Abstract
In anticipation of the emergence of more variants of bovine spongiform encephalopathy (BSE), a semiquantitative display of the following four independent molecular diagnostic prion parameters was designed: N terminus, proteinase K (PK) resistance, glycoprofile, and mixed population. One H BSE case, three L BSE cases, six C BSE cases, and one unusual classical BSE (C BSE) case are reported.
TEXT
The epidemic of bovine spongiform encephalopathy (BSE) in the United Kingdom and other countries, with the subsequent emergence of a variant form of Creutzfeldt-Jakob disease in humans, had urged the European Union (EU) in 2001 to take costly measures by active monitoring cattle for BSE and banning the recycling of mammalian offal proteins into feed (8). Eight years later, due to the effectiveness of the control measures taken, new BSE cases rarely occur.
It has become evident that atypical BSE types exist in cattle (2, 5). These variants seem to be sporadic, and all occurred in animals 8 years and older (2–7, 10, 13–15, 17–19, 1–23), except one isolated 2-year-old case (24). BSE cases are now classified into classical BSE (C BSE), L BSE, and H BSE based on Western blot (WB) analysis of PrPres, the proteinase K (PK)-resistant fragment of the disease-associated prion protein (PrP) isoform PrPSc (15). It must be anticipated that with continuing active surveillance, a majority of cases will appear as natural sporadic forms (2). Confirmation of such cases requires effective criteria.
In this study, the molecular properties of brain stem PrPres in eight Austrian and three recently reported BSE cases in the Netherlands (Table 1) were investigated by WB analysis with PrP-specific monoclonal antibodies (MAbs) 12B2, P4, L42, and SAF84. These are specific for bovine PrP epitopes 101WGQGG105, 101WGQGGSH107, 156YEDRYY161, and 174YRPVDQY180, respectively (1, 2, 9, 11, 12, 14, 20). Two WB detection methods were applied. The first method used was based on enzymatic chemiluminescent enhancement procedures followed by photographic film exposure (15), and the second WB method used was a recently developed multiplex fluorophore labeling method and was used here for glycoprofile estimations (16). This multiplex technique provided a reliable tool for glycotyping for two reasons: the absence of substrate spreading from the protein banding regions and the area of measurement is exactly the same when comparing the PrPres glycoprofiles for different MAbs. Relative antibody staining intensities between blots, between two lanes, or for PrPres glycoform estimations were performed as described previously (15, 16).
Table 1.
Details of Austrian and Dutch BSE cases until March 2011a
| Case no. | Yr of birth | Age (mo) | Screening testb | Confirmation testc | Status at death | Typing resultd |
|---|---|---|---|---|---|---|
| AU01 | 1996 | 70 | PCW | OIE-WB | Healthy | C (A1B2) |
| AU02 | 1994 | 134 | TeSeE | OIE-WB | Healthy | C (A1B2) |
| AU03 | 1992 | 155 | LIA | IHC | Healthy | C (A1B2) |
| AU04 | 2000 | 70 | LIA | IHC | Healthy | C (A1B2) |
| AU05 | 1993 | 149 | LIA | OIE-WB | Fallen st. | CP4 (A2B2) |
| AU06 | 1996 | 130 | LIA | IHC | Fallen st. | L (A4B1) |
| AU07 | 1997 | 150 | PCP | Bio-Rad-WB | Healthy | L (A4B1) |
| AU08 | 1995 | 180 | PCP | Bio-Rad-WB | Fallen st. | H (A2B4) |
| NL73 | 1996 | 91 | PCW | IHC | Healthy | C (A1B2) |
| NL74 | 1996 | 90 | PCW | IHC | Healthy | C (A1B2) |
| NL86 | 1999 | 140 | PS | IHC | Healthy | C (A1B2) |
| NL87 | 1997 | 162 | PCW | IHC | Fallen st. | C (A1B2) |
| NL88 | 1996 | 174 | PCW | IHC | Fallen st. | L (A4B1) |
All Austrian cases were investigated for the type of BSE for the first time, as well as the most recent Dutch cases (NL86 to -88), plus some confirmed C BSE cases from a previous study (NL73, NL74) in which all Dutch cases had been analyzed (15). Fallen st., fallen stock (i.e., animals that died on the farm).
PCW = Prionics-Check Western blot; TeSeE = enzyme-linked immunosorbent assay (ELISA)-like Bio-Rad TeSeE test; LIA = ELISA-like Prionics-Check luminescence immunoassay; PCP = immunochromatographic lateral diffusion Prionics-Check PrioSTRIP test; PS = Roche PrionScreen ELISA-like test.
OIE-WB = World Organisation for Animal Health (OIE)-recognized methods using scrapie-associated fibril (SAF) purification and Western blot detection; IHC = OIE-recognized method using immunohistochemistry; Bio-Rad-WB = an EU-recognized PrPres preparation method, followed by Western blotting.
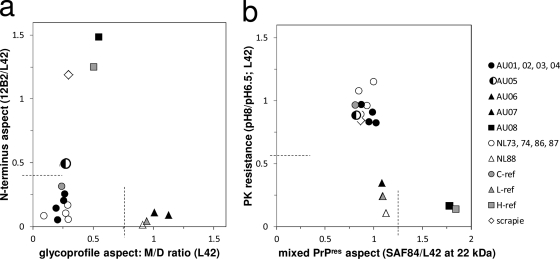
Typing results (and classifications, shown in parentheses) were derived from Fig. 1. AU05 is a C-type BSE case with intermediately high MAb 12B2 reactivity and unusually high MAb P4 binding comparable to that of 12B2. Such high P4 affinity has not been observed previously, neither for intact bovine PrP nor its fragments (Fig. 2).
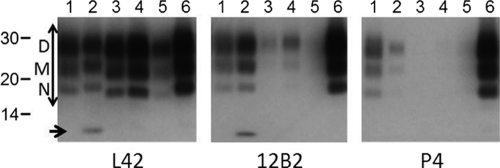
Western blot PrPres patterns obtained for the samples were similar to those reported for bovine C, L, and H BSE cases (15, 16). These patterns of MAb binding were transformed semiquantitatively as ratios of staining intensities reflecting four independent PrPres aspects in two graphs (Fig. 1a and b). The first aspect concerned the N-terminal aspect, represented by the 12B2 epitope. Most BSE samples had small amounts of this epitope (12B2/L42 ratio of <0.4), typical for C and L BSE. Sample AU08 exhibited relatively high N terminus epitope retention, as did the H BSE reference sample (Fig. 1a, vertical axis). In addition, both AU08 and the reference H sample contained an additional 7-kDa band with antibodies 12B2 and L42 (Fig. 2) (15). One Austrian case (AU05) was special since it reacted with substantial 12B2 intensity compared to MAb L42 (Fig. 2, lanes 1). Moreover, when probed with N terminus-specific MAb P4 (epitope WGQGGSH, which carries the same N-terminal amino acid as the epitope of 12B2), there was unusually high binding of this antibody to AU05, about equal to that of MAb 12B2, not seen in any of the N terminus epitope-carrying H BSE cases (Fig. 2, compare lanes 1 and 2) or in our previously studied C BSE cases (15, 16).
Fig. 1.
Combined graphical presentations of four independent discriminatory PrPres parameters for BSE isolate typing. (a) The vertical axis reflects the abundance of the N-terminal epitope of MAb 12B2 relative to the core epitope of MAb L42. On the horizontal axis, the ratio of monoglycosyl to diglycosyl forms (M/D) as probed with MAb L42 reflects a glycoprofile property of the triple-banded PrPres pattern. (b) The vertical axis reflects the resistance to PK, measured as the L42 binding ratio between samples digested at pH 8 and pH 6.5. The horizontal axis with the SAF84/L42 ratio represents another glycoprofile aspect—values of >1.25 point to the existence of two populations of PrPres triple bands in one sample (15). (a and b) By dividing the graphs each into four sectors, 1 to 4 (following the hands of the clock starting from the bottom left sector), the isolate types studied can be assigned to the following categories for C, L, and H BSE: A1B2, A4B1, and A2B4, respectively. The single classical scrapie case fits into category A2B2. The AU05 isolate is considered an exceptional C BSE case due to its N terminus aspect and, thus, having an intermediate behavior between the A1B2 and A2B2 categories.
Fig. 2.
Inspection of N-terminal epitope abundance with MAbs P4 and 12B2 relative to the PrP core L42 epitope. Lane 1, AU05; lane 2, reference H BSE; lane 3, C-type reference; lane 4, AU04; lane 5, AU02; lane 6, classical scrapie. Applied tissue equivalent (milligrams of tissue per lane) (TE) for lanes 1 to 4, 0.5 mg; for lane 5, 2.5 mg; for lane 6, 0.25 mg. The positions of the D, M, and N triple glycoprofile bands are indicated. The region for glycoprofile estimation is indicated with a double-headed arrow. The migration positions of three molecular mass standards are indicated in kDa. The M position coincides with the referred migration at 22 kDa (Fig. 1). A 7-kDa fragment is observed in H BSE samples (arrow).
A further tool for diagnosis was the analysis of resistance to PK digestion by comparing samples digested at pH 6.5 with 50 μg/ml PK and at pH 8 with 500 μg/ml PK (Fig. 1b, vertical axis). Samples AU01 to AU05 and NL86 and NL87 appeared equally resistant to PK as did the reference C BSE samples, reflected by pH 8/pH 6.5 ratios of between 0.7 and 1. Samples AU06, AU07, AU08, and NL88 compared to the poorly PK-resistant L and H BSE reference samples.
PrPres is usually characterized by a triple banding pattern that is composed of nonglycosylated (N), monoglycosylated (M), and diglycosylated (D) PrP fragments having molecular masses around 18, 22, and 28 kDa, respectively (Fig. 2). The sum of the antibody binding values for N+M+D fragments in this region was set at 1, and their fragment fractions were used for ratio calculations shown in Fig. 1a and b. When tested with MAb L42, a low M-to-D fragment ratio (≤0.3) was observed for the samples AU01, AU02, AU03, AU04, AU05, NL73, NL74, NL86, and NL87 as well as for the two reference C BSE samples (Fig. 1a, horizontal axis). Samples AU06, AU07, NL88, and reference L BSE exhibited a higher fraction of M-PrPres (M/D fragment ratio = ±1). Compared to the C and L BSE-like cases, the AU08 sample and the reference H BSE M/D fragment values were intermediate (M/D fragment ratio = ±0.5).
As a fourth criterion, glycoprofile results between those obtained with MAbs L42 and SAF84 were compared, and they were rather similar for most samples, except for case AU08 and the reference H BSE sample. This could be illustrated in the plot by the binding ratio between SAF84 and L42 at the M position (Fig. 1b, horizontal axis). This so-called dualistic behavior was previously shown to be a result of the existence of a mixture of two PrPres populations in H BSE isolates (1, 15).
When dissecting the graphs shown in Fig. 1 into four sectors, 16 categories can be constructed. The samples shown in our study fit into four of these: A1B2, A2B2, A4B1, and A2B4 for C BSE, AU05 (and scrapie), L BSE, and H BSE, respectively (Table 1). The most recent cases in Austria and the Netherlands were L type (AU06, AU07, NL88) and H type (AU08), while AU05 behaved as a C BSE case with a peculiar characteristic of an enhanced presence of the N-terminal 101WGQGG105 PrP sequence and unusually strong MAb P4 binding for bovine PrP. Unfortunately, no more AU05 tissue is available for strain typing, and the tissue was subject to strong autolysis, which might have influenced the outcome.
Considering the old ages of the BSE cases detected, it would be best to maintain BSE surveillance of cattle in the age cohort of at least 8 years and older.
Acknowledgments
We are grateful to Anne Buschmann (FLI) for providing the sample of the second Austrian BSE case and reference samples of atypical BSE cases from preceding studies and to Michaela Mannsfeldner for performing the preceding studies.
These investigations were supported by the Austrian Agency for Health and Food Safety (AGES) and the Dutch Ministry of Economic Affairs, Agriculture and Innovation, project WOT-01-002-001.01. The multiplex WB was developed under project SE1700/CSA 7335 from the Department for Environment, Food and Rural Affairs (Defra; United Kingdom).
Footnotes
Published ahead of print on 15 June 2011.
REFERENCES
- 1. Biacabe A. G., Jacobs J. G., Bencsik A., Langeveld J. P., Baron T. G. 2007. H-type bovine spongiform encephalopathy: complex molecular features and similarities with human prion diseases. Prion 1:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biacabe A. G., Laplanche J. L., Ryder S., Baron T. 2004. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 5:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biacabe A. G., Morignat E., Vulin J., Calavas D., Baron T. G. 2008. Atypical bovine spongiform encephalopathies, France, 2001–2007. Emerg. Infect. Dis. 14:298–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buschmann A., et al. 2006. Atypical BSE in Germany—proof of transmissibility and biochemical characterization. Vet. Microbiol. 117:103–116 [DOI] [PubMed] [Google Scholar]
- 5. Casalone C., et al. 2004. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. U. S. A. 101:3065–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dobly A., et al. 2010. No H- and L-type cases in Belgium in cattle diagnosed with bovine spongiform encephalopathy (1999–2008) aging seven years and older. BMC Vet. Res. 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dudas S., et al. 2010. Molecular, biochemical and genetic characteristics of BSE in Canada. PLoS One 5:e10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Union. 2001. Regulation (EC) no. 999/2001 of the European parliament and of the council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Off. J. Eur. Communities L147:1–40 [Google Scholar]
- 9. Feraudet C., et al. 2005. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J. Biol. Chem. 280:11247–11258 [DOI] [PubMed] [Google Scholar]
- 10. Gavier-Widen D., et al. 2008. Bovine spongiform encephalopathy in Sweden: an H-type variant. J. Vet. Diagn. Invest. 20:2–10 [DOI] [PubMed] [Google Scholar]
- 11. Geysen H. M., Meloen R. H., Barteling S. J. 1984. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc. Natl. Acad. Sci. U. S. A. 81:3998–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldmann W., Hunter N., Martin T., Dawson M., Hope J. 1991. Different forms of the bovine PrP gene have five or six copies of a short, G-C-rich element within the protein-coding exon. J. Gen. Virol. 72(Pt. 1):201–204 [DOI] [PubMed] [Google Scholar]
- 13. Hagiwara K., et al. 2007. Accumulation of mono-glycosylated form-rich, plaque-forming PrPSc in the second atypical bovine spongiform encephalopathy case in Japan. Jpn. J. Infect. Dis. 60:305–308 [PubMed] [Google Scholar]
- 14. Harmeyer S., Pfaff E., Groschup M. H. 1998. Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants. J. Gen. Virol. 79(Pt. 4):937–945 [DOI] [PubMed] [Google Scholar]
- 15. Jacobs J. G., et al. 2007. Molecular discrimination of atypical bovine spongiform encephalopathy strains from a geographical region spanning a wide area in Europe. J. Clin. Microbiol. 45:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobs J. G., et al. 2011. Differentiation of ruminant transmissible spongiform encephalopathy isolate types, including bovine spongiform encephalopathy and CH1641 scrapie. J. Gen. Virol. 92:222–232 [DOI] [PubMed] [Google Scholar]
- 17. Polak M. P., Zmudzinski J. F., Jacobs J. G., Langeveld J. P. 2008. Atypical status of bovine spongiform encephalopathy in Poland: a molecular typing study. Arch. Virol. 153:69–79 [DOI] [PubMed] [Google Scholar]
- 18. Richt J. A., et al. 2007. Identification and characterization of two bovine spongiform encephalopathy cases diagnosed in the United States. J. Vet. Diagn. Invest. 19:142–154 [DOI] [PubMed] [Google Scholar]
- 19. Seuberlich T., et al. 2006. Spongiform encephalopathy in a miniature zebu. Emerg. Infect. Dis. 12:1950–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Slootstra J. W., Puijk W. C., Ligtvoet G. J., Langeveld J. P., Meloen R. H. 1996. Structural aspects of antibody-antigen interaction revealed through small random peptide libraries. Mol. Divers. 1:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stack M., et al. 2009. Two unusual bovine spongiform encephalopathy cases detected in Great Britain. Zoonoses Public Health 56:376–383 [DOI] [PubMed] [Google Scholar]
- 22. Stack M. J., et al. 2009. Third atypical BSE case in Great Britain with an H-type molecular profile. Vet. Rec. 165:605–606 [DOI] [PubMed] [Google Scholar]
- 23. Vidal E., et al. 2007. Molecular profiling and comparison of field transmissible spongiform encephalopathy cases diagnosed in Catalunya. Vet. J. 174:196–199 [DOI] [PubMed] [Google Scholar]
- 24. Yamakawa Y., et al. 2003. Atypical proteinase K-resistant prion protein (PrPres) observed in an apparently healthy 23-month-old Holstein steer. Jpn. J. Infect. Dis. 56:221–222 [PubMed] [Google Scholar]