Abstract
Talin is a cytoskeletal protein that binds to integrin β cytoplasmic tails and regulates integrin activation. Talin1 ablation in mice disrupts gastrulation and causes embryonic lethality. However, the role of talin in mammalian epithelial morphogenesis is poorly understood. Here we demonstrate that embryoid bodies (EBs) differentiated from talin1-null embryonic stem cells are defective in integrin adhesion complex assembly, epiblast elongation, and lineage differentiation. These defects are accompanied by a significant reduction in integrin β1 protein levels due to accelerated degradation through an MG-132-sensitive proteasomal pathway. Overexpression of integrin β1 or MG-132 treatment in mutant EBs largely rescues the phenotype. In addition, epiblast cells isolated from talin1-null EBs exhibit impaired cell spreading and focal adhesion formation. Transfection of the mutant cells with green fluorescent protein (GFP)-tagged wild-type but not mutant talin1 that is defective in integrin binding normalizes integrin β1 protein levels and restores focal adhesion formation. Significantly, cell adhesion and spreading are also improved by overexpression of integrin β1. All together, these results suggest that talin1 binding to integrin promotes epiblast adhesion and morphogenesis in part by preventing integrin β1 degradation.
INTRODUCTION
Talin is a 270-kDa adaptor protein that is localized predominantly in macromolecular complexes formed at the junctions between cells and the extracellular matrix (ECM) where it has been shown to connect the integrin family of cell adhesion molecules to the actin cytoskeleton (4, 9, 20, 43, 50). In addition, talin is thought to be a key regulator of integrin activation (1, 45), and small interfering RNA (siRNA)-mediated knockdown of talin in various cell lines reduced the affinity of β1 and β3 integrins for their ligands (47). In vivo, conditional deletion of the talin1 gene in mouse platelet precursors inhibited ligand-induced αIIbβ3 integrin activation and platelet aggregation, leading to impaired hemostasis in the whole animal (13, 36, 40). Similarly, in B-lymphocytes, talin1 is required for α4β1 (VLA-4) and αLβ2 (LFA-1) integrin activation and thereby for homing to lymph nodes and bone marrow (31).
At the organismal level, talin is required for embryonic development and tissue morphogenesis. In Caenorhabditis elegans, RNA interference-induced talin suppression impaired distal tip cell migration and disrupted the muscle actin cytoskeleton, leading to gonad malformation and paralysis (8). In Drosophila melanogaster, talin is concentrated at the muscle attachment site in an integrin-dependent manner. Talin-null mutants die during embryogenesis, displaying a muscle detachment phenotype similar to that conferred by integrin-null mutations (3). In these invertebrates, talin functions mainly to link ECM-bound integrins to the actin cytoskeleton during tissue morphogenesis and maintenance (15). The mutant phenotype is evident when the actin cytoskeleton is under stress conditions, such as muscle contraction and cell migration. Unlike C. elegans and Drosophila, which have only one talin gene, Dictyostelium discoideum has two talin genes which play distinct roles in the single-cell and the multicellular slug phases (48). Similarly, vertebrates, including the mouse and human, have two talin genes, talin1 and talin2 (10, 33, 44). In adult mice, talin1 is expressed ubiquitously, while talin2, though widely expressed, is most abundant in the testis, brain, and muscles (10, 33, 44). Ablation of talin1 arrested embryonic development at gastrulation, and the mutant embryos were smaller in size, abnormally organized, and deficient in extraembryonic tissues. The ectoderm layer was able to form a columnar epithelium but contained fewer cells (34). In contrast, talin2 knockout mice were viable and fertile (5, 7). These data indicate that talin1 is essential for embryogenesis and show that talin2 does not compensate for loss of talin1, although whether talin2 is expressed during the early stages of embryogenesis was not established. By comparison, mice lacking integrin β1 die at the peri-implantation stage and fail to form organized germ layers (11, 46).
In this study we utilized mouse embryonic stem (ES) cell-derived embryoid bodies (EB), epithelial cysts that are structurally similar to the peri-implantation embryo, to study the mechanisms by which talin1 regulates epithelial morphogenesis and lineage differentiation. We show that talin1 ablation diminishes the assembly of integrin-based adhesion and signaling complexes and impairs epiblast elongation and lineage differentiation. These defects are likely due to the enhanced degradation of integrin β1 through an MG-132-sensitive proteasomal pathway because in the absence of talin1 (i) levels of integrin β1 protein (but not its activation) are significantly reduced, (ii) the protein half-life of integrin β1 is shortened by ∼50%, and (iii) overexpression of integrin β1 or treatment with the proteasome inhibitor MG-132 promotes the assembly of integrin adhesion complexes and epiblast epithelialization. These results uncover a new mechanism by which talin1 promotes epithelial morphogenesis by preventing integrin β1 degradation.
MATERIALS AND METHODS
Culturing of ES cell and embryoid bodies.
The ES cell lines used for this study were wild-type R1 and HM1 ES cells, talin1+/− (C39) and talin1−/− (J26 and A28) ES cells (41), and integrin β1−/− (G201) ES cells (11). Wild-type and talin1+/− and talin1−/− ES cells were cultured on mitomycin C-treated STO cells. Integrin β1-null ES cells were grown directly on plastic culture dishes (27). EB differentiation was initiated from ES cell aggregates in suspension culture as described previously (28). Integrin β1−/− EBs were cultured in poly-hydroxyethyl methacrylate (HEMA)-coated bacteriological petri dishes (29).
Reagents.
Paxillin, focal adhesion kinase (FAK), FAK pY397, integrin-linked kinase (ILK), neural cell adhesion molecule (NCAM), E-cadherin, and integrin β1 (clones 9EG7 and HMβ1-1) monoclonal antibodies (MAbs) were from BD Biosciences. Vinculin (clones hVIN1 and VIN-11-5), talin (clone 8d4) and β-tubulin MAbs were from Sigma. Mouse monoclonal antibodies specific for talin1 (97H6) and talin2 (68E7) were raised against residues 482 to 911 of each isoform. Human integrin β1 (clone HUTS-4) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) MAbs were from Chemicon. β-Dystroglycan MAb was from Novocastra. Polyclonal antibodies (pAb) to laminin α1 rG70 and nidogen were gifts from Peter Yurchenco (Robert Wood Johnson Medical School). Flk-1 pAb and interleukin-2R (IL-2R) MAbs were from Santa Cruz Biotechnologies. IL-2R pAb was from Cell Signaling Technology. α-Fetoprotein (α-FP) pAb was from ICN. Fluorescein isothiocyanate (FITC)-, Cy3-, Cy5-, and horseradish peroxidase (HRP)-conjugated secondary antibodies were from Jackson Immunochemicals. Green fluorescent protein (GFP) pAb and phycoerythrin (PE)- and Alexa 488-conjugated secondary antibodies were from Invitrogen.
Immunofluorescence.
E6.5 mouse embryos and EBs were fixed with 3% paraformaldehyde, embedded in OCT compound, and sectioned with a Leica cryostat. The experimental procedures and animal care were approved by the Institutional Animal Care and Use Committee. EB processing and immunostaining were carried out as described previously (28). The nucleus was counterstained with DAPI (4′,6-diamidino-2-phenylindole). Slides were examined with a Nikon inverted fluorescence microscope (Eclipse TE 2000), and digital images were acquired with a cooled charge-coupled-device (CCD) camera (Hamamatsu) controlled by IP Lab 4.0 software (Scanalytics). To measure the epiblast height, the phase image of an EB cross section was evenly divided into 4 parts by two perpendicular lines. The epiblast height along the lines was measured using Adobe Photoshop and averaged. Data were analyzed by the Student t test or one-way analysis of variance with Holm-Sidak comparisons with SigmaStat (version 3.5; Systat) and expressed as the mean ± the standard deviation (SD).
Immunoprecipitation and immunoblotting.
EBs were collected, washed once in phosphate-buffered saline, and lysed in radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate) containing protease and phosphatase inhibitor cocktails. Protein concentrations were determined using bicinchoninic acid (BCA) reagents (Pierce). For immunoprecipitation, cells lysates were precleared with protein G agarose and then incubated with primary antibodies and protein G beads overnight. Immunoprecipitates were washed and analyzed by SDS-PAGE. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes which were blocked with 5% nonfat dry milk. After incubation with primary antibodies, specific signals were detected with HRP-conjugated secondary antibodies and ECL reagents.
RT-PCR.
Total RNA was isolated with the TRIzol reagent (Invitrogen) and reverse transcribed to cDNA using AMV reverse transcriptase (RT) (Stratagene). PCR primers used for detecting integrin β1A mRNA in talin1+/− and talin1−/− EBs were as follows: 5′-TAGCAAATTGCCAGACGGAG (forward) and 5′-TCTCTTTCCTGCAGTAAGCGTC (reverse). The primers for detecting human and mouse integrin β1A in EBs expressing human integrin β1 were 5′-TTGTGAAGCCAGCCACGGAC (forward) and 5′-CAACCACACCAGCTACAATTGG (reverse) and 5′-ATGTCTAGCGTCAAATGGTC (forward) and 5′-CAACCACGCCTGCTACAATTGG (reverse). PCR conditions for α-FP, neurofilament protein 68 (NF68), ζ-globulin, and 18S were described previously (26, 35).
Transfection of ES cells and epiblast cells.
ES cells were transfected with pCXN2-hβ1-GFP or pCXN2 using Lipofectamine 2000 (Invitrogen). Stable cell clones were established based on GFP expression. For transient transfection of epiblast cells, endoderm was selectively removed from 5-day EBs by brief incubation in deionized water for 20 s, and the addition of 10 volumes of fresh medium followed. The remaining EBs containing basement membranes (BMs) and epiblast cells were dissociated with trypsin-EDTA. Isolated epiblast cells were cultured on laminin-111 (10-μg/ml)-coated culture dishes or glass coverslips for 12 h. The cells were transfected with phβ1-GFP (kindly provided by Martin Humphries of University of Manchester) (38), pEGFP-talin1, pEGFP-talin1 W359A, pEGFP, or pTac-β1 (kindly provided by Susan LaFlamme of Albany Medical College) (22) using Lipofectamine 2000. Twenty-four or forty-eight hours after transfection, the cells were used for immunostaining, an integrin degradation assay, or fluorescence-activated cell sorter (FACS) analysis.
Protein degradation assay.
Three-day EBs and epiblast cells transfected with Tac-β1 were treated with cycloheximide (100 μg/ml) to block new protein synthesis. Medium was changed every 4 h with fresh cycloheximide added. Cell lysates were collected at the indicated time points and analyzed by immunoblotting with integrin β1 (HMβ1-1), IL-2R, and GAPDH antibodies. The results of 3 independent experiments were plotted, and the integrin β1 (Tac-β1)/GAPDH ratio at the 0-hour time point was designated 1.
FACS.
Epiblast cells were surface labeled with either the HMβ1-1 or 9EG7 integrin β1 monoclonal antibody in Hank's solution containing 2 mg/ml bovine serum albumin for 1 h at 4°C. Isotype-matched hamster or rat IgG was used as a control. The cells were washed and fixed in 3% paraformaldehyde for 10 min. The primary antibody bound to the cell surface was detected with FITC- or PE-conjugated secondary antibodies. To assess the ligand binding of α5β1 integrin, the glutathione S-transferase (GST)-tagged fibronectin fragment FN9-11 (the expression vector was kindly provided by Mark Ginsberg of University of California, San Diego) was produced by bacterial expression and biotinylated using an EZ-link biotinylation kit (Pierce). The cells were incubated with biotinylated FN9-11 in the presence or absence of 0.5 mM MnCl2 or 5 mM EDTA. After being washed, the bound ligand was detected with FITC-conjugated streptavidin. The mean fluorescent intensity of the specific labeling was analyzed on a BD FACSCalibur flow cytometer (BD Biosciences).
RESULTS
Talin1 ablation impairs epiblast elongation and lineage differentiation.
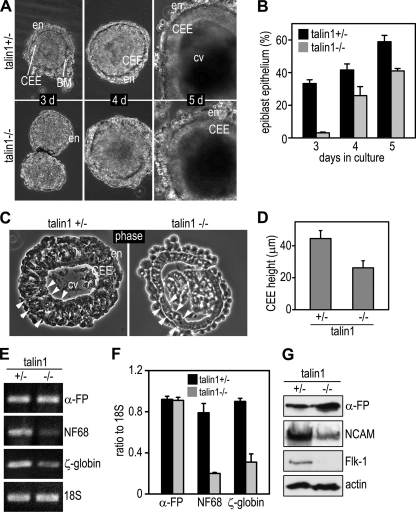
To determine the role of talin1 in embryonic epithelial morphogenesis, we analyzed EBs cultured from wild-type and talin1+/− (clone C39) and talin1−/− (clones J26 and A28) ES cells. EBs differentiated from J26 and A28 ES cells displayed similar phenotypes, and therefore, only the results obtained with J26 ES cells are reported. Talin1+/− EBs differentiated normally and began to form endoderm and underlying BM at day 2 in suspension culture, and by day 3, a distinct epiblast epithelium was observed by live phase microscopy (Fig. 1A). By day 5, the EBs were fully cavitated and had developed into epithelial cysts. Although talin1−/− EBs did form endoderm and BM, epiblast epithelial morphogenesis was delayed and was less efficient compared to that for talin1+/− EBs (Fig. 1B). Particularly, the epiblast height of 5-day talin1−/− EBs was only 50% that of the heterozygous controls (Fig. 1C and D). These results suggest that the loss of talin1 selectively affects epiblast epithelial morphogenesis.
Fig. 1.
Talin1 ablation impairs epiblast elongation and lineage differentiation. (A) EBs were cultured from talin1+/− and talin1−/− ES cells for 3 to 5 days. Phase micrographs of live EBs show the formation of endoderm (en), the columnar epiblast epithelium (CEE), and a central proamniotic-like cavity (cv). (B) EBs with the polarized epiblast epithelium were counted and plotted as a percentage of total EBs examined (n = 4, with the total of 500 to 800 EBs counted for each group). Talin1 ablation decreased the efficiency of CEE formation. (C) Phase micrographs of 5-day EB sections show that the CEE is shorter in talin1−/− EBs. The endodermal cells are also less well spread. Arrowheads define the epiblast epithelium. (D) Epiblast height was measured in 5-day EB sections (n = 60 for each group, talin1+/− versus talin1−/−, P < 0.001). (E) Total RNAs were extracted from 5-day EBs, and the mRNAs for α-fetoprotein (α-FP), neurofilament protein 68 (NF68), and ζ-globin were analyzed by RT-PCR. The 18S RNA served as a loading control. (F) Ethidium bromide-stained gels were analyzed by densitometry, and the ratio of the lineage markers to 18S was plotted (n = 4). (G) Talin1+/− and talin1−/− EBs cultured for 8 days were analyzed by immunoblotting for α-FP, the neural marker neural cell adhesion molecule (NCAM), and the vascular/cardiac marker Flk-1. Actin acted as a loading control. The expression of NCAM and Flk-1 was reduced in the absence of talin1.
The epiblast is the precursor of the three definitive germ layers that give rise to all the cell types of the body. To examine the consequence of defective epiblast morphogenesis on lineage differentiation, we performed RT-PCR for transcripts of the visceral endoderm marker α-FP, the neuronal marker NF68, and the mesoderm marker ζ-globin. The α-FP mRNA level was not altered in the absence of talin1, which is in agreement with the morphological evidence of endoderm differentiation. However, the transcripts of NF68 and ζ-globin were decreased in 5-day talin1−/− EBs (Fig. 1E and F). We also carried out immunoblot analysis for the expression of the neuronal marker NCAM and the cardiovascular lineage marker Flk-1 in 8-day EBs. As shown in Fig. 1G, NCAM expression was significantly reduced in talin1−/− EBs, while Flk-1 was detected only in talin1+/− EBs. These results suggest that talin1 ablation inhibits the differentiation of ectoderm and mesoderm lineages.
Both talin1 and talin2 locate to the epithelium-BM adhesion site depending on integrin.
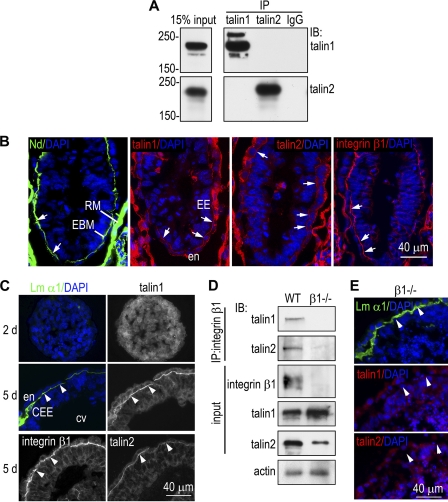
Under standard cell culture conditions, talin is localized primarily to integrin-containing focal adhesions (FAs). To gain insight into the mechanisms by which talin1 regulates embryonic epithelial morphogenesis, we studied the spatial expression of talin1 and talin2 in both E6.5 mouse embryos and EBs by immunofluorescence microscopy. We utilized isotype-specific monoclonal antibodies raised against mouse talin1 (97H6) or talin2 (68E7) (Fig. 2A). Both antibodies stained the basal side of the endoderm/epiblast adjacent to the embryonic BM as well as the apex of the endoderm (Fig. 2B). In the BM zone, talin1 and talin2 colocalized with integrin β1. In the early stages of EB differentiation before endoderm and BM formation, talin1 and talin2 were distributed mainly in the cytoplasm (Fig. 2C and data not shown). BM formation led to the recruitment of both talin1 and talin2 to the endoderm/epiblast-BM adhesion site, where they colocalized with integrin β1, and both talin1 and talin2 coimmunoprecipitated with integrin β1 (Fig. 2D). Interestingly, in β1-null EBs, talin1 expression was increased, while talin2 was markedly decreased, suggesting that the two talins are differentially regulated by integrins (Fig. 2D). We further showed that talin1 and talin2 were absent from the cell-BM adhesion site in integrin β1−/− EBs (Fig. 2E). Taken together, these results indicate that both talin1 and talin2 interact with β1 integrins at epithelium-BM adhesions during embryonic epithelial morphogenesis.
Fig. 2.
Talin binds to integrin β1 and is recruited to the epithelium-BM adhesion site in early mouse embryos and EBs. (A) Lysates of 5-day normal EBs were subject to immunoprecipitation (IP) with either talin1 or talin2 monoclonal antibodies and then to immunoblotting with the same antibodies. The talin1 antibody immunoprecipitates talin1 but not talin2 and vice versa. (B) E6.5 mouse embryos were immunostained for BM nidogen (Nd), talin1, talin2, and integrin β1. Both talin1 and talin2 accumulated in the embryonic BM (EBM) zone and colocalized with integrin β1 (arrows). They were also detected at the apex of endoderm (en). RM, Reichert's membrane; EE, embryonic ectoderm. (C) Normal EBs were cultured for 2 and 5 days and immunostained for talin1, talin2, and integrin β1. BM was identified by laminin α1 chain (Lm α1) immunofluorescence. Both talin1 and talin2 were recruited to the BM zone and colocalized with integrin β1 (arrowheads). (D) Normal and integrin β1−/− EBs were cultured for 5 days. Cell lysates were subjected to immunoprecipitation with anti-integrin β1 antibody and to immunoblotting (IB) for talin1 and talin2. Integrin β1, talin1, and talin2 in the cell lysates were also determined directly by immunoblotting (input). Actin served as a loading control. Talin1 and talin2 were coimmunoprecipitated with integrin β1. (E) Integrin β1−/− EBs were cultured for 5 days and coimmunostained for Lm α1 and talin1. An adjacent section was stained for talin2. Both talin1 and talin2 were absent from the BM zone in integrin β1−/− EBs (arrowheads).
Talin1 maintains integrin β1 protein expression and integrin-based epithelium-BM adhesion complexes.
Next, we tested whether talin, as in Drosophila, might regulate the assembly of integrin-based cell-BM adhesion complexes (3). Five-day talin1+/− and talin1−/− EBs were immunostained for integrin β1 and classic FA proteins, including talin, FAK, paxillin, vinculin, and ILK. In talin1+/− EBs, both integrin β1 and the FA proteins colocalized at cell-BM adhesions in a continuous fashion (Fig. 3A and data not shown). Furthermore, activated phospho-FAK was localized in the BM zone. These results suggest the formation of integrin-based adhesion and signaling complexes at the epithelium-BM interface. In contrast, talin1−/− EBs showed a marked reduction of integrin β1 in the BM zone as detected using both the 9EG7 antibody that is specific for activated integrin β1 and the HMβ1-1 antibody that recognizes both the active and inactive forms (24, 37). There was an accompanying reduction of FA protein staining at cell-BM adhesions, although interestingly, talin2 appeared relatively unaffected. These results suggest that talin1 is required for the assembly of integrin-based epithelium-BM adhesion complexes and that talin2 does not compensate for the loss of talin1 in this respect. Despite the diminished assembly of integrin-based cell-BM adhesion complexes in talin1−/− EBs, we did not observe any detachment of endoderm or epiblast cells from the BM. However, the endoderm cells appeared much more rounded in the null EBs, suggesting weakened integrin-BM adhesions (Fig. 1C).
Fig. 3.
Talin1 ablation diminishes the assembly of integrin adhesion complexes and reduces integrin β1 protein levels. (A) Five-day EBs were immunostained for talin1, talin2, phospho-FAK Y397 (pFAK), paxillin, and integrin β1. The last was detected using the 9EG7 monoclonal antibody that recognizes the active conformation of integrin β1 or the HMβ1-1 antibody that recognizes both the active and inactive conformations. The BM was identified by Lm α1 immunofluorescence. The accumulation of integrin β1 and other FA proteins in the BM zone was reduced in talin1−/− EBs (arrowheads). Talin1 was absent from the epithelium-BM interface in the null mutant, while talin2 remained unchanged (arrowheads). (B) Five-day EBs were lysed in SDS buffer and analyzed by immunoblotting for talin, integrins, FAK, paxillin, β-dystroglycan (β-DG), and E-cadherin. Actin served as a loading control. Note that both intact talin (top band) and the talin rod liberated by protease cleavage (bottom band) are detected by the isoform-specific talin monoclonal antibodies. (C) EBs were cultured for 1 to 5 days and analyzed for integrin β1 by immunoblotting. (D) Total and activated integrin β1 on the epiblast cell surface were labeled with 10 μg/ml anti-integrin β1 (HMβ1-1 and 9EG7, respectively) for 1 h on ice. Isotype-matched hamster and rat IgG was used as a nonspecific control. The cells were then fixed and stained with FITC-conjugated secondary antibody. The specific labeling was detected by FACS analysis. The mean fluorescence intensity of total (the HMβ1-1 epitope) and active (the 9EG7 epitope) integrin β1 on the cell surface was plotted. The total and active integrin were decreased proportionally in talin1−/− epiblast cells. (E) Activated α5β1 integrin was labeled with the biotinylated fibronectin fragment FN9-11 in the presence or absence of 0.5 mM MnCl2 or 5 mM EDTA. FN9-11 binding was determined by FACS analysis using FITC-streptavidin. The surface binding of FN9-11 was reduced in talin1−/− epiblast cells. MnCl2 slightly increased, while EDTA inhibited binding to both talin1+/− and talin1−/− cells.
To test whether the diminished assembly of adhesion complexes might be caused by the reduced expression of integrins and FA proteins, we performed immunoblot analysis on 5-day EBs (Fig. 3B). We confirmed that talin1 was not expressed in talin1−/− EBs and that talin2 was expressed in both talin1+/− and talin1−/− EBs and was not upregulated in the absence of talin1. Significantly, integrin β1 expression was reduced by ∼70% as detected using the HMβ1-1 antibody. Moreover, time course studies showed that although talin1+/− EBs expressed integrin β1 at all stages of the differentiation process, it was completely absent from talin1−/− EBs at day 1, although it was present at day 2 (Fig. 3C). Integrin α5 and α6, the two major α subunits that pair with the β1 subunit in EBs (29), were also significantly downregulated in talin1−/− EBs (Fig. 3B). In contrast, β-dystroglycan, a major BM receptor that is upregulated in integrin β1−/− EBs (27), showed a significant increase in talin1−/− EBs, while the expression of the cell-cell adhesion protein E-cadherin was unchanged. These results suggest that talin1 is required for the maintenance of the normal integrin level and that the increased dystroglycan expression might be a compensatory response to decreased integrin protein levels. We also analyzed the expression and phosphorylation of other FA proteins in talin1−/− EBs by immunoblotting (Fig. 3B). FAK activation, as assessed by phosphorylation at Tyr397, was decreased in parallel with reduced FAK protein expression. ILK expression was also reduced by ∼50% in talin1−/− EBs (data not shown). Paxillin phosphorylation at Tyr118, which has been shown to be catalyzed by activated FAK (2), was inhibited in the absence of talin1, although the expression of paxillin was slightly upregulated. All together, these results suggest that talin1 promotes the assembly of epithelium-BM adhesion and signaling complexes likely by regulating the expression of integrin β1, FAK, and ILK at the protein level.
To assess the effect of talin1 ablation on integrin β1 activation, we performed FACS analysis on epiblast cells isolated from 5-day EBs. Activated β1 and total integrin β1 on the cell surface were labeled with the monoclonal antibody 9EG7 and HMβ1-1, respectively. Isotype-matched preimmune IgG was used as a nonspecific binding control. As shown in Fig. 3D, the total integrin β1 was reduced by 57% in talin1−/− epiblast cells, and this was accompanied by a proportional reduction in activated β1 by 55%. These results suggest that the loss of talin1 reduces integrin β1 expression but not activation. To confirm the above finding, we assessed the ligand binding activity of α5β1 integrin using the fibronectin fragment FN9-11. Surface binding of FN9-11 was reduced by 54% in talin1−/− epiblast cells compared to control talin1+/− cells (Fig. 3E). The specificity of the assay is indicated by the fact that EDTA treatment abolished much of the FN9-11 binding. In the presence of MnCl2, which activates integrins independent of cellular activators, such as talin, FN9-11 binding was only slightly increased in both talin1+/− and talin1−/− epiblast cells. Again, this indicates that the bulk of integrin β1 expressed on talin1−/− cells is already in the activated form. Collectively, these results suggest that talin1 ablation reduces integrin β1 expression but not its activation in epiblast cells.
Integrin β1 protein degradation is accelerated in the absence of talin1.
To test whether the reduced integrin β1 protein in talin1−/− EBs might be due to decreased gene transcription, we performed RT-PCR using primers specific for mouse integrin β1. We found that the integrin β1 mRNA level was slightly increased in talin1−/− EBs, suggesting that the decrease of integrin β1 protein must be the result of posttranscriptional events (Fig. 4A and B). Next, we investigated whether talin1 might regulate the stability of the integrin β1 protein. Three-day talin1+/− and talin1−/− EBs were treated with 100 μg/ml cycloheximide to block new protein synthesis, and EB extracts were collected at 4, 8, 12, and 24 h after cycloheximide treatment. As shown in Fig. 4C and D, the rate of integrin β1 protein degradation was significantly accelerated in talin1−/− EBs compared to the talin+/− control. Based on this assay, we estimate the integrin β1 protein half-life to be 23.1 ± 5.0 h in talin1+/− EBs and 10.6 ± 0.5 h in talin1−/− EBs (P < 0.01, n = 3). These results suggest that the interaction of talin1 with β1 integrins prevents integrin β1 protein degradation.
Fig. 4.
Degradation of the integrin β1 protein is accelerated in the absence of talin1. (A) Total RNA was extracted from 5-day EBs, and the integrin β1 mRNA was determined by RT-PCR. The 18S RNA served as a control. (B) Ethidium bromide-stained gels were analyzed by densitometry. The ratio of integrin β1 mRNA to 18S RNA was plotted (n = 4). (C) Talin1+/− and talin1−/− EBs were cultured for 3 days and then treated with 100 μg/ml cycloheximide to block new protein synthesis. EB lysates were prepared at designated time points after cycloheximide treatment and analyzed by immunoblotting for integrin β1 using the monoclonal antibody HMβ1-1. GAPDH served as a loading control. (D) The blots were analyzed by densitometry, and the ratio of integrin β1 to GAPDH was plotted (n = 3).
Proteasome inhibition prevents integrin β1 degradation and promotes epiblast morphogenesis in talin1−/− EBs.
To elucidate the proteolytic pathways that might be involved in the degradation of the integrin β1 protein, we incubated 3-day talin1+/− and talin1−/− EBs for 16 h with the proteasome inhibitor MG-132, the calpain inhibitor ALLN, or the lysosome inhibitor chloroquine. Immunoblot analysis revealed that in the solvent control, the integrin β1 protein level of talin1−/− EBs was only 35.8% of that of talin1+/− EBs when normalized to actin. However, treatment of talin1−/− EBs with MG-132 restored the integrin β1 protein to the talin1+/− level (Fig. 5A). In contrast, in ALLN- and chloroquine-treated talin1−/− EBs the integrin β1 protein levels remained 34.6 and 28.4% of the talin1+/− control level. These results suggest that talin1 ablation increases the turnover rate of integrin β1 mainly through an MG-132-sensitive degradation pathway, although the fact that chloroquine treatment slightly increased the integrin β1 protein in both talin1+/− and talin1−/− EBs suggests that lysosomes may also be involved in integrin turnover. The restoration of integrin β1 protein levels in MG-132-treated talin1−/− EBs resulted in increased accumulation of integrin β1, paxillin, and phospho-FAK Tyr397 in the BM zone (Fig. 5B). The efficiency of epiblast epithelialization was also improved by MG-132 treatment (Fig. 5C). Furthermore, the epiblast cells of the treated EBs appeared more elongated compared to those of the solvent control. To confirm this, we quantified the epiblast height with IP Lab image analysis software and showed that inhibition of integrin β1 degradation by MG-132 increased the epiblast height by 60% in talin1−/− EBs (Fig. 5D). Similarly, lineage differentiation was restored by MG-132 treatment as indicated by increases in the mRNAs for NF68 and ζ-globin (Fig. 5E and F). All together, these results suggest that talin1 regulates adhesion assembly and epiblast morphogenesis in part by preventing integrin β1 degradation through an MG-132-sensitive proteasomal pathway.
Fig. 5.
Proteasome inhibition prevents integrin β1 degradation and improves epiblast morphogenesis in talin1-null EBs. (A) EBs were cultured for 3 days and then treated with the proteasome inhibitor MG-132 (100 nM), the calpain inhibitor ALLN (5 μM), or the lysosome inhibitor chloroquine (100 μM) for 18 h. After treatment, EBs were subject to immunoblotting for integrin β1. Actin serves as a loading control. Immunoblots shown are representatives of 3 experiments. (B) Three-day EBs were treated with 100 nM MG-132 for 2 more days and immunostained for integrin β1, paxillin, and phospho-FAK Y397 (pFAK). BM was identified by Lm α1 immunofluorescence. Arrowheads point to positive staining in the BM zone. MG-132 treatment markedly increased the accumulation of integrin β1, paxillin, and pFAK in the BM zone in talin1−/− but not talin1+/− EBs. en, endoderm; CEE, columnar epiblast epithelium. (C) Two-day EBs were treated with 0, 50, or 100 nM MG-132 for 2 more days. EB differentiation was observed by live phase microscopy, and the percentage of EBs with a polarized epiblast epithelium was plotted (n = 4, with the total of 400 to 600 EBs counted for each group). (D) The CEE heights of control and MG-132 (100 nM)-treated EBs were quantitated and plotted (n = 60 for each group). (E) Three-day EBs were treated with or without 100 nM MG-132 for 3 more days. RT-PCR was performed using primers that specifically amplify mouse NF68, ζ-globin, and 18S RNA. (F) The ethidium-stained gels were analyzed by densitometry, and the ratio of the lineage markers to 18S was plotted (n = 4).
Talin1-integrin interaction is required for the maintenance of integrin stability and focal adhesion formation.
To determine whether talin1 binding to integrin β1 is required for the maintenance of normal integrin β1 levels, we transfected talin1+/− and talin1−/− epiblast cells with GFP-tagged talin1 or a mutant talin1 that is defective in integrin binding (talin1 W359A) (12, 14). Compared with talin1+/− epiblast cells which spread nicely on the laminin-111 substrate and formed prominent FAs, talin−/− cells spread poorly and did not assemble FAs (Fig. 6A). However, transfection with wild-type GFP-talin1 completely rescued cell spreading and FA formation in talin1−/− cells. GFP-talin1 colocalized with paxillin in FAs. In contrast, transfection of the talin1−/− cells with GFP-talin1 W359A did not improve cell spreading or FA formation, and the integrin-binding talin1 mutant remained largely in the cytoplasm. Interestingly, the GFP-talin1 W359A mutant was incorporated into the FAs assembled by talin1+/− epiblast cells. These results suggest that the talin1-integrin interaction is required for epiblast spreading and FA formation but is dispensable for talin1 recruitment to preexisting FAs.
Fig. 6.
Talin1-integrin binding is required for the maintenance of integrin levels. Talin1+/− and talin1−/− epiblast cells were transfected with wild-type (WT) talin1 or the integrin-binding mutant (talin1 W359A) fused with GFP. (A) The transfected cells were cultured on the laminin-111 substrate for 16 h and immunostained using anti-GFP and anti-paxillin antibodies. Wild-type but not integrin-binding mutant talin1 rescued spreading and focal adhesion formation in talin1−/− epiblast cells. (B) The expression of integrin β1 on the surface of the transfected GFP-positive cells was analyzed by FACS using the HMβ1-1 antibody with isotype-matched hamster preimmune IgG as a control. Transfection of talin1−/− epiblast cells with wild-type talin1, but not the integrin-binding mutant, restored surface integrin expression to levels similar to those in talin1+/− cells.
We next examined the surface expression of integrin β1 in the transfected cells by FACS analysis. Forty-eight hours after transfection, cells were fixed and immunostained with the HMβ1-1 antibody followed by a phycoerythrin-conjugated secondary antibody. Analysis of the transfected, GFP-positive cells revealed that integrin β1 on the cell surface was reduced by 58% in talin1−/− epiblast cells compared with that of talin1+/− controls (Fig. 6B). Transfection of talin1−/− cells with wild-type talin1 largely restored integrin β1 expression, whereas transfection with talin1 W359A had no effect. We also performed immunoblot analysis of the transfected cells and obtained a similar result (data not shown). These data establish that a talin1-integrin interaction is required for the maintenance of normal integrin β1 levels in epiblast cells.
Overexpression of integrin β1 in talin1−/− EBs improves epithelial morphogenesis.
To test whether the reduced integrin β1 expression in talin1−/− EBs contributes to the defect in the assembly of cell adhesion complexes and epiblast morphogenesis, we stably transfected talin1−/− EBs with GFP-tagged human integrin β1 (hβ1-GFP), which has been shown to support cell attachment and FA assembly when expressed in integrin β1−/− fibroblasts (38). RT-PCR analyses of 5-day EBs using primers for a nonhomologous region of the human and mouse integrin β1 mRNA revealed that the transgene was expressed at twice the level of the endogenous mouse gene (Fig. 7A). Immunoblotting and immunofluorescence confirmed the expression and appropriate localization of the hβ1-GFP fusion protein (Fig. 7B and C). As a consequence of hβ1-GFP overexpression, the recruitment of paxillin and phospho-FAK to epithelium-BM adhesions was significantly increased in talin1−/− EBs compared to the vector control, indicating enhanced cell-BM adhesion assembly and signaling (Fig. 7D). Furthermore, hβ1-GFP overexpression in talin1−/− EBs increased both the efficiency of epiblast epithelialization and the epiblast height (Fig. 7E and F). We also determined the stability of hβ1-GFP in talin1−/− EBs and found its half-life to be 10 h, slightly shorter than endogenous integrin β1. These results suggest that the reduction in integrin β1 expression in talin1−/− EBs contributes to impaired epiblast morphogenesis and that the talin1−/− phenotype can be partially rescued by overexpressing integrin β1 to compensate for its increased degradation.
Fig. 7.
Overexpression of integrin β1 in talin1-null EBs improves cell-BM adhesion assembly and epithelial morphogenesis. Talin1−/− EBs were stably transfected with human integrin β1-GFP (hβ1-GFP) or the GFP control vector (ctl). (A) Total RNA was extracted from 5-day EBs and analyzed by RT-PCR for human and mouse integrin β1 mRNAs with 18S RNA as a loading control. The hβ1 mRNA was expressed at twice the level of that of endogenous integrin β1. (B) Lysates of 5-day EBs were analyzed by immunoblotting for the expression of the human integrin β1-GFP fusion protein. (C) Five-day EBs expressing hβ1-GFP were immunostained with anti-laminin α1 (Lm α1) antibody to reveal the basement membrane and with the monoclonal antibody HUTS-4 that specifically recognizes activated human integrin β1 (hβ1). Arrowheads point to the BM and hβ1 staining. en, endoderm; CEE, columnar epiblast epithelium. (D) Five-day EBs were immunostained for pFAK and paxillin. hβ1-GFP transfection increased the accumulation of pFAK and paxillin in the BM zone. (E) Four-day EBs were observed by live phase microscopy, and the percentage of EBs with a polarized epiblast epithelium was plotted (n = 4, with the total of 400 EBs counted for each group). (F) The height of the columnar epiblast epithelium (CEE) was measured using imaging software and plotted (n = 40 for each group). (G) Epiblast cells were isolated form 5-day normal EBs and cultured on the laminin-111 substrate. FA formation was assessed by paxillin immunostaining. (H) Talin1−/− epiblast cells were transiently transfected with hβ1-GFP or GFP alone. After 24 h, cells were immunostained using anti-GFP (β1-GFP), anti-human integrin β1 (hβ1), or anti-paxillin antibody. Expression of hβ1-GFP but not GFP alone improved spreading and FA formation in talin1−/− epiblast cells. Arrows indicate transfected cells. Arrowheads indicate untransfected cells. (I) Talin1+/− and talin1−/− cells were transiently transfected with an integrin mutant containing the extracellular and the transmembrane domains of IL-2 receptor and the intracellular domain of integrin beta1 (Tac-β1). After 48 h, cells were immunostained for IL-2R and ILK. Tac-β1 expression failed to rescue the spreading of talin1−/− epiblast cells, although it could be targeted to focal adhesions in talin1+/− cells. (J) Epiblast cells transfected with Tac-β1 were treated with cycloheximide to block new protein synthesis. Cell lysates were analyzed by immunoblotting for IL-2R and GAPDH at designated time points. (K) The blots were scanned by densitometry, and the ratio of Tac-β1 to GAPDH was plotted. There is no significant difference in Tac-β1 degradation between talin1+/− and talin1−/− epiblast cells. n = 3.
To further assess whether the reduced integrin expression after talin1 ablation causes impaired epiblast-matrix interactions, we transiently transfected EB-derived epiblast cells with either hβ1-GFP or the control vector. After transfection, cells were plated onto the laminin-111 substrate for 24 h and immunostained for human integrin β1 and paxillin. Normal epiblast cells spread nicely on laminin-111 and formed FAs (Fig. 7G). In contrast, talin1−/− epiblast cells transfected with GFP alone attached but failed to spread or form FAs (Fig. 7H). However, forced expression of hβ1-GFP in the mutant cells partially rescued cell spreading and FA formation compared to those of the untransfected or GFP-transfected cells. The exogenous integrin β1-GFP could be detected at the FAs with the monoclonal antibody HUTS-4 which reacts with the activated human integrin β1 (30). These results confirm that the phenotype of talin1−/− epiblast cells is due at least in part to reduced integrin β1 expression.
Next, we assessed whether the ligand binding site in the integrin β1 ectodomain is required for the rescue effect. To this end, we transiently transfected epiblast cells with a construct that consists of the extracellular and the transmembrane domains of IL-2 receptor and the intracellular domain of integrin β1 (Tac-β1) (22). Expression of Tac-β1 failed to rescue cell spreading and FA formation in talin1−/− cells (Fig. 7I). However, Tac-β1 could be detected at FAs when expressed at low or intermediate levels in talin1+/− cells. High-level transgene expression caused cells to round up. These results suggest that reduced integrin β1 protein levels at least partially account for the defective assembly of epiblast-BM adhesions and that an intact ligand binding domain is required for integrin β1 to rescue the talin1−/− epiblast phenotype.
To test whether the interaction between talin1 and integrin β1 tails is sufficient to regulate integrin turnover, we treated Tac-β1-transfected epiblast cells with cycloheximide to block new protein synthesis and analyzed Tac-β1 protein levels over time by immunoblotting. In contrast to the accelerated degradation of endogenous integrin β1 and transfected hβ1-GFP in talin1−/− EBs, the degradation rate of Tac-β1 in talin1−/− epiblast cells was not increased compared to that in the talin1+/− control (Fig. 7J and K). This result shows that the integrin extracellular domain plays a key role in determining the rate of integrin turnover in the absence of talin1.
DISCUSSION
In the present study, we report that talin1 ablation in EBs impairs epiblast epithelial formation and lineage differentiation. These defects correlate with a substantial reduction in integrin β1 expression and in the absence of talin1, integrin β1 was rapidly degraded through an MG-132-sensitive pathway. Rescue experiments show that talin1 binding to integrin is required for the maintenance of normal integrin β1 levels and FA formation. Moreover, overexpression of a human integrin β1-GFP fusion protein partially rescued the talin1-null phenotype. These results uncover a new mechanism by which talin1 regulates integrin function in epithelial cells and thereby epithelial morphogenesis.
Previous studies in Drosophila have showed that talin colocalizes with integrin at FA-like structures formed at the basal surface of the wing imaginal disc epithelia (3). Talin mutations resulted in the loss of integrin from these structures and vice versa. This is in agreement with our findings in talin1−/− and integrin β1−/− EBs, which suggests that talin and integrin are interdependent for the assembly of these FA-like structures. Loss of talin in fly wing epithelia causes the separation of the dorsal and ventral epithelial layers and leads to a wing blister phenotype (3, 42). In fly tracheal epithelia, talin mutations do not affect terminal bud formation and lumen morphogenesis early in development but result in convoluted lumens and branch degeneration during larval life, indicating that talin is required for tracheal epithelial maintenance (25). Our analyses of talin1−/− EBs extend these findings and demonstrate that talin1 is required for epiblast elongation and efficient epiblast epithelialization. Taken together, these results establish a critical role for talin1 in the formation and maintenance of epithelial tissues.
Conditional deletion of the talin1 gene in mouse megakaryocytes inhibited platelet adhesion and aggregation and led to severe defects in hemostasis (13, 36, 40). This phenotype was attributed to the low affinity of platelet integrins in the absence of talin1 (there is no talin2 in platelets), and they could not be activated in response to platelet agonists. In contrast, β1 integrin activation in differentiating EBs is unaffected in the absence of talin1 when assessed by FACS analysis using the 9EG7 antibody which recognizes only activated integrin β1 and using the α5β1 integrin ligand FN9-11 fragment. This could be due to the compensation by talin2, which is expressed in normal and talin1−/− EBs and continues to localize to the epithelium-BM adhesion. Indeed, cell spreading and focal adhesion assembly in talin1−/− fibroblasts is dependent on upregulation of talin2 (50). Moreover, defects in cell spreading and FA that result from talin1 knockdown in endothelial cells (which express only talin1), can be rescued by expressing talin2 (21). However, integrin β1 activation measured both with the 9EG7 antibody and via adhesion assays was also reported to be unaffected in primary cultures of fetal myoblasts in which both talin1 and talin2 were deleted, although myoblast fusion was severely compromised (7). The kindlin family of FERM domain proteins has been shown to synergize with talin to activate integrins, and ILK has also been shown to support integrin activation (16, 23). Therefore, it is possible that talin2 synergizes with other pathways to activate integrin β1 in the talin1−/− epiblast.
In contrast to platelets, skeletal muscle, and fibroblasts where integrin β1 expression was not affected by deleting the talin1 gene (6, 40, 50), talin1−/− EBs had a significant reduction of integrin β1 at the protein level. A similar finding has been reported for undifferentiated talin1−/− ES cells, but not for differentiated ES cells (41). While the reason for this difference is unclear, it is unlikely to be due simply to compensation by talin2 since fibroblasts express much less talin2 than EBs and platelets lack talin2 altogether (data not shown). One possible explanation is that integrins are highly dynamic and have faster turnover rates in rapidly expanding epiblast and undifferentiated ES cells. We found that the half-life of the integrin β1 protein is 23 h in EBs versus 96 h in confluent embryonic fibroblasts. In these undifferentiated cell types, talin1 may actively participate in the regulation of integrin turnover. In this study, we further show that the enhanced degradation of the integrin β1 protein is sensitive to the proteasome inhibitor MG-132 and that MG-132 partially reversed the epithelial defects observed with talin1−/− EBs. There is growing evidence that E3 ligases play an important role in regulating cell adhesion and migration (17), and focal adhesion proteins, including talin and paxillin, have been shown to be ubiquitinated (18, 19). In addition, ILK has been shown to regulate ubiquitination of Notch1. However, we were unable to detect ubiquitination of integrin β1, α5, or α6 using anti-hemagglutinin (HA) antibody in epiblast cells transiently transfected with HA-tagged ubiquitin (data not shown). This suggests that α5β1 and α6β1 integrin may not be the direct targets for ubiquitination in EBs. Nonetheless, transfection of the talin1−/− epiblast cells or EBs with human integrin β1 largely rescued cell spreading, FA adhesion assembly, and epiblast epithelial morphogenesis. Our data suggest that talin1 promotes epiblast cell adhesion and embryonic epithelial formation at least in part by regulating integrin turnover.
The data presented in this study may also impact the interpretation of the mouse talin1 knockout phenotype which is characterized by defects in the organization and size of the pregastrulation (E6.5) embryo (34). While the phenotype is slightly less severe than that of the integrin β1 knockout (11, 46), it was sufficiently early to lead to the conclusion that talin2 was probably not expressed in these early embryos and was therefore unable to compensate for loss of talin1. However, the current study demonstrates that both talin1 and talin2 are expressed in the same locations in cells of the epiblast at around this time (Fig. 2B). Therefore, it now seems possible that the talin1−/− embryonic phenotype is due, at least in part, to a reduction of integrin β1 levels in the epiblast as a result of increased integrin degradation. The presence of some β1 integrins in the talin1−/− epiblast (as in the EBs) could account for the decreased severity of the talin1−/− phenotype compared to the integrin β1−/− phenotype. We are currently investigating this possibility.
How talin1 regulates integrin stability is not yet known. One possibility is that talin1 may stabilize integrin clusters at the epithelium-BM adhesions by linking ligand-bound integrins to the actin cytoskeleton. Indeed, our immunofluorescence results showed that the clustering of integrin β1 at these FA-like structures was significantly reduced in talin1−/− EBs. In the absence of talin1, unbound integrins on the cell surface may be internalized and degraded, causing a reduction of integrin levels (39). Second, talin has been suggested to facilitate the exit of integrin from a subplasma membrane compartment to the cell surface. Antisense-induced suppression of talin in HeLa cells led to retention of integrin in this compartment (32). In this case, the immature integrin accumulated inside the cell may undergo proteasome-mediated degradation (49). Our immunostaining results do not support this hypothesis because no such intracellular pool of integrin β1 was found in either differentiated EBs or isolated epiblast cells. Lastly, we found that microtubules on the basal side of epiblast cells were disoriented in the mutant EBs. Since microtubules are required for membrane protein recycling (39), changes in the microtubule cytoarchitecture may compromise integrin trafficking and lead to a reduction in integrin levels. This intriguing possibility warrants further investigation.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01GM081674) to S.L. and by grants from the Wellcome Trust (077532/Z/05/Z) and Cancer Research UK to D.R.C.
We thank Ashwini Kumar for his assistance in the FACS analysis and Uta Praekelt for the generation of the isoform-specific talin1 and talin2 monoclonal antibodies.
Footnotes
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Anthis N. J., Campbell I. D. 2011. The tail of integrin activation. Trends Biochem. Sci. 36:191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bellis S. L., Miller J. T., Turner C. E. 1995. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J. Biol. Chem. 270:17437–17441 [DOI] [PubMed] [Google Scholar]
- 3. Brown N. H., et al. 2002. Talin is essential for integrin function in Drosophila. Dev. Cell 3:569–579 [DOI] [PubMed] [Google Scholar]
- 4. Calderwood D. A., Ginsberg M. H. 2003. Talin forges the links between integrins and actin. Nat. Cell Biol. 5:694–697 [DOI] [PubMed] [Google Scholar]
- 5. Chen N. T., Lo S. H. 2005. The N-terminal half of talin2 is sufficient for mouse development and survival. Biochem. Biophys. Res. Commun. 337:670–676 [DOI] [PubMed] [Google Scholar]
- 6. Conti F. J., et al. 2008. Progressive myopathy and defects in the maintenance of myotendinous junctions in mice that lack talin 1 in skeletal muscle. Development 135:2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conti F. J., Monkley S. J., Wood M. R., Critchley D. R., Muller U. 2009. Talin 1 and 2 are required for myoblast fusion, sarcomere assembly and the maintenance of myotendinous junctions. Development 136:3597–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cram E. J., Clark S. G., Schwarzbauer J. E. 2003. Talin loss-of-function uncovers roles in cell contractility and migration in C. elegans. J. Cell Sci. 116:3871–3878 [DOI] [PubMed] [Google Scholar]
- 9. Critchley D. R. 2009. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu. Rev. Biophys. 38:235–254 [DOI] [PubMed] [Google Scholar]
- 10. Debrand E., et al. 2009. Talin 2 is a large and complex gene encoding multiple transcripts and protein isoforms. FEBS J. 276:1610–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fässler R., et al. 1995. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 128:979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García-Alvarez B., et al. 2003. Structural determinants of integrin recognition by talin. Mol. Cell 11:49–58 [DOI] [PubMed] [Google Scholar]
- 13. Haling J. R., Monkley S. J., Critchley D. R., Petrich B. G. 2011. Talin-dependent integrin activation is required for fibrin clot retraction by platelets. Blood 117:1719–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han J., et al. 2006. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr. Biol. 16:1796–1806 [DOI] [PubMed] [Google Scholar]
- 15. Helsten T. L., et al. 2008. Differences in regulation of Drosophila and vertebrate integrin affinity by talin. Mol. Biol. Cell 19:3589–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honda S., et al. 2009. Integrin-linked kinase associated with integrin activation. Blood 113:5304–5313 [DOI] [PubMed] [Google Scholar]
- 17. Huang C. 2010. Roles of E3 ubiquitin ligases in cell adhesion and migration. Cell Adh. Migr. 4:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C., et al. 2009. Talin phosphorylation by Cdk5 regulates Smurf1-mediated talin head ubiquitylation and cell migration. Nat. Cell Biol. 11:624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iioka H., Iemura S., Natsume T., Kinoshita N. 2007. Wnt signalling regulates paxillin ubiquitination essential for mesodermal cell motility. Nat. Cell Biol. 9:813–821 [DOI] [PubMed] [Google Scholar]
- 20. Jiang G., Giannone G., Critchley D. R., Fukumoto E., Sheetz M. P. 2003. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature 424:334–337 [DOI] [PubMed] [Google Scholar]
- 21. Kopp P. M., et al. 2010. Studies on the morphology and spreading of human endothelial cells define key inter- and intramolecular interactions for talin1. Eur. J. Cell Biol. 89:661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LaFlamme S. E., Akiyama S. K., Yamada K. M. 1992. Regulation of fibronectin receptor distribution. J. Cell Biol. 117:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larjava H., Plow E. F., Wu C. 2008. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 9:1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lenter M., et al. 1993. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc. Natl. Acad. Sci. U. S. A. 90:9051–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levi B. P., Ghabrial A. S., Krasnow M. A. 2006. Drosophila talin and integrin genes are required for maintenance of tracheal terminal branches and luminal organization. Development 133:2383–2393 [DOI] [PubMed] [Google Scholar]
- 26. Levinson-Dushnik M., Benvenisty N. 1997. Involvement of hepatocyte nuclear factor 3 in endoderm differentiation of embryonic stem cells. Mol. Cell Biol. 17:3817–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li S., et al. 2002. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157:1279–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li S., Yurchenco P. D. 2006. Matrix assembly, cell polarization, and cell survival: analysis of peri-implantation development with cultured embryonic stem cells. Methods Mol. Biol. 329:113–125 [DOI] [PubMed] [Google Scholar]
- 29. Liu J., et al. 2009. Integrins are required for the differentiation of visceral endoderm. J. Cell Sci. 122:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luque A., et al. 1996. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J. Biol. Chem. 271:11067–11075 [DOI] [PubMed] [Google Scholar]
- 31. Manevich-Mendelson E., et al. 2010. Talin1 is required for integrin-dependent B lymphocyte homing to lymph nodes and the bone marrow but not for follicular B-cell maturation in the spleen. Blood 116:5907–5918 [DOI] [PubMed] [Google Scholar]
- 32. Martel V., et al. 2000. Talin controls the exit of the integrin alpha 5 beta 1 from an early compartment of the secretory pathway. J. Cell Sci. 113(11):1951–1961 [DOI] [PubMed] [Google Scholar]
- 33. Monkley S. J., Pritchard C. A., Critchley D. R. 2001. Analysis of the mammalian talin2 gene TLN2. Biochem. Biophys. Res. Commun. 286:880–885 [DOI] [PubMed] [Google Scholar]
- 34. Monkley S. J., et al. 2000. Disruption of the talin gene arrests mouse development at the gastrulation stage. Dev. Dyn. 219:560–574 [DOI] [PubMed] [Google Scholar]
- 35. Murray P., Edgar D. 2000. Regulation of programmed cell death by basement membranes in embryonic development. J. Cell Biol. 150:1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nieswandt B., et al. 2007. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J. Exp. Med. 204:3113–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noto K., Kato K., Okumura K., Yagita H. 1995. Identification and functional characterization of mouse CD29 with a mAb. Int. Immunol. 7:835–842 [DOI] [PubMed] [Google Scholar]
- 38. Parsons M., Messent A. J., Humphries J. D., Deakin N. O., Humphries M. J. 2008. Quantification of integrin receptor agonism by fluorescence lifetime imaging. J. Cell Sci. 121:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pellinen T., Ivaska J. 2006. Integrin traffic. J. Cell Sci. 119:3723–3731 [DOI] [PubMed] [Google Scholar]
- 40. Petrich B. G., et al. 2007. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J. Exp. Med. 204:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Priddle H., et al. 1998. Disruption of the talin gene compromises focal adhesion assembly in undifferentiated but not differentiated embryonic stem cells. J. Cell Biol. 142:1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prout M., Damania Z., Soong J., Fristrom D., Fristrom J. W. 1997. Autosomal mutations affecting adhesion between wing surfaces in Drosophila melanogaster. Genetics 146:275–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberts G. C., Critchley D. R. 2009. Structural and biophysical properties of the integrin-associated cytoskeletal protein talin. Biophys. Rev. 1:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Senetar M. A., McCann R. O. 2005. Gene duplication and functional divergence during evolution of the cytoskeletal linker protein talin. Gene 362:141–152 [DOI] [PubMed] [Google Scholar]
- 45. Shattil S. J., Kim C., Ginsberg M. H. 2010. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11:288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stephens L. E., et al. 1995. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9:1883–1895 [DOI] [PubMed] [Google Scholar]
- 47. Tadokoro S., et al. 2003. Talin binding to integrin beta tails: a final common step in integrin activation. Science 302:103–106 [DOI] [PubMed] [Google Scholar]
- 48. Tsujioka M., Yoshida K., Inouye K. 2004. Talin B is required for force transmission in morphogenesis of Dictyostelium. EMBO J. 23:2216–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoshida Y., et al. 2002. E3 ubiquitin ligase that recognizes sugar chains. Nature 418:438–442 [DOI] [PubMed] [Google Scholar]
- 50. Zhang X, et al. 2008. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 10:1062–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]