Abstract
Thirty-one antimicrobial-resistant, extended-spectrum-β-lactamase-producing strains of Vibrio cholerae O1 serotype Ogawa associated with an outbreak of cholera in South Africa (2008) were investigated. Ten selected cholera strains were PCR positive for the SXT element, harbored mutations in the quinolone resistance-determining regions of GyrA (Ser83-Ile) and ParC (Ser85-Leu), and produced TEM-63 β-lactamase.
TEXT
Cholera is transmitted via the fecal-oral route and occurs in developing regions with a problematic food or water supply, overcrowding, and inadequate sanitation (12). This diarrheal disease is endemic in parts of Africa, Asia, and South and Central America (12). The etiological agents responsible are toxin-producing strains of Vibrio cholerae belonging to serogroups O1 and O139 (9). Aside from rehydration and electrolyte replacement, treatment with antimicrobial agents can decrease the severity of the illness and bacterial shedding (9). However, antimicrobial-resistant epidemic strains of V. cholerae have been described, consequently raising concerns over the availability and options for antimicrobial treatment (9). Here we report on an outbreak of cholera due to toxigenic, antimicrobial-resistant, extended-spectrum-β-lactamase (ESBL)- producing strains of V. cholerae O1 associated with illegal miners at a gold mine in the Ehlanzeni district of the Mpumalanga Province of South Africa between May and July 2008.
Thirty-four laboratory-confirmed cases of cholera, including 5 deaths, were reported. The Enteric Diseases Reference Unit (EDRU) received bacterial strains from 31 of the outbreak cases. These cases included 18 strains from illegal miners (53%), 6 strains from close contacts (18%), and 10 strains from the remaining cases (29%). Confirmatory identification was done using standard microbiological techniques. MIC testing and agar dilution methods for each strain were performed as per the Clinical and Laboratory Standards Institute (CLSI) 2008 guidelines (1). Antimicrobial agents used included ampicillin, amoxicillin-clavulanic acid (Augmentin), co-trimoxazole, chloramphenicol, nalidixic acid, ciprofloxacin, tetracycline, kanamycin, streptomycin, imipenem, ceftazidime, and erythromycin. Standard CLSI breakpoints for erythromycin do not exist. MIC interpretation for erythromycin was performed using a previously published method (8). MIC breakpoints conferring resistance are listed in Fig. 1. Nalidixic acid resistance was investigated using agar dilution MIC testing in the presence and absence of two efflux pump inhibitors (EPIs), reserpine and phenylalanyl arginine-β-naphthylamide (PAβN). Reserpine (20 μg/ml) and PAβN (40 μg/ml) were tested independently of each other.
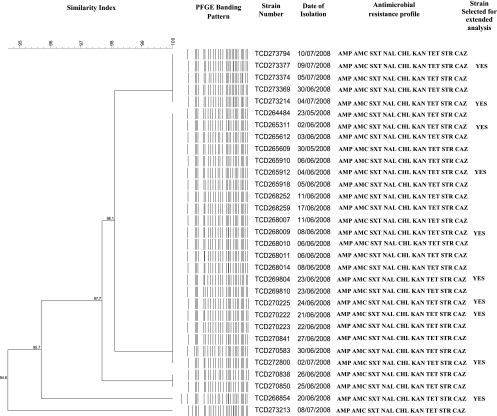
Fig. 1.
Dendrogram of PFGE banding patterns of the cholera outbreak strains. Strains were determined to be antimicrobial resistant at the following MIC breakpoints: ampicillin (AMP), MIC of ≥16 μg/ml; amoxicillin-clavulanic acid (AMC), MIC of ≥16 μg/ml; co-trimoxazole (SXT), MIC of ≥16 μg/ml; nalidixic acid (NAL), MIC of ≥32 μg/ml; chloramphenicol (CHL), MIC of ≥16 μg/ml; kanamycin (KAN), MIC of ≥32 μg/ml; tetracycline (TET), MIC of ≥8 μg/ml; streptomycin (STR), MIC of ≥64 μg/ml; and ceftazidime (CAZ), MIC of ≥16 μg/ml.
Strains were characterized by pulsed-field gel electrophoresis (PFGE) analysis using the PulseNet standardized protocol for V. cholerae (2). PFGE banding patterns were analyzed using BioNumerics (version 5.1) software (Applied Maths, Sint-Martens-Latem, Belgium).
Crude DNA extracts were prepared, and these served as template DNA in PCR assays. Conventional PCR was performed on all 31 strains for the detection of the cholera toxin (CT) gene ctxA and the gene encoding the toxin coregulated pilus (TCP) tcpA, as previously described (7). Ten strains were selected for further analysis involving conventional PCR screening for class 1 integrons (3′ conserved segment [3′-CS] and 5′-CS), class 2 integrons (intI2), plasmid-mediated quinolone resistance (PMQR) genes (qnrA, qnrB, qnrS, qnrC, and qepA), a quinolone resistance determinant (qnrVC3), ESBL-producing genes (blaTEM, blaSHV, and blaCTX-M), genes coding for the quinolone resistance-determining region (QRDR) of DNA gyrase (gyrA, gyrB) and topoisomerase IV (parC, parE), the SXT element-integrase gene (SXT int) and associated SXT resistance genes (floR, sul2, dfrA1, dfr18, strA, and strB), and the class A tetracycline resistance determinant (tetA). PCR primer sets and PCR positive-control strains used in this study are described in Table S1 in the supplemental material.
For DNA sequencing of genes, purified blaTEM, gyrA, gyrB, parC, and parE PCR-positive amplicons served as template DNA in the cycle sequencing PCR using the BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) and an Applied Biosystems model 3130 automated genetic analyzer. DNASTAR Lasergene (version 8.0) software (DNASTAR, Inc., Madison, WI) was used to analyze the nucleotide sequences. Sequence identity was determined at the DNA database of the National Center for Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Strains that displayed ESBL activity were grown in Luria-Bertani medium containing 2 μg/ml ceftriaxone (Sigma-Aldrich Chemical Co., St. Louis, MO).
Intact plasmid DNA was prepared as described by Kado and Liu (6). Forty microliters of plasmid DNA were resolved by PFGE using the CHEF-DR III system (Bio-Rad, Hercules, CA). Sizes of the plasmid DNA were estimated by comparison to a supercoiled molecular size standard, BAC-Tracker (EPICENTRE Biotechnologies, Madison, WI). Southern blot hybridizations were performed with digoxigenin (DIG)-labeled gene probes generated with the PCR DIG probe synthesis kit (Roche Diagnostics, Mannheim, Germany). Nylon membranes together with the DIG DNA labeling and detection kit (Roche) were used to perform Southern blot hybridizations and probing of plasmid DNA to determine whether specific genes were located on plasmid DNA.
All 31 cholera outbreak strains were characterized as V. cholerae O1 serotype Ogawa, a serotype associated with cholera outbreaks across Africa (11). PCR analysis revealed that 30/31 (97%) strains were positive for the CT, and all strains were positive for the El Tor variant of the TCP, which is required for pathogenesis (7), suggesting these were of the El Tor biotype. All 31 strains had the same antimicrobial susceptibility profile and were resistant to more than nine antimicrobial agents tested. All were susceptible to ciprofloxacin, imipenem, and erythromycin but were resistant to ampicillin, co-trimoxazole, chloramphenicol, nalidixic acid, tetracycline, kanamycin, and streptomycin. All strains displayed ESBL activity with further resistance to ceftazidime. All strains showed a very similar PFGE NotI profile and were determined to be clonal at a 95% pattern similarity value upon dendrogram analysis (Fig. 1). This suggests that this outbreak arose from the dissemination of a highly clonal strain of V. cholerae O1 from a point source. PFGE banding patterns resulting from NotI restriction were shown to be an effective genotypic tool to characterize strains of V. cholerae O1 (2).
Ten strains were selected for extended analysis and screening for antimicrobial resistance determinants. Strains were selected to ensure that all PFGE banding patterns were represented, and the selected strains are indicated in Fig. 1. Extended analysis and screening of the 10 strains showed the following results. Class 1 integrons, class 2 integrons, and PMQR genes were not detected by PCR. PCR and nucleotide sequence analysis showed that all strains produced TEM-63 β-lactamase, coinciding with a ceftazidime MIC of 64 μg/ml. Data obtained in our study have revealed the first incidence of TEM-63 β-lactamase-producing multidrug-resistant toxigenic V. cholerae O1 outbreak strains in South Africa. TEM-63 β-lactamase has previously been seen in Enterobacteriaceae from South Africa. The isolation of TEM-63 β-lactamase-producing strains of Klebsiella pneumoniae between 1994 and 1996 was first reported from Durban, South Africa, in 2001 by Essack et al. (3). Our V. cholerae O1 strains displayed the presence of a single plasmid of ∼140 kb in size, as shown in Fig. S2 in the supplemental material. Southern blotting and DNA probing analysis demonstrated that blaTEM encoding the TEM-63 β-lactamase, as shown in Fig. S2 in the supplemental material, was located on the plasmid in all 10 strains investigated. This finding correlates with plasmid profiling analysis done by Essack et al.: strains of K. pneumoniae contained plasmids of similar sizes encoding TEM-63 β-lactamase (3).
Our study showed that all strains harbored the integrative and conjugative element (ICE) known as the SXT element, since all strains were PCR positive for the integrase gene, SXT int, and associated SXT resistance genes showing intermediate- to high-level resistance to chloramphenicol (floR), sulfamethoxazole (sul2), trimethoprim (dfrA1), and streptomycin (strA and strB) (5). Variant types of the SXT element have previously been described in other Vibrio species, including Vibrio vulnificus, Vibrio metschnikovii, Vibrio fluvialis, and Vibrio parahaemolyticus, which harbored one to six resistance genes (10). Our strains were also PCR positive for the tetA gene, which confers tetracycline resistance. Tetracycline has been extensively used for the treatment of cholera across Africa (11). In our study, we found no evidence of active efflux conferring quinolone resistance, as there was no difference in nalidixic acid MIC values following agar dilution MIC testing in the presence and absence of EPIs. This suggests that the mechanism for nalidixic acid resistance in these strains is due to the accumulation of two mutations detected in the QRDRs of GyrA (Ser83-Ile) and ParC (Ser85-Leu). No mutations in GyrB and ParE were observed. Treatment with fluoroquinolones has until recently been a fail-safe choice in the management of cholera and other diarrheal diseases (11).
This report highlights the rapidity and ease with which drug resistance is developing in southern Africa. To conclude, we cannot speculate how a plasmid-borne ESBL-based (TEM-63 β-lactamase) resistance mechanism previously associated with nosocomial infections became so prominent in this community-based cholera outbreak. Given the ease with which plasmids can spread from one bacterial species to another, this could have major implications for the transfer of an ESBL-based resistance mechanism to bacteria associated with other community-acquired infections (unrelated to cholera) and could severely impact the management of these other community-acquired infections, should expanded-spectrum cephalosporins be the treatment of choice. From an epidemiological point of view, further investigation of our outbreak strain using genotyping techniques employing gene sequencing technology (4) and comparison of sequence data to those found in publically available databases is required to give clarity about the origin and dissemination of our outbreak strain.
Nucleotide sequence accession number.
The TEM-63 nucleotide sequence for strain 265311 was deposited in NCBI GenBank under accession number HQ904076.
Supplementary Material
Acknowledgments
This study was generously supported by the NHLS Research Trust (NHLSRT), GERMS-SA, and EDRU.
We thank staff from the Outbreak Response Unit, Epidemiology Unit, NICD, NHLS, South Africa, for their assistance in the outbreak investigation. We are very grateful to George A. Jacoby of the Lahey Clinic, Burlington, MA, for providing control strains positive for PMQR genes. Thanks to Maria Colombo of the Dip. Biologia Cellulare e dello Sviluppo, Rome, Italy, and Carlo Pazzani of the Dip. Di Genetica e Microbiologia, Bari, Italy, for providing control strains positive for class 1 integrons and the SXT element. Thanks to the team from the Antimicrobial Resistance Reference Unit (AMRRU), NICD, NHLS, South Africa, for providing control strains positive for ESBL genes. Special thanks to Daniela Ceccarelli of the Dip. Biologia Cellulare e dello Sviluppo, Rome, Italy, for her valuable advice and technical assistance.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Clinical Laboratory Standards Institute (CLSI), 2008. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2. Cooper K. L., et al. 2006. Development and validation of a PulseNet standardized pulsed-field gel electrophoresis protocol for subtyping of Vibrio cholerae. Foodborne Pathog. Dis. 3:51–58 [DOI] [PubMed] [Google Scholar]
- 3. Essack S. Y., Hall L. M., Pillay D. G., McFadyen M. L., Livermore D. M. 2001. Complexity and diversity of Klebsiella pneumoniae strains with extended-spectrum beta-lactamases isolated in 1994 and 1996 at a teaching hospital in Durban, South Africa. Antimicrob. Agents Chemother. 45:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grim C. J., et al. 2010. Genome sequence of hybrid Vibrio cholerae O1 MJ-1236, B-33, and CIRS101 and comparative genomics with V. cholerae. J. Bacteriol. 192:3524–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwanaga M., et al. 2004. Antibiotic resistance conferred by a class I integron and SXT constin in Vibrio cholerae O1 strains isolated in Laos. Antimicrob. Agents Chemother. 48:2364–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kado C. I., Liu S. T. 1981. Rapid procedure for detection and isolation of large and small plasmids. J. Bacteriol. 145:1365–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keasler S. P., Hall R. H. 1993. Detecting and biotyping Vibrio cholerae O1 with multiplex PCR. Lancet 341:1661. [DOI] [PubMed] [Google Scholar]
- 8. Ng L. K., et al. 2003. Can. Etest be used to determine Vibrio cholerae susceptibility to erythromycin? Antimicrob. Agents Chemother. 47:1479–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ngandjio A., et al. 2009. Antimicrobial resistance and molecular characterization of Vibrio cholerae O1 during the 2004 and 2005 outbreak of cholera in Cameroon. Foodborne Pathog. Dis. 6:49–56 [DOI] [PubMed] [Google Scholar]
- 10. Okoh A., Igbinosa E. 2010. Antibiotic susceptibility profiles of some Vibrio strains isolated from wastewater final effluents in a rural community of the Eastern Cape Province of South Africa. BMC Microbiol. 10:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Opintan J. A., Newman M. J., Nsiah-Poodoh O. A., Okeke I. N. 2008. Vibrio cholerae O1 from Accra, Ghana carrying a class 2 integron and the SXT element. J. Antimicrob. Chemother. 62:929–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zuckerman J. N., Rombo L., Fisch A. 2007. The true burden and risk of cholera: implications for prevention and control. Lancet Infect. Dis. 7:521–530 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.