Abstract
We have developed a Salmonella genoserotyping array (SGSA) which rapidly generates an antigenic formula consistent with the White-Kauffmann-Le Minor scheme, currently the gold standard for Salmonella serotyping. A set of 287 strains representative of 133 Salmonella serovars was assembled to validate the array and to test the array probes for accuracy, specificity, and reproducibility. Initially, 76 known serovars were utilized to validate the specificity and repeatability of the array probes and their expected probe patterns. The SGSA generated the correct serovar designations for 100% of the known subspecies I serovars tested in the validation panel and an antigenic formula consistent with that of the White-Kauffmann-Le Minor scheme for 97% of all known serovars tested. Once validated, the SGSA was assessed against a blind panel of 100 Salmonella enterica subsp. I samples serotyped using traditional methods. In summary, the SGSA correctly identified all of the blind samples as representing Salmonella and successfully identified 92% of the antigens found within the unknown samples. Antigen- and serovar-specific probes, in combination with a pepT PCR for confirmation of S. enterica subsp. Enteritidis determinations, generated an antigenic formula and/or a serovar designation consistent with the White-Kauffmann-Le Minor scheme for 87% of unknown samples tested with the SGSA. Future experiments are planned to test the specificity of the array probes with other Salmonella serovars to demonstrate the versatility and utility of this array as a public health tool in the identification of Salmonella.
INTRODUCTION
Food-borne salmonellosis is an important public health concern worldwide and continues to be one of the leading causes of gastroenteritis in North America. Since salmonellae are primarily found in the intestinal tracts of animals, most infections are the result of drinking contaminated water or eating improperly prepared foods of animal origin, including meat, poultry, eggs, and dairy products (45). Salmonella can also be found on fresh produce, including tomatoes (1, 3), and on dry foods such as pet food (4). Salmonella infections commonly present with watery diarrhea, abdominal cramps, fever, headache, nausea, and vomiting. In approximately 1 to 4% of immunocompetent patients, bacteremia occurs, and in 5 to 10% of those individuals, other extraintestinal complications, including central nervous system infections, endocarditis, reactive arthritis, and urinary tract infections, may occur (26).
It is estimated that, in the United States, 1.2 million nontyphoidal Salmonella infections occur annually, resulting in 19,336 hospitalizations and 378 deaths (56). The annual cost of these infections, including medical expenses and loss of productivity, has been estimated to range between $0.5 and $2.3 billion dollars (19). Salmonellosis is significantly underreported; therefore, it is very difficult to precisely determine the actual public health burden of Salmonella worldwide (59). Canadian studies suggest that the ratio of salmonellosis infections per reported case ranges from 13 to 37, highlighting the need to develop rapid, accessible, and economical assays to facilitate clinical diagnosis and reporting strategies (62).
Currently, Salmonella isolates are typed using the White-Kauffmann-Le Minor scheme. This classification scheme is utilized by public health organizations worldwide and is considered the gold standard for the determination of Salmonella serotypes. The White-Kauffmann-Le Minor scheme subtypes Salmonella into serotypes on the basis of surface antigen identification using polyclonal antiserum to determine the O (somatic) and H (flagellar) antigenic epitopes (20). Serotyping is essential for human disease surveillance and outbreak detection, as both the virulence and host range of Salmonella isolates can be serotype specific (15, 67).
Many of the genes required for the biosynthesis of the O antigen are organized in a large regulon called the rfb cluster, which is located between the galF and gnd genes in both Salmonella and Escherichia coli (53, 55). Differences among the 46 Salmonella O serogroups described in the White-Kauffmann-Le Minor scheme are mainly due to genetic variations in their respective rfb clusters. While the sequences of sugar transferase genes within the rfb cluster are relatively conserved (68), the O-antigen flippase (wzx) and polymerase (wzy) genes are highly variable and are considered specific with respect to the serogroup (2).
There are 114 H antigens used to serotype this bacterium. The antigenic portion of the flagellar structure is encoded by two genes, fliC (phase 1 flagellin) and fljB (phase 2 flagellin). These genes are typically highly conserved at their 5′ and 3′ ends, whereas the central region is generally quite variable (39, 43). Flagellar antigens that are immunologically related are known as antigen complexes, and antigens within these complexes often exhibit very homologous gene sequences (43). Most Salmonella serotypes exhibit diphasic flagellar antigen expression, alternately expressing fliC and fljB genes; however, serovars that express only one flagellar antigen are considered monophasic. This genetic switching mechanism is regulated by the invertible element hin (58).
Despite its usefulness, traditional serotyping is labor-intensive and expensive and can take up to 5 days to complete. It requires specialized expertise and a set of more than 250 stringently quality-assured reagents to characterize the more than 2,500 Salmonella serovars. Many hospital and private laboratories rely on the use of a limited number of commercially available antisera, covering only a restricted number of serotypes (35). These laboratories are forced to ship isolates to reference laboratories for full serotyping, causing delays in isolate identification that ultimately impede progress in outbreak investigations and containment.
Drawbacks to traditional Salmonella serotyping have prompted many groups to investigate alternative molecular methods. In recent years, molecular typing assays have been developed based upon multiplex real-time PCR (31, 49), primer extension (5), microarrays (61, 66, 69), DNA sequence-based approaches (47), and bead-based suspension arrays (16, 41). To date, many of the molecular techniques have been able to type only a very small subset of the thousands of Salmonella serovars. Some do not provide an antigenic formula that mimics the globally understood White-Kauffmann-Le Minor scheme, and others are considered too expensive to be implemented in public or private diagnostic laboratories. In 2007, we described the development of a fluorescence-based glass slide microarray for the classification of prevalent Salmonella serovars (69). Shortly thereafter, we switched to the ArrayTube platform (2) (Alere Technologies [formerly Clondiag], Germany), which offered a rapid and more economical alternative to the expensive and time-consuming glass slide array system.
Here we describe an ArrayTube-based Salmonella genoserotyping array (SGSA) that generates an antigenic formula. Validation and testing of the array was completed with 287 Salmonella strains representative of 133 Salmonella serovars, including the most prevalent Salmonella serovars from human and nonhuman isolates within North America, the United Kingdom, and Austria, to ensure the development of a comprehensive assay with an international scope.
MATERIALS AND METHODS
Bacterial strains and culture methods.
Salmonella enterica strains used in this study were obtained primarily from animal isolates submitted to the Salmonella OIE Reference Laboratory at the Laboratory for Foodborne Zoonoses (LFZ; Guelph, Ontario, Canada) and were serotyped using conventional methods. In brief, serotyping at the LFZ utilizes slide agglutination for the determination of somatic antigens (14) and a mechanized microtechnique for flagellar antigenic determination (57). In order to designate serotypes based on an antigenic formula, the White-Kauffmann-Le Minor classification scheme was utilized (20).
The Salmonella strains in the blind study were provided by the Animal Health and Veterinary Laboratories Agency (AHVLA; Addlestone, Surrey, United Kingdom). All Salmonella strains were grown overnight at 37°C on Luria-Bertani agar (BD Canada, Mississauga, ON, Canada).
Array layout and design.
All 66 oligonucleotide probes (Table 1) (18 to 35 bp in length) were designed using PrimerSelect (DNASTAR, Madison, WI), and sequence specificity was assessed using GenBank's Basic Local Alignment Search Tool (BLASTN). Probes were synthesized and printed in triplicate onto ArrayTube strips by Alere Technologies (Jena, Germany). Biotinylated oligonucleotides were spotted on the microarray as staining controls and for use as reference spots by the image analysis software. The Salmonella-specific gene invA is utilized as a positive Salmonella control (52).
Table 1.
Probe target list
| Control | Capsular or O antigen | Flagellar phase 1 antigen(s) | Flagellar phase 2 antigen(s) | Additional characteristics |
|---|---|---|---|---|
| Biotin | A (Paratyphi) (O:2) | a | 1,2 | RHS-E |
| invA | B (O:4) | b | 1,2_1,5_1,2,7 | f subcomplex |
| C1 (O:6,7) | c | 1,5 | RHS-GP | |
| C2 (O:8) | d | 1,5-2 | S. Pullorum | |
| D (O:9) | e,h | 1,5-4 | ||
| E (O:3) | f,g | 1,5 (Kottbus) | ||
| G (O:13) | f,g,s | 1,5_1,2,7 | ||
| H (O:6,14) | f,g,t_f,g_g,m,t | 1,6 | ||
| J (O:17) | f,g,t | 1,7 | ||
| K (O:18) | g,m,s_g,m,p,s | e,n,x | ||
| L (O:21) | g,m,q_g,q | e,n,x,z15 | ||
| M (O:28) | gp | e,n,z15_e,n,x,z15 | ||
| O (O:35) | g,p_g.p.s | l,w | ||
| P (O:38) | g,s,t_g,t | |||
| V (O:44) | g,z51 | |||
| Y (O:48) | i | |||
| O:58 | k | |||
| O:61 | k (O:61) | |||
| Vi | l,z13_l,v | |||
| l,z28 | ||||
| m,t_g,m,t | ||||
| r_r,i | ||||
| y | ||||
| z | ||||
| z4,z23 | ||||
| z6_z67 | ||||
| z10 | ||||
| z29 | ||||
| z38 |
Multiplex PCR.
Genomic DNA was isolated from Salmonella grown overnight at 37°C using LB agar (BD Canada) and an EZ1 DNA tissue kit and BioRobot (Qiagen Ltd., Mississauga, ON, Canada) according to the manufacturer's instructions and with the addition of 100 μg of lysozyme (Sigma-Aldrich Canada Ltd., Oakville, ON, Canada) (10 mg/ml) in the cell lysis incubation. DNA was assessed for quality and quantified spectrophotometrically (Nanodrop ND-1000; Nanodrop Technologies Inc., Wilmington, DE). A minimum recovery of 60 ng/μl of DNA was required for subsequent use as a PCR template.
A multiplex PCR was developed to amplify targeted somatic genes within the Salmonella rfb cluster, the capsular Vi antigen encoded by the viaB gene, and unique sequences within rhs and rhs-like genes (Table 2). A phase 1 flagellar gene (fliC) and a Salmonella-specific gene (invA) were coamplified in a second reaction, and the phase 2 flagellar gene (fljB) antigen was amplified separately for optimum performance (Table 2). Additional somatic genes within the Salmonella rfb cluster were also amplified and tested in the SGSA; however, they were not amplified as part of the multiplex PCR described above (Table 2). All of the gene targets tested on the array were amplified using a Qiagen multiplex PCR kit according to the manufacturer's instructions. Each 25-μl PCR mixture contained 1× multiplex master mix, 0.2 μM each primer, and 1.75 μl (approximately 100 ng/μl) of genomic DNA. Amplification conditions were 15 min at 95°C, 35 cycles of 30 s at 95°C, 90 s at 57°C, and 90 s at 72°C, with a final elongation of 5 min at 72°C performed using a T Gradient thermocycler (Biometra, Montreal Biotech Inc., Montreal, QC, Canada). For validation purposes only, the presence of appropriately sized bands was verified using 1.2% agarose Flashgel DNA cassettes (Lonza, Rockland, ME).
Table 2.
Primer sequences for Salmonella genoserotyping array (SGSA) target amplification
| Target | Gene | Accession no. | Primer | Primer sequence (5′-3′) | Amplicon size (bp) | Multiplex | Reference or source |
|---|---|---|---|---|---|---|---|
| A (O:2)/D (O:9) | prt | NC_006511 | rfbS (D) | TCACGACTTACATCCTAC | 720 | 1 | Luk et al. (38) |
| rfbS (D) | CTGCTATATCAGCACAAC | ||||||
| B (O:4) | rfbJ | X56793 | B_rfbJ_F | TGAAAGAATATGTAATTGTCAGTGG | 789 | 1 | This study |
| B_rfbJ_R | TTTCATTATCTCTTTGCTCTATCG | ||||||
| C1 (O:6,7) | wbaA | M84642 | C1_wbaA_F3 | TTGGCAGACTGGTACTGATTGG | 976 | 1 | This study |
| C1_wbaA_R | GCAGGAATCCGTGTAAAAATTC | ||||||
| C2 (O:8) | rfbJ | X61917 | C2_rfbJ_F | GAACCCCTATATCTGAACAAT | 593 | 1 | This study |
| C2_rfbJ_R | CTCGGCACTCCAACCTAATC | ||||||
| E1/E4 (O:3) | wzx | X60665 | E_wzx_F | ATGCAAGTATATCCCCTGAAAATC | 1,000 | 1 | This study |
| E_wzx_R | CCGATTTAAGGGCATTTTTGTA | ||||||
| G (O:13) | wzx | EF204526 | G_wzx_F | CTGAAAAATGGTTTAGATTG | 502 | 1 | This study |
| G_wzx_R | ACCATTGGATACTGTAACTG | ||||||
| H (O:6,14) | wzy | AY334017.1 | H_wzy_F | GTCTCCGCTAAGCTATTTCGGTTTGTA | 501 | naa | Fitzgerald et al. (18) |
| H_wzy_R | CCCTTGTGATTGAATTATTGCGGTA | ||||||
| J (O:17) | wzy | EF032635 | J_wzy_F | GGCTGGGTTGTGGCTTTTT | 565 | na | Fitzgerald et al. (17) |
| J_wzy_R | CTTCCGAAATCAATAGAAAAATCAA | ||||||
| K (O:18) | wzx | EF032634 | K_wzx_F | CTCTAGGATCAACTGAAGGTGGTC | 370 | 1 | Fitzgerald et al. (17) |
| K_wzx_R | CAACCCAGCAATAAAGCAGAA | ||||||
| L (O:21) | wzx | HQ291553 | L_wzx_F2 | AAGGATGGGACTACCGTAAG | 810 | na | This study |
| L_wzx_R2 | ATTCCCCAATGTAATAACCA | ||||||
| M (O:28) | wzx | HQ291554 | M_wzx_F | GCTGGCTATGCTAGGACTTA | 704 | na | This study |
| M_wzx_R | ACCCAGATACTTCCCAAGAT | ||||||
| O (O:35) | wzy | AF285969 | O_wzy_F | ATGTCTATTGATTTTCTTT | 684 | na | This study |
| O_wzy_R | CAACTTGTAATAATAATAAAC | ||||||
| P (O:38) | wzx | HQ291552 | P_wzx_F | AGGGAAAGTAACGCTCAGTA | 707 | na | This study |
| P_wzx_R | CAAGTGCAGGAATCAACATA | ||||||
| V (O:44) | wbcM | HQ416970 | V_wbcM_F | ACGATCTAAGTAATTCAGGTGGTA | 1,031 | na | This study |
| V_wbcM_R | TCATAGTAAAACCTCGTCCAGTA | ||||||
| Y (O:48) | wzx | HQ291555 | Y_wzx_F | TTTTTCGAGCATTTATCACA | 708 | na | This study |
| Y_wzx_R | TCGCATAGCATATAGAGCAA | ||||||
| O:58 | wfbE | EU825757 | O58_wfbE_F | AGTTAGTGTTTGTATTATTTCGTA | 746 | na | This study |
| O58_wfbE_R | ACAAGTCAATGAGTTTATCCA | ||||||
| O:61 | wzx | HQ416969 | O61_wzx_F_2 | CGAGAGCAATGGGGATGGATG | 599 | 1 | This study |
| O61_wzx_R_2 | AGGAAAAGCGAAAGAAATAACAAT | ||||||
| Vi | viaB | X67785 | ViaBF | CACGCACCATCATTTCACCG | 738 | 1 | Kumar et al. (31) |
| ViaBR | AACAGGCTGTAGCGATTTAGG | ||||||
| RHS-GP | SG1045 | NC_011274 | RHS_GP_F | GACGACCAGAGAAATGAG | 437 | 1 | This study |
| RHS_GP_R3 | CGTACCACGTCACTTCC | ||||||
| RHS-E | SEN0272 | NC_011294 | E_RHSfam_F | GTGCTGTATGAAGTGTGC | 569 | 1 | This study |
| E_RHSfam_R | CAGGTGTAGTAATACCGTTC | ||||||
| H1-fliC | fliC | AY353389 | fliC_13 | GCGCGGAATAATGAGGCATAAAGC | 1,700 | 2 | McQuiston et al. (43) |
| fliC_14 | GCTTTCGCTGCCTTGATTGTGT | ||||||
| Salmonella- | invA | M90846 | invA_F1 | CTGCTTTCTCTACTTAACAGTGCTCG | 495 | 2 | Yoshida et al. (69) |
| specific | invA_R1 | CGCATCAATAATACCGGCCTTC | |||||
| H2-fljB | fljB | AY353269 | Ph2_8_F | GAAAAGATCATGGCACAAGTAATCAACACT | 1,500 | 3 | Yoshida et al. (69) |
| AllRev3_R | GGAATCTTCGATACGGCTACG | ||||||
| pepT | pepT | NC_011294 | pepT_F2 | GTTTGCCATATTGCTGCGAGGC | 2,061b/150c | na | This study |
| pepT_R2 | GCGCTATCTCGGCGGCTG |
na, currently used only in singleplex PCRs.
Amplicon size for Salmonella Enteritidis.
Amplicon size for Salmonella Nitra.
Processing of SGSA.
In a 20-μl reaction mixture containing 2-μl aliquots of each of the three multiplex PCRs with 4 μl of shrimp alkaline phosphatase (SAP) buffer 1 (Roche Diagnostics, Indianapolis, IN), 3 μl (3 U) of SAP was added to dephosphorylate the remaining nucleotides. After incubation for 10 min at 37°C, the enzyme was denatured by 5 min of incubation at 75°C in a T Gradient thermocycler (Biometra).
The SAP-treated PCR products were then biotin labeled by a sequence-specific end labeling of oligonucleotides (SSELO) method modified from Kostić et al. (30). The labeling method uses reverse-complement oligonucleotides lacking the 3′ terminal nucleotide. Each 20-μl reaction mixture consisted of 1× PCR buffer (Applied Biosystems, Branchburg, NJ), 1.5 mM MgCl2, 0.5 pmol of each reverse complement primer/μl multiplexed with primers corresponding to each of the ArrayTube probes, 0.8 pmol of each biotinylated ddCTP (dideoxy-CTP), ddTTP, and ddGTP/μl (Perkin Elmer Life and Analytical Sciences, Boston, MA), 8 pmol of ddATP/μl (Roche), 0.125 units of AmpliTaq Gold DNA polymerase/μl (Applied Biosystems), and 5 μl of SAP-treated PCR product. The cycling conditions were 15 min at 95°C and then 25 cycles of 30 s at 95°C and 75 s at 60°C in a T Gradient thermocycler (Biometra). The dephosphorylation and SSELO reactions were optimized for robustness, eliminating the need for standardized DNA concentrations. Once labeled, the samples were used directly on the ArrayTubes without the need for further purification.
Samples were hybridized to the SGSA using a hybridization kit (Alere Technologies) and processed according to the manufacturer's instructions, except the hybridization was carried out for 1 h at 60°C and bound with D1 substrate reagent for 15 min at room temperature.
Signal intensities were detected using an ArrayMate ArrayStrip reader (Alere Technologies). Biotin signal values must be greater than 0.7 for the experiment to be considered valid. Positive signal values correspond to spot intensities above a minimum cutoff value of 0.2. This value was determined after analyzing the signal intensity range, based on the 95% central interval for each of the probes designed to detect the serovars in subset 1 of the validation panel (see Table S1 in the supplemental material). After the probe patterns of the validation strains were tested and confirmed, it was determined that, in the case of antigenic complexes with high sequence homology, the probe with the highest signal intensity was to be used for antigenic formula determinations. A Microsoft Excel macro was developed to automate data analysis and generate an antigenic formula for determination of simple and nonsubjective results. Each Salmonella sample was characterized by a unique probe pattern based on the identification of an O antigen and of phase 1 and phase 2 flagellar antigens. The antigenic formula was then used to designate serotype according to the White-Kauffmann-Le Minor scheme (20).
Validation of the array.
A strain set of 82 serovars was selected as our validation panel, which was composed of three subsets. The first subset contained a group of 43 S. enterica serovars representing the most prevalent human and nonhuman S. enterica isolates identified from North America, the United Kingdom, and Austria. The second subset comprised 33 subspecies I serovars used to validate additional probes. The third subset of six serovars was used solely to analyze the detection of additional O serogroups in the SGSA. Flagellar antigens were not tested for the third subset of serovars, as flagellum-specific array probes were designed using only subspecies I Salmonella sequence data and the probe set does not represent subspecies sequence variability (47). The remaining 76 serovars were analyzed based on their full antigenic formulas. The full panel of 82 serovars served as the fundamental strain set to test the specificity and the repeatability of the SGSA probe and probe pattern results. This panel was utilized to evaluate expected probe patterns, and the results were based solely on the known samples within the panel (Table 3 and Table 4). Hybridization of the 82 serovars was performed in triplicate, and antigenic formulas were derived using the SGSA and compared to those obtained by traditional serotyping.
Table 3.
Salmonella enterica strains used to validate the Salmonella genoserotyping array (SGSA) probes and probe patterns
| Salmonella serovar | Serogroup as determined by traditional serotyping | Antigenic formula as determined by traditional serotyping | SGSA-identified antigenic formula | SGSA antigenic formula correlation with traditional serotyping | Correct SGSA serovar designation (based on known samples) |
|---|---|---|---|---|---|
| Subset 1 | |||||
| 1,4,5,12:i:- | B | 4,5,12:i:- | B:i:- | Yes | Yes |
| Abony | B | 4,12:b:e,n,x | B:b:e,n,x | Yes | Yes |
| Agona | B | 4,12:f,g,s:- | B:f,g,s:- | Yes | Yes |
| Anatum | E1 | 10:e,h:1,6 | E:e,h:1,6 | Yes | Yes |
| Orion var. Binza | E1 | 3,15:y:1,5 | E:y:1,5 | Yes | Yes |
| Braenderup | C1 | C1 6,7:e,h:e,n,z15 | C1:e,h:e,n,z15 | Yes | Yes |
| Cerro | K | 18:z4,z23:- | K:z4,z23:- | Yes | Yes |
| Corvallis | C2 | 8,20:z4,z23:- | C2:z4,z23:- | Yes | Yes |
| Derby | B | 4,12:f,g:- | B:f,g:- | Yes | Yes |
| Dublin | D1 | 9,12:g,p:- | A/D:g,p:- | Yes | Yes |
| Enteriditis | D1 | 9,12:g,m:- | A/D:-:-, RHS-E | Partial | Yes |
| Gallinarum | D1 | 9,12:-:- | D:-:-, RHS-GP | Yes | Yes |
| Give | E1 | 10:l,v:1,7 | E:l,v:1,7 | Yes | Yes |
| Hadar | C2 | 6,8:z10:e,n,x | C2:z10:e,n,x | Yes | Yes |
| Heidelberg | B | 4,12:r:1,2 | B:r:1,2 | Yes | Yes |
| Indiana | B | 4,12:z:1,7 | B:z:1,7 | Yes | Yes |
| Infantis | C1 | 6,7,14:r:1,5 | C1:r:1,5 | Yes | Yes |
| Javiana | D1 | 9,12:l,z28:1,5 | D:l,z28:1,5 | Yes | Yes |
| Kedougou | G | 13,23:i:l,w | G:i:l,w | Yes | Yes |
| Kentucky | C2 | 8,20:i:z6 | C2:i:z6 | Yes | Yes |
| Kiambu | B | 4,12:z:1,5 | B:z:1,5 | Yes | Yes |
| Kottbus | C2 | 6,8:e,h:1,5 | C2:e,h:1,5 | Yes | Yes |
| Mbandaka | C1 | 6,7,14:z10:e,n,z15 | C1:z10:e,n,z15 | Yes | Yes |
| Mississippi | G | 13,23:b:1,5 | G:b:1,5 | Yes | Yes |
| Montevideo | C1 | 6,7:g,m,s:- | C1:g,m,s:- | Yes | Yes |
| Muenchen | C2 | 6,8:d:1,2 | C2:d:1,2 | Yes | Yes |
| Newport | C2 | 6,8,20:e,h:1,2 | C2:e,h:1,2 | Yes | Yes |
| Oranienburg | C1 | 6,7:m,t:- | C1:m,t:- | Yes | Yes |
| Paratyphi A | A | 2,12:a:1,5 | A:a:1,5 | Yes | Yes |
| Paratyphi B var. Java | B | 4,12:b:1,2 | B:b:1,2 | Yes | Yes |
| Pullorum | D1 | 9,12:-:- | D1:-:-, P | Yes | Yes |
| Rissen | C1 | 6,7,14:f,g:- | C1:f,g:e,n,x,z15 | Partial | Yes |
| Saintpaul | B | 4,5,12:e,h:1,2 | B:e,h:1,2 | Yes | Yes |
| Schwarzengrund | B | 4,12,27:d:1,7 | B:d:1,7 | Yes | Yes |
| Senftenberg | E4 | 3,19:g,s,t:- | E:g,s,t:- | Yes | Yes |
| Stanley | B | 4:d:1,2 | B:d:1,2 | Yes | Yes |
| Stanleyville | B | 4:z4,z23:- | B:z4,z23:- | Yes | Yes |
| Tennessee | C1 | 6,7:z29:- | C1:z29:- | Yes | Yes |
| Thompson | C1 | 6,7,14:k:1,5 | C1:k:1,5 | Yes | Yes |
| Typhi | D1 | 9,12,Vi:d:- | D,Vi:d:- | Yes | Yes |
| Typhimurium | B | 4,5,12:i:1,2 | B:i:1,2 | Yes | Yes |
| Virchow | C1 | 6,7,14:r:1,2 | C1:r:1,2 | Yes | Yes |
| subsp. IIIb 61:k:1,5,(7) | O:61 | 61:k:1,5,7 | 61:k:1,5 | Yes | Yes |
| Subset 2 | |||||
| Alachua | O | 35:z4,z23:- | O:z4,z24:- | Yes | Yes |
| Amsterdam | E1 | 15,34:g,m,s:- | E:g,m,s:- | Yes | Yes |
| Berlin | J | 17:d:1,5 | J:d:1,5 | Yes | Yes |
| Berta | D1 | 9,12:f,g,t:- | D:f,g,t:- | Yes | Yes |
| Blegdam | D1 | 9,12:g,m,q:- | D:g,q/g,m,q:- | Yes | Yes |
| Blijdorp | H | 1,6,14,25:c:1,5 | H:c:1,5 | Yes | Yes |
| Blockley | C2 | 6,8:k:1,5 | C2:k:1,5 | Yes | Yes |
| Brandenburg | B | 4,12:l,v:e,n,z15 | B:l,v:e,n,z15 | Yes | Yes |
| Bredeney | B | 4,12,27:l,v:1,7 | B:l,v:1,7 | Yes | Yes |
| Breukelen | C2 | 6,8: l,z13:enz15 | C2:l,z13:e,n,z15 | Yes | Yes |
| Budapest | B | 4,12:g,t:- | B:g,t/g,s,t:- | Yes | Yes |
| California | B | 4,12:g,m,t:- | B:g,m,t | Yes | Yes |
| Carrau | H | 6,14:y:1,7 | H:y:1,7 | Yes | Yes |
| Choleraesuis | C1 | 6,7:c:1,5 | C1:c:1,5 | Yes | Yes |
| Cubana | G | 1,13,23:z29:- | G:z29:- | Yes | Yes |
| Ealing | O | 35:g,m,s:- | O:g,m,s:- | Yes | Yes |
| Inverness | P | 38:k:1,6 | P:k:1,6 | Yes | Yes |
| Kiel | A | 1,2,12:g,p:- | A/D:g,p:- | Yes | Yes |
| Lansing | P | 38:i:1,5 | P:i:1,5 | Yes | Yes |
| Lille | C1 | 6,7,14:z38:- | C1:z38:- | Yes | Yes |
| Manhattan | C2 | 6,8:d:1,5 | C2:d:1,5 | Yes | Yes |
| Minnesota | L | 21:b:e,n,x | L:b:e,n,x | Yes | Yes |
| Morotai | J | 17:l,v:1,2 | J:l,v:1,2 | Yes | Yes |
| Moscow | D1 | 9,12:g,q:- | D:g,q/g,m,q:- | Yes | Yes |
| Naestved | D1 | 9,12:g,p,s:- | D:g,p,s:- | Yes | Yes |
| Ohio | C1 | 6,7,14:b:l,w | C1:b:l,w | Yes | Yes |
| Panama | D1 | 9,12:l,v:1,5 | A/D:l,v/l,z13:1,5 | Yes | Yes |
| Pomona | M | 28:y:1,7 | M:y:1,7 | Yes | Yes |
| Poona | G | 13,22:z:1,6 | G:z:1,6 | Yes | Yes |
| Reading | B | 4,12:e,h:1,5 | B:e,h:1,5 | Yes | Yes |
| Ruiru | L | 21:y:e,n,x | L:y:e,n,x | Yes | Yes |
| Uganda | E | 10:l,z13:1,5 | E:l,v/l,z13:1,5 | Yes | Yes |
| Westhampton | E1 | 3,10:g,s,t:- | E;g,s,t:- | Yes | Yes |
Table 4.
Additional Salmonella serovars used to validate serogroup probes on the Salmonella genoserotyping array (SGSA)
| Somatic serogroup of interest | Somatic antigen of interest | Salmonella serovar tested | Antigenic formula | Successful serogroup detection |
|---|---|---|---|---|
| V | O:44 | Subsp. IV | 44:z4,z23:- | Yes |
| V | O:44 | Subsp. IIIa | 44:z4,z23:- | Yes |
| Y | O:48 | Subsp. IV | 48:g,z51:- | Yes |
| Y | O:48 | Subsp. IIIb | 48:k:e,n,x,z15 | Yes |
| O:58 | O:58 | Subsp. II | 58:d:z6 | Yes |
| O:58 | O:58 | Subsp. II | 58:l,z13,z28:- | Yes |
In order to further validate the specificity of the SGSA, 20 five monophasic serovars and eight rough strains were selected to assess the genoserotyping results compared to those obtained by traditional antibody-based methods. E. coli EDL 933 and Campylobacter jejuni NCTC 11168 were also tested as negative controls.
Amplification and detection of pepT.
The genome sequences of S. enterica subsp. Enteritidis and S. enterica subsp. Nitra are highly homologous and currently cannot be differentiated on the SGSA. Whole-genome sequence alignments revealed a large deletion of the pepT gene in Salmonella Nitra (JN081866) compared to Salmonella Enteritidis (NC_011294). Based on the sequence of the latter, forward and reverse primers flanking pepT were designed (Table 2). These primers amplified a 2,061-bp target in Salmonella Enteritidis and a 106-bp fragment in Salmonella Nitra. The target was amplified using a Qiagen multiplex PCR kit according to the manufacturer's instructions. Each 25-μl PCR mixture contained 1× multiplex master mix, 0.2 μM each primer, and 1.75 μl (approximately 100 ng/μl) of genomic DNA. Amplification conditions matching those of the multiplex reactions were 15 min at 95°C, 35 cycles of 30 s at 95°C, 90 s at 57°C, and 90 s at 72°C, with a final elongation of 5 min at 72°C performed using a T Gradient thermocycler (Biometra). The amplicons were sized using 0.8% SeaKem LE agarose (Cambrex, Rockland, ME) gels with 1× Tris-borate buffer (Invitrogen, Carlsbad, CA). Sixty Salmonella Enteritidis strains and three Salmonella Nitra strains, isolated from human, animal, and environmental samples of multinational origin, were used as a validation panel for the pepT PCR. All 63 samples were correctly differentiated using the pepT assay. The validated pepT PCR was utilized to confirm the serovar identification of all unknown samples that were typed by the SGSA as representing either Salmonella Enteritidis or Salmonella Nitra.
Blind study.
Once validated, the SGSA was assessed against a blind panel of 100 S. enterica subsp. I strains (see Table S2 in the supplemental material) obtained from and serotyped using traditional methods of the AHVLA (36). Serovar designations were determined according to serovars identified in the 2007 antigenic formulae of the Salmonella serovars, 9th ed. (WHO Collaborating Centre for Reference and Research on Salmonella) (20).
RESULTS
Salmonella genoserotyping array design and layout.
Probes were designed to detect each of the antigens required to characterize the 82 serovars within the validation panel. Unique sequences were identified based on alignments of homologous sequences performed using SeqMan software (Lasergene 8; DNASTAR Inc.). Somatic probes were designed based on publicly available sequences and on sequences determined in-house for gene targets (Table 2) within the Salmonella rfb cluster. The probes printed on the current array are capable of detecting 18 somatic serogroups: A (O:2); B (O:4); C1 (O:6,7); C2 (O:8); D (O:9); E (O:3); G (O:13); H (O:6,14); J (O:17); K (O:18); L (O:21); M (O:28); O (O:35); P (O:38); V (O:44); Y (O:48); O58; and O61. Serogroup A and D Salmonella serovars cannot currently be differentiated with a single somatic probe due to the high level of sequence homology between their rfb loci (63). Salmonella Paratyphi A, which belongs to serogroup A, can be differentiated from serogroup A and D serovars by the use of a probe [A (Paratyphi) (O:2)] designed to target a 2-bp mismatch within the prt gene (16). In order to differentiate many of the serogroup A and D Salmonella serovars, alternative probes outside the rfb cluster have been designed (Table 1). Three alternative probes have been designed to differentiate Salmonella Enteritidis (RHS_E), Salmonella Gallinarum (RHS_GP), and Salmonella Pullorum (Pullorum), as they are genetically indistinguishable on the basis of the fliC allele. A probe was designed to identify a unique sequence within an rhs-like gene in Salmonella Enteritidis (SEN0271) due to the absence of an H1:g,m-specific probe on the array. The unique sequence within the rhs-like gene was discovered by comparing the whole-genome sequence of Salmonella Enteritidis (NC_011294) to sequences of other Salmonella serovars by the use of PanSeq software (32). Similarly, a unique rhs-like gene sequence (SG1045) of Salmonella Gallinarum (NC_011274) was used to design a serovar-specific probe. A Salmonella Pullorum-specific probe was designed using sequences within the prt gene (38).
Flagellar probes were designed using unique antigenic sequences within the phase 1 (fliC) and phase 2 (fljB) flagellar genes. Some probes have been designed to identify multiple antigens; thus, the SGSA has 42 flagellar probes which identify 41 antigens. The following flagellar antigens can be identified on the SGSA: a; b; c; d; e,h; e,n,x; e,n,z15; e,n,x,z15; f,g; f,g,t; f,g,s; g,m,s; g,m,t; g,t; g,m,q; g,q; g,p; g,p,s; g,s,t; g,t; g,z51; i; k; l,w; l,v; l,z13; l,z28; m,t; r; [r]; y; z10; z29; z38; z4,z23; z6; z67 and 1,2; 1,5; 1,6; and 1,7 from the 1 complex.
An additional three probes were present for further identification, including the control invA probe that confirms the identity of Salmonella species, a probe for the Vi capsular antigen, and a probe that detects G-complex antigens containing the H1:f epitope (Table 1).
Validation of the array.
A set of 82 Salmonella serovars of known serotype were assembled to validate the array for specificity and reproducibility. The strain set included a subset of 43 serovars representing a combination of the 15 most prevalent human and nonhuman serovars isolated in North America, the United Kingdom, and Austria. The remaining 39 Salmonella serovars are significantly less prevalent and were included to (i) confirm the efficacy and specificity of the antigens covered in the above-described subset (as 11 probes could detect multiple antigens), (ii) test potential cross-reactivity of probes with similar sequences, and/or (iii) test the detection of 10 additional serogroups and three flagellar antigens to increase the number of serovars detected by the SGSA. These samples included Salmonella subspecies II, IIIa, IIIb, and IV, which were tested only for determination of serogroup probe efficacy. Prior to being tested using the SGSA, each strain was serotyped using classical antibody-based methods and characterized using the White-Kauffmann-Le Minor scheme by the LFZ Salmonella OIE Reference Laboratory. Each serovar was tested a minimum of three times using the SGSA to ensure consistent results and to confirm the identification of the unique and reproducible serovar-specific probe patterns used in the macro design. Probe signals required for the detection of subset 1 Salmonella serovars were analyzed, and the signal mean, median, and 95% central interval range for each probe were calculated (see Table S1 in the supplemental material). Whereas most of the probe signal ranges differed only slightly, some of the ranges displayed a larger variation. For example, probe d-2 had a minimum signal intensity of 0.21 and a maximum intensity of 0.87. This variation in probe signal intensity may have been due to the efficiency of DNA isolation and target amplification, as these parameters were not standardized within the array protocol due to the robustness of the assay. Large variations within probe signal intensity ranges may also have been dependent on the efficiency of labeling and hybridization of individual samples. That said, the minimum value for all signal ranges was above the positive signal cutoff value of 0.2; therefore, regardless of the range, all probe signals measuring above 0.2 are considered to represent a positive result.
The SGSA generated the correct serovar designations for the 76 known serovars in subset 1 and subset 2 of the validation panel and an antigenic formula consistent with the White-Kauffmann-Le Minor scheme for 74 of the 76 known serovars (Table 3). Complete antigenic formulas were not generated for Salmonella Enteritidis because of the absence of an H1:g,m probe on the array. Designation of serovars of Salmonella Enteritidis required the use of a probe pattern rather than positive confirmation of the presence of H1:g,m. Furthermore, the array identified the H2:e,n,z15/e,n,x,z15 probe for Salmonella Rissen, a result which was not consistent with the monophasic formula generated with traditional serotyping; however, the presence of the H2 allele was confirmed by sequencing.
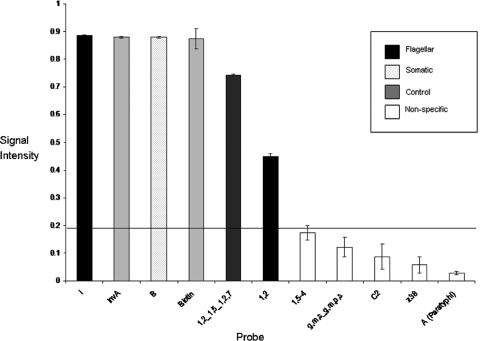
The majority of the antigenic formulas generated were identified using a combination of two to three probe signals representative of the serogroup and flagellar antigen(s). Exceptions occurred in some instances where additional probes were utilized for the identification of particular antigens as part of a serovar pattern. In the example of Salmonella Typhimurium, two probes were used for positive identification of the H2:1,2 antigen (Fig. 1), one unique to the H2:1,2 antigen and one shared with the H2:1,5 and H2:1,2,7 antigens. In the case of Salmonella Typhi, a positive signal for the Vi probe encoding the capsular virulence antigen was required as part of its pattern. Other exceptions included Salmonella serovars Pullorum, Gallinarum, and Enteritidis, whose serovar identification is based on the detection of additional probes in combination with their unique serovar probe patterns to differentiate them from each other.
Fig. 1.
Probe patterning and signal intensities for Salmonella Typhimurium. The graph depicts average signal intensities generated from triplicate probes used to identify a unique probe pattern for Salmonella Typhimurium on the SGSA array. The standard deviations of the means of the probe signal have been depicted using error bars. The assay cutoff for positive signals has been set at 0.2 (horizontal line), allowing clear differentiation of positive probe signals from background and nonspecific hybridization.
E. coli EDL 933 and C. jejuni 11168 as negative controls were also tested using the SGSA. The Salmonella-specific control, invA, did not produce any signal when hybridized with either sample (data not shown).
Monophasic and rough Salmonella.
Monophasic and rough Salmonella strains were tested to examine the correlation between traditional serotyping methods and the genoserotyping results. SGSA results classified 16 of the 25 monophasic samples as diphasic; therefore, only 36% of the serovar designations were consistent with those generated using traditional serotyping (data not shown). Furthermore, the SGSA identified serogroups from five of the eight rough mutants assessed, thus generating an antigenic formula consistent with the White-Kauffmann-Le Minor scheme for only 38% of samples tested. Antigens characterized as phenotypically absent using traditional serotyping methods but detected on the array were sequenced to confirm the presence of the probe sequence. In all cases, the probe sequence was present and therefore accounted for the difference between the genotypically and the phenotypically derived antigenic formulas (data not shown).
Blind study.
Once validated, the SGSA was used to genoserotype a blind panel of 100 Salmonella enterica subsp. I samples provided by the AHVLA. Table S2 in the supplemental material illustrates the antigenic formula generated using the SGSA and the serovar designation for each sample based on antigen-specific probes and probe patterns.
In summary, the results from the 100 blind samples tested revealed that the SGSA correctly identified all of the samples as Salmonella and was successful in identifying 92% of the antigens found within the blind samples. The SGSA generated an antigenic formula consistent with the White-Kauffmann-Le Minor scheme for 76 out of the 100 blind panel samples. The antigenic formula generated by the SGSA consists of a serogroup designation and does not include the detection of individual somatic factors. Thus, in instances in which the serogroup designation generated by the SGSA matched the serogroup identified using traditional serotyping methods, antigenic formulas were considered correct when analyzing the blind panel samples.
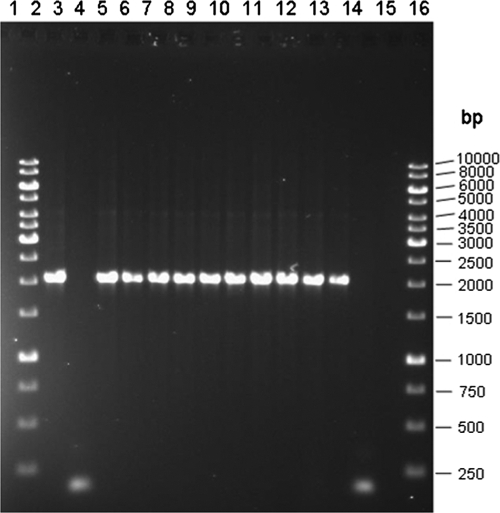
The inability of the array to differentiate Salmonella Enteritidis from the rarely isolated Salmonella Nitra accounted for 11 of the 24 samples not assigned a complete antigenic formula. All 11 samples were further analyzed using the pepT PCR for serovar confirmation. The pepT PCR was able to correctly identify 10 of the samples as Salmonella Enteritidis and 1 as Salmonella Nitra, thus increasing the number of correctly identified blind samples to 87 out of 100 (Fig. 2).
Fig. 2.
Agarose gel of pepT amplicons from PCR used to differentiate blind samples identified as either Salmonella Enteritidis or Salmonella Nitra on the basis of SGSA results. Lanes 1 and 16 represent 1-kb DNA ladders (Fermentas, Burlington, ON). Lanes 2 (blind sample 20), 4 (blind sample 21), 5 (blind sample 22), 6 (blind sample 23), 7 (blind sample 24), 8 (blind sample 25), 9 (blind sample 26), 10 (blind sample 27), 11 (blind sample 28), and 12 (blind sample 29) show the 2,061-bp pepT band corresponding to Salmonella Enteritidis. Lane 3 (blind sample 60) shows the 150-bp band corresponding to Salmonella Nitra. Lane 13 represents the Salmonella Enteritidis control strain, and lane 14 represents the Salmonella Nitra control strain. Lane 15 is a negative PCR control.
Partial antigenic formulas were generated for 13 of the 100 blind panel samples. Two of the blind panel samples could not be fully characterized due to the absence of antigen-specific probes on the array required to classify the O:47 antigen present in Salmonella Bergen and the H1:g,p,u antigen required to identify Salmonella Rostock.
The remaining 11 blind panel samples produced antigenic formulas indicative of two or more serovars due to single probes targeting multiple antigens and/or the inability of the array to detect single somatic factors. The remaining two samples were only partially characterized. The SGSA was not able to differentiate between Salmonella Blegdam and Salmonella Moscow, because there is currently only a single probe on the array that targets both H1:g,m,q and H1:g,q. Furthermore, the SGSA was unable to differentiate the two Salmonella Panama samples from Salmonella Houston, Salmonella India, Salmonella Itami, and Salmonella Koessen. In order to generate definitive designations for these serovars, the SGSA would need to incorporate the ability to target individual serogroup D somatic factors and the ability to differentiate between serogroups A and D and would also require individual probes specific for H1:l,v and H1:1,z13. The four Salmonella Senftenberg samples tested as part of the blind panel could not be differentiated from Salmonella Westhampton or Salmonella Dessau because of a shared H1:g,t/g,s,t probe and the inability of the array to target individual serogroup E somatic factors. Three of the blind panel samples (two Salmonella Dublin samples and one Salmonella Kiel sample) were only partially identified due to the lack of a probe able to differentiate between serogroups A and D.
DISCUSSION
A Salmonella genoserotyping array (SGSA) has been developed for utilization as a rapid and economical tool to serotype Salmonella. Surveillance tools such as these are needed to help identify outbreaks and raise awareness among health professionals, food producers, and consumers. The implementation of a simple, robust, and cost-effective genoserotyping array may prompt those in the food industry, clinicians, and reference and small private laboratories to perform more testing, in turn increasing the reporting of Salmonella and enhancing surveillance data (25).
The SGSA is an attractive alternative to traditional serotyping, because it benefits from a simple, less expensive protocol employing a variable platform of single tubes or a 96-well plate format with automated data analysis for nonsubjective serovar designation. The Arraytube platform (Allere, Inc.) requires inexpensive equipment and reagents that can be easily incorporated into both diagnostic and research laboratories; however, the major cost benefit to using the SGSA comes from the reduction of the technician time required to process a sample. With the use of the SGSA, Salmonella isolates involved in outbreaks can be genoserotyped in 1 day versus the minimum of 3 to 4 days required for traditional serotyping, expediting downstream subtyping with methods such as pulsed-field gel electrophoresis (PFGE) and phage typing to aid in source attribution and widespread tracking. Other methods that are alternatives to traditional serotyping have been developed; however, many are not amenable to high-throughput platforms, some are much less cost-effective, and most do not generate an antigenic formula consistent with that of the globally recognized White-Kauffmann-Le Minor scheme.
To further optimize the assay for speed and simplicity, the use of cell lysates in place of purified genomic DNA as a template for the multiplex target amplification reaction was tested (data not shown). The use of cell lysates is considered advantageous, as they are amenable to high-throughput laboratories and substantially decrease the cost of the protocol. Our results indicated that genomic DNA generated target amplification of greater consistency and, subsequently, SGSA results of greater reliability. Although the cell lysates were not included in the final protocol, the results showed promise, and since several groups have demonstrated the success of optimization of multiplex PCRs for use in Salmonella molecular typing methods, the use of lysates in investigations of future layouts is planned (7, 22, 23).
A study describing a multiplex PCR assay designed to detect all of the Salmonella subspecies, including the species S. bongori, was recently reported (33). Adaptation of this method is planned to be investigated as an additional multiplex reaction in the SGSA protocol, and subspecies-specific probes are to be added to the array for verification of all six distinct subspecies. Additionally, the next layout will have an shdA-specific probe for use in further confirmation of Salmonella subspecies I serovars (29).
The SGSA described here assesses mainly subspecies I Salmonella isolates due to their prevalence in human clinical infections (50). As an exception, a subspecies IIIb O61:k:1,5,(7) isolate was examined since it represented the third-most-prevalent animal isolate in the United Kingdom in 2007 (54). In order to test this serovar, our array also includes unique probes specific to subspecies IIIb H1:k and H2:1,5,(7).
The subspecies I Salmonella serovars tested in this study represent only a small subset of the 1,532 Salmonella subsp. I serovars classified in the White-Kauffmann-Le Minor scheme; however, the 59 targets detected by the array are theoretically capable of identifying 985 of the subspecies I Salmonella serovars. Further testing of Salmonella subsp. I serovars should result in a more accurate depiction of the array capabilities on the basis of the allelic variation within the gene targets.
Currently, 41 of 114 flagellar antigens are represented on the array, along with serogroup-specific probes for 18 of 46 Salmonella O serogroups. Publically available sequence data were used for 12 of the 21 serogroups: A (O:2) (37), B (O:4) (27), C1 (O:6,7) (34), C2 (O:8) (6), D1 (O:9) (37), E (O:3) (65), G (O:13) (17) H (O:6,14) (18), J (O:17) (17), K (O:18) (17), O (O:35) (64), and O58 (9). Six rfb cluster sequences were obtained by our laboratory: L (O:21), M (O:28), P (O:38), V (O:44), Y (O:48) and O61. Additionally, five serogroups [F (O:11), I (O:16), R (O:40), U (O:43), and Z (O:50)] have been selected for sequencing for inclusion on the next SGSA layout. The SGSA is based on the genes responsible for O-antigen and flagellar biosynthesis; thus, in most cases, it provided antigenic formulas and subsequent serovar designations comparable to those determined by traditional methods. Salmonella serovars Enteritidis, Gallinarum, and Pullorum all required the use of additional genes outside the traditional typing scheme as part of their unique pattern to ensure concise serovar identification. Other genes included in the SGSA are the invA gene (52), used as a Salmonella-specific control to ensure species detection, and the Vi antigen encoded by the viaB gene used in the detection of some serovars of Salmonella Typhi (44) and, in rare cases, Salmonella Dublin (46) and Salmonella Paratyphi C (10). In most instances, the SGSA produced an antigenic formula that corresponds to a unique serovar classified within the White-Kauffmann-Le Minor scheme. However, the inability of the array to discriminate between single somatic factors resulted in the generation of an antigenic formula common to two or more serovars in a few instances. Table S2 in the supplemental material details the antigenic formulas generated by the SGSA and the alternative serovar possibilities. For example, the table illustrates that the SGSA generated an antigenic formula of G:z:1,6, which represents both Salmonella Poona (1,13,22:z:1,6) and Salmonella Farmsen (13,23:z:1,6). Due to the lack of probes specific for single O-antigen factors defined within serogroup G (1, 13, 22, and 23), the SGSA was not able to differentiate between the two serotypes. Blind samples were found to be in agreement with traditional serotyping results as long as the serogroup designation was correct; however, all of the possible serovars based on somatic factors are listed in Table S2 in the supplemental material. This limitation is shared by all other molecular serotyping methods that rely on serogroup detection rather than individual factors (16, 49).
Four of the blind panel samples, Salmonella Bovismorbificans, Salmonella Hadar, Salmonella Manhattan, and Salmonella Newport, were unable to be differentiated from Salmonella Hindmarsh, Salmonella Istanbul, Salmonella Yovokome, and Salmonella Bardo, respectively, on the basis of the serogroups. According to the World Health Organization (WHO), these serovars, while regarded as distinct in the White-Kauffmann-Le Minor scheme, are considered to be correctly identified, as colonial form variations (the variable expression of minor antigens by different single-colony picks from the same strain) may occur in some serogroup C2 serovars (21).
Salmonella serovars Enteritidis (serogroup D) and Nitra (serogroup A) have antigenic formulas that differ only in their serogroups. The rfb region of Salmonella Paratyphi A, a representative of serogroup A, has been shown to differ from serogroup D rfb regions by only a minor modification resulting from a frameshift mutation (37). Together with Salmonella Paratyphi A, Salmonella serovars Nitra, Kiel, and Koessen represent the only serogroup A serovars. A DNA sequence alignment of the prt gene from Salmonella Paratyphi A, Salmonella Nitra, and four serogroup D sequences highlighted a 2-bp mismatch within the CDP-paratose synthase (prt) gene of Salmonella Paratyphi A (16). This region was targeted with a Salmonella Paratyphi A-specific probe to differentiate Salmonella Paratyphi A from the remaining serogroup A serovars and all serogroup D serovars. Currently, there is not a serogroup probe able to distinguish the rest of the serogroup A and D isolates. Salmonella Nitra and Salmonella Kiel are rarely isolated and have been shown by PFGE cluster analysis to demonstrate molecular similarities of between 81% and 100% to the related serogroup D serovars, whereas the Salmonella Paratyphi A isolates showed only 68% similarity (16). These results are in agreement with other microarray data that have shown that serogroup A isolates are variants of serogroup D (40, 51). The SGSA generated the antigenic formula A/D:-:- RHS-E for two of the blind samples and was not able to decipher the data further to produce a single serovar designation. The combination of our inability to differentiate most serogroup A and D serovars, the lack of a g,m-positive probe, and the fact that the alternative Salmonella Enteritidis gene target is also found in Salmonella Nitra left these two serovars indistinguishable. In instances such as this, prevalence data can be examined that may support the likelihood of the serovar designation through the comparison of isolation frequency statistics. According to Canadian data from the National Enteric Surveillance Program that were collected between 2004 to 2009, Salmonella Enteritidis accounted for 27.55% of all Canadian Salmonella isolates, whereas Salmonella Nitra isolates were not reported during that same time period (11, 12, 13). The use of prevalence data would suggest that the 11 samples designated A/D:-:- RHS-E all represent Salmonella Enteritidis; within the blind panel, however, one of the samples designated A/D:-:- RHS-E was in actuality Salmonella Nitra. We propose that having prevalence data for geographic location can be of interest; however, this is not used as part of the final serovar designation derived from the SGSA or its macro, as it can lead to misidentification. Although the positive predicted value is extremely high when using the prevalence data to predict a serovar, it is important that, in critical situations, samples be sent for traditional serotyping to confirm the serovar designation. Fitzgerald et al. published a similar result, stating that serogroup A isolates other than Salmonella Paratyphi A tested positive with their serogroup D probe on their bead-based suspension array but noting that they were extremely rare serovars (16). Although the SGSA was unable to discriminate between these two serovars, a newly designed pepT PCR was used to confirm that 10 of the 11 samples were Salmonella Enteritidis and one of the blind samples was serovar Nitra. The pepT PCR is to be added to the current sample preparation protocol, and the next layout of the SGSA is to be expanded to include pepT probes to provide direct differentiation of Salmonella Enteritidis from Salmonella Nitra.
We are currently subjecting Salmonella serovars Kiel and Koessen to whole-genome pyrosequencing in order to identify serovar-specific genes for definite identification of these closely related serovars on the array.
In other instances, the SGSA was unable to definitively derive a single serovar designation because a small number of probes on the array are specific for multiple antigens. For example, the array was unable to differentiate between Salmonella Blegdam and Salmonella Moscow, as currently there is only a single probe that detects both the H1;g,q and H1:g,m,q antigens, which have highly homologous gene sequences. These results align with the sequencing results generated in a previous study by Sonne-Hanson and Jenabian in 2005 (60). Sequence identity and high sequence similarity have often been reported as obstacles to the development of probes for the differentiation of antigens within the g-complex, as their sequences are highly homologous and sequence variation is often seen among single alleles (48). Currently, both Salmonella Blegdam and Salmonella Moscow are sequenced in order to identify serovar specific-probe targets. More sequence data from Salmonella serogroup D serovars would also aid in the development of an SGSA scheme to more easily differentiate serogroup A from serogroup D serovars.
The SGSA currently utilizes shared probes to identify H1:m,t/g,m,t, H1:g,s,t/g,t, H1:l,v/l,z13, H1:g,q/g,m,q, and, finally, H1:z6/z67.
Antigens present in the blind samples but not represented on the array are H1:g,p,u and serogroup O:47, and their absence resulted in an only partial antigenic formula identification (see Table S2 in the supplemental material). With the addition of these antigens to the array, it should be possible to identify serovars Salmonella Rostock and Salmonella Bergen along with other serovars sharing these antigens. Ongoing sequencing continues to reveal additional single nucleotide polymorphisms among H1 and H2 alleles and will be highlighted with newly designed probes on future layouts. Lastly, the SGSA generated an incorrect antigenic formula (C1:f,g:e,n,x,z15) for Salmonella Rissen in the validation panel, which included H2:e,n,z,x15, and another incorrect antigenic formula (D:g,p:1,5) for Salmonella Dublin in the blind panel, which included H2:1,5 (Table 3 and Table S2 in the supplemental material, respectively). The fljB gene from the Salmonella Rissen strain tested on the SGSA array was sequenced, and the results confirmed the presence of our e,n,z,x15 probe sequence. These results supported data from a 2010 study done by Jong et al. in which amplification of an fljB gene from Salmonella Rissen yielded a positive PCR fragment (28). Similarly, the fljB gene from the Salmonella Dublin confirmed the presence of our 1,5 probe sequence.
Several monophasic and rough Salmonella serovars were tested on the array in order to compare the genetically derived antigenic formula generated by the SGSA to the antigenic formula identified by traditional antibody-based methods. The SGSA identified alleles that were not phenotypically expressed in 64% of monophasics and 62% of rough serovars. Note that the lack of Phase 2 flagellar expression of some serologically monophasic strains can be due to a variety of mechanisms, ranging from point mutations to partial or complete deletions in fljB and adjacent genes (42), and therefore may be missed by the use of a single probe on the array. Although the SGSA generated antigenic formulas that did not always correlate with traditional serotyping results, this ability could be advantageous, as it identifies the uncharacterized allele (8).
The SGSA has 66 probes printed in triplicate, and the platform can accommodate an additional 156 probes in triplicate or up to 268 probes if printed in duplicate. Future layouts are planned to include newly sequenced O serogroup-specific probes, somatic factor probes, new phase 1 and phase 2 flagellum-specific probes (41), and new serovar-specific probes to aid in finite designation of serovars. Moreover, future layouts may also include subspecies-specific probes and relevant Salmonella virulence and antimicrobial resistance markers for additional surveillance information (24). Further studies are planned to include large-scale multiple site validation, together with a comparison to traditional serotyping as an assessment of the feasibility of implementing the SGSA as a public health tool to aid in Salmonella outbreak identification and surveillance.
Supplementary Material
ACKNOWLEDGMENTS
We thank Linda Cole, Betty Wilkie, and Ketna Mistry (Public Health Agency of Canada, Guelph, Ontario, Canada) and members of the Salmonella Reference Laboratory team (Animal Health and Veterinary Laboratories Agency, New Haw, Addlestone, Surrey, United Kingdom) for providing Salmonella strains. We also thank Alere Technologies for printing the ArrayStrips. We are grateful to Ralph Ehricht (Alere Technologies) for continued consultation and expertise with the ArrayTube platform. We are also grateful for the assistance of Paulina Konczy, Christine Wessman, and Andre Villegas for their bioinformatics expertise. Finally, we thank Adam Crossley, Stewart Loker, and Jeffery Yang for their assistance in the preliminary development of the SGSA and the probe statistical analysis.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 22 June 2011.
REFERENCES
- 1. Ansingkar V., Kulkarni N. 2010. Incidences of endophytic human pathogens in fresh produce. Webmed Cent. Microbiol. 1: WMC001299 http://www.webmedcentral.com/wmcpdf/Article_WMC001299.pdf [Google Scholar]
- 2. Ballmer K., et al. 2007. Fast DNA serotyping of Escherichia coli by use of an oligonucleotide microarray. J. Clin. Microbiol. 45: 370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barton Behravesh C., et al. 2011. 2008 Outbreak of Salmonella Saintpaul infections associated with raw produce. N. Engl. J. Med. 364: 918–927 [DOI] [PubMed] [Google Scholar]
- 4. Behravesh C. B., et al. 2010. Human Salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics 126: 477–483 [DOI] [PubMed] [Google Scholar]
- 5. Ben-Darif E., et al. 2010. Development of a multiplex primer extension assay for rapid detection of Salmonella isolates of diverse serotypes. J. Clin. Microbiol. 48: 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown P. K., Romana L. K., Reeves P. R. 1992. Molecular analysis of the rfb gene cluster of Salmonella serovar Muenchen (strain M67): the genetic basis of the polymorphism between groups C2 and B. Mol. Microbiol. 6: 1385–1394 [DOI] [PubMed] [Google Scholar]
- 7. Cardona-Castro N., Sanchez-Jimenez M., Lavalett L., Munoz N., Moreno J. 2009. Development and evaluation of a multiplex PCR assay to identify Salmonella serogroups and serotypes. Diagn. Microbiol. Infect. Dis. 65: 327–330 [DOI] [PubMed] [Google Scholar]
- 8. Chadfield M., Christensen J. P., Madsen M., Sonne-Hansen J., Bisgaard M. 2002. Application of molecular methods for identification of strains classified as Salmonella enterica serovar 6, 7:-:- by conventional serotyping. Avian Pathol. 31: 271–276 [DOI] [PubMed] [Google Scholar]
- 9. Clark C. G., et al. 2009. Escherichia coli O123 O antigen genes and polysaccharide structure are conserved in some Salmonella enterica serogroups. J. Med. Microbiol. 58: 884–894 [DOI] [PubMed] [Google Scholar]
- 10. Daniels E. M., Schneerson R., Egan W. M., Szu C., Robbins J. B. 1989. Characterization of the Salmonella paratyphi C Vi polysaccharide. Infect. Immun. 57: 3159–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demczuk W., Pankhurst R. 2007. Laboratory surveillance data for enteric pathogens in Canada—annual summary 2006. Public Health Agency of Canada, Ottawa, Ontario, Canada: http://www.nml-lnm.gc.ca/NESP-PNSME/assets/pdf/2006AnnualReport.pdf [Google Scholar]
- 12. Demczuk W., Pankhurst R. 2007. Laboratory surveillance data for enteric pathogens in Canada—annual summary 2005. Public Health Agency of Canada, Ottawa, Ontario, Canada: http://www.nml-lnm.gc.ca/NESP-PNSME/assets/pdf/2005%20Annual%20Report%20Final.pdf [Google Scholar]
- 13. Demczuk W., Boyd M. 2006. Laboratory surveillance data for enteric pathogens in Canada—annual summary 2004. Public Health Agency of Canada, Ottawa, Ontario, Canada: http://www.nml-lnm.gc.ca/NESP-PNSME/assets/pdf/2004AnnualSummary.pdf [Google Scholar]
- 14. Ewing W. H. 1986. Edwards and Ewing's identification of Enterobacteriaceae. Elsevier Science Publishing Co., Inc., New York, NY [Google Scholar]
- 15. Fierer J., Guiney D. G. 2001. Diverse virulence traits underlying different clinical outcomes of Salmonella infection. J. Clin. Invest. 107: 775–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzgerald C., et al. 2007. Multiplex, bead-based suspension array for molecular determination of common Salmonella serogroups. J. Clin. Microbiol. 45: 3323–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzgerald C., Gheesling L., Collins M., Fields P. I. 2006. Sequence analysis of the rfb loci, encoding proteins involved in the biosynthesis of the Salmonella enterica O17 and O18 antigens: serogroup-specific identification by PCR. Appl. Environ. Microbiol. 72: 7949–7953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fitzgerald C., Sherwood R., Gheesling L. L., Brenner F. W., Fields P. I. 2003. Molecular analysis of the rfb O antigen gene cluster of Salmonella enterica serogroup O:6,14 and development of a serogroup-specific PCR assay. Appl. Environ. Microbiol. 69: 6099–6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frenzen P. D., et al. 1999. 1999 Salmonella cost estimate update using FoodNet data. Food Rev. 22: 10–15http://www.ers.usda.gov/publications/foodreview/May1999 [Google Scholar]
- 20. Grimont P. A. D., Weill (ed.) F. X. 2007. Antigenic formulae of the Salmonella serovars, 9th ed. WHO Collaborating Centre for Reference and Research on Salmonella Institut Pasteur, Paris, France: http://www.pasteur.fr/ip/portal/action/WebdriveActionEvent/oid/01s-000036-089 [Google Scholar]
- 21. Hendriksen R. S., et al. 2009. WHO Global Salm-Surv external quality assurance system for aerotyping of Salmonella isolates from 2000 to 2007. J. Clin. Microbiol. 47: 2729–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrera-León S., et al. 2007. Blind comparison of traditional serotyping with three multiplex PCRs for the identification of Salmonella serotypes. Res. Microbiol. 158: 122–127 [DOI] [PubMed] [Google Scholar]
- 23. Herrera-León S., et al. 2004. Multiplex PCR for distinguishing the most common phase-1 flagellar antigens of Salmonella spp. J. Clin. Microbiol. 42: 2581–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huehn S., et al. 2010. Virulotyping and antimicrobial resistance typing of Salmonella enterica serovars relevant to human health in Europe. Foodborne Pathog. Dis. 7: 523–535 [DOI] [PubMed] [Google Scholar]
- 25. Hutwagner L. C., Maloney E. K., Bean N. H., Slutsker L., Martin S. M. 1997. Using laboratory-based surveillance data for prevention: an algorithm for detecting Salmonella outbreaks. Emerg. Infect. Dis. 3: 395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janda J. M., Abbott S. L. 2006. Nontyphoidal Salmonella, p. 81–103 In The Enterobacteria. ASM Press, Washington DC [Google Scholar]
- 27. Jiang X. M., et al. 1991. Structure and sequence of the rfb (O antigen) gene cluster of Salmonella serovar typhimurium (strain LT2). Mol. Microbiol. 5: 695–713 [DOI] [PubMed] [Google Scholar]
- 28. Jong H. Y., et al. 2010. PCR-based restriction fragment length polymorphism for subtyping of Salmonella from chicken isolates. Kasetsart J. (Nat. Sci.). 44: 79–83 [Google Scholar]
- 29. Kingsley R. A., van Amsterdam K., Kramer N., Baumler A. J. 2000. The shdA gene is restricted to serotypes of Salmonella enterica subspecies I and contributes to efficient and prolonged fecal shedding. Infect. Immun. 68: 2720–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kostić T., et al. 2007. A microbial diagnostic microarray technique for the sensitive detection and identification of pathogenic bacteria in a background of nonpathogens. Anal. Biochem. 360: 244–254 [DOI] [PubMed] [Google Scholar]
- 31. Kumar S., Balakrishna K., Batra H. V. 2006. Detection of Salmonella enterica serovar Typhi (S. Typhi) by selective amplification of invA, viaB, fliC-d and prt genes by PCR in multiplex format. Lett. Appl. Microbiol. 42: 149–154 [DOI] [PubMed] [Google Scholar]
- 32. Laing C. R., et al. 2010. Pan-genome sequence analysis using Panseq: an online tool for the rapid analysis of core and accessory genomic regions. BMC Bioinformatics 11: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee K., et al. 2009. A novel multiplex PCR assay for Salmonella subspecies identification. J. Appl. Microbiol. 107: 805–811 [DOI] [PubMed] [Google Scholar]
- 34. Lee S. J., Romana L. K., Reeves P. R. 1992. Sequence and structural analysis of the rfb (O antigen) gene cluster from a group C1 Salmonella enterica strain. J. Gen. Microbiol. 138: 1843–1855 [DOI] [PubMed] [Google Scholar]
- 35. Lim B. K., Thong K. L. 2009. Application of PCR-based serogrouping of selected Salmonella serotypes in Malaysia. J. Infect. Dev. Ctries. 3: 420–428 [DOI] [PubMed] [Google Scholar]
- 36. Lindberg A. A., Le Minor L. 1984. Serology of Salmonella, p. 1–14 In Bergman T. E. (ed.), Methods Microbiol., vol. 15 Academic Press, London, United Kingdom [Google Scholar]
- 37. Liu D., Verma N. K., Romana L. K., Reeves P. R. 1991. Relationships among the rfb regions of Salmonella serovars A, B, and D. J. Bacteriol. 173: 4814–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luk J. M., Kongmuang U., Reeves P. R., Lindberg A. A. 1993. Selective amplification of abequose and paratose synthase genes (rfb) by PCR for identification of Salmonella major serogroups (A, B, C2, and D). J. Clin. Microbiol. 31: 2118–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masten B. J., Joys T. M. 1993. Molecular analyses of the Salmonella g. flagellar antigen complex. J. Bacteriol. 175: 5359–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McClelland M., et al. 2004. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 36: 1268–1274 [DOI] [PubMed] [Google Scholar]
- 41. McQuiston J. R., Waters R. J., Dinsmore B. A., Mikoleit M. L., Fields P. I. 2011. Molecular determination of H antigens of Salmonella by use of a microsphere-based liquid array. J. Clin. Microbiol. 49: 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McQuiston J. R., Fields P. I., Tauxe R. V., Logsdon J. M., Jr 2008. Do Salmonella carry spare tyres? Trends Microbiol. 16: 142–148 [DOI] [PubMed] [Google Scholar]
- 43. McQuiston J. R., et al. 2004. Sequencing and comparative analysis of flagellin genes fliC, fljB, and flap from Salmonella. J. Clin. Microbiol. 42: 1923–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mehta G., Arya S. C. 2002. Capsular Vi polysaccharide antigen in Salmonella enterica serovar typhi isolates. J. Clin. Microbiol. 40: 1127–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller S. L., Pegues D. A. 2000. Salmonella species, including Salmonella Typhi, p. 2344–2363 In. Mandell G. L., Bennett J. E., Dolin R. (ed.), Principles and practice of infectious diseases, 5th ed., vol. 2 Churchill Livingstone, New York, NY [Google Scholar]
- 46. Morris C., Tam C. K., Wallis T. S., Jones P. W., Hackett J. 2003. Salmonella enterica serovar Dublin strains which are Vi antigen-positive use type IVB pili for bacterial self-association and human intestinal cell entry. Microb. Pathog. 35: 279–284 [DOI] [PubMed] [Google Scholar]
- 47. Mortimer C. K., Gharbia S. E., Logan J. M., Peters T. M., Arnold C. 2007. Flagellin gene sequence evolution in Salmonella. Infect. Genet. Evol. 7: 411–415 [DOI] [PubMed] [Google Scholar]
- 48. Mortimer C. K., Peters T. M., Gharbia S. E., Logan J. M., Arnold C. 2004. Towards the development of a DNA-sequence based approach to serotyping of Salmonella enterica. BMC Microbiol. 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muñoz N., Diaz-Osorio M., Moreno J., Sanchez-Jimenez M., Cardona-Castro N. 2010. Development and evaluation of a multiplex real-time PCR procedure to clinically type prevalent Salmonella enterica serovars. J. Mol. Diagn. 12: 220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Petersen A., et al. 2002. WHO global salm-surv external quality assurance system (EQAS): an important step toward improving the quality of Salmonella serotyping and antimicrobial susceptibility testing worldwide. Microb. Drug Resist. 8: 345–353 [DOI] [PubMed] [Google Scholar]
- 51. Porwollik S., et al. 2004. Characterization of Salmonella enterica subspecies I genovars by use of microarrays. J. Bacteriol. 186: 5883–5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rahn K., et al. 1992. Amplification of an invA gene sequence of Salmonella Typhimurium by PCR as a specific method of detection of Salmonella. Mol. Cell Probes 6: 271–279 [DOI] [PubMed] [Google Scholar]
- 53. Reeves P. P., Wang L. 2002. Genomic organization of LPS-specific loci. Curr. Top. Microbiol. Immunol. 264: 109–135 [PubMed] [Google Scholar]
- 54. Salmonella Surveillance Team 2008. Salmonella in Livestock Production in GB 2007. CERA. Veterinary Laboratories Agency, Weybridge, New Haw, Addlestone, Surrey KT153NB, United Kingdom [Google Scholar]
- 55. Samuel G., Reeves P. 2003. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr. Res. 338: 2503–2519 [DOI] [PubMed] [Google Scholar]
- 56. Scallan E., et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17: 7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shipp C. R., Rowe B. 1980. A mechanized microtechnique for Salmonella serotyping. J. Clin. Pathol. 33: 595–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Silverman M., Zieg J., Hilmen M., Simon M. 1979. Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc. Natl. Acad. Sci. U. S. A. 76: 391–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith DeWaal C., Klein S. A., Catella C., Roberts C., Armanda X. 2011. Introduction, p. 1–2 In All over the map: a 10-year review of State outbreak.reporting. Center for Science in the Public Interest, Washington, DC: http://cspinet.org/new/pdf/alloverthemap.pdf [Google Scholar]
- 60. Sonne-Hansen J., Jenabian S. M. 2005. Molecular serotyping of Salmonella: identification of the phase 1 H antigen based on partial sequencing of the fliC gene. APMIS 113: 340–348 [DOI] [PubMed] [Google Scholar]
- 61. Tankouo-Sandjong B., et al. 2008. Development of an oligonucleotide microarray method for Salmonella serotyping. Microb. Biotechnol. 1: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thomas M. K., et al. 2006. Estimated numbers of community cases of illness due to Salmonella, Campylobacter and verotoxigenic Escherichia coli: pathogen-specific community rates. Can. J. Infect. Dis. Med. Microbiol. 17: 229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Verma N. K., Quigley N. B., Reeves P. R. 1988. O-antigen variation in Salmonella spp.: rfb gene clusters of three strains. J. Bacteriol. 170: 103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang L., Reeves P. R. 2000. The Escherichia coli O111 and Salmonella enterica O35 gene clusters: gene clusters encoding the same colitose-containing O antigen are highly conserved. J. Bacteriol. 182: 5256–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang L., Romana L. K., Reeves P. R. 1992. Molecular analysis of a Salmonella enterica group E1 rfb gene cluster: O antigen and the genetic basis of the major polymorphism. Genetics 130: 429–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wattiau P., et al. 2008. Evaluation of the Premi Test Salmonella, a commercial low-density DNA microarray system intended for routine identification and typing of Salmonella enterica. Int. J. Food Microbiol. 123: 293–298 [DOI] [PubMed] [Google Scholar]
- 67. Wollin R. 2007. A study of invasiveness of different Salmonella serovars based on analysis of the Enter-net database. Eurosurveillance 12: E070927. [DOI] [PubMed] [Google Scholar]
- 68. Xiang S. H., Haase A. M., Reeves P. R. 1993. Variation of the rfb gene clusters in Salmonella enterica. J. Bacteriol. 175: 4877–4884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoshida C., et al. 2007. Methodologies towards the development of an oligonucleotide microarray for determination of Salmonella serotypes. J. Microbiol. Methods 70: 261–271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.