Abstract
The tyrosine kinase c-Abl is required for full activation of T cells, while its role in T-cell differentiation has not been characterized. We report that c-Abl deficiency skews CD4+ T cells to type 2 helper T cell (Th2) differentiation, and c-Abl−/− mice are more susceptible to allergic lung inflammation. c-Abl interacts with and phosphorylates T-bet, a Th1 lineage transcription factor. c-Abl-mediated phosphorylation enhances the transcriptional activation of T-bet. Interestingly, three tyrosine residues within the T-bet DNA-binding domain are the predominant sites of phosphorylation by c-Abl. Mutation of these tyrosine residues inhibits the promoter DNA-binding activity of T-bet. c-Abl regulates Th cell differentiation in a T-bet-dependent manner because genetic deletion of T-bet in CD4+ T cells abolishes c-Abl-deficiency-mediated enhancement of Th2 differentiation. Reintroduction of T-bet-null CD4+ T cells with wild-type T-bet, but not its tyrosine mutant, rescues gamma interferon (IFN-γ) production and inhibits Th2 cytokine production. Therefore, c-Abl catalyzes tyrosine phosphorylation of the DNA-binding domain of T-bet to regulate CD4+ T cell differentiation.
INTRODUCTION
Naïve CD4+ T cell differentiation is initiated when the T cell receptor (TCR) encounters its cognate antigen bound to major histocompatibility complex (MHC) class II molecules on an antigen-presenting cell (APC) (6). The stimulus delivered via TCR, in conjunction with activation of costimulatory pathways, is essential for a naïve helper T (Th) cell to progress along the differentiation pathway. Th1 cells are typically generated by activating naïve T cells via TCR cross-linking in the presence of interleukin-12 (IL-12), while Th2 cells are typically generated by activating naïve T cells through TCR cross-linking in the presence of exogenous IL-4 (7, 12, 13, 30). Considerable progress has been made in the study of the molecular mechanisms of Th1/Th2 differentiation and in particular the identification of Th1- or Th2-specific/lineage transcription factors. Activation of T-bet and STAT4 are involved in Th1 differentiation (14, 26), while GATA-3, c-MAF, STAT6, and JunB mediate Th2 differentiation (20, 28, 29). However, how TCR and the costimulatory signaling regulate CD4 T cell differentiation remains largely unknown.
T-bet, also known as Tbx21, belongs to the T-box family of transcription factors (26). T-bet is the only known T-box gene specifically expressed in the lymphoid system, with its expression largely restricted to the spleen, thymus, and lymph nodes. In CD4 T cells, T-bet is rapidly and specifically induced in Th1 but not Th2 cells (26). Gene-targeted mutation of T-bet in mice results in impaired Th1 but elevated Th2 differentiation, and T-bet−/− mice spontaneously develop allergic lung inflammation (9, 13). Although the exact molecular mechanisms remain unclear, T-bet probably regulates Th1 cell differentiation by directly initiating gamma interferon (IFN-γ) transcription and by suppressing Th2-specific transcription factor GATA-3 (23, 27). It has been reported that the tyrosine phosphorylation of T-bet, mediated by the Tec family kinase, ITK, plays important roles in Th1 differentiation. However, even in the ITK-knockout T cells, the tyrosine phosphorylation of T-bet is still detectable (13), suggesting that additional tyrosine kinases might be involved in catalyzing T-bet phosphorylation to regulate T-bet functions.
c-Abl kinase has been known as a regulator of immune response for many years. Targeted mutation of the c-abl gene in mice leads to reduced B-cell population and slightly impaired activation of both T and B cells. c-Abl-deficient mice die at weaning age due to bacterial lung infections (25). It has been reported that Abl kinases, including c-Abl and Arg (also called Abl2), regulate T-cell activation by directly phosphorylating Zap70 and the transmembrane adaptor linker for activation of T cells (LAT) (31). More recently, we have demonstrated a role of c-Abl tyrosine kinase in T cell activation. Tyrosine phosphorylation of the transcription factor c-Jun by c-Abl protects c-Jun from Itch-mediated ubiquitination and degradation. Therefore, loss of c-Abl expression results in elevated c-Jun degradation and, therefore, reduced T cell activation (10).
In the current study, we report that loss of c-Abl functions skews CD4+ T cells to Th2 differentiation. c-Abl regulates T cell differentiation by phosphorylating the Th1-lineage-specific transcription factor, T-bet, upon TCR/CD28 stimulation. Thus, c-Abl kinase-mediated phosphorylation appears to directly link TCR/CD28 signaling to the decision of T cell differentiation.
MATERIALS AND METHODS
Cell line, Abs, and reagents.
Human embryonic kidney (HEK) 293 cells were maintained in Dulbecco's modification of Eagle's medium (DMEM) (Invitrogen Life Technologies, San Diego, CA), and Jurkat cells were cultured with RPMI 1640. Media were supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 200 μg/ml streptomycin, and 0.25 μg/ml amphotericin B. Polyclonal antibodies (Abs) against T-bet and c-Abl were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CD3 and anti-CD28 were from eBioscience (San Diego, CA). The antiactin and anti-Flag antibodies were from Sigma (St. Louis, MO). Antiphosphotyrosine antibody (4G10) was purchased from Upstate (Lake Placid, NY).
Plasmids.
Luciferase expression plasmids for Flag–T-bet, c-Abl and its mutants, and 3XT-bet were generous gifts from Reiji Kannagi (Aichi Cancer Center, Nagoya, Japan) (5), Giulio Superti-Furga (European Molecular Biology Laboratory, Heidelberg, Germany) (2), and Richard M. Gronostajski (State University of New York at Buffalo) (3). IFN-γ luciferase plasmids were used as reported previously (15). The point mutations of T-bet were generated using a PCR-based point mutation kit according to the protocol provided by the manufacturers. All the newly generated plasmids in this study were verified by DNA sequencing. T-bet and its YF mutants were subcloned into retroviral vector MSCV-Thy1.1 for gene transfection into primary CD4 T cells.
Mice.
c-Abl+/− mice were used as reported previously (25). c-Abl-null mice backcrossed to a C57/B6 genetic background for 4 to 5 generations were used in this study since further backcrossing led to postbirth lethality. T-bet-null mice on a C57/BL6 genetic background were purchased from the Jackson Laboratory. Homozygous mice were produced by breeding heterozygous pairs, and their genotypes were confirmed by PCR. All mice used in this study were maintained and used at the Northwestern University mouse facility under pathogen-free conditions according to institutional guidelines and animal study proposals approved by the institutional animal care and use committees.
Isolation of mouse naïve CD4+ T cells, cell proliferation assay, and cytokine production.
T cells were isolated from the lymph nodes and spleens of 4- to 6-week-old c-Abl−/− and c-Abl+/− mice. CD4+ CD25− CD44low CD62hi naïve T cells were purified using a naïve CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA). These cells were maintained in RPMI medium supplemented with 10% FBS, 100 U/ml penicillin, 200 μg/ml streptomycin, and 0.25 μg/ml amphotericin and stimulated with anti-CD3 plus anti-CD28 (1 μg/ml each). Upon stimulation with anti-CD3 or anti-CD3 plus anti-CD28 antibodies, the proliferation of stimulated cells was determined by [3H]thymidine incorporation assay. The cytokine production levels in the culture supernatants of cultured cells were examined by enzyme-linked immunosorbent assay (ELISA) as described previously (19). For intracellular cytokine staining, activated or polarized T cells were restimulated with phorbol myristate acetate (PMA; 10 ng/ml) plus ionomycin (1 μM) in the presence of 10 mg/ml brefeldin A for 4 h. Cells were fixed and permeabilized, and intracellular staining with anti-IFN-γ–fluorescein isothiocyanate (FITC) and IL-4–phycoerythrin (PE) was performed as described previously (15).
Assessment of promoter activities.
Jurkat cells in 12-well plates were transfected with pRL-TK (Promega, Madison, WI) and IFN-γ or IL-4 luciferase plasmids, along with various expression plasmids (c-Abl, c-Abl mutants, T-bet, and T-bet mutants) as indicated. The pRL-TK plasmid contains the Renilla reniformis (sea pansy) luciferase gene under the transcriptional control of the herpesvirus thymidine kinase promoter and constitutively expresses low levels of renillar luciferase. Therefore, it can be used as an ideal control. Transfected cells were lysed, and the luciferase activities in the cell lysates were analyzed using a Dual Luciferase Reporter assay kit (Promega, Madison, WI). Luciferase activity was measured using a luminometer (Turner BioSystems Inc., Sunnyvale, CA) and expressed in relative light units (RLUs).
Transfection, immunoprecipitation, and Western blotting.
Transient transfections of plasmid DNA into HEK 293 cells were performed by using Lipofectamine 2000 (Invitrogen Life Technologies, San Diego, CA) according to the manufacturer's instructions, with 60-mm dishes and 2 to 5 μg of total DNA per transfection. Transfected cells were pelleted and resuspended in 1× Nonidet P-40 lysis buffer (1% Nonidet P-40, 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 5 mM NaPiP, 2 mM Na3VO4, and 10 μg/ml each of aprotinin and leupeptin). For immunoprecipitation, lysates (∼1 × 107 cells) were mixed with antibodies (1 μg) for 2 h, followed by the addition of 30 μl of protein G-Sepharose beads (Santa Cruz Biotechnology) for an additional 2 h at 4°C. Immunoprecipitates were washed four times with Nonidet P-40 lysis buffer and boiled in 20 μl of 2× Laemmli buffer. Samples were subjected to 8% or 10% SDS-polyacrylamide gel electrophoresis analysis and electrotransferred onto polyvinylidene difluoride membranes (Millipore). Membranes were probed with the indicated primary antibodies (usually 1 μg/ml), followed by horseradish peroxidase-conjugated secondary antibodies. Membranes were then washed and visualized with an enhanced chemiluminescence detection system (ECL; Amersham Pharmacia Biotech). When necessary, membranes were stripped by incubation in stripping buffer (Bio-Rad, Hercules, CA), washed, and then reprobed with other antibodies as indicated.
In vitro kinase assay.
In vitro phosphorylation of T-bet by c-Abl tyrosine kinase was determined using a kinase assay kit (Clontech, Mountain View, CA) according to the manufacturer's procedure. Briefly, c-Abl or its mutant plasmids were transfected into HEK 293 cells, and their proteins expressed in the transfected cells were immunoprecipitated with antihemagglutinin (anti-HA) antibody-conjugated protein Sepharose G beads. The antibody-kinase complexes were used as the kinase for T-bet. Five micrograms of purified glutathione S-transferase (GST)–T-bet or GST–T-bet/YF fusion proteins were incubated with Sepharose-bound c-Abl or its mutant proteins for 30 min in the presence of 2 μCi [32P]ATP. Samples were then subjected to SDS-PAGE analysis; gels were dried and exposed to X-ray films. The parallel prepared samples in the absence of [32P]ATP were used for Western blotting as controls.
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay was performed as we recently reported (28). Briefly, primary T cells from c-Abl−/− and c-Abl+/+ mice were stimulated with anti-CD3 plus anti-CD28 for 24 h, cross-linked with 1% formaldehyde, and lysed with SDS lysis buffer. Cell lysates were sonicated, and 10% of cell lysate was removed and used to determine the total amount of target DNA in input. Remaining cell lysates were diluted in ChIP dilution buffer. Immunoprecipitation was performed with 4 μg of polyclonal anti-T-bet antibodies at 4°C overnight. Immune complexes were then mixed with a salmon sperm DNA-protein agarose (50% slurry) at 4°C for 1 h. After immunoprecipitates were washed sequentially with low-salt buffer, high-salt buffer, LiCl wash buffer, and Tris-EDTA (TE) buffer, DNA-protein complexes were eluted with elution buffer and cross-linking was reversed. Genomic DNA was extracted using phenol-chloroform, and ethanol-precipitated DNA was resuspended in TE buffer. PCR was performed with specific primers for mouse IFN-γ promoter. PCR primer sequences are 5-CCCCTGAACCTGAAACATAAAA-3 and 5-GCATGCAAGCTCGCGTAAGA-3.
Oligonucleotide pulldown assay.
Nuclear extract (20 μg) from c-Abl+/+ and c-Abl−/− T cells was incubated with streptavidin-coated agarose beads preincubated with biotinylated double-strand oligonucleotide for 30 min at 4°C on a rotator in 1× binding buffer (30 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 5% glycerol, 1 mg/ml bovine serum albumin [BSA], and 1 mM dithiothreitol [DTT]) with 1 μg poly(dI-dC). Beads were then washed in 1× binding buffer 5 times prior to SDS-PAGE and immunoblotted for T-bet.
Induction and clinical assessment of allergic lung inflammation in mice.
A standard protocol for induction of pulmonary inflammation via antigen sensitization and aerosol challenge was used as reported previously (26). Briefly, mice were sensitized by intraperitoneal injection of 200 μg chicken ovalbumin (OVA) protein adsorbed to 2 mg aluminum hydroxide (Alum) in phosphate-buffered saline (PBS) on day 0. Unsensitized (naïve) mice receiving 2 mg Alum in PBS were used as controls. On day 20 or later, mice were aerosol challenged via the airways with 5% OVA for 30 min, once a day for three consecutive days, by ultrasonic nebulization. Mice were then euthanized; their lung tissues were collected for histological analysis. To analyze lung inflammation in immunized mice, lung tissues were collected and frozen in optimal cutting temperature (OCT) medium. Lung sections at 5 μm were stained with hematoxylin and eosin (H&E). In addition, the bronchoalveolar lavage (BAL) fluid samples were collected by lavaging the airways (bronchial) and air sacs (alveolar) with saline (2 ml each). Total cell numbers were counted, followed by analysis by flow cytometry. The numbers of eosinophils, monocytes, and lymphocytes were calculated.
Retrovirus production and transduction.
Recombinant retrovirus was produced by transient transfection of the ectopic packaging cell line Platinum-E (Plat-E) (18, 22), using Lipofectamine 2000 transfection reagent (Invitrogen, Life Technologies, San Diego, CA). Viral supernatants were harvested 48 and 72 h after transfection. Primary CD4+ CD25− T cells were cultured with anti-CD3 plus anti-CD28 for 24 h, and 1 × 106 cells/well in 6-well plates were centrifuged with 2 ml of the viral supernatants at 1,200 × g at 33°C for 60 min. After incubation at 33°C for 6 h, cells were cultured with complete RPMI 1640 for the indicated periods before experimentation.
RESULTS
c-Abl deficiency enhances Th2 but impairs Th1 cytokine production.
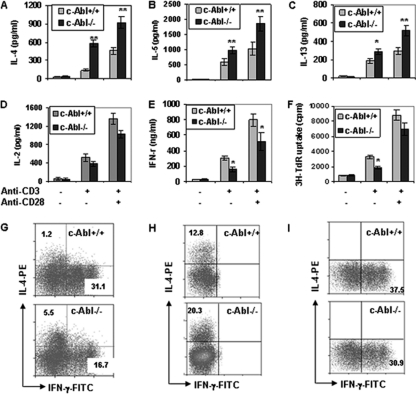
During the analysis of cytokine production profiles by c-Abl−/− T cells, we observed significant increases in the production of Th2 cytokines, including IL-4, IL-5, and IL-13, by naïve CD4+ T cells from c-Abl−/− mice compared to those from c-Abl+/+ mice (Fig. 1A to C). In contrast, the production of a Th1 cytokine, IFN-γ, by c-Abl−/− T cells was reduced (Fig. 1E). Consistent with previous studies (10, 25), the production of IL-2 and cell proliferation of c-Abl−/− T cells were slightly decreased compared to those of c-Abl+/+ T cells (Fig. 1D and F). These results indicate that the loss of c-Abl functions in CD4+ T cells upregulates Th2 cytokine production but suppresses Th1 cytokine production.
Fig. 1.
Cytokine production and proliferation of c-Abl−/− and wild-type T cells. (A to E) Naïve CD4+ CD25− CD44lo CD62Lhi T cells from c-Abl−/− and wild-type mice were isolated and stimulated with or without anti-CD3 (1 μg/ml) or anti-CD3 plus anti-CD28 (1 μg/ml) for 4 days. The production of IL-4 (A), IL-5 (B), IL-13 (C), IL-2 (D), and IFN-γ (E) was determined by ELISA. (F) The proliferation of c-Abl−/− and wild-type naïve T cells upon TCR/CD28 stimulation was examined by [3H]thymidine incorporation assay. Error bars represent data from three independent experiments. Student's t test was used for statistical analysis. *, P < 0.05; **, P < 0.01. (G) Naïve T cells from c-Abl+/+ and c-Abl−/− mice were stimulated with soluble anti-CD3 plus anti-CD28 for 5 days followed by an additional 4-hour stimulation with precoated anti-CD3 in the presence of brefeldin A (10 μg/ml). Intracellular staining for IL-4 and IFN-γ was performed, and cells were analyzed by fluorescence-activated cell sorting. (H and I) Naïve CD4+ T cells were cultured for 5 days under Th2 (1 μg/ml anti-CD3, 1 μg/ml anti-CD28, 5 μg/ml anti-IFN-γ, 5 μg/ml anti-IL-12, and 1 U/ml of IL-2) or Th1 (1 μg/ml anti-CD3, 1 μg/ml anti-CD28, 5 μg/ml anti-IL-4, and 1 U/ml of IL-2) polarization conditions, respectively. Cells were analyzed by intracellular staining and flow cytometry.
To further determine the regulatory roles of c-Abl in Th1/Th2 differentiation, we examined the percentage of IL-4- versus IFN-γ-containing CD4+ T cells from c-Abl−/− and wild-type mice in an in vitro culture system as previously reported (28). After 5 days of stimulation with anti-CD3 plus anti-CD28, the de novo synthesis of IFN-γ and IL-4 in naïve CD4+ T cells was examined by intracellular staining. Similar to previous studies, CD4 T cells were predominantly skewed to IFN-γ-producing Th1 cells (31%) with a small percentage of IL-4-producing Th2 cells (1.2%) when stimulated under nonpolarization conditions with anti-CD3 plus anti-CD28 (4, 8). In contrast, c-Abl−/− T cells stimulated under the same condition produced more IL-4+ cells (5.5%), while the percentage of IFN-γ+ cells was decreased (17%) (Fig. 1G). We then examined cell differentiation of naïve CD4+ T cells cultured under Th1 or Th2 polarization conditions. We cultured T cells under Th2 conditions and observed the enhanced generation of IL-4+ Th2 cells derived from c-Abl−/− T cells compared to wild-type T cells (Fig. 1H). In addition, when cells were cultured under Th1 conditions, the percentage of IFN-γ+ Th1 cells from c-Abl−/− T cells was lower than that of wild-type T cells (Fig. 1I). Therefore, c-Abl deficiency skews CD4+ T-cell differentiation toward Th2. However, we also noticed that the changes in cytokine production caused by c-Abl deficiency under Th1- and Th2-skewing conditions were rather modest, implying that a stronger polarization condition can partially rescue the phenotypes.
c-Abl catalyzes T-bet tyrosine phosphorylation.
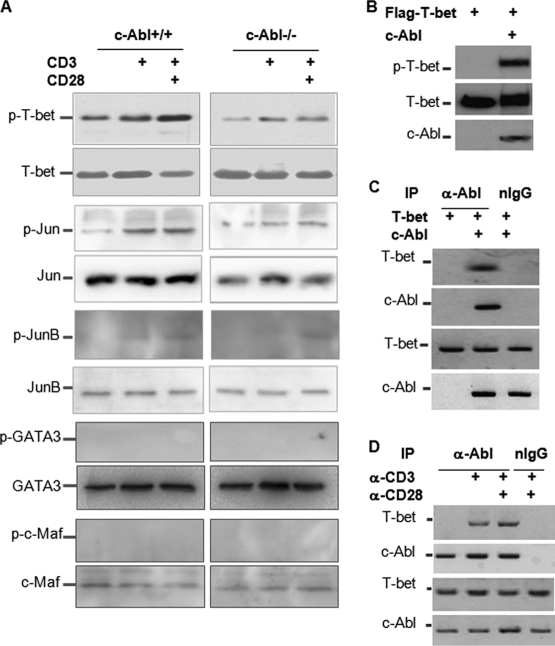
To investigate the molecular mechanisms of c-Abl tyrosine kinase in Th1/Th2 differentiation, we determined whether c-Abl deficiency affects tyrosine phosphorylation of transcription factors that are involved in Th1/Th2 differentiation. Upon TCR and CD28 stimulation, the tyrosine phosphorylation of T-bet, but not the total T-bet protein expression levels, was significantly reduced but not abolished in c-Abl−/− T cells, suggesting that c-Abl is a tyrosine kinase of T-bet (Fig. 2A). In contrast, the tyrosine phosphorylation of GATA-3 and c-Maf was not detected by Western blotting in polarized Th2 cells upon restimulation with anti-CD3 or anti-CD3 plus anti-CD28 (Fig. 2A). Consistent with our previous studies, both the total protein and the phosphorylated c-Jun levels were reduced in c-Abl-null T cells (10). We also detected a slightly reduced JunB protein expression level in c-Abl−/− T cells, but JunB phosphorylation was detected only at a background level (Fig. 2A). Given the fact that T-bet deficiency leads to impaired Th1 but elevated Th2 cytokine production by CD4 T cells, our data suggest that the reduced T-bet phosphorylation is likely responsible for the increased Th2 and impaired Th1 cytokine production by c-Abl-null T cells.
Fig. 2.
c-Abl is a tyrosine kinase of T-bet in T cells. (A) Unpolarized (for T-bet, c-Jun, and JunB) or Th2-polarized (for c-Maf and GATA-3) CD4+ T cells from c-Abl−/− and wild-type mice were stimulated with anti-CD3 or anti-CD3 plus anti-CD28. Each transcription factor was immunoprecipitated using antibodies as indicated. The immunoprecipitates were analyzed by Western blotting with the 4G10 anti-p-Y antibody. The same membrane was reprobed with antibodies specific to each transcription factor as controls. (B) Flag–T-bet expression plasmids were cotransfected with or without c-Abl. T-bet protein in the lysates of transfected cells was immunoprecipitated with anti-T-bet antibody. The tyrosine phosphorylation of T-bet was detected with anti-p-Y antibody (top panel). The same membrane was reprobed with anti-Flag antibody (middle panel). The expression levels of c-Abl in the whole-cell lysates were determined with anti-c-Abl antibody (bottom panel). (C) T-bet expression plasmid was cotransfected with or without c-Abl plasmid into HEK 293 cells. c-Abl protein in the lysates of transfected cells was immunoprecipitated with anti-c-Abl antibody or with normal rabbit immunoglobulin G (nIgG) as a negative control. The interaction of T-bet with c-Abl was detected with anti-T-bet antibody (top panel). The same membrane was reprobed with anti-c-Abl antibody (second panel). The protein expression levels of T-bet and c-Abl in the whole-cell lysates were examined by Western blotting as controls (bottom two panels). (D) Primary T cells were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 for 2 h. The interaction of c-Abl with T-bet was determined as described for panel C.
We then sought to determine whether c-Abl catalyzes T-bet tyrosine phosphorylation. T-bet expression plasmids were cotransfected into HEK 293 cells with or without c-Abl. T-bet protein in the cell lysates of transfected cells was immunoprecipitated with anti-T-bet antibody. The tyrosine phosphorylation of T-bet was detected with antiphosphotyrosine antibody. When c-Abl was cotransfected, a strong band was detected in the anti-T-bet immunoprecipitates, indicating that c-Abl induces T-bet tyrosine phosphorylation (Fig. 2B). Since a tyrosine kinase often binds to its substrates, we then tested whether c-Abl interacts with T-bet. T-bet proteins were detected in anti-c-Abl immunoprecipitates when c-Abl expression plasmids were cotransfected but not detected in the nontransfected control or in the control immunoprecipitated with normal rabbit immunoglobulin (rIgG), indicating that c-Abl interacts with T-bet in transiently transfected HEK 293 cells (Fig. 2C). In addition, we determined whether c-Abl interacts with T-bet in T cells upon stimulation with anti-CD3 or anti-CD3 plus anti-CD28. The interaction of c-Abl with T-bet was not detected in unstimulated mouse primary CD4+ T cells. Stimulation with anti-CD3 for 2 h significantly enhanced the interaction of c-Abl with T-bet (Fig. 2D), suggesting that c-Abl interacts with T-bet in T cells and that TCR-mediated activation signals enhance their interaction. We reproducibly detected that TCR stimulation alone appears to be sufficient to induce c-Abl/T-bet interaction, while a full-scale T-bet phosphorylation could be achieved only with TCR and CD28 stimulation (Fig. 2A), suggesting an involvement of additional factors during this process.
c-Abl catalyzes phosphorylation of the tyrosine residues in T-bet DNA-binding domain.
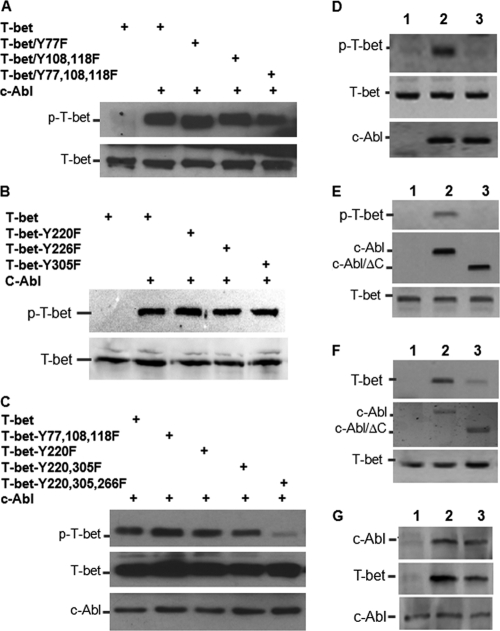
To further determine the molecular mechanisms underlying c-Abl-mediated tyrosine phosphorylation of T-bet in CD4+ T cell differentiation, we attempted to pinpoint the tyrosine residues in T-bet that can be phosphorylated by c-Abl. Using a Scansite program, three conserved c-Abl tyrosine residues (Y77, Y108, and Y118), which can be potentially phosphorylated by Src kinases, were identified (13). However, mutations of any of these three tyrosines did not affect c-Abl-mediated T-bet tyrosine phosphorylation, nor did mutation of all three tyrosine residues to phenylalanine (Y to F) (Fig. 3A). We then reanalyzed the T-bet amino acid sequence using an ELM program for functional sites of proteins (http://elm.eu.org.) and found three tyrosine sites, Y220, Y266, and Y305, which can be potentially phosphorylated by Src family kinases. Unexpectedly, all three tyrosine residues are located in the T-box DNA-binding domain of T-bet. Replacement of any one or two of these tyrosine residues with phenylalanine had little effect on T-bet phosphorylation (Fig. 3B and C). However, when all three tyrosines were mutated, the c-Abl-mediated phosphorylation of T-bet was significantly reduced (Fig. 3C), indicating that these three tyrosine residues in T-bet are the major sites of phosphorylation by c-Abl kinase in T cells.
Fig. 3.
Identification of the tyrosine residues in T-bet that are phosphorylated by c-Abl. (A to C) Flag–T-bet or each of its YF mutants was cotransfected with or without c-Abl. T-bet protein in the lysates of transfected cells was immunoprecipitated with anti-T-bet antibody. The tyrosine phosphorylation of T-bet was detected with anti-p-Y antibody (top panels). The total T-bet protein levels were determined by Western blotting (middle panel). The same membrane was reprobed with anti-Flag antibody (bottom panels). (D) Two micrograms each of GST–T-bet (lanes 1 and 2) or GST–T-bet/Y220/266/305F (lane 3) proteins was incubated without (lane 1) or with (lanes 2 and 3) c-Abl proteins immunoprecipitated from transiently transfected HEK 293 cells together with in vitro kinase assay reagents, including 2 μCi [32P]ATP, for 30 min at 37°C. Samples were subjected to SDS-PAGE analysis. Gels were dried, and the phosphorylation of GST–T-bet was detected. The protein levels of T-bet (middle panel) and c-Abl and c-Abl/ΔC mutant (bottom panel) were tested by Western blotting as controls. (E) T-bet expression plasmids were cotransfected without (lane 1) or with (lane 2) c-Abl or with c-Abl/ΔC mutant (lane 3) into HEK 293 cells. T-bet phosphorylation was analyzed as described for panels A to C (top panel). The expression levels of c-Abl, c-Abl mutant, and T-bet in the whole-cell lysates were analyzed as controls (middle and bottom panels). (F) HEK 293 cells were transfected with plasmid DNA as indicated in panel E. The interaction of T-bet with c-Abl and c-Abl/ΔC mutant was analyzed by coimmunoprecipitation with anti-c-Abl and Western blotting with anti-T-bet antibody (top panel). The same membrane was reprobed with anti-c-Abl antibody (middle panel). T-bet protein expression in the whole-cell lysates was analyzed as control (bottom panel). (G) Flag–T-bet expression plasmid was cotransfected with c-Abl or with c-Abl/R171L mutant. T-bet in the lysates was immunoprecipitated with anti-Flag antibody, and the bound c-Abl proteins were detected with anti-c-Abl antibody (top panel). The same membrane was reblotted with anti-T-bet (middle panel). The expression levels of c-Abl and c-Abl/R171L mutant in the whole-cell lysates were determined by Western blotting as a loading control (bottom panel).
To further determine whether c-Abl-mediated T-bet tyrosine phosphorylation is a direct event, we performed an in vitro kinase assay using GST-fused T-bet or its Y220/266/305F mutant proteins as substrates. As shown in Fig. 3D, GST–T-bet, but not its YF mutant, was phosphorylated by adding c-Abl kinase immunoprecipitated from transiently transfected HEK 293 cells, suggesting that c-Abl appears to directly catalyze T-bet phosphorylation and that the tyrosine residues 220, 266, and 305 of T-bet are likely the predominant phosphorylation sites. CD4+ T cells from the c-Abl mutant mice still carry a truncated c-Abl protein with an intact kinase domain (25); it is possible that this truncated mutant form of c-Abl can still catalyze T-bet phosphorylation, as T-bet tyrosine phosphorylation was detectable in c-Abl mutant T cells, despite a reduction compared to that of wild-type T cells. However, deletion of the C terminus of c-Abl (c-Abl/ΔC) completely abolished its ability to catalyze T-bet phosphorylation (Fig. 3E). This is likely due to the C terminus of c-Abl being required for its interaction with T-bet, because deletion of the C terminus significantly inhibited c-Abl interaction with T-bet (Fig. 3F). Since a weak interaction of c-Abl/ΔC with T-bet is still detected, we reasoned that the N-terminal SH2 domain, which mediates protein-protein interactions by recognizing phosphotyrosine-based motifs, is also involved in its interaction with T-bet. However, a point mutation that disrupted c-Abl SH2 domain structures, R171L, did not affect c-Abl/T-bet interaction (Fig. 3G). Collectively, our findings indicate that c-Abl is a tyrosine kinase of T-bet in T cells.
c-Abl activates T-bet-driven IFN-γ luciferase activation.
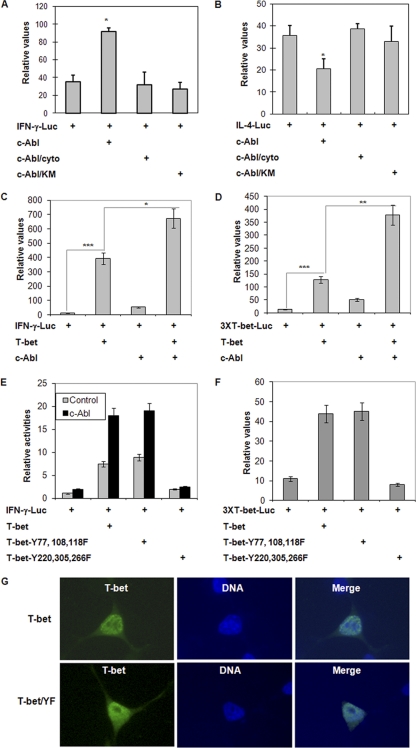
As a tyrosine kinase of T-bet, c-Abl may regulate Th1/Th2 differentiation by modulating T-bet transcriptional activation through catalyzing the phosphorylation of tyrosine residues in T-bet. Therefore, we determined the effects of c-Abl kinase on the reporter activities of IFN-γ and IL-4, respectively. The IFN-γ or IL-4 luciferase plasmid DNA was cotransfected into Jurkat T cells with c-Abl or with each of its mutants. The luciferase activity in the lysates of transfected cells was determined. Expression of c-Abl, but not its kinase-negative mutant (c-Abl/KM), significantly enhanced IFN-γ luciferase activity, suggesting that c-Abl is involved in upregulating IFN-γ transcription. Nuclear translocation of c-Abl seems to be required to promote IFN-γ luciferase activity, because mutations of the nuclear localization signals of c-Abl abolished its ability to enhance IFN-γ reporter (Fig. 4A). On the other hand, c-Abl slightly inhibited IL-4 luciferase activity, but both the kinase-dead and the nuclear localization mutations of c-Abl failed to suppress IL-4 luciferase activity (Fig. 4B). These results suggest that c-Abl tyrosine kinase could be a positive regulator of Th1 differentiation and a negative regulator of Th2 differentiation.
Fig. 4.
c-Abl-mediated phosphorylation of T-bet regulates its transcriptional activity. (A and B) IFN-γ (Α) or IL-4 (B) luciferase plasmids were cotransfected with c-Abl or with each of its mutants. (C and D) IFN-γ (C) or 3XT-bet (D) luciferase expression plasmids were cotransfected with T-bet or c-Abl or both. Student's t test was used for statistical analysis. *, P < 0.05; **, P < 0.01. (E and F) IFN-γ (E) or 3XT-bet (F) luciferase reporter plasmids were cotransfected with T-bet or each of the T-bet YF mutants in the absence or presence of c-Abl plasmids into Jurkat cells. Two days after transfection, the luciferase activities in the transfected cells were determined. Error bars represent data from three independent experiments. (G) The subcellular localization of T-bet and its YF mutant. Expression plasmids for T-bet or its Y220/266/305F mutant (T-bet/YF) were transfected to 293T cells. Transfected cells were fixed and immunostained with anti-Flag antibody for detecting the protein expression of T-bet or its Y220/266/305F mutant. Nucleic DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). Cells were visualized with fluorescence microscopy. Representative images are shown.
T-bet has been identified as a lineage-specific factor that drives Th1 cytokine production and suppresses Th2 differentiation (13, 27). Together with the fact that c-Abl catalyzes T-bet phosphorylation, we asked whether c-Abl enhances IFN-γ and suppresses IL-4 reporters via T-bet. Expression of T-bet significantly promoted IFN-γ luciferase activity, which was further enhanced by c-Abl coexpression (Fig. 4C). In addition to T-bet, the IFN-γ promoter contains specific binding sites for other Th1 transcription factors, such as STAT4. We then used a reporter plasmid that contains only three copies of T-bet-binding elements (3). As shown in Fig. 4D, the increase in T-bet-driven luciferase activity by c-Abl was even more robust when this 3XT-bet luciferase plasmid was used, suggesting that c-Abl regulates T-bet transcriptional activity in IFN-γ expression.
Mutation of tyrosines 220, 266, and 305 of T-bet completely abolished T-bet transcriptional activation as tested by IFN-γ reporter assay. In contrast, replacing the tyrosine residues 77, 108, and 118 in the N terminus of T-bet had no effect on its reporter activity (Fig. 4E and F). Coexpression of c-Abl further enhanced T-bet transcription activity, while this enhancement was abolished when these three tyrosine residues were replaced by phenylalanines (Fig. 4E and F). With the concern that mutation of these three tyrosine residues in the T-bet DNA-binding domain may affect its nuclear localization, we compared the subcellular distributions of T-bet with this mutant. As shown in Fig. 4G, the subcellular distribution patterns of T-bet and the T-bet/Y220/266/305F mutant were indistinguishable from those in HEK 293 cells. Therefore, c-Abl promotes T-bet transcriptional activity by phosphorylating T-bet at these three tyrosine residues in the T-bet DNA-binding domain, suggesting that c-Abl may facilitate T-bet binding to IFN-γ promoter DNA.
c-Abl-mediated tyrosine phosphorylation regulates the promoter DNA-binding activity of T-bet.
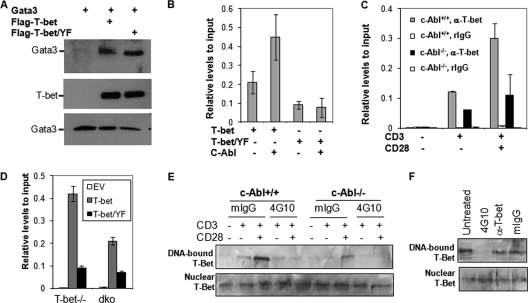
Phosphorylation of tyrosine residue 405 in the C terminus of T-bet by Tec kinase allows T-bet to recruit GATA-3. Thus, T-bet suppresses the binding of GATA-3 with IL-4 promoter to inhibit Th2 differentiation (13). c-Abl appears to regulate Th1/Th2 differentiation via a different mechanism, because overexpression of c-Abl does not affect T-bet/GATA-3 interaction (Fig. 5A). Since the tyrosine residues phosphorylated by c-Abl are in the DNA-binding domain of T-bet, this tyrosine phosphorylation event may affect the binding of T-bet to IFN-γ promoter. Indeed, c-Abl overexpression dramatically enhanced the binding of T-bet with IFN-γ promoter DNA in Jurkat T cells as measured by ChIP assay. In support of this, mutation of these three tyrosine residues, which reduced c-Abl-mediated phosphorylation, dramatically impaired T-bet-binding to IFN-γ promoter even in the presence of c-Abl (Fig. 5B).
Fig. 5.
c-Abl-mediated tyrosine phosphorylation regulates T-bet DNA binding. (A) c-Abl-mediated phosphorylation does not affect T-bet interaction with GATA-3. T-bet, GATA-3, and c-Abl expression plasmids were cotransfected into HEK 293 cells as indicated. The interaction of T-bet with GATA-3 was analyzed by immunoprecipitation of T-bet and Western blotting detection of GATA-3 (top panel). The same membrane was reprobed with anti-T-bet antibody (middle panel). The expression of GATA-3 in the whole-cell lysates was detected as controls (bottom panel). (B) Jurkat T cells were transfected with expression plasmids for Flag-tagged T-bet or its Y220/226/305F mutant (T-bet/YF) in the presence or absence of c-Abl. The binding of T-bet and its YF mutant to IFN-γ promoter was analyzed by ChIP assay with anti-Flag antibody followed by real-time PCR analysis. (C) Primary CD4+ T cells from c-Abl+/+ and c-Abl−/− mice were stimulated with anti-CD3 plus anti-CD28 for 24 h. Stimulated cells were subjected to ChIP assay using anti-T-bet antibody, and the binding of T-bet to IFN-γ promoter DNA was analyzed by real-time PCR. ChIP analysis with normal rabbit IgG (rIgG) was performed as a negative control. (D) Primary T cells from T-bet−/− and T-bet/c-Abl double-knockout (dko) mice were infected with retrovirus that carries T-bet or Tbet/Y220/266/305F (T-bet/YF). Infected cells were subjected to ChIP analysis with anti-T-bet antibody. Error bars represent data from three independent experiments (average ± standard deviation). EV, empty vector. (E) Nuclear extracts from total T cells from c-Abl+/+ and c-Abl−/− mice upon TCR/CD28 stimulation were isolated and incubated with 1 μg antiphosphotyrosine antibody (4G10) or with 1 μg control normal mouse IgG (mIgG) for 30 min. An oligonucleotide pulldown assay was performed. The bound T-bet (top panel) as well as T-bet in nucleic extracts (bottom panel) was detected by Western blotting. (F) Nucleic extracts from stimulated wild-type T cells were incubated without (Untreated) or with mIgG, 4G10, or anti-T-bet Abs (1 μg each), and an oligonucleotide pulldown assay was performed as described for panel E.
The fact that loss of c-Abl functions impairs the tyrosine phosphorylation of T-bet in T cells upon TCR/CD28 stimulation implies that T-bet may bind to the IFN-γ promoter insufficiently in c-Abl−/− T cells. ChIP assay revealed that the binding of T-bet to IFN-γ promoter, but not total T-bet protein levels (Fig. 2A), is decreased in c-Abl-null T cells with a 60 to 80% reduction compared to that in wild-type T cells (Fig. 5C). Therefore, T-bet tyrosine phosphorylation by c-Abl appears to enhance the promoter DNA-binding activity of T-bet in T cells upon TCR/CD28 stimulation. Furthermore, we used a retroviral infection approach to reconstitute T-bet-null T cells with T-bet or T-bet Y220/266/305F mutant and compared their promoter binding activities. As expected, the promoter binding activity of T-bet Y220/266/305F mutant was dramatically reduced compared to that of wild-type T-bet. When T-bet/c-Abl double-knockout T cells were reconstituted with T-bet, its binding to IFN-γ promoter was also impaired (Fig. 5D). Taken together, our data collectively suggest that c-Abl-mediated T-bet tyrosine phosphorylation is involved in enhancing T-bet binding to IFN-γ promoter in T cells.
To further investigate the effects of c-Abl-mediated tyrosine phosphorylation on the promoter DNA-binding activity, we used an oligonucleotide pulldown assay. Biotin-labeled double-strand oligonucleotide corresponding to T-bet-binding element pulled down T-bet from the nuclear extracts of c-Abl+/+ T cells upon TCR/CD28 stimulation; the level of T-bet pulldown was significantly reduced from the nuclear extracts of c-Abl−/− T cells, further confirming that loss of c-Abl functions impairs the promoter-binding activity of T-bet in T cells (Fig. 5E). Notably, incubation of nuclear extracts with antiphosphotyrosine antibody (4G10) blocked T-bet/DNA binding. As controls, anti-T-bet antibody and normal mouse IgG did not affect the promoter-binding activity of T-bet (Fig. 5E and F), indicating that 4G10 antibody (Ab) binds to the phosphorylated tyrosine residues in the T-box domain of T-bet and blocks its accessibility to DNA.
c-Abl regulates CD4+ T cell differentiation in a T-bet-dependent manner.
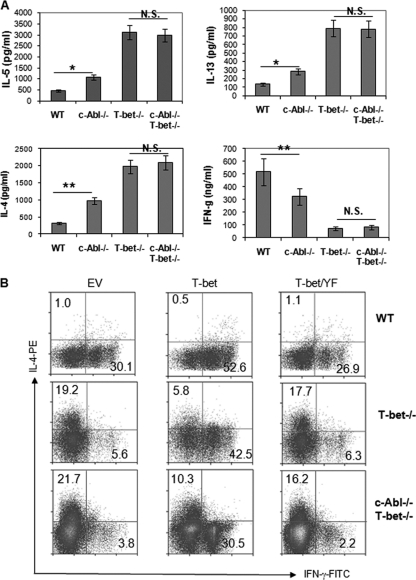
To investigate the physiological functions of c-Abl-mediated phosphorylation of T-bet, we generated c-Abl and T-bet double-knockout mice by breeding c-Abl−/− and T-bet−/− mice and analyzed Th1/Th2 cytokine production by their CD4+ T cells. Consistent with previous studies (23, 26), loss of T-bet functions leads to increased Th2 but impaired Th1 cytokine production by CD4+ T cells (Fig. 6A). Similar to what we found in Fig. 1, increased Th2 cytokine production, but reduced IFN-γ production, by c-Abl−/− T cells was confirmed. Notably, when stimulated with anti-CD3 plus anti-CD28 antibodies, the production of both Th1 and Th2 cytokines was indistinguishable between c-Abl+/+ T-bet−/− and c-Abl−/− T-bet−/− CD4+ T cells, indicating that the regulation of CD4+ T cell differentiation by c-Abl depends on T-bet (Fig. 6A).
Fig. 6.
Th1 and Th2 cytokine production by c-Abl and T-bet double-knockout CD4+ T cells. (A) Naïve CD4 T cells were isolated from wild-type (WT), c-Abl−/−, T-bet−/−, and c-Abl−/− T-bet−/− mice. Cells were cultivated in the presence of anti-CD3, anti-CD28, and IL-2 for 3 days. The production of IL-4, IL-5, IL-13, and IFN-γ was analyzed by ELISA. Error bars represent data from three independent experiments. Student's t test was used for statistical analysis. *, P < 0.05; **, P < 0.01; NS, not significant. (B) Naïve CD4+ T cells isolated from wild-type (WT), T-bet-knockout (T-bet−/−), or c-Abl/T-bet double-knockout (c-Abl−/− T-bet−/−) mice were cultured under Th1 polarization conditions (1 μg/ml anti-CD3, 1 μg/ml anti-CD28, 5 μg/ml anti-IL-4, and 1 U/ml of IL-2) and infected with retrovirus carrying empty vector (EV), T-bet, or the Y220/305/266F mutant of T-bet (T-bet/YF) twice during the first 12- to 24-h culture period. Cells were cultured under Th1 polarization conditions for an additional 3 days. Cells were stained with anti-Thy1.1-Cy5 followed by intracellular staining with IFN-γ–FITC and IL-4–PE. Thy1.1+ cells were gated for the analysis of IFN-γ and IL-4 expressions by flow cytometry.
Since c-Abl also regulates AP-1 transcriptional activity by stabilizing c-Jun (10), a transcription factor involved in T cell development (5), c-Abl deficiency may affect Th cell differentiation during T cell developmental stages. To elucidate the intrinsic functions of c-Abl in peripheral CD4+ T cell differentiation, we tested the ability of T-bet/YF mutant to rescue IFN-γ production by T-bet-null T cells using a retrovirus-based gene transfection approach as described previously (16). As shown in Fig. 6B, ectopic expression of wild-type T-bet rescued IFN-γ and inhibited IL-4 production by T-bet-null CD4+ T cells. However, reintroduction of the T-bet/YF mutant failed to rescue Th1 cytokine production by T-bet−/− CD4 T cells. When T-bet/c-Abl double-knockout CD4+ T cells were reconstituted with T-bet, T-bet's activities in suppressing IL-4 production and promoting IFN-γ production were impaired compared with that in T-bet-null T cells (Fig. 6B). We also noticed that under Th1 polarization conditions, c-Abl-null T cells, while their IFN-γ-producing cells were reduced, did not show any IL-4-producing cells (Fig. 1I). However, reintroduction of T-bet into T-bet-null and c-Abl/T-bet double-knockout T cells failed to completely suppress Th2 cytokine production (Fig. 6B). This is likely because, during a 12-hour preactivation period before retroviral infection, the Th2 cytokine transcription process had been initiated in some of these cells. Collectively, our results indicate that c-Abl functions as a tyrosine kinase of T-bet to promote Th1 cytokine production and that loss of c-Abl functions skews CD4 T cell differentiation toward Th2. In addition, the fact that expression of T-bet still significantly rescues IFN-γ production in the c-Abl/T-bet double-knockout T cells strongly implies that other tyrosine kinases, such as Arg or Abl2, are also involved in catalyzing T-bet tyrosine phosphorylation. In fact, we detected a reduced but not completely abolished tyrosine phosphorylation of T-bet in c-Abl-null T cells (Fig. 2B).
c-Abl−/− mice are more susceptible to allergen stimulation.
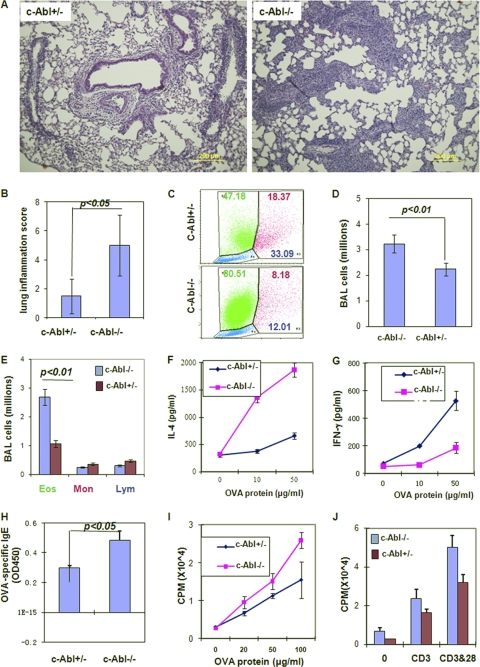
Allergic lung inflammation is associated with Th2 responses to environmental allergens. Thus, c-Abl deficiency may promote allergic lung inflammation due to elevated Th2 cytokine production. We compared the development of experimental allergic inflammation between c-Abl+/− and c-Abl−/− mice. We first analyzed lung inflammation in mice after three aerosol challenges with OVA, which induced severe lung inflammations in both c-Abl+/− and c-Abl−/− mice. Although the average severity score of c-Abl−/− mice was about 30% higher, statistical analysis by Student's t test did not show a significant difference (P = 0.056). After aerosol challenges with OVA once, modest lung inflammation was observed in wild-type mice, whereas c-Abl−/− mice developed severe lung inflammation (P < 0.01) (Fig. 7A and B), suggesting that loss of c-Abl functions in mice increases the susceptibility to allergic lung inflammation. An average 50% increase of total cells in the BAL fluid was detected in c-Abl−/− mice compared to c-Abl+/− mice after one aerosol challenge. The increased BAL fluid cells in c-Abl−/− mice were predominantly eosinophils, while the numbers of monocytes and lymphocytes were indistinguishable between c-Abl−/− and c-Abl+/− mice (Fig. 7C to E). These results indicate that loss of c-Abl functions promotes lung eosinophilic inflammation in mice.
Fig. 7.
The development of allergic lung inflammatory disease in c-Abl−/− and c-Abl+/− mice. c-Abl−/− mice and their heterozygous littermates were immunized with OVA/Alum and aerosol challenged once with OVA. (A) The lung sections were analyzed by H&E staining, and representative images were selected. (B) The inflammation of lung sections was scored. Error bars represent data from five mice per group. Student's t test was used for the statistical analysis. (C) Forward-scatter and side-scatter profiles of cell populations in bronchoalveolar lavage (BAL) fluid. Numbers indicate percentages of eosinophils (green), monocytes (magenta), and lymphocytes (blue). (D and E) Total numbers of BAL fluid cells (D) and the numbers of eosinophils (Eos), monocytes (Mon), and lymphocytes (Lym) (E) were calculated based on their average percentages. (F and G) Total splenocytes from immunized mice were stimulated with different amounts of OVA antigen for 24 h. The production of IL-4 (F) and IFN-γ (G) was analyzed by ELISA. (H) The levels of OVA-specific IgE in the sera from immunized mice were analyzed. (I and J) Total lymphocytes from immunized mice were cultivated with different amounts of OVA antigen for 3 days (I). Purified T cells from these mice were stimulated with anti-CD3 or anti-CD3 plus anti-CD28 for 3 days (J). The proliferation of these T cells was analyzed by [3H]thymidine incorporation.
The elevated lung inflammation in c-Abl−/− mice appears to be a consequence of the increased Th2 cytokine production, because IL-4 production by c-Abl−/− T cells from OVA-immunized mice was significantly increased (Fig. 7F). In contrast, the production of IFN-γ by c-Abl−/− T cells was impaired when stimulated with OVA antigen (Fig. 7G). These results suggest that c-Abl−/− mice have a Th2-biased immune response when challenged with specific antigens. To support this conclusion, we further demonstrated increased levels of antigen-specific IgE, but not other types of immunoglobulins, in the sera of immunized c-Abl−/− mice compared to those in c-Abl+/− mice (Fig. 7H).
c-Abl−/− T cells from immunized mice showed a more vigorous proliferation, with an about 30 to 40% increase compared to c-Abl+/− T cells upon OVA stimulation (Fig. 7I). This increase is probably due to the profound Th2 differentiation in c-Abl−/− mice when immunized with OVA/Alum. Indeed, the proliferation of total T cells from these immunized c-Abl−/− mice as stimulated with anti-CD3/anti-CD28 or PMA/ionomycin was slightly decreased (Fig. 7J). Taken together, the enhanced Th2 differentiation in c-Abl−/− mice is likely a major factor responsible for elevated lung inflammation.
DISCUSSION
Our findings lead us to propose a model for the tyrosine kinase c-Abl in CD4+ T cell differentiation. TCR/CD28 stimulation translocates c-Abl into the nucleus, where c-Abl interacts with and phosphorylates the Th1 lineage transcription factor, T-bet. This phosphorylation event promotes the binding activity of T-bet to IFN-γ promoter for Th1 differentiation. Thus, loss of c-Abl functions results in reduced Th1 and elevated Th2 differentiation. Mice deficient in c-Abl are more susceptible to allergic lung inflammation. Therefore, c-Abl-mediated T-bet tyrosine phosphorylation directly links TCR/CD28 signaling to the decision of Th cell differentiation.
c-Abl deficiency impairs Th1 cytokine production and globally enhances the production of Th2 cytokines, including IL-4, IL-5, and IL-13. This phenotype is similar to T-bet−/− CD4+ T cells (23, 26), providing a possibility that c-Abl kinase may cross talk with T-bet. Indeed, our data showed that c-Abl activates T-bet-driven IFN-γ promoter activity. In addition, genetic deletion of T-bet in CD4+ T cells abolished c-Abl-deficiency-mediated upregulation in Th2 cytokine production. Therefore, c-Abl likely regulates Th1/Th2 differentiation predominantly by targeting T-bet. Gu et al. (11) observed an unaltered IL-4 production by c-Abl/Arg double-knockout T cells upon 3-day in vitro TRC/CD28 stimulation. However, the proliferation of those T cells was reduced by about 90%, and this reduced cell growth was not due to increased apoptosis (11). Therefore, the “unchanged” Th2 cytokine production actually reflects a 4- to 5-fold increase if the actual total cell number is considered. We and others have observed a modest reduction in the proliferation of c-Abl-null T cells with intact Arg functions (10, 25), suggesting a redundant role of c-Abl in T cell proliferation.
c-Abl promotes Th1 differentiation by phosphorylating T-bet. As one of the few transcription factors that can be tyrosine phosphorylated, T-bet has been found as a substrate of the Tec family kinases, particularly ITK. ITK-mediated phosphorylation of T-bet controls the interaction of two opposing transcription factors, T-bet and GATA-3, in the suppression of Th2 lineage development (13). In contrast, c-Abl-mediated T-bet phosphorylation does not affect the interaction of T-bet with GATA-3. Additionally, loss of c-Abl functions in vivo affects CD4+ T cell differentiation in an opposite fashion from ITK. Loss of c-Abl functions skews CD4+ T cells toward Th2, while ITK deficiency impairs Th2 cytokine production in mice (17, 21). This is possibly because ITK has dual roles in regulating Th1/Th2 differentiation. ITK suppresses the transcriptional activity of GATA-3 by phosphorylating T-bet (13); on the other hand, it also promotes Th2 differentiation by negatively regulating T-bet transcription (1, 9). In contrast, c-Abl enhances promoter DNA-binding activities of T-bet without altering T-bet gene expression, since the protein levels of T-bet are indistinguishable between wild-type and c-Abl-null T cells.
An unexpected finding is that c-Abl phosphorylates the tyrosine residues within the T-box domain, which is the DNA-binding domain of T-bet. This phosphorylation appears to play a crucial role in the IFN-γ promoter-binding activity of T-bet. Multiple calcium-dependent phosphorylations of serine/theronine residues within the transcription activator Ets-1 have been found to dynamically change the conformation and the DNA-binding activity of ETS-1 (24). Similarly, c-Abl-mediated T-bet phosphorylation may modulate IFN-γ transcription at the level of DNA binding during Th1 differentiation. Unlike ETS-1, whose phosphorylation sites are located in the unstructured linker region (24), c-Abl phosphorylates the tyrosine residues within the DNA-binding domains of T-bet. This phosphorylation event probably leads to conformational changes of the T-box domain to facilitate the DNA-binding activity of T-bet. Further studies are needed to elucidate the precise mechanisms underlying how this tyrosine phosphorylation event impacts T-bet DNA binding. Our data show that T-bet tyrosine phosphorylation is partially impaired in c-Abl-null T cells, suggesting that other tyrosine kinases, such as ITK and Abl2, are involved in T-bet phosphorylation. Mutation of the tyrosine residues 220, 266, and 305 completely abolished T-bet's ability to bind IFN-γ promoter and failed to suppress Th2 cytokine production, suggesting that phosphorylation of these tyrosine residues is critical for T-bet transcription activity. However, our current study cannot exclude the possibility that replacing tyrosines with phenylamine causes conformational changes rather than abolishing T-bet tyrosine phosphorylation, leading to impaired T-bet promoter DNA-binding activity. This appears to be less likely, since antiphosphotyrosine antibody, but not anti-T-bet (this T-bet antibody recognize the N terminus of T-bet), blocks T-bet promoter binding activity, suggesting that a tyrosine phosphorylation event is involved in T-bet promoter DNA binding. Nevertheless, further studies are still needed to define the molecular nature of the tyrosine phosphorylation in the DNA-binding domain of T-bet in regulating its transcription activity.
ACKNOWLEDGMENTS
We thank Reiji Kannagi (Aichi Cancer Center, Nagoya, Japan), Giulio Superti-Furga (European Molecular Biology Laboratory, Heidelberg, Germany), and Richard M. Gronostajski (State University of New York at Buffalo) for reagents as described in Materials and Methods and Stephen Goff (Columbia University) for c-Abl knockout mice.
This research was supported by grants R21HL092353 and NIH R01AI079056 to D.F., a National Natural Science Foundation of China grant (30872311) to A.C., and NIH grant HL079349 to Z.Z.
A.C. and S.-M.L. contributed equally to this study.
A.C., S.-M.L., B.G., and S.S. performed experiments. A.C., Z.Z., and D.F. analyzed data, designed the research, and wrote the manuscript.
We declare that no conflict of interest exists.
Footnotes
Published ahead of print on 20 June 2011.
REFERENCES
- 1. Atherly L. O., et al. 2006. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity 25:79–91 [DOI] [PubMed] [Google Scholar]
- 2. Barila D., et al. 2000. A nuclear tyrosine phosphorylation circuit: c-Jun as an activator and substrate of c-Abl and JNK. EMBO J. 19:273–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Butz N. V., Gronostajski R. M., Campbell C. E. 2006. T-box proteins differentially activate the expression of the endogenous interferon gamma gene versus transfected reporter genes in non-immune cells. Gene 377:130–139 [DOI] [PubMed] [Google Scholar]
- 4. Chen A., et al. 2009. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol. Cell. Biol. 29:5348–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J., et al. 1994. Generation of normal T and B lymphocytes by c-jun deficient embryonic stem cells. Immunity 1:65–72 [DOI] [PubMed] [Google Scholar]
- 6. Cohen I. R. 1984. The interactions between antigen-presenting cells (APC) and T lymphocytes. Ann. Immunol. (Paris) 135C:400–402 [DOI] [PubMed] [Google Scholar]
- 7. Elias J. A., Lee C. G., Zheng T., Shim Y., Zhu Z. 2003. Interleukin-13 and leukotrienes: an intersection of pathogenetic schema. Am. J. Respir. Cell Mol. Biol. 28:401–404 [DOI] [PubMed] [Google Scholar]
- 8. Fang D., Kerppola T. K. 2004. Ubiquitin-mediated fluorescence complementation reveals that Jun ubiquitinated by Itch/AIP4 is localized to lysosomes. Proc. Natl. Acad. Sci. U. S. A. 101:14782–14787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finotto S., et al. 2002. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science 295:336–338 [DOI] [PubMed] [Google Scholar]
- 10. Gao B., Lee S. M., Fang D. 2006. The tyrosine kinase c-Abl protects c-Jun from ubiquitination-mediated degradation in T cells. J. Biol. Chem. 281:29711–29718 [DOI] [PubMed] [Google Scholar]
- 11. Gu J. J., Zhang N., He Y. W., Koleske A. J., Pendergast A. M. 2007. Defective T cell development and function in the absence of Abelson kinases. J. Immunol. 179:7334–7343 [DOI] [PubMed] [Google Scholar]
- 12. Hsieh C. S., et al. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547–549 [DOI] [PubMed] [Google Scholar]
- 13. Hwang E. S., Szabo S. J., Schwartzberg P. L., Glimcher L. H. 2005. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307:430–433 [DOI] [PubMed] [Google Scholar]
- 14. Jacobson N. G., et al. 1995. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J. Exp. Med. 181:1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaminuma O., et al. 2002. Vav-induced activation of the human IFN-gamma gene promoter is mediated by upregulation of AP-1 activity. FEBS Lett. 514:153–158 [DOI] [PubMed] [Google Scholar]
- 16. Kong S., et al. 2011. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J. Biol. Chem. 286:16967–16975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kosaka Y., Felices M., Berg L. J. 2006. Itk and Th2 responses: action but no reaction. Trends Immunol. 27:453–460 [DOI] [PubMed] [Google Scholar]
- 18. Lee S. M., Gao B., Fang D. 2008. FoxP3 maintains Treg unresponsiveness by selectively inhibiting the promoter DNA-binding activity of AP-1. Blood 111:3599–3606 [DOI] [PubMed] [Google Scholar]
- 19. Lee S. M., et al. 2011. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia 54:1136–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li B., Tournier C., Davis R. J., Flavell R. A. 1999. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J. 18:420–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller A. T., Wilcox H. M., Lai Z., Berg L. J. 2004. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity 21:67–80 [DOI] [PubMed] [Google Scholar]
- 22. Morita S., Kojima T., Kitamura T. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7:1063–1066 [DOI] [PubMed] [Google Scholar]
- 23. Mullen A. C., et al. 2001. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292:1907–1910 [DOI] [PubMed] [Google Scholar]
- 24. Pufall M. A., et al. 2005. Variable control of Ets-1 DNA binding by multiple phosphates in an unstructured region. Science 309:142–145 [DOI] [PubMed] [Google Scholar]
- 25. Schwartzberg P. L., et al. 1991. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell 65:1165–1175 [DOI] [PubMed] [Google Scholar]
- 26. Szabo S. J., et al. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655–669 [DOI] [PubMed] [Google Scholar]
- 27. Usui T., et al. 2006. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J. Exp. Med. 203:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J., et al. 2009. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Invest. 119:3048–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng W., Flavell R. A. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89:587–596 [DOI] [PubMed] [Google Scholar]
- 30. Zhu J., Yamane H., Cote-Sierra J., Guo L., Paul W. E. 2006. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 16:3–10 [DOI] [PubMed] [Google Scholar]
- 31. Zipfel P. A., Zhang W., Quiroz M., Pendergast A. M. 2004. Requirement for Abl kinases in T cell receptor signaling. Curr. Biol. 14:1222–1231 [DOI] [PubMed] [Google Scholar]