Abstract
In recent years, the well-known plant pathogens of the Colletotrichum genus were increasingly reported to cause ophthalmic infections in humans. Among 66 species in the Colletotrichum genus, only a few are known to be pathogenic for humans. We report here five cases of ophthalmic infections due to Colletotrichum truncatum, a species never reported earlier to cause human infection. The isolates were identified by morphological characteristics and the sequencing of internal spacer regions of ribosomal DNA. The progress of lesions in those patients was slow compared to that of lesions caused by Aspergillus or Fusarium infections. The surgical management included total penetrating keratoplasty in patients with keratitis and pars plana vitrectomy in endophthalmitis. Two patients were treated additionally with intravitreal amphotericin B deoxycholate, one patient with oral itraconazole, and another patient with oral and topical fluconazole therapy. The present series therefore highlights the expanding spectrum of agents causing eye infections and the inclusion of C. truncatum as a human pathogen.
INTRODUCTION
Colletotrichum species are well-known plant pathogens in tropical and subtropical countries. The taxonomy and nomenclature of Colletotrichum species are confusing, even to scientists working in this field (11). Initially, the species were identified by the characteristic host specificity, location of isolation, and conidial morphology. Subsequently, based on morphological characteristics, host range (of plants), and phylogenetic analysis, the fungi in the genus Colletotrichum were systematically classified. Currently there are 66 species, including 19 species for which the identification is doubtful (11).
Human infection due to Colletotrichum spp. is rare and has been reported from ophthalmic and subcutaneous infections (5, 8, 10, 13-15, 17-24, 26, 30, 32, 38, 41). The latter group of infections generally are reported in immunocompromised hosts (4). The species implicated in human infections include C. dematium (5, 8, 10, 13, 15, 17, 21, 32), C. coccodes (reported as C. atramentarium) (18, 24, 27), C. gloeosporioides (8, 20, 23, 24, 30, 40), C. graminicola (26, 41), C. crassipes (4), C. capsicii (38) (currently named C. truncatum) (7), and an unknown species (8, 22). Only a few cases of C. dematium eye infections are reported from India (5, 13-15, 21, 32). In all of those reports, the fungus was identified on the basis of morphological features. Here, we report for the first time five cases of ophthalmic infections due to C. truncatum, which initially were misidentified morphologically as C. dematium. The study also emphasizes the need of nucleic acid sequence analysis for the accurate identification of these difficult-to-identify fungi.
MATERIALS AND METHODS
The records of all patients with culture-proven fungal eye infections during 2003 to 2009 at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, were retrospectively reviewed. The clinical details of patients with eye infections due to Colletotrichum species were noted. The age, sex, eye involved, risk factors, antibiotic and antifungal agents used for treatment, clinical course, and outcome for each patient were recorded.
Mycological investigation.
The direct microscopy of corneal scrapings from patients with corneal ulcers and vitreous/aqueous aspirate from patients with endophthalmitis were performed under a fluorescent microscope (Olympus BX51; Tokyo, Japan) using 1% calcofluor (Sigma-Aldrich, St. Louis, MO) and 10% KOH. Sabouraud dextrose agar (SDA) (Hi Media, Mumbai, India) was used for the primary isolation of the fungi. The morphological features of the isolates were recorded after growth on potato dextrose agar (PDA) and oatmeal agar. The isolates are deposited at the National Culture Collection of Pathogenic Fungi (NCCPF), PGIMER, Chandigarh, India.
Phylogeny and molecular identification.
Molecular identification was done by sequencing the internal transcribed spacer (ITS) region of the ribosomal DNA (rDNA). The genomic DNA from the mycelia was extracted by following a modified protocol of Lee and Taylor (16). Briefly, Colletotrichum spp. were allowed to grow in potato dextrose broth at 37°C on a rotary shaker (Innova, Ependroff, Hamburg, Germany) at 120 rpm for 3 to 5 days. The growth was filtered, and the harvested mycelial mat was washed with sterile normal saline and crushed in the presence of liquid nitrogen. The powder was transferred to a 1.5-ml microcentrifuge tube containing 600 μl of lysis buffer (100 mM Tris-HCl, pH 8.0, 50 mM EDTA, 3% SDS). After a brief vortexing, proteinase K (Sigma St. Louis, MO) was added at a final concentration of 20 μg/ml, and the solution was incubated at 56°C for 1 h. The DNA was extracted using a phenol-chloroform extraction procedure. The precipitation of DNA was done with an equal volume of isopropanol in the presence of 3 M sodium acetate. The pellet was washed with 70% alcohol and dissolved in 100 μl of TE (10 mM Tris-HCl, pH 7.5, 1 mM EDTA) and stored at −20°C until further use.
The amplification of the ITS region of the rRNA gene was performed by PCR. Amplification reactions were done as a 10-μl reaction mixture in the presence of 2 mM MgCl2, 200 μM each deoxynucleotide triphosphate (dNTP), 0.25 μM each primer (ITS1, GCATATCAATAAGCGGAGGAAAAG; ITS4, GGTCCGTGTTTCAAGACGG) (39), 0.25 U of Taq polymerase (Bangalore Genei, Bengaluru, India), and 5 to 10 ng of fungal genomic DNA. The PCR cycling conditions consisted of an initial denaturation step for 5 min at 94°C, followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 30 s and 72°C for 1 min, and a final extension step at 72°C for 5 min. The gene fragments were visualized on an agarose gel and excised with a clean sharp scalpel, and the DNA was retrieved by using a Q/A quick-spin column (QIAquick; Qiagen, Hilden, Germany) per the manufacturer's instructions. DNA sequencing was performed with the primers described above using a BigDye terminator cycle sequencing kit, version 3.1 (Applied Biosystems, Foster City, CA) per the manufacturer's instructions. The sequencing reactions were analyzed by an ABI Genetic Analyzer (Applied Biosystems, Foster City, CA).
For each isolate, the DNA sequences obtained from forward and reverse primers were used to obtain consensus sequences using the Seqman software (DNA Star; Lasergene, Madison, WI). The isolates were identified by blasting the sequences in the NCBI GenBank. Based on the ITS sequence data from the ex-type or ex-epitype cultures of different Colletotrichum species, a phylogenetic tree was constructed (2). DNA sequences obtained from the database and the sequences of our isolates were aligned using Clustal X (37), and gaps were treated as missing data. Phylogenetic analysis was carried out by MEGA4 (35) based on the neighbor-joining method (28). The evolutionary distances were computed using the maximum composite likelihood method and were expressed as numbers of base substitutions per site (36). All positions containing alignment gaps and missing data were eliminated in pairwise sequence comparisons (using the pairwise deletion option). The resulting tree was evaluated with 1,000 bootstrap replications to test the stability.
Nucleotide sequence accession numbers.
The sequences of all of the isolates determined in the course of this work were deposited in NCBI GenBank, and the accession numbers are given in Table 1.
Table 1.
Clinical and mycological details of cases with ophthalmic infections caused by Colletotrichum a
| Case no. | NCCPF | GenBank accession no. | Age/sex | Mode of infection | Clinical specimen type | Direct smear type | Presumptive morphological identification | Identification by ITS sequencing | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 112002 | HQ437661 | 60/F | Trauma with wooden stick | Corneal scraping | Septate hyphae | Colletotrichum dematium | C. truncatum | Therapeutic keratoplasty, oral and topical fluconazole | Atrophic bulbitis with staphyloma |
| 2 | 112003 | HQ437662 | 38/M | Subconjunctival injection of dexamethasone | Vitreous tap | Septate hyphae | Colletotrichum dematium | C. truncatum | Amphotericin B, dexamethasone, itraconazole, pars plana vitrectomy | Clinical improvement |
| 3 | 112004 | HQ437663 | 70/M | No trauma | Corneal scraping | Septate hyphae | Colletotrichum dematium | C. truncatum | Therapeutic keratoplasty | Clinical improvement |
| 4 | 112006 | ND | 18/M | Vegetable matter | Corneal scraping | Septate hyphae | Colletotrichum dematium | ND | Therapeutic keratoplasty | Clinical improvement |
| 5 | 112007 | HQ437660 | 58/M | Insect fall | Corneal scraping | Septate hyphae | Colletotrichum dematium | C. truncatum | Therapeutic keratoplasty | Corneal opacity |
| 6 | 112008 | HQ437664 | 50/M | Trauma with wooden stick | Vitreous tap | Septate hyphae | Colletotrichum dematium | C. truncatum | Amphotericin B, pars plana vitrectomy | Partial improvement in CF (1.5 m) |
F, female; M, male; ND, not done because the strain could not be revived from culture collection; CF, counting finger (value represents the maximum distance at which the patient was able to count fingers on a hand).
RESULTS
During the 7-year study period (2003 to 2009), 717 ophthalmic samples (from 608 patients with fungal keratitis and 109 patients with endophthalmitis) were positive for the isolation of fungi. Only six isolates (0.83%) were identified as Colletotrichum spp., out of which four were isolated from corneal scrapings (0.65%) and two from vitreous tap (1.83%). The age of these patients ranged from 18 to 70 years; five were male and one was female. A history of trauma was recorded for four patients due to wooden stick injury for two patients, fall of insect for one patient, and trauma due to vegetable matter for one patient. One patient had the subconjunctival injection of dexamethasone, which might be a risk factor for the development of Colletotrichum infection. No obvious history of trauma could be elicited for the sixth patient. As the patient was 70 years old, trivial unnoticed trauma could not be excluded. The visual acuity of the affected eye was less in all patients at the time of presentation than that of the unaffected eye. The clinical details of these six patients are summarized in Table 1.
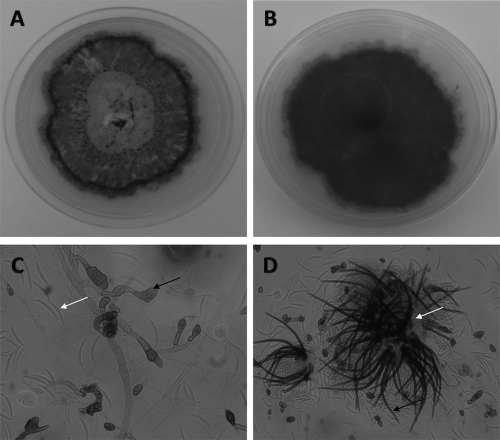
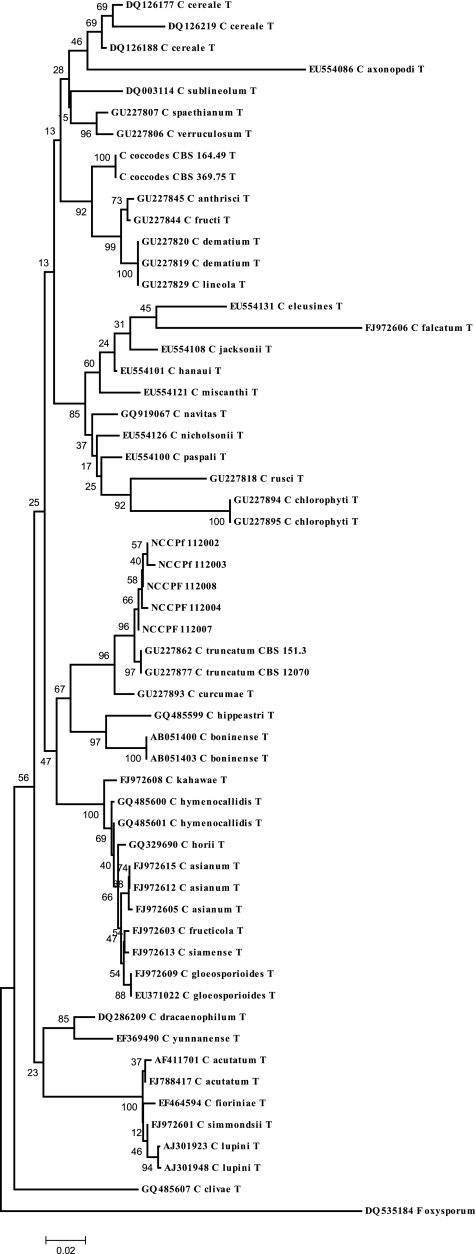
The direct microscopy of the samples revealed the presence of septate, acutely angled branching hyphae. Fungal growth on SDA had pinkish aerial mycelia that became gray-black with dark brown on the reverse. Colonies on oatmeal agar were flat, and the entire margin did not have any aerial mycelia; the surface was buff color and covered with olivaceous gray to iron gray acervuli, with the reverse side buff to pale olivaceous gray (Fig. 1 A and B). The examination of the slide cultures after 7 days of growth at 25°C revealed hyaline, septate hyphae. Conidiomata were acervular, with 2 to 5 septate setae appearing hyaline to pale brown and smooth to verruculose, with tapering toward the tip. Conidia were hyaline, smooth-walled to verruculose, aseptate, and long, and the central part was slightly curved with parallel walls, ending abruptly at the round and truncated base, with tapering toward the acute and more strongly curved apex. Appressoria were light brown, with the entire lobed edge having a roundish to ellipsoidal outline (Fig. 1C and D). The culture isolates were presumptively identified as C. dematium based on these morphological findings. Subsequently, BLAST results of the sequences showed 99 to 100% identity with the C. truncatum isolated from the environment in India (GU227878, CBS 172.48). The phylogram constructed by using the ITS data showed our strains clustering with the ex-type strains of C. truncatum (CBS 151.35 and CBS 120709) (Fig. 2). The molecular identification of one strain was not possible, as the strain could not be revived and the stored culture was contaminated.
Fig. 1.
Colonies of Colletotrichum truncatum (NCCPF 112007) as noted on oatmeal agar after 10 days of incubation. (A) Obverse image; (B) reverse image. (C) Lactophenol cotton blue mount of Colletotrichum truncatum (NCCPF 112007) showing the appresoria (black arrow) and falcate nonseptate conidia (white arrow). (D) Acervuli (white arrow) containing setae (black arrow).
Fig. 2.
Phylogram generated using the maximum composite likelihood method based on rDNA ITS sequence data. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Fusarium oxysporum was used as an outgroup.
DISCUSSION
Although Colletotrichum spp. are considered important plant pathogens, the taxonomy of this group of fungi is confusing even to plant pathologists. Several efforts have been made to revise the classification by using a polyphasic approach incorporating morphological, biochemical, physiological, and phylogenetic characteristics (2). The epitypification of this fungus is important, as the type strain might have been lost or might be in poor condition (12, 29). Certain positive developments in the epitypification has helped to solve many taxonomic problems and the understanding of species, genera, families, or orders of this group of fungi (6, 11, 12, 25, 29). Presently, Colletotrichum spp. have gained importance as human pathogens causing keratitis (8, 13-15, 17, 21, 23, 26, 27, 30, 38, 40, 41). Of 66 currently known species, C. dematium has been reported from most human cases (5, 8, 10, 13, 15, 17, 21, 32). Other rare species implicated in human infections include C. gloeosporioides (20, 23, 40), Glomerulla cingulata (teleomorph state of C. gloeosporioides) (30), C. graminicola (26, 41), and C. coccodes (18, 24). Eye infections due to Colletotrichum spp. have been reported in many countries, including C. gloeosporioides (8), C. graminicola (26), C. dematium (8, 10), and unknown Colletotrichum spp. (8, 19) in the United States, C. gloeosporioides (40, 20, 23) in Japan, C. dematium (13-15, 21) and C. graminicola (41) in India, C. dematium (17) in China, C. coccodes (18) in South Africa, and C. capsicii (38) in Nepal. To the best of our knowledge, no infection in humans due to C. truncatum had been reported before the present series. However, C. capsicii isolated from a patient with keratitis (38) was later identified as C. truncatum based on molecular methods (7). The present series describes five cases of ophthalmic infection due to C. truncatum.
The identification of Colletotrichum to the species level may be important for epidemiological investigations. However, morphological identification on the basis of the appearance of conidia, appressoria, and acervuli on routine medium is both time-consuming and technically challenging. To induce characteristic morphological features, such as aseptate conidia, appressoria, and acervuli, growth on PDA is useful. Once the culture is actively sporulating, plugs of around 4 mm are cut from the actively sporulating areas and are grown on PDA plates under alternating 12 h of UV light and 12 h of no light (33). Still, the major difficulty for morphological identification is the close resemblance of the curved conidia of Fusarium and Colletotrichum spp. However, Colletotrichum spp. can be identified by nonseptate conidia, the presence of appressoria, and (in the later stage) acervuli with setae.
Due to the difficulty of the morphological identification of Colletotrichum spp. in the diagnostic laboratory, the molecular approach seems to be more applicable and accurate. Phylogenetic analysis based on the nucleic acid sequence of the internal transcribed spacer region of the rDNA is the most suitable method for the identification of Colletotrichum isolates to the species level, although many sequences might have been deposited under different names in GenBank due to the repeated revisions of the taxonomy of Colletotrichum spp. Concerns were expressed occasionally when using ITS sequence data alone for the identification of Colletotrichum spp (2). Crouch et al. reported a high error rate (86%) for comparing ITS sequence data within the C. graminicola species complex (6). Similarly, more than 86% evolutionary divergence was noted from type species of C. gloeosporioides for the analysis of 343 ITS sequences (3). In spite of this limitation, only sequences of the ITS region of the rDNA are available for all of the ex-type and epitype cultures of Colletotrichum spp. Hence, we attempted to compare the sequences of our strains to the typified and epitypified strains of the recently described Colletotrichum spp. In the phylogenetic study, all of our strains clustered together with C. truncatum (CBS 151.3 and CBS 120709) with 96% bootstrap agreement. C. curcumae is the only species that is closely related to C. truncatum (7). The need for molecular techniques for species identification is emphasized despite the limitation of the present database, as we misidentified all of our strains as C. dematium due to its close morphological resemblance to C. truncatum. Note that all C dematium clinical isolates from India were identified by morphological characteristics only, and no sequence study was attempted.
The present case series describes infections of the cornea and also endophthalmitis due to C. truncatum. No case of endophthalmitis due to Colletotrichum spp. was reported earlier. It would be pertinent to study the virulence properties of C. truncatum, as two patients developed endophthalmitis. Colletotrichum spp. have been reported to cause infections in skin, subcutaneous tissue, and eye, and it rarely causes systemic disease in humans. The first case of keratitis due to C. dematium was reported in 1983, and subsequently the majority of infections due to Colletotrichum spp. were reported in the eye (5, 8, 13-15, 17-21, 23, 26, 27, 30, 32, 38, 40, 41). Although Colletotrichum eye infections are increasingly reported, the rate of isolation still is low (0.83% in the present series). However, it would require close observation, as the identification of the fungus is difficult and many centers may not attempt it. Colletotrichum spp. usually enter tissue by trauma caused by vegetable matter or the contamination of a wound with soil (8, 15, 20, 21, 24). The traumatic implantation is important for the initiation of infection, as the conidia are contained in the asexual fruiting body (acervular conidiomata) and are not freely released. Trauma/insect fall/iatrogenic injury were noted in affected eyes in all of our patients except one.
C. truncatum has ubiquitous distribution and is reported to cause the anthracnose of bean, soybean, peanut, chili pepper, and pepper. The agent has been isolated from the United States, India, and Thailand (2, 9, 25). In India, C. truncatum is known to infect kharif crops such as paddy, sugarcane, groundnuts, maize, and a variety of pulses, which are grown during kharif season (summer and rainy seasons) (9), and it is reported from the northwestern Himalayas of the Maratwada region of Maharashtra state and in Tamil Nadu (reported as C. capsicii in chili fruit) (29). The present cases, except one (case 6), also were from the northwestern part of India and were reported with eye infections at the end of the kharif season. Although no attempt was made to correlate environmental and clinical isolates in the present study, it would be important for a future detailed epidemiological study of these infections.
As is the case for infection with other types of fungal keratitis, patients with Colletotrichum spp. keratitis present with a history of pain, redness, decreased vision, photophobia, and discharge. The ulcers usually are central or paracentral in position with irregular or serrated edges. Colletotrichum keratitis progresses slowly compared to Aspergillus or Fusarium keratitis, and brown pigmentation may be noted in the ulcer bed as the fungus is a dematiaceous mold (1).
Like other fungal eye infections, Colletotrichum spp. infections are best managed by the surgical removal of the fungal mass and the infected tissue. The patients in the present series were treated by total penetrating keratoplasty for keratitis or pars plana vitrectomy for endophthalmitis. The majority (67%) of the patients had partial or complete clinical improvement. Two of the patients who had endophthalmitis were treated additionally with intravitreal amphotericin B deoxycholate. One of the Colletotrichum keratitis patients received oral and topical fluconazole. There is no consensus on the medical therapy of Colletotrichum ophthalmic infection in the literature (8, 14, 21). In a 21-year-long study, good response to natamycin was noted in nine patients despite exhibiting high minimum inhibitory concentrations (MIC) by in vitro susceptibility testing. The in vitro amphotericin B MIC for all of those strains was uniformly low (8). Similarly, the antifungal susceptibility laboratory of the University of Texas reported that the amphotericin B MICs for most of their Colletotrichum spp. were low (34). In contrast, Shukla et al. have reported that Colletotrichum strains were more sensitive to clotrimazole and micanozole than amphotericin B (31). In the review by Kaliamurthy et al., the complete resolution of Colletotrichum ulcers was reported for the combination of medical and surgical therapy. The average duration of therapy for complete resolution ranged from 2 to 6 weeks (14). It appears that the early removal of the fungal mass by keratoplasty or pars plans vitrectomy is the best management option available until a consensus develops on additional medical therapy.
In conclusion, although Colletotrichum infection in humans is rare, the current study highlights the expanding spectrum of infectious agents due to Colletotrichum spp. and reports C. truncatum as a human pathogenic species. It is too early to judge whether species identification is necessary for the management of this infection, but it may be important for the epidemiology of the disease. As the morphological identification of the agent is difficult, molecular techniques may help in accurate diagnosis. Future studies are required to understand its epidemiology as well as the management of this disease.
ACKNOWLEDGMENT
We thank the Indian Council of Medical Research, New Delhi, India, for their support in purchasing the sequencer for the Centre of Advance Research in Medical Mycology.
We report no association that might pose a conflict of interest relevant to this report.
Footnotes
Published ahead of print on 8 June 2011.
REFERENCES
- 1. Berger S. T., Katsev D. A., Mondino B. J., Pettit T. H. 1991. Macroscopic pigmentation in a dematiaceous fungal keratitis. Cornea 10: 272–276 [DOI] [PubMed] [Google Scholar]
- 2. Cai L., et al. 2009. A polyphasic approach for studying Colletotrichum. Fungal Divers. 39: 183–204 [Google Scholar]
- 3. Cannon P. F., Buddie A. G., Bridge P. D. 2008. The typification of Colletotrichum gloeosporioides. Mycotaxon 104: 189–204 [Google Scholar]
- 4. Castro L. G. M., et al. 2001. Phaeohyphomycotic cyst caused by Colletotrichum crassipes. J. Clin. Microbiol. 39: 2321–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakrabarti A., et al. 2008. Fungal endophthalmitis: fourteen years' experience from a centre in India. Retina 28: 1400–1407 [DOI] [PubMed] [Google Scholar]
- 6. Crouch J. A., Clarke B. B., White J. F., Hillman B. I. 2009. Systematic analysis of the falcate-spored graminicolous Colletotrichum and a description of six new species from warm-season grasses. Mycologia 101: 717–732 [DOI] [PubMed] [Google Scholar]
- 7. Damm U., Woudenberg J. H. C., Cannon P. F., Crous P. W. 2009. Colletotrichum species with curved conidia from herbaceous host. Fungal Divers. 39: 45–87 [Google Scholar]
- 8. Fernandez V., Dursun D., Miller D., Alfonso E. C. 2002. Colletotrichum keratitis. Am. J. Ophthalmol. 134: 435–438 [DOI] [PubMed] [Google Scholar]
- 9. Gawade D. B., Suryawanshi A. P., Pawar A. K., Apet K. T., Devgire S. S. 2009. Field evaluation of fungicides, botanicals and bioagents against anthracnose of soybean. Agr. Sci. Digest. 29: 174–177 [Google Scholar]
- 10. Giaconi J. A., Marangon F. B., Miller D., Alfonso E. C. 2006. Voriconazole and fungal keratitis: a report of two treatment failures. J. Ocul. Pharmacol. Ther. 22: 437–439 [DOI] [PubMed] [Google Scholar]
- 11. Hyde K. D., et al. 2009. Colletotrichum-names in current use. Fungal Divers. 39: 147–182 [Google Scholar]
- 12. Hyde K. D., Zhang Y. 2008. Epitypification: should we epitypify? J. Zhejiang Univ. Sci. 9: 842–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Joseph J., Fernandes M., Sharma S. 2004. Colletotrichum dematium keratitis. J. Postgrad. Med. 50: 309–310 [PubMed] [Google Scholar]
- 14. Kaliamurthy J., et al. 2004. Keratitis due to a Coelomycetous fungus: case reports and review of the literature. Cornea 23: 3–12 [DOI] [PubMed] [Google Scholar]
- 15. Kaliamurthy J., Thomas P. A. 2005. Keratitis due to Colletotrichum dematium. Indian J. Med. Microbiol. 23: 206. [DOI] [PubMed] [Google Scholar]
- 16. Lee S. B., Taylor J. W. 1990. Isolation of DNA from fungal mycelia and single spores, p. 282–287 In Innis M. A., Gelfand D. H., Sninsky J. J., White T. J. (ed.), PCR protocols: a guide to methods and applications. Academic Press, New York, NY [Google Scholar]
- 17. Liao W. Q., et al. 1983. Colletotrichum dematium causing keratitis. Chin. Med. J. 96: 391–394 [PubMed] [Google Scholar]
- 18. Liesegang T. J., Forster R. K. 1980. Spectrum of microbial keratitis in South Florida. Am. J. Ophthalmol. 90: 38–47 [DOI] [PubMed] [Google Scholar]
- 19. Marangon F. B., Miller D., Giaconi J. A., Alfonso E. C. 2004. In-vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am. J. Ophthalmol. 137: 820–825 [DOI] [PubMed] [Google Scholar]
- 20. Matsuzaki O., Yasuda M., Ichinohe M. 1988. Keratomycosis due to Glomerella cingulata. Rev. Iber. Micol. 5(Suppl. 1):30 [Google Scholar]
- 21. Mendiratta D. K., Thamke D., Shukla A. K., Narang P. 2005. Keratitis due to Colletotrichum dematium-a case report. Indian J. Med. Microbiol. 23: 56–58 [DOI] [PubMed] [Google Scholar]
- 22. Midha N. K. Y., Mirzanejad Y., Soni M. 1996. Colletotrichum spp: plant or human pathogen? Antimicrob. Infect. Dis. Newsl. 15: 26–27 [Google Scholar]
- 23. Mitani A., et al. 2004. In vivo and in vitro investigations of fungal keratitis caused by Colletotrichum gloeosporioides. J. Ocul. Pharm. Ther. 25: 563–565 [DOI] [PubMed] [Google Scholar]
- 24. O'Quinn R. P., Hoffmann J. L., Boyd A. S. 2001. Colletotrichum species as emerging opportunistic fungal pathogens: a report of 3 cases of phaeohyphomycosis and review. J. Am. Acad. Dermatol. 45: 56–60 [DOI] [PubMed] [Google Scholar]
- 25. Prihastuti H., Cai L., Chen H., McKenzie E. H. C., Hyde K. D. 2009. Characterization of Colletotrichum species associated with coffee berries in Chiang Mai, Thailand. Fungal Divers. 39: 89–109 [Google Scholar]
- 26. Ritterband D. C., Shah M., Seedor J. A. 1997. Colletotrichum graminicola: a new corneal pathogen. Cornea 16: 362–364 [PubMed] [Google Scholar]
- 27. Rosa R. H., Miller D., Alfonso E. C. 1994. The changing spectrum of fungal keratitis in south Florida. Ophthalmology 101: 1005–1013 [DOI] [PubMed] [Google Scholar]
- 28. Saitou N., Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425 [DOI] [PubMed] [Google Scholar]
- 29. Shenoy B. D., et al. 2007. Morpho-molecular characterisation and epitypification of Colletotrichum capsicii (Glomerellaceae, Sordariomycetes), the causative agent of anthracnose chilli. Fungal Divers. 27: 197–211 [Google Scholar]
- 30. Shukla P. K., Khan Z. A., Lal B., Agrawal P. K., Srivastava O. P. 1983. Clinical and experimental keratitis caused by the Colletotrichum state of Glomerella cingulata and Acrophialophora fusispora. Sabouraudia 21: 137–147 [PubMed] [Google Scholar]
- 31. Shukla P. K., Kumar M., Keshava G. B. S. 2008. Mycotic keratitis: an overview of diagnosis and therapy. Mycoses 51: 183–199 [DOI] [PubMed] [Google Scholar]
- 32. Singh R., et al. 2006. Fungal endophthalmitis complicating subconjunctival injection of triamcinolone acetonide in anterior scleritis. Ann. Ophthalmol. 38: 253–256 [DOI] [PubMed] [Google Scholar]
- 33. Sutton B. C. 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew, Surrey, United Kingdom [Google Scholar]
- 34. Sutton D. A. 1999. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Rev. Iberoam. Micol. 16: 171–179 [PubMed] [Google Scholar]
- 35. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- 36. Tamura K., Nei M., Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U. S. A. 101: 11030–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Upadhyay M. P., et al. 1991. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am. J. Ophthalmol. 111: 92–99 [DOI] [PubMed] [Google Scholar]
- 39. White T. J., Bruns T. D., Lee S., Taylor J. W. 1990. Amplification and direct sequencing of fungal rRNA genes for phylogenetics, p. 315–322 In Innis M. A., Gelfand D. H., Sninsky J. J., White T. J. (ed.), PCR protocols: a guide to methods and applications. Academic Press, New York, NY [Google Scholar]
- 40. Yamamoto N., Matsumoto T., Ishibashi Y. 2001. Fungal keratitis caused by Colletotrichum gloeosporioides. Cornea 20: 902–903 [DOI] [PubMed] [Google Scholar]
- 41. Yegneswaran P. P., Pai V., Bairy I., Bhandary S. 2010. Colletotrichum graminicola keratitis: First case report from India. Indian J. Ophthalmol. 58: 415–417 [DOI] [PMC free article] [PubMed] [Google Scholar]