Abstract
The Russian Federation is a high-tuberculosis (TB)-burden country with high rates of drug resistance, including multidrug and extensive drug resistance to TB (M/XDRTB). Rapid diagnosis of resistance to fluoroquinolones (FQs) using molecular assays is essential for the implementation of appropriate drug regimens and prevention of the transmission of XDR strains. A total of 51 individual MDRTB strains were tested by pyrosequencing of the quinolone resistance determining region of the gyrA gene and the GenoType MTBDRsl assay (Hain Lifescience, GmbH, Nehren, Germany), and the results were evaluated against those obtained by phenotypic drug susceptibility testing (DST). Mutations were detected in 25 (78.1%) FQ-resistant strains, with the majority of mutations (n = 19 [73.0%]) found in codon 94 of the gyrA gene; the novel mutation 1457 C→Τ was found in the gyrB gene. Three mixed allelic variants were detected, which is a well-known phenomenon in areas with high TB and drug-resistant TB rates. The sensitivity and specificity of pyrosequencing (86.2 and 100%, respectively) and MTBDRsl (86.2 and 100%, respectively) were high; however, the results for 5.9% of the analyzed strains were unreadable when MTBDRsl was used. The MTBDRsl and pyrosequencing assays offer a rapid and accurate means for diagnosing resistance to FQs in high-TB-burden areas.
INTRODUCTION
Tuberculosis (TB) remains a major threat to public health worldwide and in the developing world in particular, causing almost 2 million deaths annually. The real magnitude of drug resistance is not yet known, although the Indian subcontinent, China, and Russia are believed to account for the majority of cases globally (48).
Multidrug-resistant TB (MDRTB), caused by TB bacilli resistant to both isoniazid (INH) and rifampin (RIF), poses difficulties in diagnosis and treatment, with lower survival rates (especially in HIV-infected persons) and the associated high costs of TB control programs. The World Health Organization (WHO) estimates current MDRTB rates in new and previously treated cases globally to be 2.9 and 15.3%, respectively (50).
The Russian Federation is a high-TB-burden country with high rates of TB drug resistance, dominated by TB strains of the Beijing family reported to be associated with MDRTB (7, 42). The Samara Oblast (Central Russia) is a hot spot for both TB and HIV epidemics; ca. 20% of new TB cases are MDR, with rates even higher in previously treated cases and in the prison sector (8). Converging TB, drug-resistant TB, and HIV epidemics pose a serious problem for low- and middle-income countries such as Russia where access to second- and third-line drug therapy is limited (2, 8).
The rate of extensively drug-resistant TB (XDRTB) (which is caused by MDRTB bacilli with additional resistance to a fluoroquinolone [FQ] and one or more injectable drugs) has been increasing in Russia (36) due to multiple incomplete treatment regimens and poor infection control practice. Cases of XDRTB show a high rate of treatment failure and mortality especially in XDRTB patients with concomitant HIV infection (13).
Increasing MDRTB and XDRTB rates require the development and implementation of rapid diagnostic systems for the detection of microbial resistance to prevent further nosocomial transmission and promptly implement appropriate drug regimes.
Automated liquid culture systems have significantly shortened turnaround times for drug susceptibility tests (DSTs) compared to solid media, but bacteriological assays are technically demanding and still require approximately 7 to 10 days (34). Detection of genetic mutations that correlate with resistance to certain antimicrobial agents represents a more rapid alternative.
Resistance to RIF and INH is defined by mutations in a number of genes, including rpoB (codons 508 to 533), katG (codon 315), and the mabH-inhA regulatory regions (6, 38). The principal target of the FQs is the DNA gyrase composed of two A and two B subunits (47) encoded by gyrA and gyrB, respectively, and resistance to FQs is mediated by mutations in a short discrete region of the gyrA and gyrB genes termed the quinolone resistance determining region (QRDR) (3, 46).
Recently, developed line-probe assays (4, 16) for RIF and/or INH have been extensively validated and currently are recommended by the WHO for implementation globally (49). Assays for the detection of mutations in genes associated with resistance to ethambutol (EMB), aminoglycosides/cyclic peptides (AG/CP), and FQ have been developed by Hain Lifescience, GmbH (Nehren, Germany), evaluated in low-incidence settings in two recent studies. and demonstrated good sensitivity and specificity (5, 17). Pyrosequencing assays have also proved effective for the detection of mutations in genes associated with the drug resistance (19). However, larger studies in high TB burden and drug-resistant TB setting are required to demonstrate the feasibility of molecular assay usage for the routine detection of strains resistant to FQs and also to evaluate their sensitivity and specificity, which could be potentially affected by the prevalence of certain types of mutations in regions where Beijing and other highly conserved genotypes dominate.
In the present study we sought to compare the sensitivity and specificity of two assays for the molecular analysis of target genes that could be used for the detection of resistance to FQs and explore associations between FQ-resistant phenotypes and Mycobacterium tuberculosis genotypes in a high TB burden and drug resistant settings.
MATERIALS AND METHODS
Bacterial cultures and phenotypic drug susceptibility testing.
We analyzed 51 consecutive individual MDR M. tuberculosis clinical strains isolated from pulmonary TB patients (23 new cases and 28 that had received treatment in the past) treated at TB clinics in the Samara Region, Russian Federation, in 2008. These cultures were derived from sputum samples taken from consecutive patients; two sputum samples were collected from each patient. Specimens were processed using the NaOH-NALC method and were inoculated on both solid Lowenstein-Jensen medium (LJ) and the BACTEC MGIT 960 automated liquid medium system (Becton Dickinson, Cockeysville, MD) according to a standard protocol (24). First-line drug susceptibility testing (FLD DST) on LJ was performed in Samara according to the absolute concentration method, utilizing the following drug concentrations: streptomycin, 10.0 μg/ml; INH, 1.0 μg/ml; RIF, 40.0 μg/ml; and EMB, 2.0 μg/ml (23, 24, 33). Identification of the M. tuberculosis complex was performed as part of the GenoType MTBDRsl assay (see below). Patients identified as MDR were consecutively recruited into the study.
All identified MDR TB strains were subcultured to test sensitivity to second line drugs. Second-line DST (SLD DST) was performed using the automated BACTEC MGIT 960 system at the National Mycobacterium Reference Laboratory, London, England. The drug concentrations used in the MGIT system were as follows: ofloxacin, 2.0 μg/ml; and moxifloxacin, 0.25 μg/ml (23).
Molecular methods.
Crude DNA extracts were isolated from cultures by heating (95°C, 30 min), followed by sonication (20 min). Isolates were genotyped using spoligotyping and variable-number tandem-repeat (VNTR) typing with a standardized panel of 24 loci as described previously (21, 32, 44, 45). The VNTR loci 3232, 3336, 2163A, and 1982 were used, in addition to the standard panel (44).
The conventional identification of M. tuberculosis complex isolates was confirmed by spoligotyping and analysis of resistance to FQs, AG/CP, and EMB was performed using GenoType MTBDRsl kits (Hain Lifescience) according to the manufacturer's instructions. Reading and interpretation of results was performed by two independent operators blinded to the known SLD DST results. Specimens producing poor results (no bands and/or faint bands on the membrane preventing the operator from unambiguous reading) were tested one more time in independent cycles of the PCR and hybridization and were considered unreadable if the operator was still unable to read the results.
Identification of nucleotide sequences of the QRDR of the gyrA and gyrB genes (associated with resistance to FQs) was carried out using conventional DNA sequencing (capillary electrophoresis using an ABI 3170 analyzer [Applied Biosystems]) and pyrosequencing (PyroMarkID, Biotage; Qiagen, United Kingdom) in London.
Sequencing of the QRDRs of gyrA and gyrB genes (positions 7379 to 7699 and positions 6443 to 6963, respectively) was performed as described previously (9) with modifications using the following primers: GYRA_Seq_F, ATCGACTATGCGATGAGCG; GYRA_Seq_R, GGGCTTCGGTGTACCTCAT; GYRB_Seq_F, AGTCGTTGTGAACAAGGCTGT; and GYRB_Seq_R, CCACTTGAGTTTGTACAGCGG. Analysis of sequencing results was carried out using the DNA Dynamo program (Blue Tractor Software, United Kingdom).
Pyrosequencing of codons 88 to 100 of the gyrA gene was performed according to the manufacturer's recommendations (Qiagen) with the primers GyrAF (CCGCAGCCACGCCAAGTC), GyrAR (biotin-GTCCACCAGCGGGTAGCG), and GyrASeq (AACTACCACCCGCAC). Processing and analysis of the results were conducted using proprietary software (Qiagen) and the ClustalX and BioEdit programs.
Statistical and cluster analysis.
Statistical analysis was performed using the Fisher test with a P = 0.05 significance level. Clustering analysis of strains was performed using Bionumerics (Applied Maths, Ghent, Belgium).
RESULTS
Resistance to FQs was detected in 32 of the 51 (62.7%) MDRTB strains based on phenotypic SLD DST in liquid media. There was full agreement between the ofloxacin and moxifloxacin results, confirming well-known FQ cross-resistance. Overall, concordance between phenotypic and molecular testing results was high (91.7% for sequencing and pyrosequencing and 91.1% for MTBDRsl).
Conventional sequencing results.
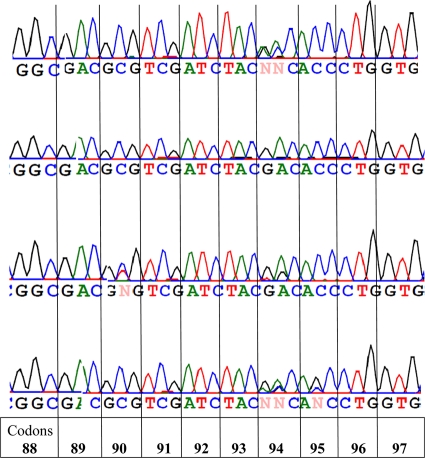
Mutations associated with FQ resistance were detected in 26 of 32 phenotypically resistant strains by conventional sequencing of gyrA and gyrB genes. Additionally, three mixed allelic variants (that have both wild-type and mutant alleles in the gyrA gene) were detected among these 32 phenotypically resistant strains (Tables 1 and 2, Fig. 1). More than half of strains with mutations (n = 19 [73.0%]) had them in codon 94 of the gyrA gene. All 26 strains had a mutation in one codon only of the gyrA (Table 2) and gyrB genes. In addition, 30 strains had polymorphisms at codon 95 AGC→ACC not associated with FQ resistance. Three phenotypically resistant strains did not have any mutations in gyrA or gyrB genes. In strains with mixed allelic variants mutations in codon 94 (GAC/GGC and GAC/AAC/GCC) and codon 90 (GCG/GTG) were identified (Fig. 1).
Table 1.
Comparison of phenotypic and molecular DST results
| Molecular method and result | No. of strainsa (%) |
Assessment characteristic (%)b |
||||
|---|---|---|---|---|---|---|
| Resistant to FQ (n = 32) | Sensitive to FQ (n = 19) | Sensitivity | Specificity | PPV | NPV | |
| Sequencing | ||||||
| Resistant | 26 (81.3) | 1 (5.3) | 89.6 | 94.7 | 96.3 | 85.7 |
| Sensitive | 3 (9.4) | 18 (94.7) | ||||
| Unreadable | 0 | 0 | ||||
| Mixed | 3 (9.4) | 0 | ||||
| Pyrosequencing | ||||||
| Resistant | 25 (78.1) | 0 | 86.2 | 100 | 100 | 82.6 |
| Sensitive | 4 (12.5) | 19 (100.0) | ||||
| Unreadable | 0 | 0 | ||||
| Mixed | 3 (9.4) | 0 | ||||
| MTBDRsl | ||||||
| Resistant | 25 (78.1) | 0 | 86.2 | 100 | 100 | 82.6 |
| Sensitive | 4 (12.5) | 19 (100.0) | ||||
| Unreadable | 3 (9.4) | 0 | ||||
| Mixed | 0 | |||||
Strains harboring mutations in QRDRs were considered resistant to FQ.
Compared to the gold standard (phenotypic) for readable results only. Resistance results for mixed cultures are excluded. PPV, positive predictive value; NPV, negative predictive value.
Table 2.
Distribution of mutations in QRDRs of the gyrA gene in M. tuberculosis resistant to FQ strainsa
| Sequencing result | Molecular assay result |
|
|---|---|---|
| Pyrosequencing | MTBDRsl | |
| G88C (88 GGC-GCC), n = 1 | G88C (88 GGC-GCC), n = 1 | Unreadable |
| A90V (90 GCG-GTG), n = 4 | A90V (90 GCG-GTG), n = 4 | Δwt2 + MUT1 (codon 90), n = 2; Δwt2 (codon 90), n = 1; unreadable n = 1 |
| S91P (91 TCG-CCG), n = 1 | S91P (91 TCG-CCG), n = 1 | Δwt2 + MUT2 (codon 91), n = 1 |
| D94G (94 GAC-GGC), n = 13 | D94G (94 GAC-GGC), n = 13 | Δwt3 + MUT3C (codon 94), n = 12; Δwt3 + MUT3C + MUT3A (codon 94), n = 1 |
| D94A (94 GAC-GCC), n = 2 | D94A (94 GAC-GCC), n = 2 | Δwt3 + MUT3A (codon 94), n = 1; unreadable, n = 1 |
| D94N (94 GAC-AAC), n = 3 | D94N (94 GAC-AAC), n = 3 | Δwt3 + MUT3B (codon 94), n = 3 |
| D94Y (94 GAC- TAC), n = 1 | D94Y (94 GAC-TAC), n = 1 | Δwt3 + MUT3B (codon 94), n = 1 |
| Wild type, n = 3 | Wild type, n = 3 | Wild type, n = 3 |
| Mixed 90 GCG/GTG + 94 GAC/GGC, n = 1 | Mixed | wt + MUT1 + MUT3C, n = 1 |
| Mixed 94 GAC/AAC/GCC, n = 2 | Mixed | Δwt3 + MUT3A + MUT3B, n = 1; wt + MUT3A + MUT3B + MUT3C, n = 1 |
All results are presented as the amino acid change(s), followed by the nucleotide change(s) in parentheses, in gyrA. n, Number of mutations.
Fig. 1.
Histograms of the DNA sequences in codons 88 to 97 of the gyrA gene in four strains with mixed allelic variants.
Three phenotypically resistant strains were identified as sensitive by all molecular methods used in the study. One more phenotypically resistant strain identified as sensitive by pyrosequencing and MTBDRsl had a 1453 C→Τ mutation in the gyrB gene.
All 19 phenotypically sensitive strains had no mutations in gyrA. A novel (previously unreported) mutation in gyrB (1457 C→Τ) was detected in one sensitive strain, probably indicating that it was not associated with resistance to FQ.
Pyrosequencing results.
By pyrosequencing of the QRDR of gyrA gene, mutations associated with FQ resistance were detected in 25 of 32 phenotypically resistant strains. In addition, 3 mixed allelic variants were found among these 32 phenotypically resistant strains (Tables 1 and 2). There was complete agreement between sequences of the gyrA gene obtained using conventional sequencing and pyrosequencing (Table 2). Of the 32 resistant strains, 30 had the mutation AGC→ACC in codon 95 (which is not associated with FQ resistance) and a wild type in this codon was registered in two strains. Mutations in gyrA were not detected in four phenotypically resistant strains.
No mutations in the QRDR of gyrA in 19 FQ-susceptible strains were found by pyrosequencing apart from the natural polymorphism 95 AGC→ACC. Therefore, the sensitivity and specificity of pyrosequencing were 86.2 and 100.0%, respectively, compared to the standard phenotypic DST method.
MTBDRsl results.
Readable MTBDRsl results were obtained for 48 strains (94.1%). FQ resistance was detected in 25 of 32 phenotypically resistant strains using the MTBDRsl assay (Tables 1 and 2). Mutations in codons 90 (gyrA MUT1), 91 (gyrA MUT2), and 94 (gyrA MUT3A, gyrA MUT3B, and gyrA MUT3C) were found in 3, 1, and 21 strains, respectively. In three strains mixed genotypes were seen (Table 2); these have been interpreted as resistant according to the manufacturer's recommendations. Four phenotypically resistant strains had no mutations in gyrA according to the MTBDRsl assay. The phenotypic and MTBDRsl results for susceptible strains were identical.
The sensitivity and specificity of the MTBDRsl assay were 86.2 and 100.0%, respectively, compared to the standard DST method. However, the results for three phenotypically resistant strains were unreadable even when the test was repeated twice. Overall, the concordance between MTBDRsl and pyrosequencing was high (95.8%).
Results of genotyping and association with TB family.
The vast majority (49 isolates, 96.0%) of tested MDRTB strains were identified as belonging to the Beijing genotype family using spoligotyping; two other strains (4.0%) belonged to the T1 genotype family. All Beijing family strains had the natural mutation 95 (AGC-ACC) in the gyrA gene associated with the Beijing genotype and the principal genetic group 1 (12, 40). Genotyping using 28-locus VNTR, followed by cluster analysis, allowed identification of four clusters with cluster sizes varying from 2 to 25 strains and 10 unique profiles. The largest cluster included 16 phenotypically FQ-resistant and 9 phenotypically FQ-susceptible strains (64.0 and 36.0%, respectively) (Table 3). There was no statistical association between FQ resistance and the VNTR profile of the strain. A double allele (four and five copies) was found in the ETR-C locus in one strain with a mixed allelic variant 94 GAC/AAC/GCC, suggesting it was probably a mixed culture.
Table 3.
Genetic characteristics of M. tuberculosis FQ-sensitive and -resistant strains
| Clustera | VNTR profile | No. of MDR strains |
|
|---|---|---|---|
| FQ sensitive (n = 19) | FQ resistant (n = 32) | ||
| Cluster 1 | 223325153533424448443(12)726296 | 9 | 16 |
| Cluster 2 | 223325173533424446443(14)227296 | 4 | 7 |
| Cluster 3 | 223325153533424448443(14)728296 | 1 | 2 |
| Cluster 4 | 223325173533424446443(14)722296 | 1 | 1 |
| Unique profile | 4 | 6 | |
Cluster genotype as determined by the VNTR typing using the following loci: MIRU 2, 4, 10, 16, 20, 23, 24, 26, 27, 31, 39, and 40; ETR-A; ETR-B; ETR-C; and VNTR 424, 1955, 1982, 2347, 2401, 3171, 3232, 3336, 3690, 4052, 4156, 2163A, and 2163B.
DISCUSSION
Rapid and accurate detection of resistance to second-line anti-TB drugs is the key to successful therapy and interruption of the chain of MDRTB strain transmission. It is extremely important in areas with high rates of drug resistance, including cases of coinfection with HIV (37). Bacteriological methods are highly sensitive and specific but very labor-intensive and time-consuming. Consequently, molecular diagnostic methods are being introduced into routine use for the rapid detection of FQ resistance by identification of mutations in specific gyrA and gyrB genes regions (QRDR) (17, 46).
This study is the first to assess the effectiveness of both a noncommercial DNA sequencing and a commercially available molecular diagnostics system (GenoType MTBDRsl) for the rapid detection of M. tuberculosis resistance to FQ, conducted in the Russian Federation (a middle-incidence region with high rates resistance to anti-TB drugs). Pyrosequencing was performed to examine correlations between presence of mutations encoding resistance to FQ and phenotypic DST. Molecular results were verified by using conventional sequencing of QRDRs in gyrA and gyrB (3, 35, 46).
The sensitivity and specificity of the MTBDRsl assay were 86.2 and 100%, respectively, compared to the standard MGIT method. This is similar to previously reported data (5, 17), suggesting that this assay is feasible for use in areas with high rates of drug resistance. There was 95.5% concordance between results of conventional sequencing and the MTBDRsl assay, excluding unreadable results and mixed allelic variants, demonstrating a high specificity for the assay. However, the assay performance and sensitivity are limited by analyzed genes and unreadable results. For example, in our study resistance in two strains was determined by mutations in codon 88 of the gyrA and, in one more strain, by a mutation in gyrB probes, which are absent in the MTBDRsl assay. We conclude that the MTBDRsl assay provides a relatively accurate and rapid diagnostic tool for FQ resistance with 100% specificity (although 5.9% of analyzed strains were unreadable), but slower culture-based diagnostics using liquid media are still needed to confirm the assay results.
We have also evaluated pyrosequencing (which has recently been proposed for the effective diagnosis of M/XDR-TB [1, 15, 19]) for the detection of mutations associated with resistance to FQs as an alternative to conventional sequencing. All results of pyrosequencing of gyrA QRDR codons 88 to 100 were in complete agreement with results of conventional sequencing. However, the overall sensitivity of this method was lower (86.2%), probably due to other undetected mechanisms of FQ resistance. Mixed allelic variants were detected correctly by pyrosequencing. Pyrosequencing can be used as an effective alternative to the MTBDRsl assay in reference laboratories because it identifies any mutations in the observed region and can be utilized for other analyses requiring short sequencing reads. Pyrosequencing is not particularly time-consuming and takes approximately one working day to analyze up to 100 strains for one mutation. Despite the simplicity, low cost, and relative rapidity of pyrosequencing, the methodology requires intensive staff training and more expensive equipment.
We have determined a spectrum of mutations associated with FQ resistance in strains circulating in the Samara region by using molecular assays. In our study, polymorphisms in codons 88, 90, 91, 94, and 95 of gyrA were identified, with mutations in codon 94 being the most frequent (73.0% of all mutant strains). D94G (94 GAC-GGC) was the most prevalent mutation in codon 94 of gyrA (46.4% of all mutant strains). In addition, two strains had mutations in gyrB (1457 C→Τ and 1453 C→Τ), but one of these strains was phenotypically sensitive.
Our data are in agreement with previous studies where FQ resistance was reported to be associated largely with mutations in codons 90 and 94 of gyrA (A90V, D94G, and D94Y [20, 35]) and, more rarely, with mutations D94A, D94N, T80A, G88C, and D89 N gyrA (3, 26, 46). The distribution of mutations in the analyzed strains was in agreement with that reported in a recent study from St. Petersburg (26), suggesting a similar mechanism of drug resistance development in areas where the Beijing strains family dominates. Mutations in gyrB are less common (3), and their association with resistance to FQ is not known. The gyrB mutations found in our study are novel; however, further studies, including molecular cloning of the novel mutation into a susceptible strain with subsequent detection of increased MICs, are required to prove the role of these mutations in drug resistance development. It is known that multiple mutations in gyrA and/or gyrB cause higher levels of anti-TB resistance (14, 22). Nevertheless, our study confirms earlier observations that significant resistance levels can be reached by having single mutations in observed genes.
The majority of analyzed XDR strains (96.0%) belonged to the Beijing genotype family. Associations between this genotype family and resistance to anti-TB drugs, including RIF, INH, and FQs, have been reported previously (9, 10, 25, 27–30, 43). In our study only two strains belonged to the T1 family strains, and both of them had the mutation D94N detected in the Beijing family strains as well. No conclusion about associations between specific mutations and family genotypes could be made. The majority of the mutations in the Beijing family genotype can be successfully detected by the MTBDRsl assay (and presumably for other TB families). A relatively small number of unique genotypes were identified (Table 3), suggesting high rates of current transmission of XDR strains in Samara region. This is supported by previous and current evidence that only limited numbers of strain types, primarily belonging to the Beijing family, are responsible for the transmission of drug resistant TB in Samara (7; unpublished data). Despite recent reports demonstrating significant associations between mutations conferring drug resistance and VNTR profiles of Beijing family strains (20, 35), no such correlations were seen here, suggesting independent acquisition of FQ resistance by Beijing family strains in Samara.
Interestingly, we found three mixed allelic variants in FQ-resistant strains (9.4% of all resistant strains) in the present study. All of these strains were isolated from retreatment patients and could therefore be explained by a mixed infection with sensitive and resistant strains. Mixed infections are seen in areas with high rates of TB and MDRTB (11, 41). However, double alleles were identified by VNTR typing in one of three mixed strains only. Therefore, mixed allelic variants could be explained by heteroresistance (the presence of FQ-susceptible and -resistant populations of bacteria as a result of ongoing resistance development) (39). Heteroresistance and mixed allelic variants in gyrA codons have been previously reported in areas with high rates of drug resistance (9, 18, 31). Such cases should be interpreted clinically with caution.
ACKNOWLEDGMENTS
This study was supported by the EU FP7 grant 223681 TB PAN-NET.
We thank all of the doctors, nurses, and laboratory persons involved in this study for their essential work and support.
Footnotes
Published ahead of print on 1 June 2011.
REFERENCES
- 1. Arnold C. 2010. Rapid analysis of resistant mutant genotypes using pyrosequencing. Methods Mol. Biol. 642:217–223 [DOI] [PubMed] [Google Scholar]
- 2. Atun R. A., Lebcir R., Drobniewski F., Coker R. J. 2005. Impact of an effective multidrug-resistant tuberculosis control programme in the setting of an immature HIV epidemic: system dynamics simulation model. Int. J. STD AIDS 16:560–570 [DOI] [PubMed] [Google Scholar]
- 3. Aubry A., et al. 2006. Novel gyrase mutations in quinolone-resistant and -hypersusceptible clinical isolates of Mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob. Agents Chemother. 50:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bang D., Bengard Andersen A., Thomsen V. O. 2006. Rapid genotypic detection of rifampin- and isoniazid-resistant Mycobacterium tuberculosis directly in clinical specimens. J. Clin. Microbiol. 44:2605–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brossier F., Veziris N., Aubry A., Jarlier V., Sougakoff W. 2010. Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 48:1683–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown T. J., Herrera-Leon L., Anthony R. M., Drobniewski F. A. 2006. The use of macroarrays for the identification of MDR Mycobacterium tuberculosis. J. Microbiol. Methods 65:294–300 [DOI] [PubMed] [Google Scholar]
- 7. Drobniewski F., et al. 2005. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA 293:2726–2731 [DOI] [PubMed] [Google Scholar]
- 8. Drobniewski F. A., et al. 2005. Tuberculosis, HIV seroprevalence and intravenous drug abuse in prisoners. Eur. Respir. J. 26:298–304 [DOI] [PubMed] [Google Scholar]
- 9. Duong D. A., et al. 2009. Beijing genotype of Mycobacterium tuberculosis is significantly associated with high-level fluoroquinolone resistance in Vietnam. Antimicrob. Agents Chemother. 53:4835–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis, 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg. Infect. Dis. 12:736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang R., et al. 2008. Mixed infections of Mycobacterium tuberculosis in tuberculosis patients in Shanghai, China. Tuberculosis (Edinb.) 88:469–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagneux S., et al. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:2869–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandhi N. R., et al. 2006. Extensively drug-resistant tuberculosis as a cause of death in patients coinfected with tuberculosis and HIV in a rural area of South Africa. Lancet 368:1575–1580 [DOI] [PubMed] [Google Scholar]
- 14. Ginsburg A. S., Grosset J. H., Bishai W. R. 2003. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect. Dis. 3:432–442 [DOI] [PubMed] [Google Scholar]
- 15. Halse T. A., et al. 2010. Combined real-time PCR and rpoB gene pyrosequencing for rapid identification of Mycobacterium tuberculosis and determination of rifampin resistance directly in clinical specimens. J. Clin. Microbiol. 48:1182–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hillemann D., Rusch-Gerdes S., Richter E. 2007. Evaluation of the GenoType MTBDRplus assay for rifampin and isoniazid susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 45:2635–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hillemann D., Rusch-Gerdes S., Richter E. 2009. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 47:1767–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hofmann-Thiel S., et al. 2009. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur. Respir. J. 33:368–374 [DOI] [PubMed] [Google Scholar]
- 19. Jureen P., et al. 2006. Rapid detection of rifampin resistance in Mycobacterium tuberculosis by pyrosequencing technology. J. Clin. Microbiol. 44:1925–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kam K. M., et al. 2006. Stepwise decrease in moxifloxacin susceptibility amongst clinical isolates of multidrug-resistant Mycobacterium tuberculosis: correlation with ofloxacin susceptibility. Microb. Drug Resist. 12:7–11 [DOI] [PubMed] [Google Scholar]
- 21. Kamerbeek J., et al. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kocagoz T., et al. 1996. Gyrase mutations in laboratory-selected, fluoroquinolone-resistant mutants of Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 40:1768–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kruuner A., Yates M. D., Drobniewski F. A. 2006. Evaluation of MGIT 960-based antimicrobial testing and determination of critical concentrations of first- and second-line antimicrobial drugs with drug-resistant clinical strains of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ministry of Health of the Russian Federation, 2003. On improvement of TB control activities in the Russian Federation. Document 109. Ministry of Health of the Russian Federation, Moscow, Russian Federation. [Google Scholar]
- 25. Mokrousov I., et al. 2002. High prevalence of KatG Ser315Thr substitution among isoniazid-resistant Mycobacterium tuberculosis clinical isolates from northwestern Russia, 1996 to 2001. Antimicrob. Agents Chemother. 46:1417–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mokrousov I., et al. 2008. Molecular characterization of ofloxacin-resistant Mycobacterium tuberculosis strains from Russia. Antimicrob. Agents Chemother. 52:2937–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mokrousov I., et al. 2003. PCR-based methodology for detecting multidrug-resistant strains of Mycobacterium tuberculosis Beijing family circulating in Russia. Eur. J. Clin. Microbiol. Infect. Dis. 22:342–348 [DOI] [PubMed] [Google Scholar]
- 28. Mokrousov I., Otten T., Vyshnevskiy B., Narvskaya O. 2002. Detection of embB306 mutations in ethambutol-susceptible clinical isolates of Mycobacterium tuberculosis from Northwestern Russia: implications for genotypic resistance testing. J. Clin. Microbiol. 40:3810–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niemann S., et al. 2009. Genomic diversity among drug sensitive and multidrug resistant isolates of Mycobacterium tuberculosis with identical DNA fingerprints. PLoS One 4:e7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nikolayevsky V., et al. 2004. Detection of mutations associated with isoniazid and rifampin resistance in Mycobacterium tuberculosis isolates from Samara Region, Russian Federation. J. Clin. Microbiol. 42:4498–4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nikolayevskyy V., et al. 2009. Performance of the Genotype MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation. BMC Clin. Pathol. 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nikolayevskyy V., et al. 2006. Differentiation of tuberculosis strains in a population with mainly Beijing-family strains. Emerg. Infect. Dis. 12:1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pfyffer G. E., Palicova F., Rusch-Gerdes S. 2002. Testing of susceptibility of Mycobacterium tuberculosis to pyrazinamide with the nonradiometric BACTEC MGIT 960 system. J. Clin. Microbiol. 40:1670–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piersimoni C., Olivieri A., Benacchio L., Scarparo C. 2006. Current perspectives on drug susceptibility testing of Mycobacterium tuberculosis complex: the automated nonradiometric systems. J. Clin. Microbiol. 44:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pitaksajjakul P., et al. 2005. Mutations in the gyrA and gyrB genes of fluoroquinolone-resistant Mycobacterium tuberculosis from TB patients in Thailand. Southeast Asian J. Trop. Med. Public Health 36(Suppl. 4):228–237 [PubMed] [Google Scholar]
- 36. Punga V. V., et al. 2009. Prevalence of extensively drug-resistant tuberculosis in Vladimir and Orel regions, Russia. Int. J. Tuberc. Lung Dis. 13:1309–1312 [PubMed] [Google Scholar]
- 37. Rajasekaran S., et al. 2009. HIV coinfection among multidrug resistant and extensively drug-resistant tuberculosis patients: a trend. J. Indian Med. Assoc. 107:281–286 [PubMed] [Google Scholar]
- 38. Ramaswamy S., Musser J. M. 1998. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update. Tuberc. Lung Dis. 79:3–29 [DOI] [PubMed] [Google Scholar]
- 39. Rinder H., Mieskes K. T., Loscher T. 2001. Heteroresistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 5:339–345 [PubMed] [Google Scholar]
- 40. Sreevatsan S., et al. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. U. S. A. 94:9869–9874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stavrum R., et al. 2009. High diversity of Mycobacterium tuberculosis genotypes in South Africa and preponderance of mixed infections among ST53 isolates. J. Clin. Microbiol. 47:1848–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Toungoussova O. S., Mariandyshev A. O., Bjune G., Caugant D. A., Sandven P. 2005. Resistance of multidrug-resistant strains of Mycobacterium tuberculosis from the Archangel oblast, Russia, to second-line anti-tuberculosis drugs. Eur. J. Clin. Microbiol. Infect. Dis. 24:202–206 [DOI] [PubMed] [Google Scholar]
- 43. Tungusova O. S., Mar'iandyshev A. O., Bewne H., Sandwen P. 2004. Drug resistance of Mycobacterium tuberculosis of the genotype Beijing in imprisonment places in the Arkhangelsk Region. Probl. Tuberk. Bolezn. Legk. 2004:35–41 (In Russian.) [PubMed] [Google Scholar]
- 44. Velji P., Nikolayevskyy V., Brown T., Drobniewski F. 2009. Discriminatory ability of hypervariable variable number tandem repeat loci in population-based analysis of Mycobacterium tuberculosis strains, London, UK. Emerg. Infect. Dis. 15:1609–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vitol I., Driscoll J., Kreiswirth B., Kurepina N., Bennett K. P. 2006. Identifying Mycobacterium tuberculosis complex strain families using spoligotypes. Infect. Genet. Evol. 6:491–504 [DOI] [PubMed] [Google Scholar]
- 46. Von Groll A., et al. 2009. Fluoroquinolone resistance in Mycobacterium tuberculosis and mutations in gyrA and gyrB. Antimicrob. Agents Chemother. 53:4498–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang J. C. 1985. DNA topoisomerases. Annu. Rev. Biochem. 54:665–697 [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization, 2008. Anti-tuberculosis drug resistance in the world, fourth global report. WHO/HTM/TB/2008.394. World Health Organization, Geneva, Switzerland [Google Scholar]
- 49. World Health Organization, 2008. Molecular line probe assays for rapid screening of patients at risk of multidrug resistant tuberculosis (MDR-TB): policy statement. World Health Organization, Geneva, Switzerland [Google Scholar]
- 50. Wright A., et al. 2009. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet 373:1861–1873 [DOI] [PubMed] [Google Scholar]