Abstract
Although the sensitivity and specificity of nucleic acid amplification assays are high with smear-positive samples, the sensitivity with smear-negative and extrapulmonary samples for the diagnosis of tuberculosis in suspicious tuberculosis cases still remains to be investigated. This study evaluates the performance of the GenoType Mycobacteria Direct (GTMD) test for rapid molecular detection and identification of the Mycobacterium tuberculosis complex and four clinically important nontuberculous mycobacteria (M. avium, M. intracellulare, M. kansasii, and M. malmoense) in smear-negative samples. A total of 1,570 samples (1,103 bronchial aspiration, 127 sputum, and 340 extrapulmonary samples) were analyzed. When we evaluated the performance criteria in combination with a positive culture result and/or the clinical outcome of the patients, the overall sensitivity, specificity, and positive and negative predictive values were found to be 62.4, 99.5, 95.9, and 93.9%, respectively, whereas they were 63.2, 99.4, 95.7, and 92.8%, respectively, for pulmonary samples and 52.9, 100, 100, and 97.6%, respectively, for extrapulmonary samples. Among the culture-positive samples which had Mycobacterium species detectable by the GTMD test, three samples were identified to be M. intracellulare and one sample was identified to be M. avium. However, five M. intracellulare samples and an M. kansasii sample could not be identified by the molecular test and were found to be negative. The GTMD test has been a reliable, practical, and easy tool for rapid diagnosis of smear-negative pulmonary and extrapulmonary tuberculosis so that effective precautions may be taken and appropriate treatment may be initiated. However, the low sensitivity level should be considered in the differentiation of suspected tuberculosis and some other clinical condition until the culture result is found to be negative and a true picture of the clinical outcome is obtained.
INTRODUCTION
Acid-fast smear examination and culture (liquid- and solid-based media, automated and semiautomated systems) are conventional techniques for microbiological detection of mycobacteria causing tuberculosis (TB) (14, 24). However, the sensitivity of smear has been variable (range, 20 to 80%) (1). In some smear-negative cases, TB might be difficult to differentiate from a number of other clinical pictures. Therefore, invasive medical procedures are necessary for sampling of patients from whom a qualified sputum sample cannot be obtained or in case of extrapulmonary TB, which requires histopathological, cytopathological, and microbiological examination of tissue specimens and body fluids. Nucleic acid amplification (NAA) techniques have been used for early detection of causative mycobacteria in clinical samples and also to support the clinical and radiological diagnosis in patients with presumptive Mycobacterium tuberculosis infection (3, 19, 33). Although the specificity values obtained with both smear-positive and smear-negative samples are high, the sensitivities of molecular assays are rather less with smear-negative samples than the values found with smear-positive samples and were reported to cover a wide range of from 50% to 80% in previous studies (7, 8). Recently, a combined system of the nucleic acid sequence-based amplification (NASBA; a registered trademark of bioMérieux) technique (6) and reverse hybridization method based on the GenoType Mycobacteria Direct (GTMD; Hain Lifescience GmbH, Nehren, Germany) test has been used in routine practice to obtain better sensitivity and specificity and achieve early diagnosis. In several studies which compared the GTMD test with culture as the reference method and/or other NAA tests, the sizes of the study populations with smear-negative samples have not been sufficient for an accurate evaluation of this method under routine hospital conditions (9, 15, 20, 25, 28). The intent of this study was to evaluate the performance of the GTMD test for direct detection of the Mycobacterium tuberculosis complex and four clinically important nontuberculous mycobacteria (NTM) (Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium malmoense) in smear-negative samples obtained from patients suspected of having M. tuberculosis infection. Among these nontuberculous Mycobacterium species, M. avium, M. intracellulare, and M. kansasii have been reported to be the common agents causing disease worldwide and also in the Aegean region of Turkey, while M. malmoense has rarely been reported in most geographic regions of the world except northern Europe (2, 10).
MATERIALS AND METHODS
Clinical samples.
A total of 1,570 samples (1,230 pulmonary [1,103 bronchial aspiration and 127 sputum samples] and 340 extrapulmonary [210 urine, 29 pleural fluid, 57 gastric lavage, 18 cerebrospinal fluid {CSF}, 8 biopsy, and 18 various sterile body fluid or pus samples]) recovered from 1,462 patients between June 2006 and September 2008 were analyzed in the Microbiology Laboratory of Izmir Training and Research Hospital for Chest Diseases and Chest Surgery, which is a regional reference hospital for TB patients on the Aegean Coast of Turkey (West Anatolian region). The patients included in the study had not received antituberculous treatment within the last 12 months and were evaluated as having suspected M. tuberculosis infection. The bronchial aspiration procedure was applied to the patients in whom a positive smear of acid-fast bacilli could not be obtained from sputum or a qualified sputum sample could not be collected. All the clinical specimens were subjected to direct smear microscopy by a standard Kinyoun cold staining method (17) and evaluated by an experienced microbiologist. Additionally, two separate direct smears from morning sputum were prepared for each patient on the following days in order to increase the sensitivity of smear examination for these samples. The standard M. tuberculosis H37Rv (ATCC 27294) strain was used for quality control in the staining process. Mycobacterial cultivation, identification, and molecular detection were applied to each sample as follows.
Mycobacterial cultivation and identification.
Specimens other than sterile specimens which contained normal bacterial flora, such as sputum, and nonsterile specimens were digested and decontaminated with the N-acetyl-l-cysteine–sodium hydroxide method (17) by using a commercial decontamination kit (Mycoprosafe; Salubris AS, Istanbul, Turkey). Mycobacterial cultivation was performed by the Bactec MGIT 960 system (BD Biosciences, Sparks, MD) according to the recommendations of the manufacturer as described elsewhere (30) and in Lowenstein-Jensen slants (Salubris AS, Istanbul, Turkey). An acid-fast smear preparation by Kinyoun staining was also applied to each processed specimen. Differentiation of M. tuberculosis and NTM was performed by conventional methods (17) and the Bactec 460 p-nitro-α-acetylamino-β-hydroxypropiophenone (NAP) test (BD Biosciences, Sparks, MD). Additionally, commercially available PCR-based reverse hybridization (line probe assay [LiPA]) kits (GenoType Mycobacterium CM and AS [for additional species; Hain Lifescience GmbH, Nehren, Germany]) were used for further identification of atypical mycobacteria to species level (2).
Molecular detection.
Rapid molecular detection and identification for each sample were performed with the GTMD test, version 4.0 (Hain Lifescience GmbH, Nehren, Germany), according to the manufacturer's recommendations as described elsewhere (9). The whole procedure was divided into three steps: RNA isolation from decontaminated patient specimen using a magnetic bead capture method, amplification based on the NASBA technique, and reverse hybridization. A master mix containing 15 μl of primer/nucleotide mix and 10 μl isolated RNA in a 0.5-ml screw-cap tube was prepared for each reaction per sample. The amplification program and the hybridization procedure were carried out in a TwinCubator incubator (Hain Lifescience, Nehren, Germany) with a hybridization block. The hybridization procedure included chemical denaturation of amplification products, hybridization of single-stranded, biotin-labeled amplicons to membrane-bound probes, stringent washing, addition of a streptavidin-alkaline phosphatase (AP) conjugate, and an AP-mediated staining reaction. Band patterns which occurred as a result of hybridization were evaluated visually by use of an evaluation sheet provided by the manufacturer.
Evaluation and interpretation of results.
Results were evaluated visually on a reading chart according to the hybridization bands corresponding to the patterns of M. tuberculosis and other mycobacteria on the strips. Interpretation and evaluation of test results and quality controls were applied as recommended by the manufacturer. Thus, a conjugate control band and an internal amplification control (IAC) band were observed for quality control and validation of the assay. The other five bands determined the reaction zones corresponding to specific probes belonging to the M. tuberculosis complex and four mycobacteria (M. avium, M. intracellulare, M. kansasii, and M. malmoense). The molecular assays that had discrepant results according to the culture results and that were considered false positive or that had cross-contamination were repeated using frozen aliquots of the samples. The same result which was obtained twice (either negative or positive) repeatedly was accepted as the final result in these discrepant assays. Specimens showing IAC inhibition by the GTMD test were also retested with dilutions of 1/100 and 1/1,000, if necessary.
RESULTS
GTMD test results in accordance with the results of culture and clinical evaluation.
All the specimens tested were smear negative with Kinyoun acid-fast staining. The ratios of positive and negative results of culture and GTMD test according to sample type are shown in Table 1. GTMD tests of 93 (5.9%) samples were repeated because of discrepancies. After evaluation of GTMD test results, 34 (2.2%) samples were culture negative and GTMD test positive, whereas 86 (5.5%) samples were culture positive and GTMD test negative (Table 2). GTMD test-negative and culture-positive samples were also confirmed to contain M. tuberculosis by clinical evaluation. Among GTMD test-positive and culture-negative samples, 28 (82.4%) samples were recovered from the patients who were evaluated as having M. tuberculosis infection and given anti-TB treatment according to the clinical data and/or cultures of other separate samples positive for mycobacteria. In total, an amplification band was not observed in 74 samples and was evaluated as inhibition. In these samples, 72 and 2 samples gave valid results with the dilutions of 1/100 and 1/1,000, respectively. A representative image of the mycobacterial band patterns on the strips of the GTMD assay is shown in Fig. 1.
Table 1.
Ratios of positive and negative results of culture and GTMD test according to sample type
| Sample type | No. (%) of samples |
|||
|---|---|---|---|---|
| Culturea |
GTMD test |
|||
| Positive | Negative | Positive | Negative | |
| Bronchial aspirate (n = 1,103) | 161 (14.6) | 942 (85.4) | 122 (11.2) | 980 (88.8) |
| Sputum (n = 127) | 26 (20.5) | 101 (79.5) | 18 (14.2) | 109 (85.8) |
| Urine (n = 210) | 6 (2.9) | 204 (97.1) | 7 (3.3) | 203 (96.7) |
| Pleural fluid (n = 29) | 1 (3.4) | 28 (96.6) | 0 | 29 (100) |
| Gastric lavage (n = 57) | 6 (10.5) | 51 (89.5) | 1 (1.8) | 56 (98.2) |
| CSF (n = 18) | 0 | 18 (100) | 0 | 18 (100) |
| Biopsy (n = 8) | 0 | 8 (100) | 0 | 8 (100) |
| Otherb (n = 18) | 1 (5.6) | 17 (94.4) | 1 (5.6) | 17 (94.4) |
| Total (n = 1,570) | 201 (12.8) | 1,369 (87.2) | 149 (9.5) | 1,421 (90.5) |
Culture was done with the Bactec 960 system and in Lowenstein-Jensen medium.
Sterile body fluids (e.g., pericardial fluid, wound, and ascitic fluid).
Table 2.
Distribution of result patterns determined by GTMD test and culture among smear-negative clinical samples
| Sample type | No. (%) of samples with the following result patterns: |
|||
|---|---|---|---|---|
| GTMD test and culture positive | GTMD test and culture negative | GTMD test negative and culture positivea | GTMD test positive and culture negativeb | |
| Pulmonary (n = 1,230) | 109 (8.9) | 1,012 (82.3) | 78 (6.3) | 31 (2.5) |
| Extrapulmonary (n = 340) | 6 (1.8) | 323 (95.0) | 8 (2.3) | 3 (0.9) |
| Total (n = 1,570) | 115 (7.3) | 1,335 (85.0) | 86 (5.5) | 34 (2.2) |
GTMD test-negative and culture-positive samples were also confirmed to be positive for M. tuberculosis by clinical evaluation.
Among GTMD test-positive and culture-negative samples, 28 (82.4%) samples were recovered from the patients who were evaluated as having M. tuberculosis infection and given anti-TB treatment according to the clinical data and/or positive mycobacterial culture results for other separate samples.
Fig. 1.
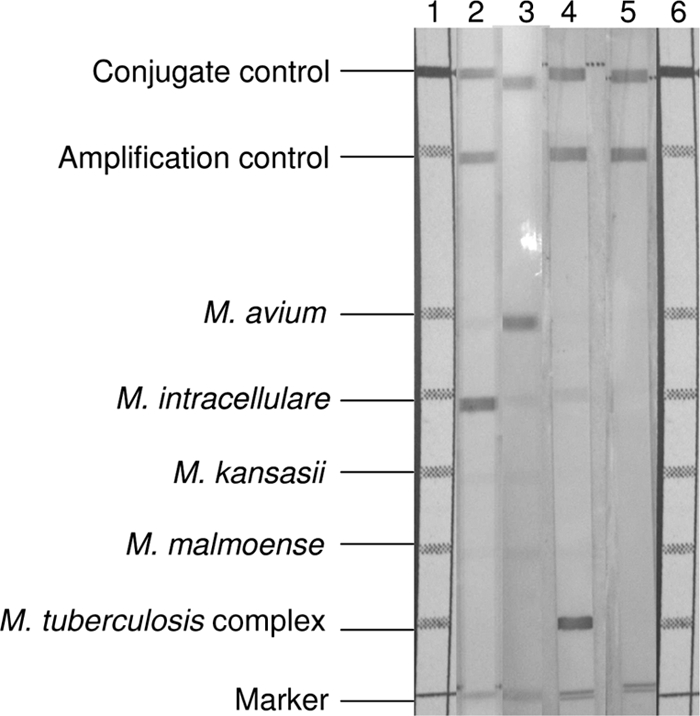
Representative image of mycobacterial band patterns on the strips of the GTMD assay. Lane 1, scale for alignment; lane 2, M. intracellulare positive; lane 3, M. avium positive; lane 4, M. tuberculosis complex positive; lane 5, negative; lane 6, scale for alignment.
Performance evaluation of GTMD test.
When we evaluated the performance criteria in combination with the results for the other culture-positive samples and/or clinical outcome of the patients for discrepant results, the overall sensitivity, specificity, and positive and negative predictive values were 62.4, 99.5 95.9, and 93.9%, respectively, whereas the values were 63.2, 99.4, 95.7, and 92.8%, respectively, for pulmonary samples and 52.9, 100, 100, and 97.6%, respectively, for extrapulmonary samples. The sensitivity, specificity, and positive and negative predictive values for the GTMD test with smear-negative clinical samples compared with the results of culture and with the results of culture in combination with the clinical diagnosis are shown in Table 3.
Table 3.
Sensitivity, specificity, and positive and negative predictive values for GTMD test with smear-negative clinical samples
| Sample type | %a |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity |
Specificity |
PPV |
NPV |
|||||
| A | B | A | B | A | B | A | B | |
| Pulmonary | 58.3 | 63.2 | 97.0 | 99.4 | 77.9 | 95.7 | 92.8 | 92.8 |
| Extrapulmonary | 42.9 | 52.9 | 99.1 | 100 | 66.7 | 100 | 97.6 | 97.6 |
| Total | 57.2 | 62.4 | 97.5 | 99.5 | 77.2 | 95.9 | 93.9 | 93.9 |
A, sensitivity, specificity, and positive and negative predictive values compared with the culture results; B, sensitivity, specificity, and positive and negative predictive values compared with the culture results in combination with the clinical diagnosis.
Nontuberculosis mycobacterial test results.
Among all samples, a total of eight nontuberculous Mycobacterium species (M. intracellulare [n = 8], M. abscessus [n = 2], M. avium [n = 1], M. kansasii [n = 1], M. scrofulaceum [n = 1], M. szulgai/M. intermedium [n = 1], and two Mycobacterium spp.) were isolated from the culture media of 16 pulmonary samples. Three Mycobacterium species isolated in 10 of these 16 pulmonary samples (M. intracellulare [n = 8], M. avium [n = 1], and M. kansasii [n = 1]) were detectable by the GTMD test. However, the GTMD test detected and accurately identified the M. intracellulare isolates in three of eight samples and the M. avium isolate in one sample, while the M. intracellulare isolates in the remaining five samples and the M. kansasii isolate in one sample could not be detected by the GTMD test and were considered negative. M. malmoense was not isolated from any of the clinical specimens included in this study.
DISCUSSION
In general, conventional laboratory methods, including microscopic examination by acid-fast staining and/or culture in solid and liquid media and automated and semiautomated systems, have been used for microbiological detection of mycobacteria for microbiological diagnosis of active TB and other mycobacterial infections. In smear-positive cases, the patients almost always have classical TB symptoms, and especially in high-incidence settings, clinicians generally do not need an additional fast molecular method for detection of M. tuberculosis other than the conventional culture techniques and liquid-based automated systems, which have a reporting time of as early as 1 week. On the other hand, acid-fast staining methods are known to have low sensitivity levels caused by technical and conditional variations, such as the duration of examination, experience of the microbiologist, sample type, immune status of patient, stage of infection, and application procedure. The sensitivity level of smear microscopy can change according to the acid-fast staining method as well (12, 16, 26). However, in our study, 89.7% (n = 1,103) of pulmonary specimens were bronchial aspirates, which were included as one of the criteria to increase the sensitivity of microscopic examination. In previous studies, bronchial aspiration by fiberoptic bronchoscopy was found to be a useful procedure to obtain a definitive diagnosis of pulmonary TB because it increased smear and culture sensitivity (5, 23). It is known that the sensitivity level of microscopic examination is very low with extrapulmonary samples (4, 21, 22), and in general, invasive medical procedures which are difficult to repeat are necessary for sampling. In patients for whom the diagnosis was not certain but who showed symptoms of active M. tuberculosis infection, the usage of NAA tests has been recommended due to a testing and an interpretation algorithm (3). If smear and NAA test results are negative, clinical evaluation for a certain diagnosis is necessary to start antituberculous treatment before resolution of the culture and additional test results. Recent studies showed that reliable molecular methods used in routine practice have led to a decision to initiate therapy for 20% to 50% of TB cases or to reduce nonindicated TB treatment (11, 29).
In previous studies, the sensitivity and the specificity levels of the GTMD test were reported to be within the ranges of 92 to 97% and 90 to 100%, respectively (9, 15, 20, 28). For samples with discrepant results, evaluation in combination with positive culture results and the clinical outcome increased the sensitivity and the specificity levels (9, 15). In this study, the evaluation of culture and clinical findings resulted in a low level of increase in the sensitivity and specificity values, but a high level of increase in the positive predictive value was observed (Table 3). The sensitivity of the GTMD test was evaluated to be as low as that in the other studies using different NAA methods with smear-negative samples, while the specificity was high. However, when we compare the results of the present study with those of the previous studies investigating the diagnostic performance of the GTMD test, the present study differs from the others, which have previously reported high sensitivity values (>90%). This difference might have occurred because of the presence of smear-positive samples in the study population, which has led to an increase in sensitivity levels. Additionally, making a decision regarding the sensitivity level of the GTMD test with samples from a few smear-negative patients, even if the results were confirmed clinically, may lead to misinterpretation of the performance evaluation results. It was recommended by Syre et al (28) that a larger study with more smear-negative, culture-positive samples would be required to evaluate the GTMD test performance with smear-negative samples. The present study has provided the previously absent data on the efficient use of the GTMD test with smear-negative samples.
The patient population selected for NAA testing for TB can vary according to the clinical findings, stage of the disease (i.e., whether they have received anti-TB treatment), the incidence of mycobacteria in that region, and the experience of the laboratory. Each TB control or treatment program should evaluate the overall costs and benefits of NAA testing in deciding the value and optimal use of the test in its setting. As the incidence of causative NTM has been reported to be too low (<1%) in our region (2), it has been considered that a molecular detection test with a low sensitivity may not be necessary for identification of NTM directly from clinical samples. Thus, the present study has shown that molecular identification of NTM from culture in Bactec 960 vials, which would be much more sensitive, would be sufficient for effective diagnosis in our setting. Although the present study and the current data (25) have determined the low performance of the GTMD test for detection and identification of four NTM directly from clinical specimens, the performance of the GTMD test for atypical mycobacteria still needs to be evaluated with larger numbers of samples.
Patients with smear-negative status are capable of transmitting M. tuberculosis, and smear-negative cases appear to be responsible for at least one-sixth of culture-positive episodes of TB transmission (1, 13, 31). Diagnostic delay related to the patient or health care provider leads to poor outcomes for individual patients and to increased spread of TB within the community. It was demonstrated that patients with extrapulmonary or smear-negative disease have been significantly more likely to be hospitalized and to have experienced treatment delay (32). Smear-negative TB often requires assessment of the response to antibiotic treatment as well as a review of the findings of radiological investigations (27). The clinical variables (i.e., HIV infection, lymphadenopathy, cavitary lung lesion, history of contact with TB, persistent cough, and weight loss) could not be used ubiquitously because of the different epidemiological characteristics of each population and are not always predictors of treatment outcome (18). Improved diagnostic tools are necessary to introduce a curative resolution for smear-negative TB patients at the proper time and also to protect the patients without TB from inappropriate, potentially toxic treatment. Nonetheless, whether to start empirical treatment or wait for the culture results and continue or stop empirical treatment if a final culture shows negative results has still been a conflict for clinicians.
The GTMD test was reliable, rapid (results were available in 5 h), practical, and easy to apply to pulmonary and extrapulmonary TB under routine hospital conditions. The findings have supported the suggestion that a positive GTMD test result for smear-negative patients would help effective precautions to be taken to prevent transmission among populations and initiate treatment against a possible M. tuberculosis infection. However, the low sensitivity level should be taken into account in the differential diagnosis in patients suspected of having TB. It would be better to follow these patients and take precautions against transmission of TB until the culture result is found to be negative and a true picture of the clinical outcome is obtained. New diagnostic tools for early detection of M. tuberculosis complex and nontuberculous mycobacteria in smear-negative TB patients and in extrapulmonary cases need to be investigated in further studies.
Footnotes
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Behr M. A., et al. 1999. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353:444–449 [DOI] [PubMed] [Google Scholar]
- 2. Bicmen C., et al. 2010. Nontuberculous mycobacteria isolated from pulmonary specimens between 2004 and 2009: causative agent or not? New Microbiol. 33:399–403 [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention 2009. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb. Mortal. Wkly. Rep. 58:7–10 [PubMed] [Google Scholar]
- 4. Chakravorty S., Sen M. K., Tyagi J. S. 2005. Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J. Clin. Microbiol. 43:4357–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan H. S., Sun A. J., Hoheisel G. B. 1990. Bronchoscopic aspiration and bronchoalveolar lavage in the diagnosis of sputum smear-negative pulmonary tuberculosis. Lung 168:215–220 [DOI] [PubMed] [Google Scholar]
- 6. Compton J. 1991. Nucleic acid sequence-based amplification. Nature 350:91–92 [DOI] [PubMed] [Google Scholar]
- 7. Dinnes J., et al. 2007. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol. Assess. 11:1–196 [DOI] [PubMed] [Google Scholar]
- 8. Flores L. L., Pai M., Colford J. M., Jr., Riley L. W. 2005. In-house nucleic acid amplification tests for the detection of Mycobacterium tuberculosis in sputum specimens: meta-analysis and meta-regression. BMC Microbiol. 5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franco-Alvarez de Luna F., Ruiz P., Gutierrez J., Casal M. 2006. Evaluation of the GenoType Mycobacteria Direct assay for detection of Mycobacterium tuberculosis complex and four atypical mycobacterial species in clinical samples. J. Clin. Microbiol. 44:3025–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griffith D. E., et al. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 11. Guerra R. L., et al. 2007. Use of the amplified Mycobacterium tuberculosis Direct Test in a public health laboratory: test performance and impact on clinical care. Chest 132:946–951 [DOI] [PubMed] [Google Scholar]
- 12. Gupta S., Prasad V., Bairy I., Muralidharan S. 2009. Comparative evaluation of two cold staining methods with the Ziehl-Neelsen method for the diagnosis of tuberculosis. Southeast Asian J. Trop. Med. Public Health 40:765–769 [PubMed] [Google Scholar]
- 13. Hernandez-Garduno E., et al. 2004. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax 59:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kehinde A. O., Obaseki F. A., Cadmus S. I., Bakare R. A. 2005. Diagnosis of tuberculosis: urgent need to strengthen laboratory services. J. Natl. Med. Assoc. 97:394–396 [PMC free article] [PubMed] [Google Scholar]
- 15. Kiraz N., Saglik I., Kiremitci A., Kasifoglu N., Akgun Y. 2010. Evaluation of the GenoType Mycobacteria Direct assay for direct detection of the Mycobacterium tuberculosis complex obtained from sputum samples. J. Med. Microbiol. 59:930–934 [DOI] [PubMed] [Google Scholar]
- 16. Kivihya-Ndugga L. E., et al. 2003. A comprehensive comparison of Ziehl-Neelsen and fluorescence microscopy for the diagnosis of tuberculosis in a resource-poor urban setting. Int. J. Tuberc. Lung Dis. 7:1163–1171 [PubMed] [Google Scholar]
- 17. Koneman E. W., Allen S. D., Janda W. M., Schreckenberger P. C., Winn W. C. 1992. Mycobacteria, p. 703–755In Color atlas and textbook of diagnostic microbiology, 4th ed J. B. Lippincott Company, Philadelphia, PA [Google Scholar]
- 18. Lee C. H., et al. 2005. Response to empirical anti-tuberculosis treatment in patients with sputum smear-negative presumptive pulmonary tuberculosis. Respiration 72:369–374 [DOI] [PubMed] [Google Scholar]
- 19. Moore D. F., Guzman J. A., Mikhail L. T. 2005. Reduction in turnaround time for laboratory diagnosis of pulmonary tuberculosis by routine use of a nucleic acid amplification test. Diagn. Microbiol. Infect. Dis. 52:247–254 [DOI] [PubMed] [Google Scholar]
- 20. Neonakis I. K., et al. 2009. Comparative evaluation of GenoType mycobacteria direct assay with Gen-Probe Mycobacterium tuberculosis amplified direct test and GenoType MTBDRplus for direct detection of Mycobacterium tuberculosis complex in clinical samples. J. Clin. Microbiol. 47:2601–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Noussair L., Bert F., Leflon-Guibout V., Gayet N., Nicolas-Chanoine M. H. 2009. Early diagnosis of extrapulmonary tuberculosis by a new procedure combining broth culture and PCR. J. Clin. Microbiol. 47:1452–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pai M., Ling D. I. 2008. Rapid diagnosis of extrapulmonary tuberculosis using nucleic acid amplification tests: what is the evidence? Future Microbiol. 3:1–4 [DOI] [PubMed] [Google Scholar]
- 23. Saglam L., Akgun M., Aktas E. 2005. Usefulness of induced sputum and fibreoptic bronchoscopy specimens in the diagnosis of pulmonary tuberculosis. J. Int. Med. Res. 33:260–265 [DOI] [PubMed] [Google Scholar]
- 24. Scarparo C., et al. 2002. Evaluation of the BACTEC MGIT 960 in comparison with BACTEC 460 TB for detection and recovery of mycobacteria from clinical specimens. Diagn. Microbiol. Infect. Dis. 44:157–161 [DOI] [PubMed] [Google Scholar]
- 25. Seagar A. L., et al. 2008. Evaluation of the GenoType Mycobacteria Direct assay for the simultaneous detection of the Mycobacterium tuberculosis complex and four atypical mycobacterial species in smear-positive respiratory specimens. J. Med. Microbiol. 57:605–611 [DOI] [PubMed] [Google Scholar]
- 26. Selvakumar N., Gomathi M., Rehman F., Narayanan P. R. 2002. Evaluation of a two-reagent cold staining method for detection of acid-fast bacilli. Int. J. Tuberc. Lung Dis. 6:728–731 [PubMed] [Google Scholar]
- 27. Siddiqi K., Lambert M. L., Walley J. 2003. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect. Dis. 3:288–296 [DOI] [PubMed] [Google Scholar]
- 28. Syre H., Myneedu V. P., Arora V. K., Grewal H. M. S. 2009. Direct detection of mycobacterial species in pulmonary specimens by two rapid amplification tests, the Gen-Probe Amplified Mycobacterium tuberculosis Direct test and the GenoType Mycobacteria Direct test. J. Clin. Microbiol. 47:3635–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taegtmeyer M., et al. 2008. The clinical impact of nucleic acid amplification tests on the diagnosis and management of tuberculosis in a British hospital. Thorax 63:317–321 [DOI] [PubMed] [Google Scholar]
- 30. Tortoli E., et al. 1999. Use of BACTEC MGIT 960 for recovery of mycobacteria from clinical specimens: multicenter study. J. Clin. Microbiol. 37:3578–3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tostmann A., et al. 2008. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in The Netherlands. Clin. Infect. Dis. 47:1135–1142 [DOI] [PubMed] [Google Scholar]
- 32. Whitehorn J., Ayles H., Godfrey-Faussett P. 2010. Extra-pulmonary and smear-negative forms of tuberculosis are associated with treatment delay and hospitalization. Int. J. Tuberc. Lung Dis. 14:741–744 [PubMed] [Google Scholar]
- 33. Woods G. L. 1999. Molecular methods in the detection and identification of mycobacterial infections. Arch. Pathol. Lab. Med. 123:1002–1006 [DOI] [PubMed] [Google Scholar]


