Abstract
Integrin-linked kinase (ILK) is an essential component of the cardiac mechanical stretch sensor and is bound in a protein complex with parvin and PINCH proteins, the so-called ILK-PINCH-parvin (IPP) complex. We have recently shown that inactivation of ILK or β-parvin activity leads to heart failure in zebrafish via reduced protein kinase B (PKB/Akt) activation. Here, we show that PINCH proteins localize at sarcomeric Z disks and costameres in the zebrafish heart and skeletal muscle. To investigate the in vivo role of PINCH proteins for IPP complex stability and PKB signaling within the vertebrate heart, we inactivated PINCH1 and PINCH2 in zebrafish. Inactivation of either PINCH isoform independently leads to instability of ILK, loss of stretch-responsive anf and vegf expression, and progressive heart failure. The predominant cause of heart failure in PINCH morphants seems to be loss of PKB activity, since PKB phosphorylation at serine 473 is significantly reduced in PINCH-deficient hearts and overexpression of constitutively active PKB reconstitutes cardiac function in PINCH morphants. These findings highlight the essential function of PINCH proteins in controlling cardiac contractility by granting IPP/PKB-mediated signaling.
INTRODUCTION
Integrin-linked kinase (ILK) is an essential component of the cardiac mechanical stretch sensor, modulating expression of stretch-responsive genes such as anf and vegf and thereby enabling the heart to adapt its force of contraction to changing hemodynamic needs. Many of the currently known ILK-mediated functions require additional cofactors and interacting proteins. Increasing focus has therefore been put on the roles of the ternary complex comprising the proteins ILK, α-, β-, or γ-parvin, and PINCH, PINCH1 or PINCH2 (5, 26, 30, 34).
The ILK-parvin-PINCH (IPP) complex provides physical linkage between integrin transmembrane receptors and the actin cytoskeleton and serves as a signaling mediator that transduces mechanical signals to downstream effectors (32). PINCH and parvins do not have catalytic activity but rather function as adapters for other signaling molecules such as Nck-2 and Ras suppressor 1 (RSU-1), through which PINCH can regulate c-Jun N-terminal kinase (JNK) (7, 25).
In cardiomyocyte cell cultures, the IPP complex is known to regulate integrin-mediated signaling pathways involved in hypertrophic and apoptotic responses (5). In vivo, a role of the IPP complex and specifically of PINCH proteins in the control of cardiac contractility was demonstrated in a recent study using PINCH-deficient mice. Cardiac muscle-specific double knockout of pinch1 and pinch2, but not single deletion of either gene, resulted in heart failure and early postnatal lethality; however, the underlying molecular pathway was ultimately not elucidated (16).
We recently found that zebrafish ILK regulates the cardiac stretch response and contractility via protein kinase B (PKB)-VEGF signaling (2). To investigate if PINCH affects IPP-PKB signaling, we analyzed PINCH1 and PINCH2 function in the zebrafish heart. We show that PINCH localizes at sarcomeric Z disks/costameres in heart and skeletal muscle. In contrast to mice, knockdown of either PINCH1 or PINCH2 in zebrafish independently leads to severe heart failure, similar to the phenotype observed in the ILK main squeeze (msq) mutant (2). Loss of PINCH leads to ILK protein instability and consequently affects ILK-mediated downstream signaling, resulting in reduced PKB phosphorylation and expression of stretch-responsive genes. Finally, we show that restoration of defective PKB signaling is sufficient to reconstitute cardiac contractility and the stretch-responsive gene program in PINCH morphants, demonstrating an important role of PINCH proteins in IPP-PKB-dependent cardiac mechanosensing and transduction.
MATERIALS AND METHODS
Zebrafish strains.
Care and breeding of zebrafish, Danio rerio, were as described previously (27). The present study was performed after appropriate institutional approvals were secured. It conforms to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (24a). For all mRNA and morpholino injection procedures, the TüAB wild-type strain was used.
Histology, transmission electron microscopy (TEM), immunostaining, immunoblotting, real-time PCR, and RNA in situ hybridization.
Histological analysis and electron micrographs of fish embryos were performed as described previously (22). For whole-mount, sectioned hematoxylin-eosin (HE) staining and immunostaining, embryos were fixed in 4% paraformaldehyde. Immunostaining was performed on 8-μm cryosections, using a polyclonal anti-PINCH1 IgG antibody (15), a monoclonal anti-PINCH1 antibody raised against amino acids (aa) 1 to 108 of recombinant human PINCH1 (R&D Systems), an anti-ILK IgG antibody (2), a polyclonal anti-vinculin antibody (Sigma), and a monoclonal anti-α-actinin IgG1 antibody (Sigma, Germany). Secondary antibodies included goat anti-mouse IgG2b-tetramethyl rhodamine isocyanate (TRITC) and goat anti-mouse IgG1-fluorescein isothiocyanate (FITC) antibodies (Southern Biotech), anti-rabbit IgG-TRITC antibody (Sigma, Germany), and anti-rabbit IgG-FITC antibody (Molecular Probes). Western blots were probed with both the monoclonal and polyclonal anti-PINCH1 antibodies, a polyclonal anti-PINCH2 antibody (28), or anti-ILK antibody as primary antibodies, and signals were detected by chemiluminescence. Loading controls included mouse anti-rabbit β-actin (Sigma-Aldrich, Germany) and rabbit anti-human pan-cadherin (Abcam). anf mRNA whole-mount in situ hybridization and anf and vegf real-time PCR were carried out as described previously (2). For real-time PCR, we used the SYBR green method with the following primers: for anf, forward primer 5′-ACAGAGACCGAGAGGAAGCA-3′ and reverse primer 5′-AGGGTGCTGGAAGACCCTAT-3′), and for vegf, forward primer 5′-ATGAACTTGGTTGTTTATTTGA-3′ and reverse primer 5′-TTCTTTGCTTTGACTTCTGC-3′). ef1a was used as the control gene.
Morpholino injection procedures and transient overexpression of protein kinase B.
Morpholino-modified antisense oligonucleotides (Gene Tools) were directed against the splice-donor site of exon 5 (MO1-pinch1 [5′-CGCTGCTCTACTGACCCGCAGTTGC-3′]) or the translational start site (MO2-pinch1 [5′-GCCATGTTACTGCCCGTCATCTCTG-3′]) of zebrafish pinch1 (zpinch1). zpinch2 was targeted using morpholinos directed against its translational start site (MO2-pinch2 [5′-GAGCTTCTGCTTCTGCTTCCATCAT-3′]). zpinch1 and zpinch2 antisense oligonucleotides or a standard control oligonucleotide (MO-control), diluted in 0.2 mol/liter KCl, were microinjected into one-cell-stage zebrafish embryos. Transient overexpression of constitutive-active or kinase-deficient PKB was performed essentially as described previously (2).
Functional assessment and statistical analysis.
Still images and video films were recorded and digitized with a Zeiss MCU II microscope. The functional assessment of cardiac contractility was carried out as described before (23). Fractional shortening (FS) and atrial and ventricular diameters were assayed with the help of the zebraFS software (http://www.benegfx.de). If not further specified, results are expressed as means ± standard deviations. Analyses were performed using unpaired Student's t test, and a P value of <0.05 was accepted as statistically significant.
RESULTS
PINCH1 localizes to the cardiac Z disk of the zebrafish heart.
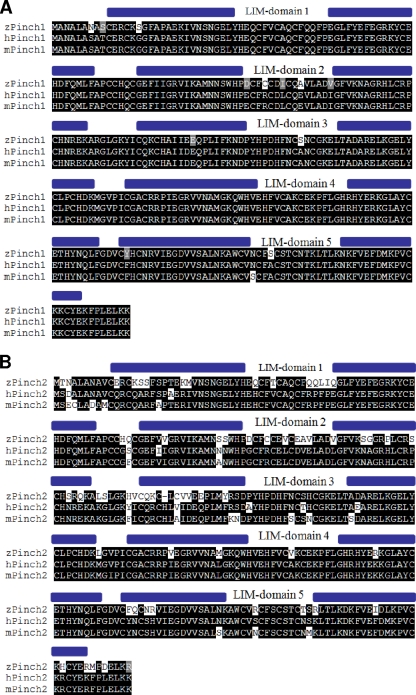
We identified the zebrafish orthologs of PINCH1 (expressed sequence tag [EST]; GenBank accession number BQ130993) and PINCH2 (GenBank accession number BC065908.1) by BLAST analysis of human PINCH protein sequences against the NCBI zebrafish EST database. Both zebrafish PINCH proteins are highly conserved, showing 96% and 78% sequence identities with human PINCH1 and PINCH2, respectively (Fig. 1A and B). Like their mammalian counterparts, zebrafish PINCH1 and PINCH2 contain 5 LIM domains, followed by a short C-terminal tail.
Fig. 1.
Identification of zebrafish PINCH1 and PINCH2. (A and B) Amino acid sequence alignments of zebrafish (z), human (h), and mouse (m) PINCH1 (A) and PINCH2 (B), demonstrating the high cross-species homology of each PINCH ortholog. Black background, amino acid identity; gray background, amino acids with similar chemical properties.
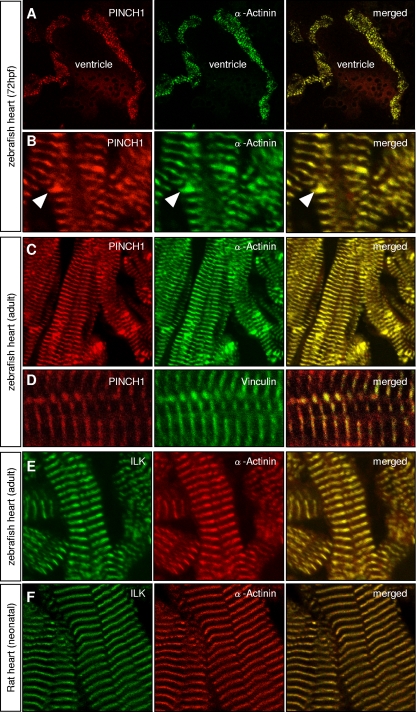
In mice, PINCH1 is expressed in various tissues, including the heart and skeletal muscle (4). To evaluate in detail the subcellular expression of PINCH1 in heart and skeletal muscle cells of zebrafish, we performed immunostainings with polyclonal (Fig. 2) and monoclonal (data not shown) antibodies directed against PINCH1. As revealed by coimmunofluorescence imaging with α-actinin, an established marker of sarcomeric Z disks, zPINCH1 shows the specific stripy pattern of sarcomeric Z-disk localization in both cardiomyocytes and skeletal muscle cells of embryonic and adult zebrafish (Fig. 2A to D). We furthermore find localization of PINCH1 at intercalated disks of zebrafish cardiomyocytes (Fig. 2B, arrowheads), whereas other sarcomeric structures are devoid of PINCH1 expression (2, 22). Colocalization with vinculin (Fig. 2D), an established marker of Z disks and costameres (29), which connect peripheral Z disks with adjoining sarcolemma, further supports our conclusion that PINCH1 localizes to Z disks.
Fig. 2.
PINCH1 is expressed at Z disks of the embryonic and adult zebrafish heart. In the embryonic (A and B) and the adult (C and D) zebrafish heart, PINCH1 protein localizes specifically to the cardiac Z disks and intercalated disks (B, arrowheads). Localization of PINCH1 at the sarcomeric Z disk of cardiomyocytes is demonstrated by coimmunostaining with α-actinin (A to C) and vinculin (D). ILK is also highly expressed at the sarcomeric Z disk, as shown by coimmunostaining with α-actinin in zebrafish (E) and rat (F) hearts.
PINCH deficiency leads to heart failure in zebrafish.
To investigate the in vivo function of zebrafish PINCH proteins, we inactivated PINCH1 or PINCH2 by blocking either the translational start site of pinch1 (MO2-pinch1) or pinch2 (MO2-pinch2) or the splice donor site of exon 5 of pinch1 (MO1-pinch1) with morpholino-modified antisense oligonucleotides.
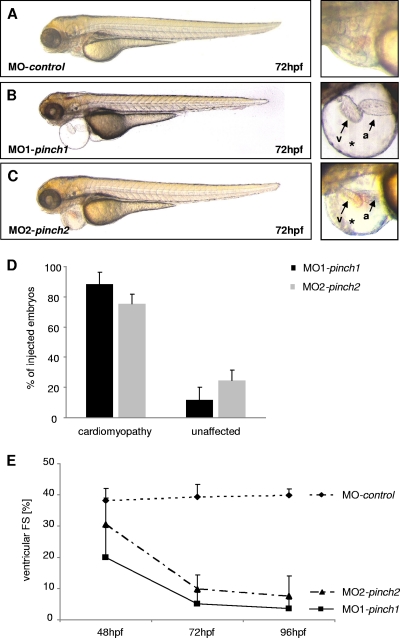
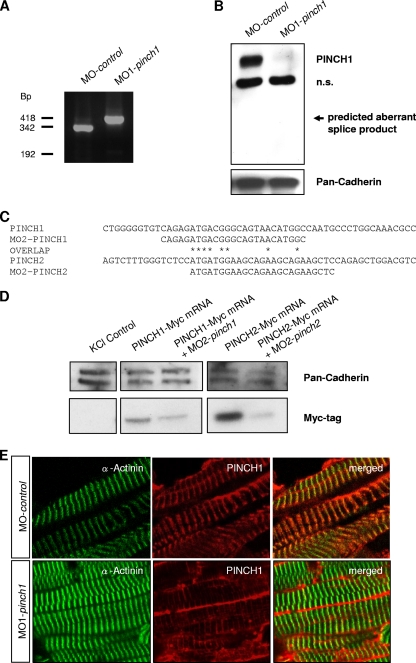
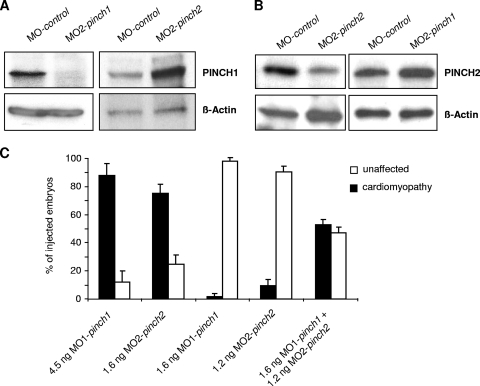
When either 4 ng of MO1-pinch1 or 4.5 ng of MO2-pinch1 was injected, up to 88% of injected embryos develop progressive heart failure (Fig. 3A to D). Starting at 48 hours postfertilization (hpf), ventricular contractility of PINCH1 morphants decreases progressively and FS of the ventricular chamber declines from 20% ± 10% at 48 hpf to 5% ± 4% at 72 hpf. By 96 hpf, the ventricular chamber becomes almost silent (Fig. 3E; see Movie S1 in the supplemental material). Similarly, when PINCH2 was targeted by injecting 1.6 ng of MO1-pinch2, 75% of injected embryos develop the same heart failure phenotype (Fig. 3A to E), with the ventricular FS declining from 30.5% ± 9% at 48 hpf to 9.8% ± 5% at 72 hpf and 7.6% ± 6.3% at 96 hpf (Fig. 3E; see Movie S2 in the supplemental material). These findings demonstrate that in contrast to mice, loss of either PINCH1 or PINCH2 independently leads to heart failure in zebrafish. To ensure that the PINCH morpholinos used are indeed (i) functional in blocking PINCH expression and (ii) specific to the respective PINCH protein targeted, we have performed a number of control experiments (Fig. 4A to E and 5A and B). Isolation of mRNA from MO1-pinch1-injected embryos confirmed the predicted effect on mRNA splicing, namely, either skipping of exon 5 (192-bp product) or integration of intron 5 (418-bp product), both leading to premature termination of protein translation. Wild-type zpinch1 cDNA (342 bp product) was not detectable in the injected embryos (Fig. 4A). This is confirmed by Western blot analysis of MO1-pinch1-injected embryos, as neither wild-type PINCH1 protein (molecular mass, approximately 37 kDa) nor aberrant PINCH1 protein (predicted molecular mass, 20.8 kDa) is detected when an antibody raised against the untargeted amino acids 1 to 108 of PINCH1 is used (Fig. 4B). The start site morpholinos MO2-pinch1 and MO2-pinch2 were chosen carefully to target cDNA sequences near the translational start sites of both proteins, which are poorly conserved, to avoid cross-reactivity (Fig. 4C). As shown in Fig. 5A and B, MO2-pinch1 specifically blocks protein translation of PINCH1 but not PINCH2, and vice versa, MO2-pinch2 specifically knocks down PINCH2 but not PINCH1. In fact, our results show that PINCH1 and PINCH2 are, rather, upregulated in zebrafish in which the respective other isoform has been knocked down, indicating an attempt to compensate for the loss of one isoform by upregulation of the other, which, however, fails to prevent the heart failure phenotype observed in morphant embryos. Lastly, the efficacy and specificity of PINCH1 knockdown via MO1-pinch1 injection were again confirmed by immunostaining of PINCH1 morphant tissue, showing significantly reduced fluorescence intensity in morphant versus control muscle tissues (Fig. 4E).
Fig. 3.
Knockdown of PINCH1 or PINCH2 leads to cardiomyopathy and heart failure. (A to C) MO1-pinch1- and MO2-pinch2-injected embryos develop pericardial edema (*) and precardial blood congestion due to disturbed cardiac contractility. Lateral views of MO-control-injected (A), MO1-pinch1-injected (B), and MO2-pinch2-injected (C) embryos at 72 hpf (v, ventricle; a, atrium). (D) After injection of MO1-pinch1 or MO2-pinch2, 88% and 75% of morphant embryos, respectively, develop heart failure. (E) FS of the ventricular chambers of MO-control-, MO1-pinch1-, and MO2-pinch2-injected embryos measured at the indicated developmental stages. FS is significantly reduced in PINCH morphants after 48 hpf and further declines by 96 hpf.
Fig. 4.
Morpholino-mediated knockdown of PINCH1 and PINCH2 is efficient and specific. (A) cDNA analysis of PINCH1 morphants after injection of MO1-pinch1 shows the expected effect of the morpholino on splicing, namely, the integration of intron 5 (418-bp product) or skipping of exon 5 (192-bp product). The wild-type form of pinch1 is undetectable in PINCH1-knockdown zebrafish. (B) Western blot analysis of MO-control- and MO1-pinch1-injected embryos. While PINCH1 levels are normal in MO-control-injected embryos, they are severely reduced after knockdown in the respective PINCH-morphant embryos. An aberrantly spliced PINCH1 fragment cannot be detected, indicating protein instability of such a truncated form. n.s., not specific. (C) Although PINCH1 and -2 are highly conserved on the protein level, the start-site morpholinos against PINCH1 (MO2-pinch1) and PINCH2 (MO2-pinch2) share only 8 overlapping homologous DNA bases, resulting in a high specificity of the antisense probes. (D) As demonstrated by Western blot analysis, MO2-pinch1 targets Myc-tagged PINCH1 mRNA and MO2-pinch2 targets Myc-tagged PINCH2 mRNA, as predicted. (E) Coimmunostaining of PINCH1 with α-actinin in MO-control- and MO1-pinch1-injected zebrafish embryos. As shown, knockdown of PINCH1 (lower panels) leads to reduced immunofluorescence at the Z disk, whereas PINCH1 is normally expressed and localized in control-injected zebrafish (upper panels).
Fig. 5.
PINCH1 and PINCH2 have synergistic functions. (A and B) Western blot analysis of MO-control-, MO2-pinch1-, and MO2-pinch2-injected embryos. While PINCH levels are normal in MO-control-injected embryos, they are severely reduced after knockdown in the respective PINCH-morphant embryos. Interestingly, in each case the untargeted PINCH form is slightly increased, indicating a partially compensatory mechanism. (C) Titrated double knockdown of PINCH1 and PINCH2. As shown, knockdown with 4.5 ng MO1-pinch1 or 1.6 ng MO2-pinch2 does not lead to a cardiomyopathic phenotype. However, in double knockdowns of PINCH1 and PINCH2 using 1.6 ng MO1-pinch1 and 1.2 ng MO2-pinch2, a high number of zebrafish embryos show the PINCH cardiomyopathic phenotype, indicating a synergistic effect of the two PINCH forms.
To further investigate if PINCH proteins may also have synergistic functions in zebrafish, we performed titrated knockdown experiments for dose-dependent inactivation of both PINCH forms. As shown in Fig. 5C, injection of either 1.6 ng MO1-pinch1 or 1.2 ng MO2-pinch2 does not induce the cardiomyopathy phenotype. However, after coinjection of 1.6 ng MO1-pinch1 and 1.2 ng MO2-pinch2, a significant number (52.8%) of zebrafish embryos display the PINCH-morphant cardiomyopathic phenotype, indicating a synergistic function of PINCH1 and -2.
In addition to the loss of cardiac contractility, skeletal muscle function is also impaired in PINCH-knockdown embryos. On tactile stimulation, MO-pinch1-injected as well as MO-pinch2-injected embryos show only an insufficient flight response and trembling skeletal muscle movements or even complete paralysis (see Movie S3 in the supplemental material).
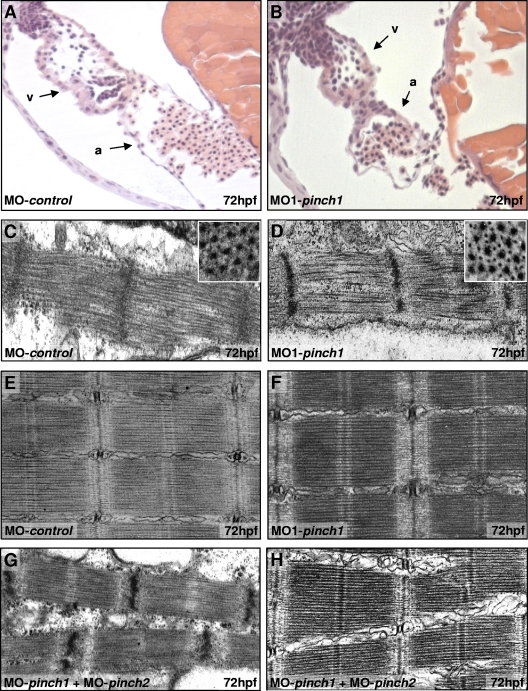
In order to evaluate how PINCH1 or PINCH2 deficiency induces impairment of cardiac contractility, we next analyzed cardiac morphology and ultrastructure in PINCH1 and PINCH2 morphant zebrafish embryos. Histological analysis of PINCH1-deficient hearts shows that cardiac chambers are well defined, with atrium and ventricle separated by the atrioventricular ring (Fig. 6A and B), and that endocardial and myocardial layers of both chambers have developed properly with a multilayered ventricular myocardium. In Drosophila melanogaster, loss of ILK function results in detachment of actin filaments from muscle ends (33). Similarly, in PINCH1 and -2 double-knockout mice, hearts exhibit disruptions of intercalated disks accompanied by interstitial fibrosis. Hence, to determine whether the contractility defects in PINCH1- or PINCH2-depleted zebrafish hearts may be caused by disturbed myofibrillogenesis or intercalated disk abnormalities and consequent myocyte detachment, we analyzed PINCH1 and PINCH2 morphant hearts and skeletal muscle by TEM. However, heart and skeletal muscle cells of PINCH1 (Fig. 6C to F) or PINCH2 (data not shown) morphant embryos show normal, highly organized arrays of maturated thick and thin myofilaments interconnected by Z disks and aligned in discernible A/I and M bands. Furthermore, there are no apparent signs of cardiac or skeletal muscle detachment. Similarly, in PINCH1 and -2 double-knockdown embryos, cardiac (Fig. 6G) and skeletal (Fig. 6H) muscle ultrastructure appears normal. Hence, although PINCH morphants suffer from severe cardiac and skeletal muscle dysfunction, there are no ultrastructural abnormalities, indicating that heart failure in zebrafish deficient in either or both PINCH isoforms is not due to structural alterations but can, rather, be attributed to functional defects.
Fig. 6.
PINCH is dispensable for sarcomere formation in heart and skeletal muscle cells. (A and B) Hematoxylin-eosin-stained sagittal histological sections of MO-control and MO1-pinch1 morphant hearts at 72 hpf. Besides collapse of the ventricular chamber, morphants display normal heart morphology with distinct endocardial and myocardial cell layers in atrium (a) and ventricle (v). (C through F) Ultrastructural analysis of ventricular cardiomyocytes (C and D) and skeletal muscle cells (E and F) of MO-control- and MO1-pinch1-injected embryos at 72 hpf shows highly organized sarcomeres with thin and thick myofilaments in well-aligned bundles and discernible A/I, M, and Z bands. (G and H) Even PINCH1 and -2 double knockdowns show aligned heart (G) and skeletal muscle (H) sarcomeres, demonstrating that structural abnormalities are not responsible for heart failure in PINCH-deficient embryos.
PINCH deficiency induces ILK protein instability and vice versa.
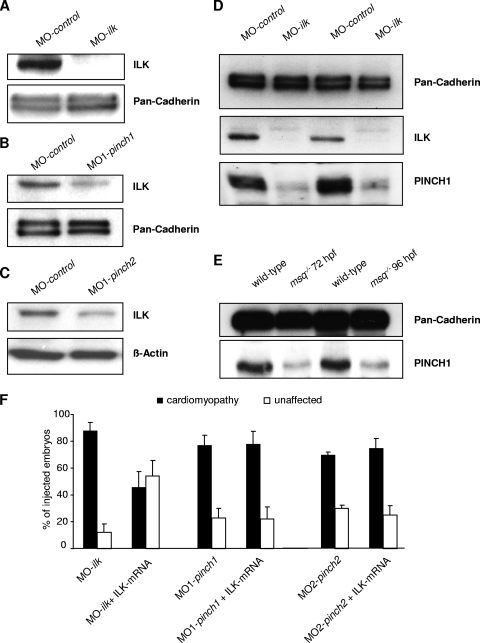
It has been previously reported that protein stability of components of the IPP complex depends on IPP complex integrity. Accordingly, PINCH binding-deficient ILK variants fail to localize to focal adhesions sites (35). Similarly, small interfering RNA-mediated depletion of PINCH1 in HeLa cells leads to downregulation of ILK protein levels in vitro (9). Hence, to test whether zebrafish PINCH1 or PINCH2 is essential for ILK protein stability in vivo, we assayed ILK protein levels in PINCH1- or PINCH2-deficient zebrafish embryos. After knockdown of PINCH1 or PINCH2, protein levels of PINCH1 or PINCH2 are significantly reduced in the respective knockdown embryos (Fig. 5A and B). As shown in Fig. 7B and C, PINCH deficiency leads to a significant reduction of ILK protein levels; however, ILK is not completely abolished as in embryos in which ILK has been knocked down using morpholino (Fig. 7A). To test whether ILK is also necessary for stability of PINCH, we next analyzed PINCH1 protein expression in both ILK-depleted zebrafish embryos (Fig. 7D) and ILK mutant msq embryos (Fig. 7E), which harbor a β-parvin binding-deficient form of ILK. Interestingly, expression of PINCH1 is reduced in both, showing that the stability of PINCH1 is strongly dependent on the presence and functionality of ILK. These results demonstrate that zebrafish PINCH proteins are necessary for ILK protein stability in vivo and vice versa. Furthermore, loss of PINCH cannot be compensated for by overexpression of ILK (Fig. 7F), indicating that a functional and self-stabilizing complex consisting of ILK and PINCH is required for unconstrained cardiac function.
Fig. 7.
Knockdown of PINCH induces ILK protein instability and vice versa. (A to C) To determine the effect of PINCH deficiency on the IPP complex and in particular on ILK protein stability, Western blot analysis of MO-control-, MO-ilk-, MO1-pinch1-, and MO1-pinch2-injected embryos probed against an ILK antibody was carried out. ILK protein levels are normal in MO-control-treated embryos, while injection of MO-ilk completely abolishes ILK protein expression (A). Interestingly, injection of either MO1-pinch1 (B) or MO1-pinch2 (C) also results in a significant reduction of ILK levels, indicating that PINCH is needed to maintain ILK protein stability. (D and E) In ILK morphants as well as in ILK msq mutant zebrafish, PINCH1 expression is also severely reduced, demonstrating that, vice versa, ILK is also mandatory for PINCH stability. (F) Bar graphs representing ventricular fractional shortening of MO-ilk-, MO1-pinch1-, and MO2-pinch2-injected embryos. While coinjection of ILK mRNA can compensate for knockdown of endogenous ILK, it cannot rescue the cardiomyopathic phenotype in PINCH morphants, underlining the fact that only a functional IPP complex is able to control stretch-responsive signaling.
PINCH proteins control cardiac contractility via PKB-mediated signaling.
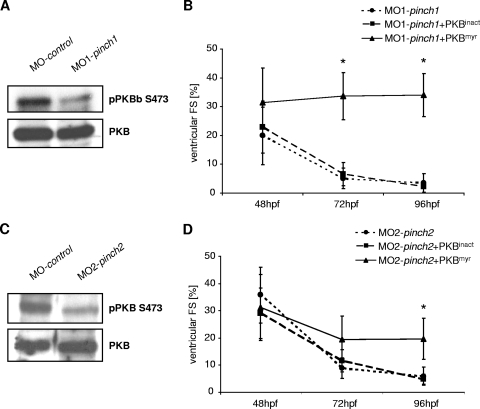
We have previously shown that ILK regulates cardiac contractility via PKB, modulating the expression of stretch-responsive genes such as anf and vegf (2). To investigate whether disturbed ILK-PKB-mediated signaling accounts for heart failure in PINCH morphants, we investigated PKB phosphorylation at serine 473. As shown in Fig. 8A and C, the amount of phosphorylated PKB in zebrafish embryos is significantly reduced after PINCH1 or PINCH2 knockdown in comparison to control-injected embryos, while overall PKB levels remain unchanged. To test if reduction in PKB phosphorylation is per se causative for heart failure in PINCH morphants, we transiently overexpressed a constitutively active PKB variant in PINCH1- or PINCH2-deficient embryos to bypass IPP-PKB signaling. Indeed, injection of 380 pg mRNA encoding constitutively active, myristoylated PKB (PKBmyr) restores cardiac contractility in PINCH1 and -2 morphants, while injection of the same amount of kinase-dead PKB mRNA (PKBinact) does not reconstitute heart function in PINCH1- and -2 morphants (Fig. 8B and D).
Fig. 8.
PINCH proteins regulate PKB activation in the zebrafish heart. (A and C) Western blot analysis of serine 473-phosphorylated PKB (pPKB S473) in comparison to whole PKB. In both PINCH1- and PINCH2-morphant zebrafish embryos, phosphorylation of PKB is strongly reduced. (B and D) Ventricular fractional shortening measurements of wild-type zebrafish embryos injected with either MO-pinch1 or MO-pinch2 alone or MO-pinch1 and MO-pinch2 in combination with constitutive active PKB (PKBmyr) or kinase dead PKB (PKBinact) mRNA. As shown, only constitutive active PKB restores cardiac contractility in PINCH morphants.
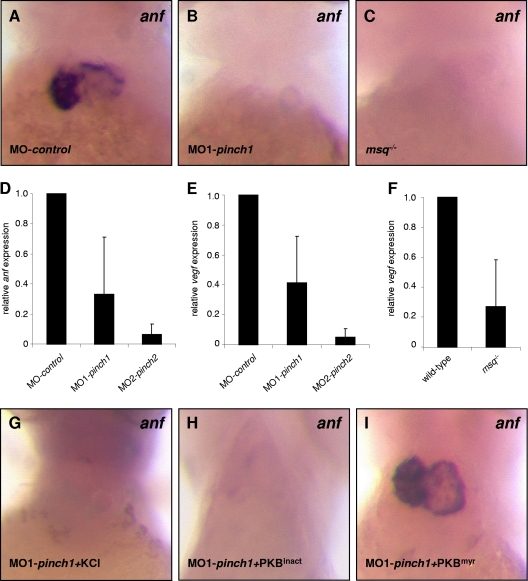
Next, we investigated the expression of stretch-responsive genes anf and vegf by whole-mount mRNA antisense in situ hybridization and quantitative real-time PCR in PINCH morphants (Fig. 9). While anf is highly expressed in atria and ventricles of control embryos, abrogation of either ILK in msq mutants (Fig. 9F) or PINCH1 and -2 via morpholino (Fig. 9D and E) leads to a loss of anf and vegf expression in affected zebrafish hearts. Importantly, we furthermore show that anf expression in PINCH1 morphants is reconstituted to almost normal levels in those zebrafish coinjected with constitutively active PKBmyr but not kinase-dead PKBinact (Fig. 9G to I), suggesting that ILK and PINCH act in a common molecular pathway.
Fig. 9.
Loss of PINCH leads to reduced expression of stretch-responsive anf and vegf. (A to C) RNA antisense in situ hybridization against atrial natriuretic factor (anf) demonstrates normal expression of the stretch-response marker anf in control-injected zebrafish in comparison to PINCH1 morphants or homozygous msq−/− embryos, where loss of anf expression is observed. (D to F) Quantitative real-time PCR of anf or vegf transcripts after injection of MO-control, MO-pinch1, or MO2-pinch2. In PINCH-morphant embryos, anf (D) as well as vegf (E) mRNA levels are significantly reduced, as observed in msq−/− mutant zebrafish (F and data not shown). (G to I) RNA antisense in situ hybridization demonstrates restored expression of anf mRNA in PINCH1 morphants injected with constitutive active PKB but not kinase-dead PKB.
DISCUSSION
The sensing of cellular mechanical stress and its transduction into molecular signals are vital for the vertebrate heart in order to adapt its contractile force and energy metabolism to changing hemodynamic needs. Several members of the cardiac stretch-sensing apparatus, such as β-integrin, melusin, and muscle LIM protein (MLP), have been identified as regulators of cardiac contractility and, when defective, lead to heart failure (3, 11). We have recently identified ILK, a β1-integrin-binding protein, to be a novel component of the cardiac stretch sensor by studying the zebrafish heart failure main squeeze (msq) mutant. ILK forms a link between integrin transmembrane receptors and sarcomeric structures at the costameres, the mechanical integration sites of cardiomyocytes, and regulates cardiomyocyte mechanotransduction and contractile force via PKB signaling. ILK contains four N-terminal ankyrin domains, which are proposed to underlie its mechanosensing ability by exhibiting tertiary structure-based elasticity and behaving as a nanospring (14).
We have investigated here the in vivo functions of PINCH1 and PINCH2 in zebrafish, which specifically bind to ILK via its ankyrin repeat domain and colocalize with ILK at the Z disk and costamere. We show that antisense oligonucleotide-mediated knockdown of PINCH1 or PINCH2 independently leads to severe heart failure, resembling the phenotype of msq mutants. The effect of PINCH knockdown also mimics that of the msq mutation on the molecular level by inducing ILK protein instability, showing decreased PKB activation and severely reduced expression of stretch-responsive genes such as anf and vegf.
PINCH and ILK not only interact with each other but also form a ternary complex with α, β, or γ-parvin, the so-called IPP complex. In mammals, this complex assembles in the cytoplasm before it is recruited to integrin adhesion sites, such as the intercalated disks of cardiomyocytes. The stability of the individual IPP components is ultimately dependent on the successful formation of the complex (9). We show here that knockdown of either PINCH1 or PINCH2 by itself leads to the destabilization of ILK and consequent heart failure in zebrafish. This stands in contrast to the situation in mice, where the single loss of either PINCH1 or PINCH2 does not immediately cause destabilization of the IPP complex and a cardiac failure, because loss of one PINCH isoform can be compensated for by upregulation of the other, which is sufficient to maintain IPP stability (16, 17, 28). While we observe a similar upregulation of one PINCH isoform in the absence of the other, this does not prevent the development of cardiomyopathy in PINCH1 or PINCH2 morphant embryos.
Interestingly, we did not observe underlying ultrastructural abnormalities in PINCH single- or double-morphant zebrafish hearts and skeletal muscle cells. This is similar to observations with the genetic ILK msq mutant, where the cardiac and skeletal muscle ultrastructures in msq mutants are also unaffected (2), but stands in contrast to recent findings from cardiac muscle-specific PINCH1 and -2 double-knockout mice, where abnormal intercalated disk structure and sarcomeric disarray were observed (16). This difference may be attributed to PINCH proteins fulfilling primarily functional rather than structural roles in the control of zebrafish heart and skeletal muscle contraction. Hence, disruption of cell-cell adhesion and sarcomere structure in PINCH-deficient mice may, at least in part, be a secondary consequence of long-standing contractile dysfunction (10), which we do not observe in PINCH-morphant zebrafish embryos, which die within 5 days after fertilization. The screening and generation of PINCH mutant zebrafish by targeting induced local lesions in genomes (TILLING) (24) or zinc-finger mediated gene silencing (1) will help to further unravel the distinct structural and functional roles of the IPP complex and its interaction partners in the beating heart.
The kinase domain of ILK lacks the highly conserved Asp-Phe-Gly (DFG) and His-Arg-Asp (HRD) motifs. Several studies, however, have shown that downstream factors such as cardiac myosin light chain 2 (CMLC-2), glycogen synthase kinase 3 beta (GSK-3β), and PKB are substrates of ILK activity, at least in vitro (6). A study by Maydan and colleagues, for example, provides the most recent evidence that ILK is a functional Mn2+-dependent protein kinase in vitro (20). However, in vivo studies in D. melanogaster, Caenorhabditis elegans, and mice showed that ILK-knockout models can be rescued using kinase-dead versions of ILK, suggesting that ILK activity may not be required in vivo and that ILK may, rather, act on its targets indirectly via associated proteins (13, 18, 19, 31, 33). Our own previous studies in zebrafish pointed toward zebrafish ILK possessing kinase activity in vitro. Furthermore, in ILK msq mutants, wild-type and constitutive kinase-active ILK variants could rescue the mutant phenotype, but ILK variants containing mutations within the kinase domain could not, indicating possible kinase activity in vivo. However, an alternative explanation could be the defective interaction of ILK with its binding partners, such as β-parvin and PINCH, which potentially mediate the downstream effects. Results in this study show the importance of the ILK-interacting PINCH proteins on PKB phosphorylation at serine 473, which is strongly reduced in PINCH-deficient cardiomyopathic zebrafish. Accordingly, ectopic expression of constitutively active PKB rescues the cardiomyopathy phenotype in PINCH morphants, showing that PKB is the main downstream effector of PINCH function in the control of cardiac contractility. Reduced phosphorylation of PKB in PINCH morphants could be due to destabilization of the IPP complex and, consequently, reduced ILK levels and ILK activity. Alternatively, PINCH proteins closely interact via their LIM5 domain (conserved KEVEF motif) with protein phosphatase 1 alpha (PP1α), a negative regulator of PKB activation (8). PINCH is responsible for keeping PP1α in an inactive state at focal adhesion sites, as shown in cell culture experiments (8). However, after loss of PINCH, PP1α switches to an activated state and dephosphorylates PKB at serine 473, possibly explaining the decreased phosphorylation of PKB in PINCH-morphant zebrafish.
Taken together, our results demonstrate that PINCH1 and PINCH2 are essential for unconstrained myocardial function by stabilizing the IPP complex and thereby regulating PKB activity. Since PINCH-morphant zebrafish exhibit a cardiomyopathy phenotype that is functionally, morphologically, and molecularly nearly identical to that of mutants with the ILK msq mutation and ILK mutations were recently found to cause human dilated cardiomyopathy (DCM) (12, 21), it will be interesting to analyze whether PINCH mutations also contribute to this heart muscle disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank H. Hosser, S. Hassel, and R. Nietsch for excellent technical assistance and Reinhard Fässler for providing us his PINCH1 and PINCH2 antibodies. We also thank Ingrid Haußer-Siller and Jacomine Krijnse-Locker (Electron Scan Core Facility of the University Heidelberg) for their helpful support.
This work was supported by grants from the Bundesministerium für Bildung und Forschung, NGFN-plus and NGFN-transfer (01GS0108, 01GS0420, 01GR0823, and 01GS0836); the European Union FP7 Programme INHERITANCE; and the Postdoc Fellowship of the Medical Faculty of the University of Heidelberg.
We have no conflicts of interest to disclose.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
Published ahead of print on 13 June 2011.
REFERENCES
- 1. Amacher S. L. 2008. Emerging gene knockout technology in zebrafish: zinc-finger nucleases. Brief. Funct. Genomic. Proteomic. 7:460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bendig G., et al. 2006. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 20:2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brancaccio M., et al. 2003. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat. Med. 9:68–75 [DOI] [PubMed] [Google Scholar]
- 4. Braun A., et al. 2003. PINCH2 is a new five LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp. Cell Res. 284:239–250 [DOI] [PubMed] [Google Scholar]
- 5. Chen H., et al. 2005. Role of the integrin-linked kinase/PINCH1/alpha-parvin complex in cardiac myocyte hypertrophy. Lab. Invest. 85:1342–1356 [DOI] [PubMed] [Google Scholar]
- 6. Dagnino L. 2011. Integrin-linked kinase: a Scaffold protein unique among its ilk. J. Cell Commun. Signal. 5:81–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delcommenne M., et al. 1998. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. U. S. A. 95:11211–11216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eke I., et al. 2010. PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha.. J. Clin. Invest. 120:2516–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukuda T., Chen K., Shi X., Wu C. 2003. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J. Biol. Chem. 278:51324–51333 [DOI] [PubMed] [Google Scholar]
- 10. Hassel D., et al. 2009. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat. Med. 15:1281–1288 [DOI] [PubMed] [Google Scholar]
- 11. Hongo M., et al. 2000. Effects of growth hormone on cardiac dysfunction and gene expression in genetic murine dilated cardiomyopathy. Basic Res. Cardiol. 95:431–441 [DOI] [PubMed] [Google Scholar]
- 12. Knoll R., et al. 2007. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation 116:515–525 [DOI] [PubMed] [Google Scholar]
- 13. Lange A., et al. 2009. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature 461:1002–1006 [DOI] [PubMed] [Google Scholar]
- 14. Lee G., et al. 2006. Nanospring behaviour of ankyrin repeats. Nature 440:246–249 [DOI] [PubMed] [Google Scholar]
- 15. Li S., et al. 2005. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J. Cell Sci. 118:2913–2921 [DOI] [PubMed] [Google Scholar]
- 16. Liang X., et al. 2009. Targeted ablation of PINCH1 and PINCH2 from murine myocardium results in dilated cardiomyopathy and early postnatal lethality. Circulation 120:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang X., et al. 2005. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol. Cell. Biol. 25:3056–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lynch D. K., Ellis C. A., Edwards P. A., Hiles I. D. 1999. Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene 18:8024–8032 [DOI] [PubMed] [Google Scholar]
- 19. Mackinnon A. C., Qadota H., Norman K. R., Moerman D. G., Williams B. D. 2002. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12:787–797 [DOI] [PubMed] [Google Scholar]
- 20. Maydan M., et al. 2010. Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3beta (GSK-3beta) phosphorylation. PLoS One 5:e12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meder B., et al. 2011. Targeted next-generation sequencing for the molecular genetic diagnostics of cardiomyopathies. Circ. Cardiovasc. Genet. 4:110–122 [DOI] [PubMed] [Google Scholar]
- 22. Meder B., et al. 2010. JunB-CBFbeta signaling is essential to maintain sarcomeric Z-disc structure and when defective leads to heart failure. J. Cell Sci. 123(Pt 15):2613–2620 [DOI] [PubMed] [Google Scholar]
- 23. Meder B., et al. 2009. A single serine in the carboxyl terminus of cardiac essential myosin light chain-1 controls cardiomyocyte contractility in vivo. Circ. Res. 104:650–659 [DOI] [PubMed] [Google Scholar]
- 24. Moens C. B., Donn T. M., Wolf-Saxon E. R., Ma T. P. 2008. Reverse genetics in zebrafish by TILLING. Brief. Funct. Genomic. Proteomic. 7:454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a. National Institutes of Health 1996. Guide for the care and use of laboratory animals. NIH publication no. 85-23. National Institutes of Health, Bethesda, MD. [Google Scholar]
- 25. Persad S., et al. 2001. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J. Biol. Chem. 276:27462–27469 [DOI] [PubMed] [Google Scholar]
- 26. Rearden A. 1994. A new LIM protein containing an autoepitope homologous to “senescent cell antigen.” Biochem. Biophys. Res. Commun. 201:1124–1131 [DOI] [PubMed] [Google Scholar]
- 27. Rottbauer W., et al. 2005. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 19:1624–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stanchi F., et al. 2005. Consequences of loss of PINCH2 expression in mice. J. Cell Sci. 118:5899–5910 [DOI] [PubMed] [Google Scholar]
- 29. Terracio L., et al. 1990. Distribution of vinculin in the Z-disk of striated muscle: analysis by laser scanning confocal microscopy. J. Cell. Physiol. 145:78–87 [DOI] [PubMed] [Google Scholar]
- 30. Tu Y., Li F., Goicoechea S., Wu C. 1999. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol. Cell. Biol. 19:2425–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wickstrom S. A., Lange A., Montanez E., Fassler R. 2010. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 29:281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu C. 2004. The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim. Biophys. Acta 1692:55–62 [DOI] [PubMed] [Google Scholar]
- 33. Zervas C. G., Gregory S. L., Brown N. H. 2001. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152:1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y., et al. 2002. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J. Cell Sci. 115:4777–4786 [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y., Guo L., Chen K., Wu C. 2002. A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J. Biol. Chem. 277:318–326 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.