective and versatile at colonization, the organism can become a pathogen of many different marine hosts, e.g., shrimp, crab, and sea otters (2, 33). In addition, as the major cause of seafood-borne gastroenteritis and occasionally a lethal agent of infection, it is a significant pathogen of humans (47, 72). The variety of conditions under which V. parahaemolyticus persists presumably require diverse mechanisms for survival. One extreme mechanism is a phase variation that profoundly alters colony morphology and, as we demonstrate here, controls global gene expression.
Prior work has established that phase variation in V. parahaemolyticus can be the consequence of genomic alterations in the locus encoding the central terminal output regulator of quorum sensing, OpaR. In this work, we demonstrate that another mutation in the quorum-sensing pathway can have the same consequence. Specifically, a single missense mutation in luxO was found to create a quorum-regulatory protein with an altered, constitutively active function. A variety of constitutive mutations (missense mutations and small deletions) have been discovered or created in other vibrio LuxO proteins; some map to the presumed site of phosphorylation, whereas others occur at various other locations in the protein and are thought to switch it to an “open” conformation (17, 52, 62). Our newly discovered allele belongs to the latter class. In V. parahaemolyticus, this luxO* allele resulted in elevated expression of three small Qrr RNAs and reduced OpaR activity. Although it is assumed to play roles similar to those investigated in other Vibrio species, the discovery of this allele in V. parahaemolyticus provides proof for the first time of the functionality of the central components of yhe quorum pathway of this species, because they can act to silence the production of OpaR.
Although the colony morphology and swarming phenotypes of LM4476 (luxO*) closely resemble the phenotype of LM5674 (a ΔopaR strain) (Fig. 1), and the kinetics of swarming gene induction in LM1017 (luxO*) resemble laf::lux induction in opaR mutants (data not shown), they are not identical. The LM4476 genetic background displays a slightly more translucent and more swarm permissive phenotype than LM5674. Correspondingly, the virulence profile of LM4476 with respect to cytotoxicity is also slightly more enhanced (Fig. 7). These differences are not large, and it seems likely that small differences in gene expression produce detectable phenotypic differences, since the effects are cumulative with time. The opacity and swarming phenotypes of the two ΔluxO strains, LM9688 (derived from LM4476) and LM9847 (derived from LM5312), differ similarly from each other. Although both are ΔluxO strains, the colonies of LM9688 (derived from LM4476) are less opaque, i.e., more glistening and swarm permissive, than those of than LM9847 (Fig. 4). As with LM4476 versus LM5674, LM9688 seems more cytotoxic than the other OP strains examined (Fig. 7). Thus, the backgrounds of strains LM4476 and LM5674 may have additional genotypic differences that will be interesting to explore. It will also be interesting to probe whether alterations of the quorum pathway at different points have particular consequences for the cell. For example, some strains of V. cholerae possess an alternate pathway bypassing the output regulator HapR (23).
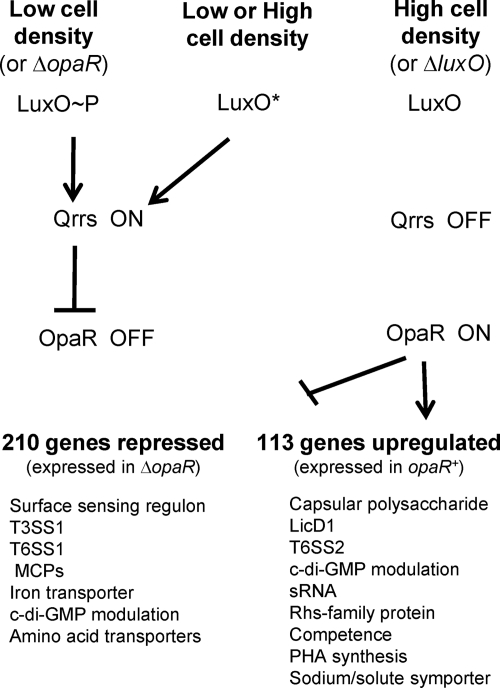
Some clues to the divergent survival strategies of the OP and TR cell types are provided by the nature and scope of the genes controlled by OpaR (summarized in Fig. 8; see also Table S1 in the supplemental material). OpaR regulates, directly or indirectly, approximately 5.2% of the genome of V. parahaemolyticus. In some respects, the quorum-sensing On and Off states in V. parahaemolyticus, surprisingly, seem inverse to those in other bacteria (1, 50). Swarming is generally considered a group activity (11), and yet it is in the Off state (high cell density and OpaR− phenotype) that V. parahaemolyticus differentiates to the swarmer cell and moves over surfaces (28, 40). We find that the TR cell type seems particularly primed for detecting and responding to environmental cues. It can sense its physical environment and program the expression of ∼70 genes in response to growth on a surface (20). The RNA abundance profiles in this work reveal that OpaR controls not only the lateral flagellar system, as had been demonstrated previously, but also the expression of the entire surface-sensing regulon. The expression of some of the genes in this regulon, e.g., those encoding the chitin and N-acetyl binding protein, collagenase, and T3SS1, may prepare this cell type for contact with a potential host. Two operons encoding predicted extracytoplasmic function (ECF)-type sigma factors are more highly expressed in the TR cell, perhaps further equipping this cell to alter global gene expression in response to environmental changes. In keeping with its capacity for mobility, 12 of the 29 genes predicted to encode methyl-accepting chemotaxis proteins have higher levels of expression in the ΔopaR cell type, and none of the remaining chemosensory genes were more highly expressed in the opaR+ background.
Fig. 8.
Quorum-sensing control in V. parahaemolyticus. The strains analyzed in this work support the general Vibrio model of the quorum-sensing pathway for information flow in V. parahaemolyticus. Low cell density can be mimicked by a constitutively active LuxO* or ΔopaR strains; high cell density is mimicked by ΔluxO. The constitutive form of LuxO results in elevated expression of at least three small RNAs (Qrrs). Functioning as the downstream regulator in the pathway, opaR is epistatic to luxO. Transcriptome analyses reveal that OpaR regulates ∼210 genes negatively and 113 genes positively (>4-fold). The selected subset of regulated genes shown in the model include those for which we have experimental evidence or those displaying very high degrees of regulation in the array analyses (i.e., 10-fold or greater changes in gene expression). Previous work showed that OpaR represses swarming and induces capsular polysaccharide gene expression; here we demonstrate that OpaR regulates the level of c-di-GMP in the cell, represses cytotoxicity for host cells in coculture, and induces competence for DNA uptake. Motility and/or virulence may drive selection toward strains in the quorum-silenced state, either via loss of function of the output regulator OpaR or via altered function of the quorum regulator LuxO.
In contrast, the OP cell type, with its quorum signaling cascade intact, seems well adapted for a sessile, community lifestyle. This cell type has increased expression levels of genes encoding several cell surface and potential bacterial cell surface/interaction molecules, e.g., capsular polysaccharide (VPA1403 to -1412; 5- to 8-fold increased) and a predicted phosphorylcholine-modifying protein, LicD1, and other proteins potentially involved in membrane biogenesis (VP1318 to -1322; ∼10-fold regulation). OpaR regulates a number of transcription factors, including an AraC-type transcription factor (VPA0606) previously identified as playing a role in biofilm development, which is 5-fold induced (13). We demonstrate, as has been shown for V. cholerae (44), that OpaR is required for cells to become competent, an activity that is probably most efficient when the local bacterial population is high.
V. parahaemolyticus harbors two T6SS, and their regulation is strikingly different. OpaR represses T6SS1 genes as much as 40-fold, while T6SS2 genes are upregulated as much as 100-fold; moreover, when these large sets are expressed, the abundance of each is as great as those of some of the most highly expressed RNAs in the cell. Although it will be challenging to discern the roles of each of these systems, it seems quite likely—given the profoundly different On/Off states in the two cell types—that the T6SS may predicate specific bacterial interactions, whether among cells of the same type, with other bacteria, or with diverse hosts. In previous work (13), we identified a biofilm mutant in the ΔopaR background with a transposon insertion in one of the T6SS1 genes, hcp (VP1393), which encodes a protein thought to be part of the extracellular secretion apparatus. This mutant produces a biofilm pellicle with altered structural integrity and is severely impaired in 3-dimensional biofilm development. We think that the phenotype probably does not reveal the full function of this T6SS1; rather, given its remarkably high level of expression, the biofilm phenotype may be indicative simply of loss of a major cell surface determinant impacting cell-cell interactions.
The expression profiles of the particular panel of sensory enzymes present in the two cell types that our microarray studies provide also underscore how diversely programmed OP and TR strains seem to be. In possessing a different complement of c-di-GMP pertinent enzymes that are coupled to diverse sensory and signal transduction domains (i.e., 18 with >2-fold expression differences), the two cell types seem wired to respond differently to their environments. To examine this, we measured cellular c-di-GMP concentrations and found a ∼3-fold higher level of this second messenger in the opaR+ cell type than in the ΔopaR cell type during growth on a surface. This trend conforms to the paradigmatic role for c-di-GMP in repressing motility and enhancing community-associated phenotypes, such as increasing the extracellular matrix, sticking, and biofilm formation (reviewed in reference 25).
Our findings lay the foundation for studying the complex relationship between quorum sensing and pathogenicity in V. parahaemolyticus. Acting as a master switch, the production of OpaR is a profound point of gene control for the expression of many potential virulence factors. It may even dictate host target cell specificity, given the distinctive T6SS expression patterns. We find that OpaR represses cytotoxicity toward host cells in culture. Although the expression of virulence factors at a low cell density rather than a high cell density seems inverse to the pattern for many pathogenic bacteria, this is the direction of control that is also observed for V. cholerae and V. harveyi. V. harveyi LuxR represses the expression of the T3SS master regulator exsA and prevents the secretion of virulence effectors (26, 66). Quorum control of virulence in V. cholerae is used to precisely tune the organism to a cycle of infection and dissemination. Many virulence factors, including cholera toxin, the toxin-coregulated pilus, and the HlyA hemolysin are expressed at a low cell density and then repressed at a high cell density by HapR. However, HapR also positively regulates the expression of other virulence genes, such as the HA/protease and a T6SS, and represses the extracellular polysaccharide locus and biofilm development (29, 34, 61, 76–78).
The nature of the genes regulated by the central output regulator of the quorum pathway has been examined for a few other Vibrio species. HapR regulates approximately 4.1% of the V. cholerae El Tor genome (73). For V. vulnificus, a 121-gene SmcR regulon has been predicted by using a consensus binding sequence that was generated by DNA footprinting assays (35). A screen of random genomic V. harveyi DNA fused to promoterless green fluorescent protein (GFP) identified 71 autoinducer-regulated genes, all of which were LuxR dependent (64). The scope of each output network is similarly wide, but the specific sets of genes controlled are different. In general, there is very little overlap of specific gene regulation when our OpaR regulon is compared to the network of genes regulated by the central output regulators of other vibrios. Comparison with V. cholerae provides the best example of how different the consequences of quorum sensing can be, even though the central wiring is similar. For example, the high-cell-density state in V. cholerae results in low c-di-GMP and extracellular polysaccharide levels (65), exactly the opposite of its effects in V. parahaemolyticus. Our analysis begins to define the scope of output control in V. parahaemolyticus, and future work must sort out direct and indirect regulation by determining the cascade of post-OpaR gene control. Furthermore, we emphasize that these studies provide only a snapshot of RNA abundance profiles at a particular time and under specific conditions (growth on a surface); moreover, the particular strain with which this work was done, BB22, is not the sequenced strain whose genome information was used to derive the GeneChip. Thus, new OpaR-controlled genes are certainly yet to be discovered.
Phase shifting and variable colony morphology are not peculiarities of strain BB22, (53). Swarming proficiency seems a dominant phenotype of the V. parahaemolyticus strains that have been studied. For example, Baumann et al. reported that 125 out of 132 strains collected throughout the world that were isolated variously from infected gastrointestinal systems, seafood, and seawater produced lateral flagella (3). In our small collection of environmental and clinical V. parahaemolyticus strains, 18 of 22 are swarming proficient. Accordingly, we predict that quorum silencing is a general phenomenon found for many V. parahaemolyticus strains. Consistent with this hypothesis, ectopic expression of opaR in some of our various swarming-proficient strains converted about half to a more opaque and swarming defective phenotype; furthermore, the introduction of an opaR deletion into the well-studied first sequenced strain RIMD2210633, isolated in Japan from a patient with traveler's diarrhea (37), had no effect on colony opacity, swarming, or cytotoxicity (data not shown). Whether these observed frequencies of the prevalence of the translucent phenotype are the consequence of isolation biases or are a true reflection of the distribution of the morphotypes in nature is not known and will be interesting to learn—as will the molecular basis for the swarming permissiveness of a variety of isolates. Sequence gazing at the few V. parahaemolyticus strains whose genomes have been sequenced suggests some variability in the coding region for luxO; comparative analysis of the opaR locus is more difficult, since the majority of the silencing mutations that we have found for strain BB22 occur in the promoter rather than the coding region. This is probably because there is a sequence repeat in this region.
We emphasize that OP/TR phase variation is also not a peculiarity of this species. Reversible switching in colony morphology has long been reported for other Vibrio species (67). In many cases, the variation in colony opacity is a consequence of the amount of extracellular polysaccharide produced. The encapsulated opaque strains of V. vulnificus are the more virulent form of the organism (56, 69, 70, 75). Interestingly, high-frequency opacity shifting is observed for V. vulnificus during oyster infections (58). Non-O1 V. cholerae strains produce interconverting OP and TR types, and the encapsulated OP form also displays increased virulence (31). In many cases, V. cholerae strains have been shown to have alterations in the quorum-sensing cascade. For example, some V. cholerae strains contain mutations in the coding region of hapR, while other strains have been found to harbor a variety of constitutive luxO alleles (17, 23, 30, 52, 62). For each Vibrio species, there may be a particular balance in the benefits conferred by the ability or inability to sense and communicate. Quorum silencing may be particularly favored in V. parahaemolyticus due to the coupling of the asocial phenotype with robust surface motility, which seems a highly powerful driving force for cell type; however, certain advantages of sociability must help preserve the reversible switching mechanism.
Supplementary Material
Acknowledgements
We thank Sandford Jaques for constructing the luxO and opaR alleles, David Weiss and Ryan Kustusch for help with the microarray data collection, Patrick Breheny for designing the analysis of the microarray data, Jessica King for sequencing and cloning luxOU, and Eric Ransom for developing the chitin transformation protocol. We also thank the University of Iowa Carver College of Medicine DNA Core Facility and the Mass Spectrometry Facility at Michigan State University for the expert resources provided.
This work was supported by NIH grant 5 R21 AI065526, NSF grant 0817593, and a Medical Research Initiative Grant from the Carver College of Medicine. C.G.-P. was supported by NIH Training Grant 5 T32 GM077973, “Statistics in Microbiology, Infectious Diseases & Bioinformatics.”
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 24 June 2011.
REFERENCES
- 1. Antunes L. C., Ferreira R. B., Buckner M. M., Finlay B. B. 2010. Quorum sensing in bacterial virulence. Microbiology 156:2271–2282 [DOI] [PubMed] [Google Scholar]
- 2. Austin B. 2010. Vibrios as causal agents of zoonoses. Vet. Microbiol. 140:310–317 [DOI] [PubMed] [Google Scholar]
- 3. Baumann P., Baumann L., Reichelt J. L. 1973. Taxonomy of marine bacteria: Beneckea parahaemolytica and Beneckea alginolytica. J. Bacteriol. 113:1144–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belas R., et al. 1982. Bacterial bioluminescence: isolation and expression of the luciferase genes from Vibrio harveyi. Science 218:791–793 [DOI] [PubMed] [Google Scholar]
- 5. Belas R., Simon M., Silverman M. 1986. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J. Bacteriol. 167:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 157:289–300 [Google Scholar]
- 7. Berger L. R., Reynolds D. M. 1958. The chitinase system of a strain of Streptomyces griseus. Biochim. Biophys. Acta 29:522–534 [DOI] [PubMed] [Google Scholar]
- 8. Boles B. R., McCarter L. L. 2000. Insertional inactivation of genes encoding components of the sodium-type flagellar motor and switch of Vibrio parahaemolyticus. J. Bacteriol. 182:1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broberg C. A., Zhang L., Gonzalez H., Laskowski-Arce M. A., Orth K. 2010. A Vibrio effector protein is an inositol phosphatase and disrupts host cell membrane integrity. Science 329:1660–1662 [DOI] [PubMed] [Google Scholar]
- 10. Croxatto A., et al. 2002. VanT, a homologue of Vibrio harveyi LuxR, regulates serine, metalloprotease, pigment, and biofilm production in Vibrio anguillarum. J. Bacteriol. 184:1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Daniels R., Vanderleyden J., Michiels J. 2004. Quorum sensing and swarming migration in bacteria. FEMS Microbiol. Rev. 28:261–289 [DOI] [PubMed] [Google Scholar]
- 12. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enos-Berlage J. L., Guvener Z. T., Keenan C. E., McCarter L. L. 2005. Genetic determinants of biofilm development of opaque and translucent Vibrio parahaemolyticus. Mol. Microbiol. 55:1160–1182 [DOI] [PubMed] [Google Scholar]
- 14. Enos-Berlage J. L., McCarter L. L. 2000. Relation of capsular polysaccharide production and colonial cell organization to colony morphology in Vibrio parahaemolyticus. J. Bacteriol. 182:5513–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira R. B., Antunes L. C., Greenberg E. P., McCarter L. L. 2008. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J. Bacteriol. 190:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fidopiastis P. M., Miyamoto C. M., Jobling M. G., Meighen E. A., Ruby E. G. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45:131–143 [DOI] [PubMed] [Google Scholar]
- 17. Freeman J. A., Bassler B. L. 1999. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31:665–677 [DOI] [PubMed] [Google Scholar]
- 18. Gentleman R., et al. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gode-Potratz C. J., Chodur D. M., McCarter L. L. 2010. Calcium and iron regulate swarming and type III secretion in Vibrio parahaemolyticus. J. Bacteriol. 192:6025–6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gode-Potratz C. J., Kustusch R. J., Breheny P. J., Weiss D. S., McCarter L. L. 2011. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 79:240–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Güvener Z. T., McCarter L. L. 2003. Multiple regulators control capsular polysaccharide production in Vibrio parahaemolyticus. J. Bacteriol. 185:5431–5441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammer B. K., Bassler B. L. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50:101–104 [DOI] [PubMed] [Google Scholar]
- 23. Hammer B. K., Bassler B. L. 2007. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 104:11145–11149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hasegawa H., Hase C. C. 2009. TetR-type transcriptional regulator VtpR functions as a global regulator in Vibrio tubiashii. Appl. Environ. Microbiol. 75:7602–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7:263–273 [DOI] [PubMed] [Google Scholar]
- 26. Henke J. M., Bassler B. L. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hiyoshi H., Kodama T., Iida T., Honda T. 2010. Contribution of Vibrio parahaemolyticus virulence factors to cytotoxicity, enterotoxicity, and lethality in mice. Infect. Immun. 78:1772–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jaques S., McCarter L. L. 2006. Three new regulators of swarming in Vibrio parahaemolyticus. J. Bacteriol. 188:2625–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jobling M. G., Holmes R. K. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023–1034 [DOI] [PubMed] [Google Scholar]
- 30. Joelsson A., Liu Z., Zhu J. 2006. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect. Immun. 74:1141–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnson J. A., Panigrahi P., Morris J. G., Jr 1992. Non-O1 Vibrio cholerae NRT36S produces a polysaccharide capsule that determines colony morphology, serum resistance, and virulence in mice. Infect. Immun. 60:864–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joseph S. W., Colwell R. R., Kaper J. B. 1982. Vibrio parahaemolyticus and related halophilic vibrios. Crit. Rev. Microbiol. 10:77–124 [DOI] [PubMed] [Google Scholar]
- 33. Kaneko T., Colwell R. R. 1973. Ecology of Vibrio parahaemolyticus in Chesapeake Bay. J. Bacteriol. 113:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kovacikova G., Skorupski K. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135–1147 [DOI] [PubMed] [Google Scholar]
- 35. Lee D. H., et al. 2008. A consensus sequence for binding of SmcR, a Vibrio vulnificus LuxR homologue, and genome-wide identification of the SmcR regulon. J. Biol. Chem. 283:23610–23618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lenz D. H., Miller M. B., Zhu J., Kulkarni R. V., Bassler B. L. 2005. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol. Microbiol. 58:1186–1202 [DOI] [PubMed] [Google Scholar]
- 37. Makino K., et al. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743–749 [DOI] [PubMed] [Google Scholar]
- 38. Manoil C., Bailey J. 1997. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J. Mol. Biol. 267:250–263 [DOI] [PubMed] [Google Scholar]
- 39. McCarter L. 1999. The multiple identities of Vibrio parahaemolyticus. J. Mol. Microbiol. Biotechnol. 1:51–57 [PubMed] [Google Scholar]
- 40. McCarter L. L. 1998. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J. Bacteriol. 180:3166–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCarter L. L., Wright M. E. 1993. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J. Bacteriol. 175:3361–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McDougald D., Rice S. A., Kjelleberg S. 2000. The marine pathogen Vibrio vulnificus encodes a putative homologue of the Vibrio harveyi regulatory gene, luxR: a genetic and phylogenetic comparison. Gene 248:213–221 [DOI] [PubMed] [Google Scholar]
- 43. McLaughlin J. B., et al. 2005. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N. Engl. J. Med. 353:1463–1470 [DOI] [PubMed] [Google Scholar]
- 44. Meibom K. L., Blokesch M., Dolganov N. A., Wu C. Y., Schoolnik G. K. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824–1827 [DOI] [PubMed] [Google Scholar]
- 45. Miller J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 46. Milton D. L. 2006. Quorum sensing in vibrios: complexity for diversification. Int. J. Med. Microbiol. 296:61–71 [DOI] [PubMed] [Google Scholar]
- 47. Nair G. B., et al. 2007. Global dissemination of Vibrio parahaemolyticus serotype O3:K6 and its serovariants. Clin. Microbiol. Rev. 20:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ng W. L., Bassler B. L. 2009. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43:197–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Park K. S., et al. 2004. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 72:6659–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parker C. T., Sperandio V. 2009. Cell-to-cell signalling during pathogenesis. Cell. Microbiol. 11:363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Putnam S. L., Koch A. L. 1975. Complications in the simplest cellular enzyme assay: lysis of Escherichia coli for the assay of beta-galactosidase. Anal. Biochem. 63:350–360 [DOI] [PubMed] [Google Scholar]
- 52. Raychaudhuri S., Jain V., Dongre M. 2006. Identification of a constitutively active variant of LuxO that affects production of HA/protease and biofilm development in a non-O1, non-O139 Vibrio cholerae O110. Gene 369:126–133 [DOI] [PubMed] [Google Scholar]
- 53. Sakazaki R., Iwanami S., Fukumi H. 1963. Studies on the enteropathogenic, facultatively halophilic bacteria, Vibrio parahaemolyticus. I. Morphological, cultural and biochemical properties and its taxonomical position. Jpn. J. Med. Sci. Biol. 16:161–188 [DOI] [PubMed] [Google Scholar]
- 54. Schweizer H. D. 1993. Small broad-host-range gentamycin resistance gene cassettes for site-specific insertion and deletion mutagenesis. Biotechniques 15:831–834 [PubMed] [Google Scholar]
- 55. Showalter R. E., Martin M. O., Silverman M. R. 1990. Cloning and nucleotide sequence of luxR, a regulatory gene controlling bioluminescence in Vibrio harveyi. J. Bacteriol. 172:2946–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Simpson L. M., White V. K., Zane S. F., Oliver J. D. 1987. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 55:269–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spangler C., Bohm A., Jenal U., Seifert R., Kaever V. 2010. A liquid chromatography-coupled tandem mass spectrometry method for quantitation of cyclic di-GMP. J. Microbiol. Methods 81:226–231 [DOI] [PubMed] [Google Scholar]
- 58. Srivastava M., Tucker M. S., Gulig P. A., Wright A. C. 2009. Phase variation, capsular polysaccharide, pilus and flagella contribute to uptake of Vibrio vulnificus by the Eastern oyster (Crassostrea virginica). Environ. Microbiol. 11:1934–1944 [DOI] [PubMed] [Google Scholar]
- 59. Stewart B. J., McCarter L. L. 2003. Lateral flagellar gene system of Vibrio parahaemolyticus. J. Bacteriol. 185:4508–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Su Y. C., Liu C. 2007. Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 24:549–558 [DOI] [PubMed] [Google Scholar]
- 61. Tsou A. M., Zhu J. 2010. Quorum sensing negatively regulates hemolysin transcriptionally and posttranslationally in Vibrio cholerae. Infect. Immun. 78:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vance R. E., Zhu J., Mekalanos J. J. 2003. A constitutively active variant of the quorum-sensing regulator LuxO affects protease production and biofilm formation in Vibrio cholerae. Infect. Immun. 71:2571–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Waters C. M., Bassler B. L. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319–346 [DOI] [PubMed] [Google Scholar]
- 64. Waters C. M., Bassler B. L. 2006. The Vibrio harveyi quorum-sensing system uses shared regulatory components to discriminate between multiple autoinducers. Genes Dev. 20:2754–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Waters C. M., Lu W., Rabinowitz J. D., Bassler B. L. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 190:2527–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waters C. M., Wu J. T., Ramsey M. E., Harris R. C., Bassler B. L. 2010. Control of the type 3 secretion system in Vibrio harveyi by quorum sensing through repression of ExsA. Appl. Environ. Microbiol. 76:4996–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. White P. B. 1938. The rugose variant of vibrios. J. Pathol, Bacteriol. 46:1–6 [Google Scholar]
- 68. Woo T. H. S., Cheng A. F., Ling J. M. 1992. An application of a simple method for the preparation of bacterial DNA. Biotechniques 13:696. [PubMed] [Google Scholar]
- 69. Wright A. C., et al. 1999. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect. Immun. 67:2250–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wright A. C., Simpson L. M., Oliver J. D., Morris J. G. 1990. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 58:1769–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu Z., Irizarry R., Gentleman R., Martinez-Murillo F., Spencer F. 2004. A model-based background adjustment for oligonucleotide expression arrays. J. Am. Stat. Assoc. 99:909–917 [Google Scholar]
- 72. Yeung P. S., Boor K. J. 2004. Epidemiology, pathogenesis, and prevention of foodborne Vibrio parahaemolyticus infections. Foodborne Pathog. Dis. 1:74–88 [DOI] [PubMed] [Google Scholar]
- 73. Yildiz F. H., Liu X. S., Heydorn A., Schoolnik G. K. 2004. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 53:497–515 [DOI] [PubMed] [Google Scholar]
- 74. Yildiz F. H., Visick K. L. 2009. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 17:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yoshida S. I., Ogawa M., Mizuguchi Y. 1985. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 47:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zheng J., Shin O. S., Cameron D. E., Mekalanos J. J. 2010. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 107:21128–21133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhu J., Mekalanos J. J. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647–656 [DOI] [PubMed] [Google Scholar]
- 78. Zhu J., et al. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 99:3129–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.