Abstract
Heat shock factor 1 (HSF1), while recognized as the major regulator of the heat shock transcriptional response, also exerts important functions during mammalian embryonic development and gametogenesis. In particular, HSF1 is required for oocyte maturation, the adult phase of meiosis preceding fertilization. To identify HSF1 target genes implicated in this process, comparative transcriptomic analyses were performed with wild-type and HSF-deficient oocytes. This revealed a network of meiotic genes involved in cohesin and synaptonemal complex (SC) structures, DNA recombination, and the spindle assembly checkpoint (SAC). All of them were found to be regulated by HSF1 not only during adult but also in embryonic phases of female meiosis. Additional investigations showed that SC, recombination nodules, and DNA repair were affected in Hsf1−/− oocytes during prenatal meiotic prophase I. However, targeting Hsf1 deletion to postnatal oocytes (using Zp3 Cre; Hsf1loxP/loxP) did not fully rescue the chromosomal anomalies identified during meiotic maturation, which possibly caused a persistent SAC activation. This would explain the metaphase I arrest previously described in HSF1-deficient oocytes since SAC inhibition circumvented this block. This work provides new insights into meiotic gene regulation and points out potential links between cellular stress and the meiotic anomalies frequently observed in humans.
INTRODUCTION
Heat shock factor 1 (HSF1) belongs to the mammalian heat shock factor family, which consists of four related members (HSF1 to -4). The role of HSF1 was first thought to be as the major regulator of the protective cellular heat shock or stress response. This response includes the activation of HSF1, which then binds to defined heat shock element sequences (HSEs) and triggers the transcription of HSF1 target genes. The best-known HSF1 target genes encode heat shock proteins (HSPs), which are mostly responsible for cell protection (3, 14, 34).
Mouse embryonic fibroblasts (MEFs) derived from Hsf1-null mice have been widely used to analyze the molecular mechanisms involved in HSF1-dependent gene regulation. This initially led to the confirmation that HSF1 was essential for the stress response (37). Additional studies revealed that HSF2 was also contributing to the stress response, and several mechanisms were proposed regarding HSF1-HSF2 interplay, in particular HSF2 bookmarking HSF1 target genes (58) and HSF1-HSF2 interacting to form heterodimers (36, 49). Interestingly, genome-wide transcript analysis performed on HSF1-deficient MEFs showed that besides Hsp genes, HSF1 was involved in the regulation of numerous other genes, even in the absence of any defined cellular stress (53). However, the biological implication of such a wide HSF1-dependent gene regulation cannot be completely assessed in MEFs and necessitates analysis in more complex systems.
The role of HSF1 was evaluated using HSF1-deficient mice that exhibited complex phenotypes, including developmental defects such as placenta anomalies associated with partial embryonic lethality and more importantly, complete female infertility (57). HSF1 was identified as one of the first maternal effect genes in mammals (13). We previously reported that HSF1-depleted oocytes exhibited multiple defects during meiotic maturation, i.e., a G2/M delay, a marked block at the metaphase I (MI) stage, and alterations in meiotic asymmetrical cytokinesis. Because of those defects, less than 16% of mutant oocytes reached the normal MII stage (40), and subsequently, most of the MII oocytes were unable to cleave to the two-cell stage after fertilization, possibly due to mitochondrial damage and altered redox homeostasis (8).
Some of these defects might possibly be attributed to low levels of several HSPs, but the complexity and the severity of the phenotype led us to hypothesize that HSF1 might specifically regulate other non-HSP genes essential for female gametes. This study, using comparative analysis of adult oocyte transcriptomes and further investigations of the newly discovered HSF1 target genes, supports a model in which HSF1 acts as a transcriptional regulator of meiotic genes during both embryonic and adult phases of female meiosis.
MATERIALS AND METHODS
Animals.
Hsf1−/− (Hsf1tm1Ijb) and Hsf2−/− (Hsf2tm1Ijb) mice were previously provided from I. J. Benjamin (Salt Lake City, UT) and are described elsewhere (37, 38). The Hsf1loxP/loxP line was generated by flanking exons 2 to 4 with loxP sequences. The experimental procedure was undertaken by contract (IR1238) with the Mouse Clinical Institute (MCI) at Strasbourg. Hsf1loxP/loxP animals were crossed with Zp3 Cre transgenic mice to obtain Zp3 Cre; Hsf1loxP/loxP females exhibiting an oocyte-targeted Hsf1 deletion expected to occur in growing oocytes by day 5 after birth based on Zp3 Cre activity (18, 32). Mice were maintained in a mixed genetic background. Protocols for animal breeding and experiments were approved by the Departmental Veterinary Office (Haute-Garonne) according to French legislation (no. 31 09 555 39).
Oocyte collection and culture.
Fully grown oocytes (germinal vesicles [GV]) were collected from ovaries of 8- to 12-week-old mice (wild type [WT]; Hsf1−/−, Hsf2−/− or Zp3 Cre; Hsf1loxP/loxP) in M2 medium (Sigma, St. Louis, MO). They were cultured in M16 medium (Sigma, St. Louis, MO) according to previously described procedures (8, 40) or stored frozen until further use.
Microarray experiments and analyses.
Total RNA from 200 to 400 fully grown oocytes from each genotype (WT, Hsf1−/−, and Hsf2−/−) were extracted using a Qiagen RNeasy microkit, and 10-ng samples of RNA were amplified using the NuGen WT ovation Pico RNA amplification system to obtain about 10 μg of single-stranded (ss) cDNA. Two micrograms of ss cDNA was converted to double-stranded cDNA using the Klenow fragment of DNA polymerase I, and the synthesized cDNAs were then incubated with 1 μl of 4 mg/ml RNase A at 37°C for 10 min and precipitated, and the pellet was dissolved with 20 μl of nuclease-free water. The cDNA of each sample (3.5 μg) was synthesized as a probe and labeled with Cy3- or Cy5-conjugated random nonamers (TriLink Biotechnologies, San Diego, CA) and hybridized to a NimbleGen Mus musculus gene expression 385K microarray, containing 42,586 probe sets with up to 9 probes of 60-mer oligonucleotides per gene, following the protocol by Roche NimbleGen, Inc. (Madison, WI). The microarrays were incubated on the NimbleGen hybridization system 4 (Roche NimbleGen) for 16 h at 42°C. The hybridized slides were washed with 10× wash buffers I, II, and III (Roche NimbleGen), dried by nitrogen gas at room temperature, and scanned with an Axon GenePix Pro 4200A microarray scanner at a 5-μm resolution, with 532-nm and 635-nm wavelengths, using the associated GenePix Pro software (Molecular Devices, Sunnyvale, CA). The scanned images of the arrays were quantified using NimbleScan software (Roche NimbleGen). The expression data for all of the samples in the study were normalized by quantile normalization across replicate on arrays as described previously (10). The gene expression values were generated by robust multichip average (RMA) analysis. Subsequent microarray data analysis was performed using ArrayStar software (DNASTAR, Inc., Madison, WI). Average ratios of expression values of WT versus Hsf1−/− and WT versus Hsf2−/− were calculated from three replicates. Genes were considered differentially expressed when the level of expression change was at least 1.5-fold (upregulation or downregulation) and the difference was significant (P < 0.05, moderated t test, in which the false discovery rate was controlled by the Benjamini Hochberg correction method).
The GenBank accession numbers of genes that showed significant differential expression were uploaded into the Babelomics platform to perform functional enrichment analysis using the Fatigo tool (http://babelomics.bioinfo.cipf.es/functional.html) (39). The genes were classified into functional groups using “GO TERM Biological process” at level 3.
RT-qPCR analysis.
Oocyte samples (20 oocytes) were mixed with up to 2 μl of lysis buffer, consisting of 0.8% IGEPAL (octylphenyl-polyethylene glycol; Sigma, St. Louis, MO), 1 U/μl RNasin (Promega, Madison, WI), and 5 mM dithiothreitol. Before reverse transcription, samples were heated at 75°C for 5 min and transferred immediately to ice. Total RNA from ovaries at 17.5 days postcoïtum (dpc) and from 17-day-old testes were extracted using TRIzol reagent following the manufacturer's protocol (Invitrogen, Carlsbad, CA). DNase treatment was performed by adding 3 μl of DNA lysis buffer (2.5 mM MgCl2, 5× buffer [Invitrogen, Carlsbad, CA], 30 U of DNase I-RNase free [Roche Applied Science, Indianapolis, IN]) to the sample followed by incubation for 1 h at 25°C and 5 min at 70°C. The reverse transcription reaction was carried out according to the manufacturer's instructions. The samples were subjected to oligonucleotide (dT)-primed first-strand cDNA synthesis in a final volume of 20 μl using the Super-ScriptII reverse transcriptase kit (Invitrogen, Carlsbad, CA). cDNA was synthesized at 42°C for 1.5 h. The quantitative PCRs (qPCRs) were performed on an iCycler device (Bio-Rad, Hercules, CA) using 1 μl cDNA and Platinum SYBR green qPCR supermix-UDG (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Reverse transcription-qPCR (RT-qPCR) results were normalized against ribosomal S16 transcript level. Primer sequences are listed in Table S2 in the supplemental material.
ChIP.
The chromatin immunoprecipitation (ChIP) extracts were prepared according to the protocol modified from reference 11. One thousand oocytes (GV stage, CD1 wild-type females) were lysed in 3 ml of lysis buffer. Primer sequences are listed in Table S2 in the supplemental material. HSE sites were identified within 10-kb of the 5′ region of HSF1-dependent genes using RSA tool patser (http://rsat.ulb.ac.be/patser_form.cgi) with an HSE matrix previously described (54).
Western blot analysis.
Fully grown oocytes and ovaries at 17.5 dpc were supplemented with sample buffer (31) and heated to 100°C for 5 min. Total protein extracts (200 oocytes or 2 ovaries per lane) were then subjected to one-dimensional SDS-PAGE followed by transfer onto nitrocellulose membranes. Blots were incubated in blocking buffer (Tris-buffered saline [TBS] containing 1% Tween 20 [TBS-T] and 5% milk) overnight at 4°C and then incubated with the following primary antibody in TBS-T containing 5% milk for 2 h at room temperature: rabbit polyclonal anti-SYCE1 (gift from Howard Cooke [9]) at a 1:500 dilution and mouse monoclonal anti-α-tubulin (T9026; Sigma) at a 1:1000 dilution.
Preparation of oocytes and chromosome spreads for analysis by confocal microscopy.
DNA staining and α-tubulin immunodetection were performed on adult oocytes as described before (8, 40). Spread chromosome preparations from fetal and newborn ovaries and 17-day-old testes were performed using the air-drying method (44). For DNA staining of metaphase I chromosomes, WT and mutant (Hsf1−/− or Zp3 Cre; Hsf1loxP/loxP) fully grown oocytes (GV) were collected from ovaries of 8- to 12-week-old females and cultured either for 8 h (WT) or overnight (mutant delayed maturation) (40) to reach the MI stage. Oocytes were then fixed in 1% paraformaldehyde (PFA) (24) and stained with TO-PRO-3 iodide (Invitrogen, Carlsbad, CA). The following antibodies were used: rabbit polyclonal anti-SYCP3 (Novus Biologicals NB300-231; 1:500), rabbit polyclonal anti-SYCE1 (gift from Howard Cooke [9]; 1:100), goat polyclonal anti-MSH4 (Santa Cruz sc-69510; 1:50), mouse polyclonal anti-MLH1 (Pharmingen 551092; 1:50), and mouse monoclonal anti-γH2AX (Millipore JBW301; 1:50). A Leica SP5 confocal microscope (Leica Microsystems GmbH, Heidelberg, Germanay) was used to capture images from stained oocytes and chromosome spreads.
Chromosome spread analysis.
Nuclei from oocytes and spermatocytes were staged using immunofluorescent detection against SYCP3 protein. The zygotene stage was defined as having stretches of SYCP3 staining and some synapsis. The pachytene stage was defined by a complete synapsis between the homologous chromosomes, and finally, the diplotene stage was defined by homologous chromosomes linked by chiasmas. Distinction between late zygotene and early diplotene stages can be further enhanced by MSH4 staining, which is present during the zygotenema and pachytenema stages but not at the diplotene stage (50). ImageJ software (16) was used to perform all the measurements carried out on chromosome spreads as synaptonemal complex (SC) length, SYCE1 intensity, MSH4 foci, and γH2AX area at 17.5 dpc and chromatid length at the metaphase I stage.
Detection of cold-stable microtubules and SAC inhibition.
WT and mutant (Hsf1−/− or Zp3 Cre; Hsf1loxP/loxP) fully grown oocytes (GV) were collected from ovaries of 8- to 12-week-old females and cultured either for 8 h (WT) or overnight (mutant delayed maturation) (40) to reach the MI stage. Oocytes were then transferred to ice-cold medium at 4°C for 20 min before being processed for DNA and α-tubulin staining to detect cold-stable microtubules (26). Mouse monoclonal anti-α-tubulin coupled to fluorescein isothiocyanate (FITC) from Sigma (F2168) was used at 1:1,000. To test the role of the SAC, MI oocytes were cultured for 2 additional hours in M16 (Sigma, St. Louis, MO) containing Mps1 inhibitor (a generous gift from N. Gray [30]) at a final concentration of 10 μm (solution of 10 mM in dimethyl sulfoxide [DMSO], at a final dilution of 1:1,000) or only DMSO (1:1,000), before further observation and staining.
Statistical analysis.
Data were acquired from samples collected from at least three different females. Control samples indicated as WT were obtained from Hsf1+/+ or Hsf1loxP/loxP females, which had normal reproductive capacity. Under some circumstances, sibling Hsf1+/− and Hsf1−/− animals were compared because Hsf1+/− mice exhibit WT characteristics. The results are presented as means ± standard errors of the means (SEM). Differences between groups were assessed either by Student's t test or chi-square test with a P value of <0.05 considered significant.
Microarray data accession number.
Microarray data have been submitted to the GEO repository to comply with the MIAME standard and can be accessed at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=drklzycgsoeeyhm&acc=GSE26240.
RESULTS
Distinct expression and roles of HSF1 and HSF2 in adult oocytes.
It was originally reported that HSF1 was highly expressed in mouse oocytes, while the related transcription factor HSF2 was barely detectable (12). A more recent study indicated that Hsf1 was uniformly expressed in various cell types, while the levels of Hsf2 fluctuated (49). To clarify those divergent descriptions and to confirm the high expression of Hsf1 in the female gamete, a semiquantitative analysis of Hsf1 and Hsf2 transcript abundance was performed using adult fully grown oocytes and a large series of somatic tissues. Fully grown oocytes exhibited a strikingly high (70:1) Hsf1/Hsf2 transcript ratio in comparison to the 0.5:1 to 4:1 ratios observed in somatic tissues or early embryonic cells (blastocysts), and this was mostly due to the very high level of Hsf1 transcripts (Fig. 1A).
Fig. 1.
Distinct expression and function of Hsf1 and Hsf2 in adult murine oocytes. (A) RT-qPCR analysis of Hsf1 and Hsf2 transcripts in various tissues and cell types showed that mouse fully grown (germinal vesicle [GV]) oocytes are characterized by a very high level of Hsf1 transcripts in comparison to a lower level of Hsf2 mRNAs. The level of transcripts from the gene encoding ribosomal protein S16 was used as an internal reference to normalize the level of Hsf1 and Hsf2 mRNAs, respectively. Experiments were performed with tissues, oocytes, and embryos collected from n = 4 animals. Bars represent means ± SEM. (B) In vitro meiotic maturation of GV oocytes demonstrated that Hsf2−/− oocytes, in contrast to Hsf1−/− oocytes, normally reach metaphase II (MII). Bars represent the mean percentages of oocytes at the GV stage, metaphase I (MI), and MII following 16 h of culture (WT, n = 541 oocytes; Hsf1−/−, n = 427 oocytes; Hsf2−/−, n = 120 oocytes). (C) Comparison of HSF1- and HSF2-dependent transcriptome in GV oocytes. The number of genes differently expressed (≥1.5-fold; P < 0.05) in Hsf1−/− and Hsf2−/− oocytes in comparison to WT ones is indicated in circles colored in red or blue when they are upregulated in Hsf1−/− and Hsf2−/− oocytes, respectively, or in circles colored in green or yellow when they are downregulated in Hsf1−/− and Hsf2−/− oocytes, respectively. The number of genes regulated by both HSF1 and HSF2 is written in the area of the intersecting circles.
Despite this unbalanced ratio, Hsf1 and Hsf2 are both expressed in oocytes, and both factors could therefore contribute to oocyte-specific functions. We have previously shown that an HSF1 deficiency perturbs adult meiosis progression (40), while Kallio and colleagues reported that HSF2 deficiency could lead to abnormal ovulated oocytes (28). Those data would suggest that both HSF1 and HSF2 could contribute to meiotic progression (28, 40). We reinvestigated this possibility using a different Hsf2 knockout line (Hsf2tmI1jb) (38) from the one used in the study by Kallio and colleagues (Hsf2tm1Mmr) (28). In contrast to Hsf1−/− oocytes, there was no difference between the ability of Hsf2−/− (Hsf2tmI1jb) and WT oocytes to complete the first meiotic division and reach metaphase II (Fig. 1B). These results were consistent with the fact that the HSF2-deficient females from the Hsf2tmI1jb line were fertile, indicating that HSF2 was not essential for oocyte development (38) (see Discussion).
It remains puzzling that HSF1 and HSF2 rarely exert completely redundant functions (3, 15), as their DNA binding domains share a high level of homology (70% amino acid sequence identity) (45, 51), suggesting that they could regulate the same target genes. Therefore, to better understand the distinct roles of HSF1 and HSF2 in adult oocytes, a comparative analysis of WT, Hsf1−/− and Hsf2−/− oocyte transcriptomes was performed. This led to the identification of 1,665 and 959 genes regulated, at least in part, by HSF1 and HSF2, respectively (Fig. 1C). Of these, 226 genes were found commonly regulated by HSF1 and HSF2. Differentially expressed genes were either down- or upregulated in HSF-deficient oocytes, suggesting that both HSF1 and HSF2 could act, either directly or indirectly, as transcriptional activators as well as repressors.
HSF1 regulates genes involved in several meiotic processes.
While a computational functional enrichment analysis did not reveal any specific biological process among HSF2-dependent genes, we discovered that numerous HSF1-specific target genes were implicated in the cell cycle (75 genes) (Fig. 2A; see Table S1 in the supplemental material), an observation directly relevant to the meiotic phenotype of Hsf1−/− oocytes (40). Therefore, we focused our attention on the largest group of misregulated genes that belonged to the “chromosome and chromatid cohesion” category (n = 17 genes).
Fig. 2.
HSF1 regulates meiotic genes. (A) Molecular functions of HSF1-dependent genes identified in the enriched biological process entitled “cell cycle” (see Table S1 in the supplemental material). (B) RT-qPCR validation of HSF1 target genes listed under the “chromosome and chromatid cohesion” process (see Table S2 in the supplemental material). RT-qPCR results were normalized against the ribosomal S16 transcript level, and the normalized expression level was compared to the WT sample, which was arbitrarily expressed as 1. Bars represent means ± SEM of at least three independent experiments performed with oocytes collected from several females (n = 5). (C) Schematic representation of the 5′ region of HSF1-dependent genes (Syce1, Stag2 and -3, and Msh4) showing the respective positions of HSE sites within the 10 kb upstream of the transcription start to be analyzed in panel D. (D) ChIP experiments performed with fully grown (GV) oocytes revealed that HSF1 and HSF2 bind distinct HSEs (see Tables S2 and S3 in the supplemental material). HSE numbers refer to panel C. HSEs indicated in panel C but not present in panel D correspond to a ChIP-negative sample or HSEs not bound. The HSP90α sample was included as a positive control (38, 40). Nonspecific antibody (NS) was used as a negative control, and acetylated histone H4 (AcH4) was used as an indicator of transcriptionally active promoters. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Real-time quantitative PCR (RT-qPCR) was used to confirm the microarray data. Those experiments validated 16 of the 17 genes as significantly differentially expressed between WT and Hsf1−/− oocytes. Most of these genes were downregulated in the absence of HSF1, with the exception of two testis-expressed genes (Tex11 and Tex12), Ppp3cb, and Rad51D, which were upregulated (Fig. 2B; see Table S2 in the supplemental material). To determine whether these genes could be directly regulated by HSF1, a search for HSF1 DNA binding sites (HSEs) within 10 kb of their 5′ regulatory region using an HSE matrix (53, 54) was performed. Among the 16 validated genes, only Sgol2 and Tex12 did not contain such sequences (see Table S3 in the supplemental material). Chromatin immunoprecipitation (ChIP) experiments were performed on large pool of adult oocytes isolated from ovaries. The results confirmed that several HSEs identified by computer analysis were indeed bound by HSF1 (Syce1) or by HSF1 and HSF2 (Stag2, Stag3, and Msh4) (Fig. 2C and D). The fact that the expression of these genes did not change in Hsf2−/− oocytes also indicated that HSF2 binding is likely not required for their transcriptional control under normal development.
Further investigation of the role played by those novel HSF1-dependent genes revealed that despite their identification in adult fully grown oocytes, most of them were better known for the functions they exert during meiotic prophase I, which is initiated before birth. In particular, STAG2 (stromal antigen 2; MGI no. 1098583) and STAG3 (stromal antigen 3; MGI no. 1355311) are subunits of the cohesin complex which maintains links between sister chromatids at the beginning of prophase I (13.5 to 14.5 days postcoitum [dpc]) until anaphase II (5, 52). SYCE1 (synaptonemal complex central element protein 1; MGI no.1921325) is a constituent of the central element of the synaptonemal complex (SC), a tripartite protein structure, which is required for chromosome synapsis and is fully assembled at the pachytene stage (17.5 dpc) (9, 60). Finally, MSH4 (MutS homolog 4 [Escherichia coli]; MGI no. 1860077) is part of the recombination/repair machinery which forms at double strand breaks (DSBs). Some of these sites evolve into late recombination nodules which mark formation of chiasmata between homologous chromosomes and crossovers (6, 41). Thus, HSF1 might also regulate these genes during the prenatal prophase I and that its loss of function could hamper the meiotic mechanisms as early as prophase I.
Female (but not male) prophase I requires meiotic transcriptional role of HSF1.
In females, meiosis is initiated around 13.5 dpc and oocytes progress in a relatively synchronous way through the four phases of prophase I (leptotene, zygotene, pachytene, and diplotene), which are mostly completed during fetal development (17, 19, 43) (Fig. 3A). While HSF1 deficiency is characterized by a partial embryonic lethality occurring mainly between 13.5 and 15.5 dpc (57), we decided to work with 17.5-dpc ovaries from viable sibling Hsf1−/− and Hsf1+/− embryos. At this time point, most oocytes should be progressing through meiotic prophase I (17, 19, 43). RT-qPCR experiments revealed that Stag2, Stag3, Syce1, and Msh4 were significantly downregulated in Hsf1−/− ovaries (Fig. 3B). To determine whether the observed meiotic gene dysregulation affected embryonic prophase I, immunostaining was performed against the lateral element protein SYCP3, a marker for SC assembly disassembly, a key cellular process characterizing the four phases of prophase I. Significant differences in the percentage of 17.5-dpc oocytes exhibiting typical zygotene, pachytene, and diplotene morphologies between control and HSF1-deficient samples were observed, as exemplified in the upper panels in Fig. 3C. In newborns (1 day postpartum [dpp]) (Fig. 3C), the zygotene stage was not present in Hsf1+/− samples and the diplotene stage was reached by 80% of Hsf1+/− oocytes, and only 51% of mutant oocytes (P < 0.001, chi-square test). This indicated that HSF1 deficiency disturbed prophase I progression.
Fig. 3.
Abnormal prenatal prophase I in Hsf1−/− oocytes. (A) Schematic representation of prenatal prophase I stages (L, leptotene; Z, zygotene; P, pachytene; D, diplotene). (B) RT-qPCR experiments showed that HSF1-dependent genes (Fig. 2B and D) are downregulated in 17.5-dpc Hsf1−/− samples. RT-qPCR results were normalized against the ribosomal S16 transcript level, and the normal- ized expression level was compared to the Hsf1+/− sample, which was arbitrarily given the value 1. Bars represent means ± SEM from at least three independent experiments performed with ovaries collected from several fetuses (n = 4). (C) SYCP3 immunostaining was used to compare prophase I progression at 17.5 dpc and 1 dpp. Representative images of observed stages at 17.5 dpc are presented in the upper panels. The percentage of zygotene, pachytene, and diplotene stages was calculated for each sample: 17.5 dpc for Hsf1+/−, control (n = 209), and Hsf1−/− (n = 224) oocytes and 1 dpp for Hsf1+/− (control, n = 228) and Hsf1−/− (n = 253) oocytes (graph, lower panel). Bars represent means ± SEM from experiments performed with several siblings (n = 4 to 17.5 dpc for embryos or −1 dpp for pups). Prophase I stages were differently represented in HSF1-deficient oocytes according to chi-square test (P < 0.001). (D) RT-qPCR experiments performed on 17-day-old testes show no changes in the expression of selected HSF1-dependent genes. Data are presented as in panel B. Bars represent means ± SEM from at least three independent experiments performed with testes collected from several males (n = 3). (E) Spreads of male germ cells were prepared from testes at 17 days postpartum. Analysis of Hsf1−/− (n = 350) and Hsf1+/− (n = 318) spermatocytes did not reveal any difference between the two genotypes (n = 3 males). **, P < 0.01; ***, P < 0.001.
The group of meiotic genes identified as regulated by HSF1 in females is also involved in prophase I during male gametogenesis (6, 9, 41, 52, 60). We therefore analyzed their expression in juvenile testes (17 dpp). At that time, spermatocytes produced by the first wave of spermatogenesis have not yet undertaken any meiotic division and are mostly at the end of prophase I (7). We did not observe any difference in Stag2, Stag3, Syce1, and Msh4 mRNA levels between Hsf1−/− and Hsf1+/− testes (Fig. 3D). Consistent with this observation, SYCP3 immunostaining revealed the same percentage of spermatocytes at the different prophase I phases between Hsf1−/− and sibling Hsf1+/− testes (Fig. 3E). This showed that, in contrast to oocytes, Hsf1−/− spermatocytes I could progress normally through prophase I of the first wave.
Chromosome structure anomalies in HSF1-deficient embryonic oocytes.
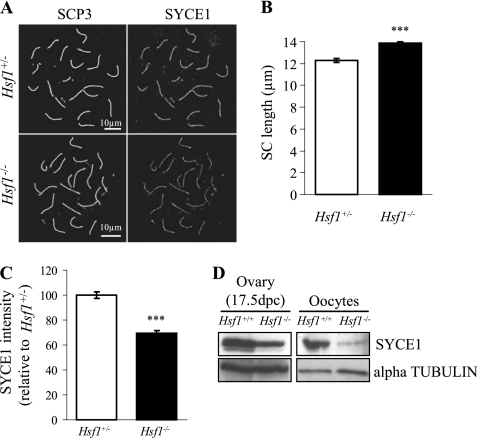
In addition to the abnormal progression through prenatal prophase I, we noted that the length of the SC measured by immunostaining using anti-SYCP3 antibody was significantly greater in Hsf1−/− oocytes than in Hsf1+/− oocytes (Fig. 4A and B), consistent with lower level of chromatin condensation. This chromosomal anomaly was correlated with a reduced staining of the SC central element, as observed using an anti-SYCE1 antibody (Fig. 4C). This suggested that the Syce1 transcriptional decrease in Hsf1−/− oocytes led to a corresponding reduced level of SYCE1 protein. This was confirmed by Western blot analysis showing that SYCE1 protein was less abundant in embryonic Hsf1−/− ovaries (Fig. 4D, left panel) but was still present in adult oocytes (Fig. 4D, right panel).
Fig. 4.
HSF1 deficiency leads to defects in central element of the synaptonemal complex (SC). (A) Immunostaining for SYCP3 and SYCE1 performed on 17.5-dpc Hsf1+/− (control) (n = 111) and Hsf1−/− (n = 107) oocytes (n = 3 fetuses). (B) Synaptonemal complex length measured after SYCP3 staining was significantly increased in Hsf1−/− (n = 53) versus Hsf1+/− (n = 56) oocytes (13.88 ± 0.14 μm and 12.28 ± 0.19 μm, respectively). Measurements were performed using ImageJ software (16). (C) SYCE1 intensity was measured from images as shown in panel A using ImageJ software (16) and plotted relative to the Hsf1+/− value arbitrarily set to 1. (D) Western blot analysis of SYCE1 expression in 17.5-dpc ovaries and fully grown (GV) adult oocytes (control for equal loading, α-tubulin). ***, P < 0.001.
Several chromosomal and cellular events are coordinated with SC assembly and disassembly. In particular, an important aspect of meiotic processes is the recombination of chromatids between homologous chromosomes that occurs following the developmentally programmed generation of double-strand DNA breaks (Fig. 5A). MSH4 participates in recombination and operates along the DSB repair pathway (Fig. 5A) (33, 41, 50). Since Msh4 transcript expression was lower in HSF1-deficient oocytes, the number of MSH4-containing foci might be reduced (41). To investigate this possibility, MSH4 foci were counted in pachytene oocytes where a close alignment of the lateral elements, revealed by SYCP3 staining, was taken as a sign of synapsis completion. Hsf1−/− oocytes exhibited significantly fewer MSH4 foci in comparison with Hsf1+/− ones (Fig. 5B and C). Through the pachynema stage of prophase I, some of the MSH4 foci become MLH1-positive recombination foci that mark crossovers (6, 41). MLH1 recombination foci were counted on pachytene oocytes. There was no significant difference between Hsf1−/− and Hsf1+/− oocytes (Fig. 5D), showing that HSF1 deficiency did not affect the establishment of crossovers during female meiosis.
Fig. 5.
Recombination and the DNA repair process are altered in 17.5-dpc HSF1-deficient oocytes. (A) Diagram illustrating MSH4-related functions and indicating endpoint analyses performed on meiotic prenatal oocytes. MSH4 is included in transformed nodules (TN) which emerge from early nodules (EN) and subsequently—for some of them—evolve as recombination nodules (RN) associated with MLH1 (41). MSH4 foci are analyzed in panels B, C, and D. DNA repair progression is assessed with γH2AX staining as the hallmark for DSB (see panels B and E). (B) SYCP3 (red), MSH4 (green), and γH2AX (blue) immunostaining performed on Hsf1+/− (control) and Hsf1−/− oocyte spreads at 17.5 dpc (n = 50 for each genotype). (C) The number of MSH4 foci calculated per oocyte was significantly different in Hsf1−/− oocytes compared to that in Hsf1+/− oocytes (number of foci, 96.5 ± 14 versus 270 ± 40, respectively). (D) SYCP3 (red) and MLH1 (green) immunostaining on pachytene oocytes showed that there was no difference between the number of late recombination nodules between Hsf1−/− (24.61 ± 1.12 foci) and Hsf1+/− (24.15 ± 1.46 foci) oocytes (n = 25). (E) The area covered by γH2AX staining was different in Hsf1−/− oocytes from that in Hsf1+/− oocytes (γH2AX area, 142 ± 11 versus 28.5 ± 3 μm2). **, P < 0.01; ***, P < 0.001.
Because MSH4 has also been shown to function in DNA repair (6), we investigated whether its reduced expression in mutant oocytes might lead to defects in this process that can be visualized using γH2AX staining together with MSH4 and SYCP3 immunodetection. Only pachytene oocytes were used in this analysis to avoid the confounding effect of stage and staining changes. Hsf1−/− oocytes displayed on average a 5-fold-larger γH2AX-positive area than sibling Hsf1+/− oocytes. This difference suggested that DNA repair processes were affected when HSF1 was absent in embryonic oocytes (Fig. 5A, B, and E).
Use of an Hsf1 conditional knockout to delineate temporal consequences of HSF1 loss of function.
Using a constitutive knockout of Hsf1 (Hsf1tm1Ijb), several defects were observed during embryonic (this study) and adult (40) meiosis. This raised the question of a possible causal effect exerted by the embryonic anomalies on the meiotic syndrome in adult oocytes.
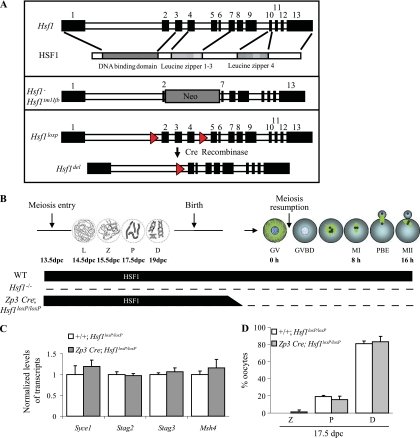
To address this question, we took advantage of a new conditional Hsf1 knockout that we had generated. Combined with Hsf1loxP/loxP, Zp3 Cre transgene induced the deletion of the genomic region corresponding to Hsf1 exons 2 to 4, in growing oocytes starting at day 5 postpartum (Fig. 6A and B) (18, 32). Under these conditions, embryonic prophase I is reached with a normal supply of HSF1, while the adult meiotic maturation is undertaken in the absence of HSF1.
Fig. 6.
Postnatal deletion of Hsf1 (Zp3 Cre; Hsf1loxP/loxP). (A) Schematic representation of Hsf1 genomic sequence and associated protein domains. The constitutive Hsf1 knockout mouse line was made by insertion of Neo cassette in place of exons 3 to 6 (37), whereas the conditional deletion leads to deletion of the exons 2 to 4, which correspond to a large part of the DNA binding domain of HSF1. (B) Phases of prenatal prophase I (L, leptotene; Z, zygotene; P, pachytene; D, diplotene) and adult female meiosis (GV, germinal vesicle; GVBD, germinal vesicle breakdown; MI, metaphase I; PBE, polar body extrusion; MII, metaphase II) illustrated with the profile of Hsf1 deletion in the Hsf1−/− (constitutive) and Zp3 Cre; Hsf1loxP/loxP (conditional) lines. (C) RT-qPCR was performed as in Fig. 3A. Ovaries collected from Zp3 Cre; Hsf1loxP/loxP female embryos (17.5 dpc) exhibited the same level of expression of HSF1 target genes as that in control samples. Bars represent means ± SEM from at least three independent experiments performed with ovaries collected from several fetuses (n = 5). (D) SYCP3 immunostaining was used to compare the different prophase I stages (L, Z, and P) in 17.5-dpc Hsf1+/+; Hsf1loxP/loxP (control, n = 128) and Zp3 Cre; Hsf1loxP/loxP (n = 111) oocytes. Bars represent means ± SEM from experiments performed with several fetuses (n = 3). There was no difference in prophase I stages between Zp3 Cre; Hsf1loxP/loxP and control Hsf1+/+; Hsf1loxP/loxP oocytes.
To confirm that the molecular and cellular events occurring during prenatal prophase I were normal in Zp3 Cre; Hsf1loxP/loxP oocytes, experiments similar to those described in Fig. 3 were performed in parallel using oocytes with constitutive or with conditional Hsf1 deletions. In contrast to Hsf1−/− embryonic/fetal oocytes, Zp3 Cre; Hsf1loxP/loxP oocytes expressed the meiotic genes Stag2, Stag3, Syce1, and Msh4 at the same level as control Hsf1loxP/loxP oocytes and did not exhibit any difference in the proportion of the different prophase I stages (Fig. 6C and D).
Comparison of metaphase I chromosome morphology in adult oocytes constitutively or conditionally deficient in HSF1.
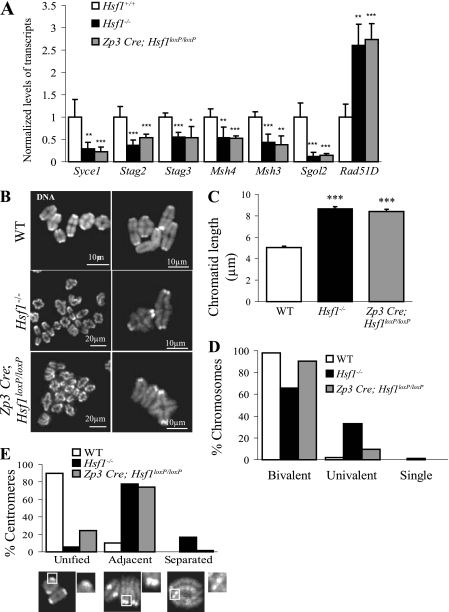
First, to determine whether adult fully grown oocytes collected from constitutive or conditional HSF1-deficient females behaved similarly at the molecular level, HSF1 target gene expression (Syce1, Stag2, Stag3, Msh4, Msh3, Sgol2, and Rad51D) was assessed by RT-qPCR. Both types of mutant oocytes exhibited similar changes in transcript levels compared to control oocytes (Fig. 7A).
Fig. 7.
Postnatal deletion of Hsf1 (Zp3 Cre; Hsf1loxP/loxP) partially rescues the defects observed in MI chromosomal structure. (A) RT-qPCR performed on adult fully grown oocytes obtained from Zp3 Cre; Hsf1loxP/loxP females revealed that HSF1 target genes are as affected as those in the constitutive knockout. Relative quantities of mRNA were normalized against the quantity of the ribosomal S16 transcripts and their relative expression levels were compared to those of the WT sample, which was arbitrarily given the value 1. Bars represent means ± SEM from at least three independent experiments performed with oocytes collected from several females (n = 3). (B) Representative images of MI chromosome shown at two different magnifications (TO-PRO-3 staining). (C) Chromatid length, measured from images as shown in panel B by ImageJ software, was similarly increased in Hsf1−/− (n = 103) and Zp3 Cre; Hsf1loxP/loxP (n = 108) oocytes compared to WT (n = 112) oocytes. (D) MI chromosomes (as shown in panel B) were classified as bivalent, univalent, or single (number of analyzed chromatids, WT, n = 880; Hsf1−/−, n = 1,494; Zp3 Cre; and Hsf1loxP/loxP, n = 1,032). (E) Proportions of unified, adjacent, and separated sister centromeres in WT, Hsf1−/−, and Zp3 Cre; Hsf1loxP/loxP oocytes (number of centromeres analyzed per genotype, n = 100). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
We next assessed the cellular consequences associated with a conditional Hsf1 mutation. Histological analysis of adult ovaries did not reveal any gross anomaly in the conditional knockout of Hsf1 similar to what was previously described for the constitutive deletion (13). The numbers of fully grown oocytes collected from WT and Hsf1 mutant ovaries were not significantly different (26 ± 6, 24 ± 5, and 31 ± 14 oocytes in WT, Hsf1−/− and Zp3 Cre; Hsf1loxP/loxP females, respectively). Chromosome morphology analyses were performed on oocytes collected and cultured such that they were expected to have reached metaphase I (see Materials and Methods). Following DNA staining with the far-red fluorescent nucleic acid stain TO-PRO-3, both constitutive and conditional HSF1-deficient oocytes similarly displayed longer chromosomes than WT oocytes. This suggested an altered chromatin organization in both types of HSF1-deficient oocytes (Fig. 7B and C).
At metaphase I, chromosomes should be bivalent, formed by a recombined parental pair that includes two chromatids per chromosome. The frequency of abnormal chromosome disjunction revealed by the percentage of univalent chromosomes in addition to the presence of single chromatids was significantly higher in Hsf1−/− than in Zp3 Cre; Hsf1loxP/loxP oocyte spreads (Fig. 7B and D), suggesting reduced cohesion. Reduced chromosomal cohesion in Hsf1−/− oocytes was further supported by the higher percentage of separated centromeres (Fig. 7E). Based on these criteria (percentage of single chromatids and separated centromeres), chromosomal alterations were less severe when HSF1 was lost after prenatal prophase I (Fig. 7D and E). This observation further supported the hypothesis that there was a link between prenatal and adult meiotic events that HSF1 contributes to.
Loss of HSF1 triggers SAC activity in maturing fully grown oocytes.
Since Zp3 Cre; Hsf1loxP/loxP oocytes displayed less severe chromosomal disjunction and greater centromere cohesion, one could predict that their ability to complete meiotic maturation would be improved in comparison to constitutively HSF1-deficient oocytes. Fully grown oocytes were collected from adult ovaries and cultured as previously described (Fig. 1B) (40). However, there were no significant differences between the two types of Hsf1 mutant oocytes; both remained significantly blocked in pro-MI/MI phase (Fig. 8A to C).
Fig. 8.
Presence of HSF1 during embryonic prophase I did not rescue maturation defects and female infertility in conditional Hsf1 knockout. (A) Histograms show the number of pups obtained by litter produced by females of the indicated genotype. Zp3 Cre; Hsf1loxP/loxP females did not produce any offspring. (B) GV oocytes were cultured during 16 h, and a similar in vitro MI block was observed in constitutive Hsf1−/− (n = 427) and conditional Zp3 Cre; Hsf1loxP/loxP (n = 296) oocytes compared to WT (n = 541) oocytes. (C) Representative images of meiotic spindles observed in both types of mutant oocytes (blue, TO-PRO-3-stained DNA; green, α-tubulin).
Taking into account the downregulation of HSF1 target genes such as Sgol2 and Bub1b (Fig. 2B) together with chromosomal abnormalities, this severe meiotic block could be a result of a persistent activation of the spindle assembly checkpoint (SAC) that monitors kinetochore-microtubule attachment and spindle integrity (23, 35, 55). One way to test this hypothesis is to examine the presence of microtubules that become cold stable through proper attachment to the kinetochores (26). Treatment at 4°C provoked limited modifications of the meiotic spindle in WT oocytes, indicating that they contained cold-stable microtubules. This was in sharp contrast with the complete disappearance of microtubules or the severe disorganization of the meiotic spindle with misaligned chromosomes observed in Hsf1−/− and Zp3 Cre; Hsf1loxP/loxP (mutant oocytes, Fig. 9A and B). To test whether metaphase I arrest was due to persistent SAC activation in the Hsf1 mutant oocytes, a small-molecule kinase inhibitor (Mps1-IN-1) was used to abolish the activity of Mps1 (TTK; MGI no.1194921), an upstream regulator of SAC effectors (1, 30). Experiments were carried out with both the constitutive and the conditional Hsf1 knockouts. The results were similar for mutant oocytes from both lines (Hsf1−/− and Zp3 cre; Hsf1loxP/loxP); therefore, the graph presented in Fig. 9C corresponds to pooled data from both types of oocytes. This treatment enabled mutant oocytes to progress beyond metaphase I to the first meiotic division with the extrusion of the first polar body (PBE). Less than 10% of refractory oocytes remained at MI, and 27% underwent degenerative fragmentation (Fig. 9C and D). Therefore, once the SAC was inactivated, meiosis progression with chromosomal segregation (data not shown) could be resumed in mutant oocytes. This result confirms that HSF1 deficiency caused SAC activation blocking meiotic maturation in adult oocytes.
Fig. 9.
Mechanisms involved in HSF1-dependent MI block. (A and B) Cold treatment. Meiotic spindles lacked cold-stable microtubules (A), and misaligned chromosomes (B) were more frequent in mutant (Hsf1−/− and Zp3 Cre; Hsf1loxP/loxP) (n = 73) oocytes compared to WT (n = 55) oocytes. (C and D) Spindle assembly checkpoint (SAC) inhibition by MspI-IN-18 in HSF1-deficient oocytes. (C) Percentage of oocytes resuming meiosis after treatment with DMSO (n = 108 oocytes) or DMSO plus Mps1-IN-18 (n = 194 oocytes); (D) representative images of MI (i), polar body extrusion (ii), and degenerated oocyte (iii).
DISCUSSION
While there is a single HSF in yeast (e.g., Saccharomyces cerevisiae) and Drosophila melanogaster, mammals have multiple HSFs, raising the question of their specific and/or redundant roles. HSF1 and HSF2 are the closest members of the family and are coexpressed in most cell types. Nevertheless, loss-of-function mutants have demonstrated very distinct roles for those factors. HSF1, which was known initially as the transcriptional regulator of the heat shock response and inducible expression of HSPs, was subsequently identified as a maternal factor essential for female reproductive success (13, 37). HSF1 was then shown to be required for oocyte meiosis completion (40). Here, we report that Hsf2 is also expressed in fully grown oocytes but at a markedly lower level in comparison to the oocyte-specific “overexpression” of Hsf1. Nevertheless, the expression of several hundred genes was found to be significantly changed in HSF2-deficient oocytes. Because HSF2-deficient oocytes (line Hsf2tm1Ijb) did not exhibit any visible defects during meiotic maturation and mutant females were fertile (38), this led to a surprising conclusion that the oocyte transcriptome can be modified to a large extent without inducing functional defects. In contrast to our observations, some meiotic defects were reported for an independent Hsf2 knockout line (28). Differences in phenotypes between two knockout lines are not uncommon and can be explained by strain-dependent combinations of modifier genes. It would be interesting to compare the oocyte transcriptome from this line in order to identify HSF2-dependent critical genes.
More importantly, data presented here further establish HSF1 as a meiotic transcription factor controlling a network of genes involved in shaping meiotic chromosomes.
Although much progress has been made in our understanding of meiotic gene functions, very little has been reported about their regulation. As far as we know, there is no global study comparing the regulatory regions of those meiotic genes, a strategy that could have helped to identify potential common regulatory sequences as DNA binding sites targeted by corresponding meiotic transcription factors. Our experiments demonstrated that HSF1 DNA binding sites (HSEs) are active and can be considered a common cis regulatory element for a group of meiotic genes. Thus, HSF1 can directly regulate some meiotic genes, while other HSF1-dependent genes such as Sgol2, that did not contain HSE in the 10-kb (5′) regulatory sequences analyzed, might be indirectly regulated by HSF1. In Drosophila, it has been shown that many or even most HSEs are located in introns (21). Further analysis is required to verify if this could apply to other meiotic genes in mammals.
Although meiosis encompasses comparable chromosome remodeling in female and male germ cells, there are numerous important differences between genders. In this particular instance, we found significantly different Hsf1/Hsf2 ratios in female and male gonads. Meiotic genes identified as critically dependent on HSF1 in oocytes did not require the factor during spermatogenesis. In agreement with our data, those genes were not found among the HSF1- or HSF2-positive ChIP candidates identified in testis (2, 4). Consistent with lack of direct molecular involvement of HSF1 in meiotic gene expression, Hsf1-null animals exhibited much less severe consequences during spermatogenesis (4, 48, 57). It is worth mentioning that double-knockout Hsf1 and Hsf2 (Hsf1.2) mice have an additive deficiency phenotype, i.e., a complete arrest of spermatogenesis (56; our unpublished data). Thus, HSF target genes are regulated differently in female and male gametes.
A major difference between female and male gametogenesis is the length of meiosis observed in oocytes, which initiate prophase I during embryonic development and complete the 1st and 2nd meiotic divisions during adult reproductive life. Our data demonstrated that HSF1 was involved in female meiosis from early stages. In the absence of HSF1, axial elements of the synaptonemal complex in Hsf1 mutant pachytene oocytes were found to be longer than control ones. This observation, in addition to reduced transcript expression of Syce1 and members of cohesin complex (Stag2 and Stag3), was in agreement with previous work by others demonstrating that combined alterations in SC protein and cohesin expression modify SC length (42).
Meiosis is characterized by a tightly regulated chromosome-chromatid separation, resulting in equal distribution of chromosomes between the oocyte and the two polar bodies. Loss of cohesion can lead to precocious chromosome disjunction (univalent) or even chromatid separation (single chromatid). The impact of the prenatal cohesion-related events on chromosome-chromatid segregation during meiotic divisions has been suggested as a cause of the increasing level of errors found in aging human oocytes (47). Adult oocytes that were constitutively deficient for HSF1 exhibited significant chromosome separation, although not as severe as that resulting from a total loss of cohesin Smc1β expression (i.e., univalent about 30% and 60%, respectively, from data presented in this paper and by others) (25). Even if the chromosomal anomalies appeared severe in oocytes with Smc1β deleted, it was recently shown that delaying postnatally Smc1β loss of function rescues the defects (47). In contrast, the same procedure applied to an Hsf1 knockout using a Zp3 Cre; Hsf1loxP/loxP line led only to limited but visible improvements. Based on our transcriptomic analysis, HSF1 did not control Smc1β expression, but it does affect other elements of the cohesin complex, such as Stag2 and Stag3. This could indicate that meiotic requirements vary for the different elements of the complex or that additional deficiencies existing in Hsf1−/− or Zp3 Cre; Hsf1loxP/loxP caused this persistent alteration of chromosomal cohesion.
Cell cycle progression is tightly regulated, and the quality of the process is under the control of several checkpoints that provoke cell arrest in case of anomalies. While male meiosis exhibits several checkpoints at defined stages, the existence of female meiosis checkpoints has been questioned because complete meiotic arrest was not observed even in the cases of severe disturbances in chromosome alignment on the spindle (22, 27). For example, Sycp3-null males did not produce any gametes, while Sycp3-null females could generate aneuploid gametes and embryos due to erroneous meiosis in their oocytes (61). HSF1 was previously shown to be required for the checkpoint induced by genotoxic stress during spermatogenesis (48). In this study, two checkpoints were investigated in HSF1-deficient oocytes. The first one is linked to the quality of DNA repair and chromosome pairing and occurs perinatally, provoking an important wave of oocyte death. In some cases, such as in Syce1- or Msh4-null mutations (9), this leads to a complete oocyte depletion from the ovary. As described in this paper, Hsf1 knockout (Hsf1ko) created a hypomorph version of those mutations by reducing the level of expression of Syce1 and Msh4 genes. This situation did not induce a massive oocyte loss around birth (data not shown), suggesting that even if a perinatal checkpoint had been triggered, Hsf1 mutant oocytes remain insensitive to this checkpoint. In contrast, the second checkpoint that is in place during meiotic maturation was maintained and a pro-MI/MI block was one of the main features of Hsf1ko phenotype (40). We found that preventing SAC activity by inhibiting MPS1 enabled HSF1-deficient oocytes to progress further through meiosis and extrude the first polar body. Our experiments not only showed that HSF1 meiotic arrest was SAC dependent but that MPS1 was essential in regulating SAC. It should be emphasized that the role of MPS1 in female meiosis has been previously demonstrated in vertebrates such as amphibians and fish (1, 20, 46) but not yet in mouse oocytes.
The present study reveals an important question: as several meiotic genes (e.g., Syce1 and Msh4) involved in embryonic prophase I were found to be expressed in adult oocytes, do those genes have a function in those postnatal/adult oocytes? One elegant but time-consuming way to address this question would be to make a conditional knockout for each of them to allow their inactivation after birth, as for HSF1 in the present study.
Altogether, our data point out an unexpected role for the well-known transcription factor HSF1. It coregulates a network of meiotic genes implicated in interconnected functions overall implied in meiotic chromosome architecture (chromosome pairing, chromosomal kinetochore-microtubule attachment) acting from prenatal prophase I to adult meiotic maturation, as summarized in Fig. 10(59). Since HSF1 activity is modulated by environmental conditions (e.g., thermal stress, heavy metal, and alcohol) (3), our findings have potentially important implications in human reproductive life and health (22) and deserve further investigation.
Fig. 10.
Schematic model illustrating HSF1 as a regulator of the meiotic gene network. The HSF1 transcriptome analyzed in GV oocytes identified a group of genes listed in the “chromosome and chromatid cohesion” category. Genes with the name written in boldface were shown to be bound by HSF1 (Fig. 2). We found that HSF1 is regulating those genes from the beginning of the meiosis process (prenatal prophase I: L, Z, P, D stages). HSF1-dependent genes are listed in boxes (rectangles) colored in a similar way to their functions (circles). These different meiotic functions have been found to be mechanistically linked, as indicated by the arrows. Numbers in parentheses refer to the following papers: 1, Novak et al. (42); 2, Zickler (62); 3, Barbero (5); and 4, Kan et al. (29). Thus, HSF1 contributes to the coordinated expression of meiotic genes acting in a coordinated way. Question marks indicate some yet unexplained observations: e.g., role of genes such as Msh4 (participating in a DNA repair/recombination function) or Syce1 (contributing to synaptonemal complex) in adult oocytes. Finally, further analyses of the related functions pointed out a series of anomalies linked to the absence of HSF1 (HSF1 knockout [HSF1ko]) during the prenatal or postnatal/adult phases of meiosis. In conclusion, HSF1 deficiency affects meiotic gene expression and functions.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Monget for providing us with biological tools, J. L. Barbero and T. K. Tang for supplying antibodies for these studies. We are indebted to L. Sistonen, M. Akerfelt, and A. Metchat for providing us with the ChIP extracts. We are very grateful to D. Morello for constant support during this project and to I. J. Benjamin for providing the appropriate environment to complete the present paper.
This work was supported by the Centre National de la Recherche Scientifique (CNRS), le Ministère de l'Education Nationale et de la Recherche (to F.L.M.), la Fondation pour la Recherche Médicale (FRM) and le Consortium National de la Recherche en Génomique (CNRG; project IR1238) (to E.S.C.), and an NSERC Discovery Grant (to J.T.W.).
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 20 June 2011.
REFERENCES
- 1. Abrieu A., et al. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106:83–93 [DOI] [PubMed] [Google Scholar]
- 2. Akerfelt M., et al. 2008. Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2. Proc. Natl. Acad. Sci. U. S. A. 105:11224–11229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akerfelt M., Morimoto R. I., Sistonen L. 2010. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 11:545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akerfelt M., et al. 2010. Heat shock transcription factor 1 localizes to sex chromatin during meiotic repression. J. Biol. Chem. 285:34469–34476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbero J. L. 2009. Cohesins: chromatin architects in chromosome segregation, control of gene expression and much more. Cell. Mol. Life Sci. 66:2025–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baudat F., de Massy B. 2007. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res. 15:565–577 [DOI] [PubMed] [Google Scholar]
- 7. Bellve A. R., et al. 1977. Spermatogenesis cells of the prepuberal mouse. J. Cell Biol. 74:68–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bierkamp C., et al. 2010. Lack of maternal heat shock factor 1 results in multiple cellular and developmental defects, including mitochondrial damage and altered redox homeostasis, and leads to reduced survival of mammalian oocytes and embryos. Dev. Biol. 339:338–353 [DOI] [PubMed] [Google Scholar]
- 9. Bolcun-Filas E., et al. 2009. Mutation of the mouse Syce1 gene disrupts synapsis and suggests a link between synaptonemal complex structural components and DNA repair. PLoS Genet. 5:e1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193 [DOI] [PubMed] [Google Scholar]
- 11. Chang Y., et al. 2006. Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression. Genes Dev. 20:836–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christians E., et al. 1997. Evidence for the involvement of mouse heat shock factor 1 in the atypical expression of the HSP70.1 heat shock gene during mouse zygotic genome activation. Mol. Cell. Biol. 17:778–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christians E., Davis A. A., Thomas S. D., Benjamin I. J. 2000. Maternal effect of Hsf1 on reproductive success. Nature 407:693–694 [DOI] [PubMed] [Google Scholar]
- 14. Christians E. S., Yan L. J., Benjamin I. J. 2002. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit. Care Med. 30:S43–S50 [PubMed] [Google Scholar]
- 15. Christians E. S., Benjamin I. J. 2006. Heat shock response: lessons from mouse knockouts. Handb. Exp. Pharmacol. 172:139–152 [DOI] [PubMed] [Google Scholar]
- 16. Collins T. J. 2007. ImageJ for microscopy. Biotechniques 43:25–30 [DOI] [PubMed] [Google Scholar]
- 17. De Felici M., et al. 2005. Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death. Reprod. Biomed. Online 10:182–191 [DOI] [PubMed] [Google Scholar]
- 18. de Vries W. N., et al. 2000. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes. Genesis 26:110–112 [PubMed] [Google Scholar]
- 19. Ghafari F., Gutierrez C. G., Hartshorne G. M. 2007. Apoptosis in mouse fetal and neonatal oocytes during meiotic prophase one. BMC Dev. Biol. 24:7–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilliland W. D., et al. 2007. The multiple roles of mps1 in Drosophila female meiosis. PLoS Genet. 3:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonsalves S. E., Moses A. M., Razak Z., Robert F., Westwood J. T. 2011. Whole-genome analysis reveals that active heat shock factor binding sites are mostly associated with non-heat shock genes in Drosophila melanogaster. PLoS One 6:e15934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Handel M. A., Schimenti J. C. 2010. Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat. Rev. Genet. 11:124–136 [DOI] [PubMed] [Google Scholar]
- 23. Hawley R. S. 2011. Oogenesis: when most is good enough. Curr. Biol. 21:R288–R290 [DOI] [PubMed] [Google Scholar]
- 24. Hodges C. A., Hunt P. A. 2002. Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma 111:165–169 [DOI] [PubMed] [Google Scholar]
- 25. Hodges C. A., Revenkova E., Jessberger R., Hassold T. J., Hunt P. A. 2005. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat. Genet. 37:1351–1355 [DOI] [PubMed] [Google Scholar]
- 26. Homer H., Gui L., Carroll J. 2009. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science 326:991–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunt P. A., Hassold T. J. 2002. Sex matters in meiosis. Science 296:2181–2183 [DOI] [PubMed] [Google Scholar]
- 28. Kallio M., et al. 2002. Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in Hsf2 null mice. EMBO J. 21:2591–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kan R., et al. 2008. Comparative analysis of meiotic progression in female mice bearing mutations in genes of the DNA mismatch repair pathway. Biol. Reprod. 78:462–471 [DOI] [PubMed] [Google Scholar]
- 30. Kwiatkowski N., et al. 2010. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat. Chem. Biol. 6:359–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 32. Lan Z. J., Xu X., Cooney A. J. 2004. Differential oocyte-specific expression of Cre recombinase activity in GDF-9-iCre, Zp3cre, and Msx2Cre transgenic mice. Biol. Reprod. 71:1469–1474 [DOI] [PubMed] [Google Scholar]
- 33. Lenzi M. L., et al. 2005. Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis I in human oocytes. Am. J. Hum. Genet. 76:112–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindquist S., Craig E. A. 1988. The heat-shock proteins. Annu. Rev. Genet. 22:631–677 [DOI] [PubMed] [Google Scholar]
- 35. Llano E., et al. 2008. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 22:2400–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loison F., et al. 2006. Up-regulation of the clusterin gene after proteotoxic stress: implication of HSF1-HSF2 heterocomplexes. Biochem. J. 395:223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMillan D. R., Xiao X., Shao L., Graves K., Benjamin I. J. 1998. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 273:7523–7528 [DOI] [PubMed] [Google Scholar]
- 38. McMillan D. R., et al. 2002. Heat shock transcription factor 2 is not essential for embryonic development, fertility, or adult cognitive and psychomotor function in mice. Mol. Cell. Biol. 22:8005–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Medina I., et al. 2010. Babelomics: an integrative platform for the analysis of transcriptomics, proteomics and genomic data with advanced functional profiling. Nucleic Acids Res. 38:W210–W213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Metchat A., et al. 2009. Mammalian heat shock factor 1 is essential for oocyte meiosis and directly regulates Hsp90alpha expression. J. Biol. Chem. 284:9521–9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moens P. B., Marcon E., Shore J. S., Kochakpour N., Spyropoulos B. 2007. Initiation and resolution of interhomolog connections: crossover and non-crossover sites along mouse synaptonemal complexes. J. Cell Sci. 120:1017–1027 [DOI] [PubMed] [Google Scholar]
- 42. Novak I., et al. 2008. Cohesin Smc1beta determines meiotic chromatin axis loop organization. J. Cell Biol. 180:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Keeffe C., Hulten M. A., Tease C. 1997. Analysis of proximal X chromosome pairing in early mouse meiosis. Chromosoma 106:276–283 [DOI] [PubMed] [Google Scholar]
- 44. Peters A. H., Plug A. W., van Vugt M. J., de Boer P. 1997. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 5:66–68 [DOI] [PubMed] [Google Scholar]
- 45. Pirkkala L., Nykanen P., Sistonen L. 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15:1118–1131 [DOI] [PubMed] [Google Scholar]
- 46. Poss K. D., Nechiporuk A., Stringer K. F., Lee C., Keating M. T. 2004. Germ cell aneuploidy in zebrafish with mutations in the mitotic checkpoint gene mps1. Genes Dev. 18:1527–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Revenkova E., Herrmann K., Adelfalk C., Jessberger R. 2010. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr. Biol. 20:1529–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Salmand P. A., Jungas T., Fernandez M., Conter A., Christians E. S. 2008. Mouse heat-shock factor 1 (HSF1) is involved in testicular response to genotoxic stress induced by doxorubicin. Biol. Reprod. 79:1092–1101 [DOI] [PubMed] [Google Scholar]
- 49. Sandqvist A., et al. 2009. Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol. Biol. Cell 20:1340–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santucci-Darmanin S., et al. 2000. MSH4 acts in conjunction with MLH1 during mammalian meiosis. FASEB J. 14:1539–1547 [DOI] [PubMed] [Google Scholar]
- 51. Sarge K. D., Zimarino V., Holm K., Wu C., Morimoto R. I. 1991. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 5:1902–1911 [DOI] [PubMed] [Google Scholar]
- 52. Suja J. A., Barbero J. L. 2009. Cohesin complexes and sister chromatid cohesion in mammalian meiosis. Genome Dyn. 5:94–116 [DOI] [PubMed] [Google Scholar]
- 53. Trinklein N. D., Murray J. I., Hartman S. J., Botstein D., Myers R. M. 2004. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol. Biol. Cell 15:1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Trinklein N. D., Chen W. C., Kingston R. E., Myers R. M. 2004. Transcriptional regulation and binding of heat shock factor 1 and heat shock factor 2 to 32 human heat shock genes during thermal stress and differentiation. Cell Stress Chaperones 9:21–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vogt E., Kirsch-Volders M., Parry J., Eichenlaub-Ritter U. 2008. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat. Res. 651:14–29 [DOI] [PubMed] [Google Scholar]
- 56. Wang G., et al. 2004. Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis 38:66–80 [DOI] [PubMed] [Google Scholar]
- 57. Xiao X., et al. 1999. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 18:5943–5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xing H., et al. 2005. Mechanism of hsp70i gene bookmarking. Science 307:421–423 [DOI] [PubMed] [Google Scholar]
- 59. Yang F., Baumann C., De La Fuente R. 2009. Persistence of histone H2AX phosphorylation after meiotic chromosome synapsis and abnormal centromere cohesion in poly (ADP-ribose) polymerase (Parp-1) null oocytes. Dev. Biol. 331:326–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang F., Wang P. J. 2009. The mammalian synaptonemal complex: a scaffold and beyond. Genome Dyn. 5:69–80 [DOI] [PubMed] [Google Scholar]
- 61. Yuan L., et al. 2002. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SYCP3. Science 296:1115–1118 [DOI] [PubMed] [Google Scholar]
- 62. Zickler D. 2006. From early homologue recognition to synaptonemal complex formation. Chromosoma 115:158–174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.