Abstract
Coxsackievirus (CV) strains CVA2, CVA4, CVA5, and CVA10 were isolated from patients with hand, foot, and mouth disease during a 2009 outbreak in China. Full genome sequences for four representative strains, CVA2/SD/CHN/09 (A2SD09), CVA4/SZ/CHN/09 (A4SZ09), CVA5/SD/CHN/09 (A5SD09), and CVA10/SD/CHN/09 (A10SD09), were determined. Phylogenetic and recombination analyses of the isolates by comparison with human enterovirus A prototype strains revealed that genetic recombination occurred during cocirculation of the viruses. The A2SD09 and A4SZ09 strains were most closely related to their corresponding prototype strains in the capsid region but shared noncapsid sequences with each other. Similarly, strains A5SD09 and A10SD09 had serotype-specific homology for the capsid proteins but shared noncapsid sequences with each other. Phylogenetic analyses of the four isolates with homotypic strains showed that CVA2 strains were divided into five genotypes. The A2SD09 strain clustered with Mongolia strains isolated in 2003, forming genotype V. The A4SZ09 strain and other isolates from mainland China and Taiwan clustered with genotype III strains and are likely related to strains that circulated in Europe and Mongolia. The A5SD09 strain is closely related to other Chinese strains isolated in 2008. The A10SD09 isolate, together with other Chinese strains isolated since 2004, formed a distinct lineage that was likely imported from Japan and South Korea. This study shows that natural recombination is a frequent event in human enterovirus A evolution. More comprehensive surveillance of enteroviruses that focus not only on EV71 or CVA16 is needed for us to understand the molecular epidemiology of enteroviruses and to track recombination events which may ultimately affect the virulence of viruses during outbreaks.
INTRODUCTION
Enteroviruses are among the most common viruses infecting humans and cause a wide spectrum of illnesses, with clinical manifestations ranging from a mild febrile illness to severe complications such as myocarditis, hepatitis, and encephalitis (41). Human enteroviruses (HEVs) belong to the genus Enterovirus, family Picornaviridae, and have originally consisted of polioviruses (PVs), coxsackie A viruses (CVAs), coxsackie B viruses (CVBs), echoviruses, and the numbered enteroviruses (22). These viruses are divided into four species, HEV-A, HEV-B, HEV-C (including poliovirus), and HEV-D, on the basis of the phylogenetic properties of the viruses (3, 13, 35). HEV-A is comprised of 12 conventional serotypes, including CVA2 to CVA8, CVA10, CVA12, CVA14, CVA16, and enterovirus 71 (EV71), and newly identified viruses (EV76 and EV89 to EV92) that are most closely related to simian enteroviruses (27).
The genome of HEV is a single-stranded, positive-sense RNA of approximately 7.4 kb which consists of a long single open reading frame flanked by 5′ and 3′ untranslated regions (UTRs) and encodes a polyprotein that is cleaved by viral proteases into the mature viral capsid proteins P1 (VP4, VP2, VP3, and VP1) and noncapsid proteins P2 and P3 (2A to 2C and 3A to 3D, respectively) (28). The VP1 sequence contains serotype-specific information that can be used for virus identification. Further, the complete or partial VP1 sequence has been employed widely in molecular epidemiological studies of enterovirus disease outbreaks (4, 25, 26). An important property of enteroviruses is their ability to undergo extensive genetic recombination that represents another mechanism, together with viral polymerase-generated mutations, through which these viruses generate genetic diversity and evolve. Recombination in enteroviruses was first described in 1962 (10, 17), and since then numerous studies have demonstrated that recombination is a significant and relatively frequent event in circulating enteroviruses and that genetic exchanges could occur both within a given serotype and between different serotypes (28, 39, 50).
Hand, foot, and mouth disease (HFMD) is a common contagious disease among children and occurs worldwide sporadically and in epidemics. In the past 3 years, there has been a large outbreak of HFMD every year in China, each involving more than 500,000 cases and an increasing number of neurologic symptoms and deaths reported (published on the website of the Ministry of Health of China). Thus, HFMD has become a significant issue in public health.
HFMD is caused by enterovirus infections, particularly by viruses in the HEV-A species (42, 48). Molecular epidemiology studies have demonstrated that a number of HEV-A viruses of the same or different serotypes cocirculate during outbreaks, and mixed infections with two or three serotypes in the same individual are common (1, 2, 19, 49, 53). Research focused on EV71 has been conducted widely due to its association with severe HFMD; however, much less attention has been paid to cocirculating non-EV71 HEV-A strains, though it is known that other HEV-A strains also cause HFMD outbreaks (2, 31) and that cocirculation of viruses during outbreaks facilitates recombination of viruses (1, 12, 14, 30, 51). Similar to previous studies (7, 46), molecular epidemiology studies of HFMD that we conducted in China revealed that a relatively high proportion of HFMD patients were positive for non-EV71 or CVA16 HEVs, in particular, for CVA2, CVA4, CVA5, and CVA10 (not published), and these viruses may play an important role in the evolution of enteroviruses associated with HFMD.
While full-length genome sequences for all HEV-A prototype strains that were isolated in as early as the 1950s from the United States are available, no new genome sequences of a modern HEV-A strain have been reported so far (29). Therefore, determination of the genetic changes to the genome over time has not been described. Further, global phylogenetic analyses of CVA2, CVA4, CVA5, and CVA10 strains are limited because few sequences from these genotypes are available in GenBank (40, 45, 47).
In this study, we present four new full-length genome sequences of modern HEV-A strains, CVA2/SD/CHN/09 (A2SD09), CVA4/SZ/CHN/09 (A4SZ09), CVA5/SD/CHN/09 (A5SD09), and CVA10/SD/CHN/09 (A10SD09), which represent enterovirus serotypes CVA2, CVA4, CVA5, and CVA10, respectively. These viruses were isolated from throat swab specimens of HFMD patients during the 2009 outbreak in China. Full-genome comparison of the four modern strains with all prototype strains of human enterovirus A was conducted. Finally, the phylogenetic relationships of the four isolates with other homotypic isolates based on the 3′ end of the VP1 sequence were analyzed.
MATERIALS AND METHODS
Enterovirus isolation and RNA extraction.
According to a standard WHO protocol (44), coxsackievirus strains A2SD09, A4SZ09, A5SD09, and A10SD09 were isolated in 2009 from throat swab specimens obtained from HFMD patients positive for CVA2, CVA4, CVA5, and CVA10, respectively, using RD and Vero cells. Identification of patient strains was performed by reverse transcription-seminested PCR (RT-snPCR) (23). As described in a previous study (16), to avoid viral mixtures, serially diluted samples were prepared and inoculated into cells in 96-well plates, and the most dilute sample that produced a cytopathic effect (CPE) was expanded. The virus serotypes were confirmed using RT-snPCR with primer pair 292-222, as described previously (23), and viral mixtures were excluded by sequencing the VP1 and 3D genome regions of individual clones, using a TA cloning kit (Invitrogen, Carlsbad, CA) (18). These regions were amplified with primer pairs 292-222 and rpol 1s-rpol 1a (5), respectively. Viral RNA was extracted from 140 μl cell culture supernatant using RNeasy minikits (Qiagen, Valencia, CA) and stored at −80°C.
Complete genome amplification and sequencing.
Overlapping fragments covering each viral genome were amplified using a one-step RT-PCR kit (Qiagen), and specific primers were designed on the basis of available genome sequences of the prototype strains, CVA2 Fleetwood (CVA2F, GenBank accession number AY421760), CVA4 High Point (CVA4H, GenBank accession number AY421762), CVA5 Swartz (CVA5S, GenBank accession number AY421763), and CVA10 Kowalik (CVA10K, GenBank accession number AY421767) (see Tables S1 to S4 in the supplemental material). To fill the gaps between the initial PCR products, additional primers were designed on the basis of the preliminary sequences. The one-step RT-PCR mixture for each tube consisted of 5 μl viral RNA, 1 μl of each primer (25 pmol/μl), 10 μl 5× RT-PCR buffer, 2 μl deoxynucleoside triphosphate mix (10 mM each), 2 μl One-Step RT-PCR enzyme mix, and 29 μl nuclease-free water up to a final volume of 50 μl/tube. One-step RT-PCR was performed under the following conditions: 30 min at 50°C and 15 min at 95°C, followed by a total of 35 cycles of 30 s at 95°C, 30 s at 55°C, and 0.5 to 3 min at 72°C. The synthesis of cDNA for 3′ rapid amplification of cDNA ends (3′ RACE) was performed as previously described (6). The PCR products were purified for sequencing using a QIAquick PCR purification kit (Qiagen). Both strands were sequenced by automated methods, using fluorescent dideoxy-chain terminators (Applied Biosystems, Foster City, CA).
Sequence analysis.
The sequenced DNA fragments were evaluated and assembled into a complete genome. Pair-wise sequence identities among the nucleotide and deduced amino acid sequences for all of the HEV-A serotypes were calculated using the MegAlign program in the Lasergene software package, version 7.2 (DNAStar, Inc., Madison, WI). Nucleotide sequences and deduced amino acid sequences were aligned using the TCoffee package (24, 38). Alignments were checked manually using JalView, version 2.6.1 (43).
Nucleotide substitution models to obtain the best fit for the data were then justified using jModeltest, version 0.1.1 (33). According to the Akaike information criterion (AIC), comparisons of model likelihoods were most favorable to the global time reversible (GTR) nucleotide substitution model with a proportion of invariant sites (+I) and gamma-distributed (+G) rate heterogeneity (GTR+I+G). The phylogenetic analyses were conducted in a maximum likelihood (ML) framework under the appropriate model (GTR+I+G) of nucleotide substitution with four of the substitution rate categories using the program PhyML, version 3.0 (9). The initial tree was determined using the BioNJ program, and the nearest-neighbor interchange (NNI) of the tree search was used. Support for the ML trees was assessed by 1,000 bootstrap replicates.
The full-length genome sequences of strains A2SD09, A4SZ09, A5SD09, and A10SD09 were aligned with 12 prototype sequences of HEV-A using TCoffee, and then similarity plots depicting the relationships among the aligned sequences were generated using SimPlot, version 3.2, software (21). Similarity was calculated in each window of 400 nucleotides (nt) by the F84 (Felsenstein, 1984) distance model with a transition-transversion ratio of 10. The window was successively advanced along the genome alignment in 30-nt increments. For bootscanning analyses, the neighbor-joining algorithm was run with 100 pseudoreplicates. Signals of 70% or more of the observed permuted trees indicate potential recombination events. Further, a model-based approach, the genetic algorithms for recombination detection (GARD) method (15, 32, 34), was employed to search for putative breakpoints delimiting sequence regions possessing distinct phylogenies. GARD analyses were implemented via the DataMonkey program (http://www.datamonkey.org/GARD/) using the multiple breakpoint detection method. Support for recombination is reflected by changes in the goodness of fit between nonrecombinant and recombinant models, as assessed by the AIC. The Shimodaira-Hasegawa (SH) test was applied to verify whether adjacent sequence fragments yield statistically different tree topologies with sequential scaling for multiple tests using the Bonferroni correction method (11).
Nucleotide sequence accession numbers.
The sequences described here have been deposited in the GenBank sequence database, and the GenBank accession numbers are HQ728259 to HQ728262. The GenBank accession numbers of the sequences used in the phylogenetic analyses are provided in the legends to Fig. 1, 2, and 3.
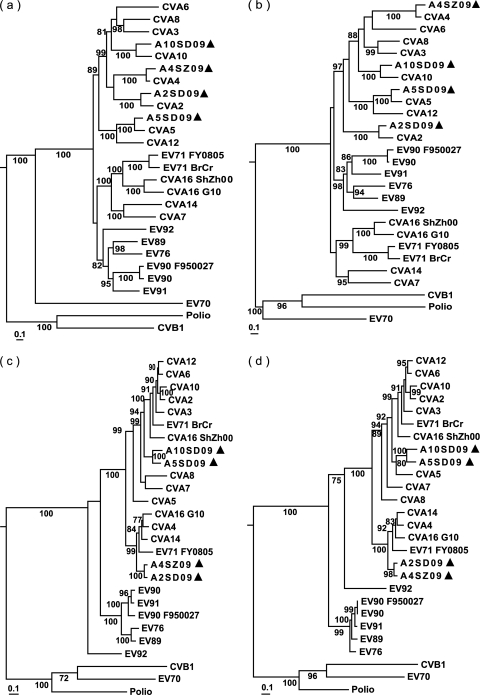
Fig. 1.
Maximum likelihood trees constructed on the basis of the comparisons of different regions of genomes of the four new 2009 enteroviruses and all other prototype strains and modern strains in HEV-A species by using the PhyML, version 3.0, program. (a) P1 region; (b) VP1 region; (c) P3 region; (d) 3D region. Bootstrap values (the percentage of 1,000 pseudoreplicate data sets) lower than 70% are not shown for clarity. Each of the trees includes a representative (CVB1, poliovirus, and EV70) of each of the other three human enterovirus species (HEV-B, -C, and -D) as reference points. The sequenced isolates are indicated by triangles. Sequences with the following GenBank accession numbers were used: CVA2 to CVA8, CVA10, CVA12, and CVA14, AY421760 to AY421769, respectively; CVA16G10, U05876; CVA16ShZh00, AY790926; EV71 BrCr, U22521; EV71, FY0805 and FJ439769; EV76, AY697458; EV89, AY697459; EV90, AB192877; EV90, F950027 and AY773285; EV91, AY697461; EV92, EF667344; CVB1, M16560; poliovirus, NC_002058; EV70, DQ201177.
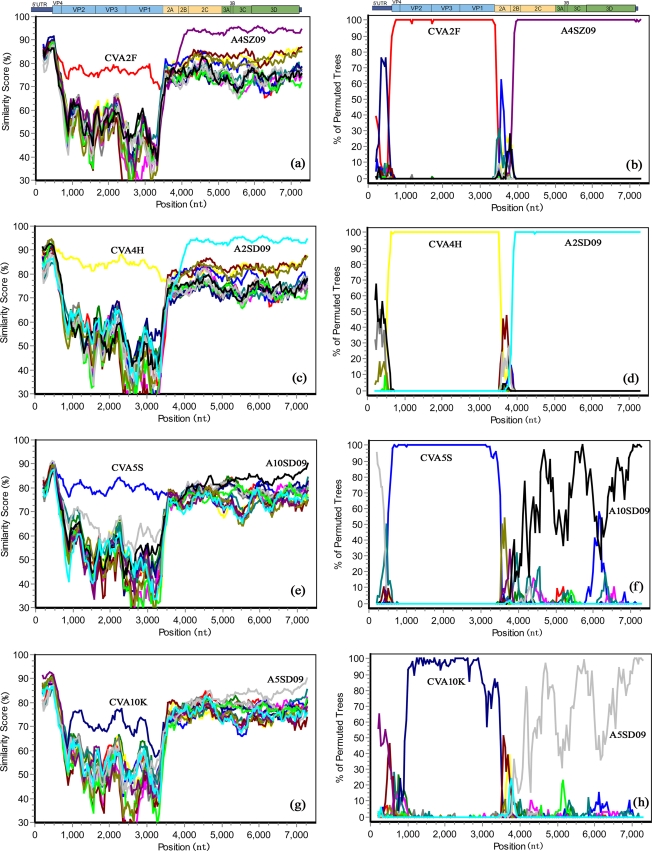
Fig. 2.
Simplot and bootscanning analyses of the four 2009 HEV-A strains and other HEV-A prototype strains on the basis of full-length genomes. Each analysis used each of the four new viruses as the query sequence. A sliding window of 400 nucleotides moving in 50-nucleotide steps was used in this analysis. (a and b) A2SD09; (c and d) A4SZ09; (e and f): A5SD09; (g and h) A10SD09.
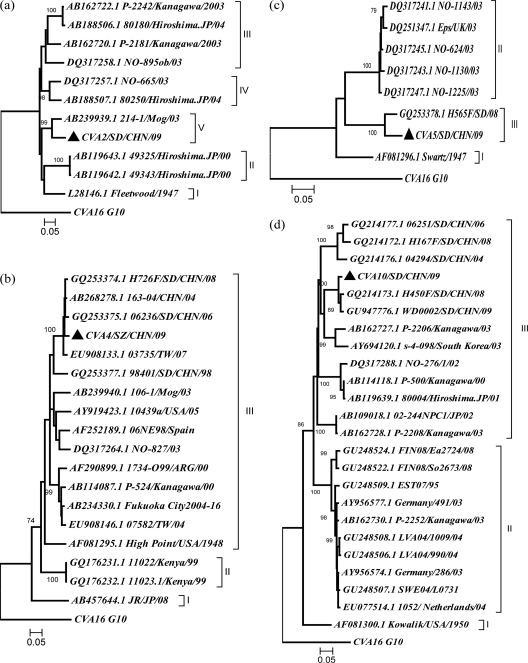
Fig. 3.
Phylogenetic dendrograms constructed by using the neighbor-joining method and the MEGA, version 4.0, program and based on the alignment of the common 3′ partial VP1 sequences of the new 2009 strains and all other corresponding strains from GenBank. The bootstrap values from 1,000 pseudoreplicates for major lineages within the tree are shown as percentages. Bootstrap values lower than 70% are not shown for clarity. The prototype CVA16 strain (G10) was used as an outgroup. The sequenced isolates are indicated by triangles. (a) A2SD09, nt 2919 to 3320; (b) A4SZ09, nt 2923 to 3250; (c) A5SD09, nt 3006 to 3273; (d) A10SD09, nt 2917 to 3301. Sequences with the following GenBank accession numbers were used: CVA2, AB162720.1, AB162722.1, DQ317258.1, DQ317257.1, L28146.1, AB239939.1, AB188507.1, AB188506.1, AB119643.1, and AB119642.1; CVA4, GQ253377.1, GQ253375.1, GQ253374.1, AB268278.1, AB268278.1, AF081295.1, AB162723.1, AB114087.1, AB234330.1, AB188508.1, AB167797.1, AB239940.1, EU908146.1, EU9081391, EU908133.1, EU908121.1, DQ317264.1, AF290899.1, AF252189.1, GQ176232.1, GQ176231.1, and AB457644.1; CVA5, GQ253378.1, DQ317241.1, DQ317243.1, DQ317245.1, DQ317247.1, DQ251347.1, and AF081296.1; CVA10, AB109018.1, AB119638.1, AB119639.1, AB126614.1, AB162727.1, AB162728.1, AB162730.1, AB167985.1, AF081296.1, AF081300.1, AY694120.1, AY956574.1, AY956577.1, DQ317288.1, DQ317289.1, EU077514.1, GQ214172.1, GQ214173.1, GQ214176.1, GQ214177.1, GU248506.1, GU248509.1, GU248522.1, GU248522.1, and GU248522.1.
RESULTS
Coxsackievirus strains CVA2, CVA4, CVA5, and CVA10 were isolated from HFMD patients during the 2009 outbreak in China. Full genomes for each of the four serotypes (A2SD09, A4SZ09, A5SD09, and A10SD09) were completed. Pair-wise sequence comparison phylogenetic, Simplot, and GARD analyses were carried out to investigate the genomic and phylogenetic properties of the newly sequenced genomes.
Genome feature.
The completed genome sequences for strains A2SD09, A4SZ09, A5SD09, and A10SD09 were found to have a typical enterovirus genome organization (see Tables S5 to S8 in the supplemental material). The genome length for A4SZ09 was identical to that of the prototype CVA4H strain, while the genome lengths for A2SD09, A5SD09, and A10SD09 were 1, 6, and 3 nt longer, respectively, than those of the corresponding prototype virus genomes. All of these differences resulted from insertion or deletion in the 5′ UTR regions. Amino acid sequence identity of complete VP1 sequences between A2SD09 and CVA2F was 96.2%, that between A4SZ09 and CVA4H was 97.6%, that between A5SD09 and CVA5S was 96.1%, and that A10SD09 and CVA10S was 94.1%, which confirmed the serotype of the corresponding virus.
A comprehensive comparison of amino acid sequence identities between the four newly sequenced viruses and the corresponding prototype strains and other HEV-A viruses is shown in Table 1. We found that the overall capsid protein sequences and sequences of the individual mature proteins, VP4 to VP1, were highly conserved within a serotype and had at least 95.2% amino acid sequence identity between homotypic viruses. This level of homology was higher than the <85.5% amino acid sequence identity observed for viruses of different serotypes. In the noncapsid region, the sequences of the four new viruses and all other HEV-A viruses analyzed were almost equidistant from each other and did not cluster with regard to serotypes. Further, amino acid sequence similarities between different serotypes for the noncapsid region were much higher than similarities seen for the capsid region. Interestingly, in the P2 and P3 regions, the amino acid sequence identities between the A2SD09 and A4SZ09 strains were 98.3% and 98.1%, respectively, which are higher than the sequence identities between each virus and its corresponding serotype prototype strain. Similarly, in the P2 and P3 regions, the amino acid sequence identities between A5SD09 and A10SD09 were 98.1% and 96.6%, respectively, which are higher than the sequence identities between each virus and its corresponding prototype strain. The 5′ UTR sequence of the four 2009 coxsackieviruses and other HEV-A viruses were closely related to one another and to the representative viruses of HEV-B, with more than 85.8% nucleotide acid sequence identity. The 3′ UTR sequences of the four 2009 viruses analyzed were similar to those of the other HEV-A viruses and more than 75.2% identical to each other but less than 52.4% identical to representatives of other HEV species.
Table 1.
Pair-wise amino acid sequence identities between coxsackievirus strains and prototype strains of the HEV-A species
| Region | % identity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A2SD09 |
A4SZ09 |
A5SD09 |
A10SD09 |
|||||||
| CVA2F | Other HEV-As | CVA4H | Other HEV-As | CVA5S | Other HEV-As | CVA10K | Other HEV-As | |||
| 5′ UTRa | 86.9 | 79.8-85.8 | 89.4 | 81.3-88.6 | 84.8 | 79.4-84.6 | 82.0 | 79.5-86.3 | ||
| Polyprotein | 96.2 | 84.9-88.3 | 97.7 | 82.5-87.2 | 96.9 | 84.8-91.6 | 95.9 | 83.6-88.9 | ||
| P1 | 97.2 | 69.2-75.5 | 98.3 | 64.7-75.0 | 97.2 | 68.8-83.5 | 95.2 | 66.3-77.5 | ||
| VP4 | 95.7 | 63.8-79.7 | 98.6 | 68.1-81.2 | 94.2 | 65.2-85.5 | 95.7 | 65.2-82.6 | ||
| VP2 | 98.5 | 73.6-79.5 | 98.5 | 74.1-86.4 | 98.2 | 73.8-86.8 | 96.7 | 76.4-82.4 | ||
| VP3 | 97.5 | 71.5-83.1 | 99.2 | 70.0-83.9 | 98.8 | 71.7-88.4 | 95.8 | 68.8-80.6 | ||
| VP1 | 96.2 | 62.1-70.4 | 97.6 | 56.5-66.7 | 96.1 | 61.0-78.9 | 94.1 | 61.9-72.9 | ||
| P2 | 96.5 | 96.2-98.3 | 97.4 | 96.5-98.3 | 97.6 | 96.4-98.1 | 96.5 | 96.0-98.1 | ||
| 2APRO | 92.7 | 92.0-94.7 | 96.7 | 92.7-95.3 | 96.0 | 94.7-97.3 | 96.0 | 96.0-98.0 | ||
| 2B | 98.0 | 96.0-98.0 | 98.0 | 96.0-99.0 | 96.0 | 96.0-100 | 97.0 | 96.0-98.0 | ||
| 2CATPase | 97.9 | 97.6-99.1 | 97.6 | 97.3-99.1 | 98.8 | 97.3-98.8 | 96.7 | 95.8-97.6 | ||
| P3 | 95.0 | 92.3-98.1 | 97.4 | 93.7-98.1 | 96.0 | 94.4-96.6 | 96.3 | 95.5-96.6 | ||
| 3A | 97.3 | 93.7-97.3 | 94.2 | 90.7-94.2 | 97.7 | 95.3-100 | 96.5 | 95.3-98.8 | ||
| 3B | 100 | 90.9-100 | 100 | 86.4-100.0 | 86.4 | 90.9-100 | 100 | 86.4-100 | ||
| 3Cpro | 95.1 | 92.9-99.5 | 98.4 | 93.4-98.4 | 94.5 | 92.3-98.9 | 96.7 | 93.4-97.8 | ||
| 3Dpol | 94.4 | 93.5-98.1 | 97.4 | 92.2-97.4 | 96.8 | 93.5-96.8 | 95.9 | 93.5-96.8 | ||
| 3′ UTRa | 95.0 | 83.8-97.1 | 95.2 | 83.8-93.3 | 95.2 | 83.8-96.2 | 84.8 | 75.2-88.6 | ||
Nucleotide acid sequence identities between the coxsackievirus strains and prototype strains of HEV-A.
Phylogenetic and recombination analysis of the four 2009 HEV-A strains and other HEV-A genomes.
To investigate the genetic relationship between the four CVs and the prototype HEV-A strains available in GenBank, phylogenetic trees based on the P1 or P3 region of the genome were constructed (Fig. 1). In the P1 capsid-coding region, A2SD09, A4SZ09, A5SD09, and A10SD09 all clustered with their corresponding prototype strains, CVA2F, CVA4H, CVA5S, and CVA10K, respectively, with strong bootstrap support, as was also the case for each of the individual mature proteins (VP1 to VP4) derived from the P1 region, which agrees well with the pair-wise amino acid sequence identities and reconfirms the serotype of each virus. However, the phylogenetic relationships of the viruses were different with respect to different positions in the genome. In the P3 region, including the 3D region, we observed that the A2SD09 and A4SZ09 clustered together separately from a cluster of A5SD09 and A10SD09, and both independent clusters had strong bootstrap support. The observed differences in the phylogenetic tree topologies between the capsid and the noncapsid regions indicate that recombination might have occurred during the evolution of these viruses.
Next, the genome sequences of the four 2009 viruses and all available HEV-A prototype strains were analyzed with Simplot software, using each of the viruses in turn as the query sequence (Fig. 2). Similarity plot analyses demonstrated that A2SD09, A4SZ09, A5SD09, and A10SD09 showed the highest degree of similarity to their respective prototype strains in the capsid region, but in the noncapsid region, A2SD09 and A4SZ09 were most similar to each other, with approximately 95% nucleotide acid identity (Fig. 2a and c). Similarly, A5SD09 and A10SD09 noncapsid region sequences were most similar to each other and more distantly related to their respective prototype strains (Fig. 2e and g).
Subsequent bootscan analyses indicated possible recombination events. A2SD09 and A4SZ09 were most closely related to their corresponding prototype strains in the 5′ half of the genome, which is consistent with the Simplot analysis results. However, after the junction sequences between VP1 and 2A, the bootscan graph exhibited a sound phylogenetic relationship between A2SD09 and A4SZ09 with an approximately 100% bootscan value (Fig. 2b and d). A5SD09 and A10SD09 were also most closely related to their corresponding prototype strains in the capsid region. However, in the P2 region, bootscanning showed that there was no reliable phylogenetic relationship among enteroviruses analyzed, but in the 3A to 3D (P3) region, A5SD09 and A10SD09 showed a close phylogenetic relationship with one another on the basis of a high bootscan value (Fig. 2f and h). Also, bootscan analyses demonstrated that A10SD09 is a mosaic virus, showing a high bootscan value (>70%) with the prototype strain of CVA4H in the 5′ UTR (Fig. 2h).
Further, the recombination in these viruses was confirmed by detecting putative breakpoints within genomes using GARD, and then GARD-estimated breakpoints were further substantiated by positive results of the SH test, which demonstrated significant incongruence between topologies before and after each breakpoint (Table 2). The results show that 10 breakpoints were found for alignment of A2SD09, A4SZ09, CVA2, and CVA4, but only topology flanking position at 3410 bp was significantly discordant via the SH test, supporting the position as a recombination breakpoint. Similarly, 11 breakpoints were found for alignment of A5SD09, A10SD09, CVA5, and CVA10 on the basis of cAIC goodness of fit, and the SH test showed that the position at 3808 bp is a breakpoint (Table 2). The results of the GARD analyses are consistent with the Simplot and bootscan analysis results described above (Fig. 2). The relative agreement of results of the two methods provides strong support for recombination events in these viruses.
Table 2.
Recombination breakpoints detected within HEV-A genome by GARD
| Alignment | Nc | Positiond (regions of genome) | ΔcAICe |
|---|---|---|---|
| A2/A4a | 10 | 101, 173, 263, 404, 654, 2489, 2523, 2606, 3410,f 3869 | 3.1 |
| A5/A10b | 11 | 406, 671, 2482, 2545, 2572, 3379, 3438, 3808, 4776, 5595, 7281 | 2.5 |
A2/A4, alignment of A2SD09, A4SZ09, CVA2, and CVA4.
A5/A10, alignment of A5SD09, A10SZ09, CVA5, and CVA10.
N, number of breakpoints detected.
Position, breakpoint positions identified via GARD.
ΔcAIC, improvement in Bozdogan's consistent AIC (cAIC) of the breakpoint-partitioned model over a no-recombination single-phylogeny model.
Data in boldface are GARD-identified breakpoints for which flanking trees were significantly discordant via an SH test (P = 0.05, sequentially scaled using the Bonferroni method of Holm [11]).
These results indicate that the cocirculating enteroviruses had undergone a recombination event that produced new virus variants that possessed serotype-specific capsid protein sequences and shared noncapsid protein sequences present in currently circulating strains of different serotypes in the same species. Because few complete modern HEV-A genome sequences are available and because of the possibility of frequent recombination, we could not determine the origin of the noncapsid protein sequences.
Molecular epidemiology of the 2009 coxsackievirus isolates.
According to the molecular epidemiology of polioviruses introduced by Rico-Hesse et al., genotypes have been defined as clusters of related strains with >85% nucleotide identity in the junction region between the VP1 and 2A genes (37). Using similar criteria, individual phylogenetic dendrograms for A2SD09, A4SZ09, A5SD09, and A10SD09 were drawn using 3′ partial VP1 sequences in the context of all of the other representative strains available in GenBank, excluding very similar strains in the same region. This is the first reported description of the phylogenetic relationships of each of the globally circulating CVA2, CVA4, CVA5, and CVA10 isolates with sequences available from GenBank. The phylogenetic trees are presented in Fig. 3. CVA2 strains were divided phylogenetically into five genotypes (Fig. 3a). Genotype I contains only one prototype strain, isolated in 1947. Genotypes II to IV consisted of strains isolated from 2000 to 2004 in Japan and Norway, in which strains of different genotypes cocirculated within a given region. The A2SD09 strain clustered with a Mongolia strain isolated in 2003, forming an independent genotype, genotype V.
The sequences of global CVA4 isolates formed three genotypes (Fig. 3b). Genotype I contained one strain isolated from Japan in 2008 with less than 85% nucleotide identity with other CVA4 sequences. Genotype II was comprised of two strains isolated from Kenya in 1999. Genotype III consisted of strains isolated in the Americas, Europe, and Asia (including China) from 1947 to 2009. The A4SZ09 strain was most closely related to the isolates from mainland China and Taiwan, forming a cluster different from that of other genotype III strains.
Though the number of CVA5 sequences available in GenBank is limited, the phylogenetic tree showed that CVA5 evolved into three genotypes (Fig. 3c). Genotype I contained only the prototype CVA5 Swartz strain, which was isolated from the United States in 1947. Genotype II was comprised of strains isolated from Norway and England in 2003. Genotype III consisted of the A5SD09 isolate and one other strain isolated from the same region in 2008, which suggested that CVA5 isolates circulating in China belonged to a unique genotype different from the genotypes of CVA5 isolates in other regions of the world.
As described in previous research studying an HFMD outbreak caused by cocirculation of CVA6 and CVA10 in Finland, in the present study CVA10 strains were phylogenetically divided into three branches. A10SD09 clustered with isolates circulating in China since 2004, which were related to strains from Japan in 2003 (Fig. 3d).
DISCUSSION
In the present study, we report complete genome sequences of four strains representing four different serotypes. These strains are CVA2/SD/CHN/09, CVA4/SZ/CHN/09, CVA5/SD/CHN/09, and CVA10/SD/CHN/09 and were isolated in 2009 from throat swab specimens from HFMD patients in China. The applied enterovirus typing scheme required the amino acid sequence identity of the complete VP1 sequence for strains within the same serotype to be no lower than 85% (>75% VP1 nucleotide acid identity), whereas isolates of different serotypes had less than 85% identity with VP1 (<70% nucleotide acid identity) (25). The whole VP1 protein sequences of A2SD09, A4SZ09, A5SD09, and A10/SD09 were 96.2%, 97.6%, 96.1%, and 94.1% identical to those of the corresponding prototype strains, respectively. These are the first complete genome sequences for modern HEV-A isolates associated with HFMD in China.
Recombination is a well-known phenomenon for enteroviruses. Ten outbreaks of poliomyelitis caused by pathogenic circulating vaccine-derived polioviruses (cVDPVs) have been reported so far, and most cVDPVs were recombinants of vaccine strains and other cocirculating HEV-C viruses, such as CVA13 and CVA17 (14, 36). Analyses of complete genomes for all prototype strains of HEV-B species suggested that RNA recombination is a common evolutionary event resulting in mosaic enteroviruses and that recombination usually occurs within noncapid regions (P2, P3) of the genome (28). Many other studies have demonstrated that natural genetic recombination among cocirculating HEV-B viruses is frequent (16, 20, 30, 52). While many recombinant enteroviruses have been observed in isolates derived from vaccine polioviruses and cocirculating HEV-C viruses, as well as from naturally circulating wild-type HEV-B viruses, fewer examples of recombinant HEV-A viruses have been described (12, 29, 49).
In our study, the incongruent phylogenies, the Simplot and Bootscan analyses, and GARD analysis clearly indicate that recombination has occurred during the evolution of each of the 2009 strains. In the capsid region, the four field isolates were most closely related to their respective homotypic prototype strains, but in the noncapsid region, the epidemic A2SD09 and A4SZ09 strains were highly similar to each other and the epidemic A5SD09 and A10SD09 strains were most closely related to each other. These data suggest that a genetic rearrangement between the A2SD09 and A4SZ09 strains and between the A5SD09 and A10SZ09 strains may have occurred. It has been reported that multiple HEV-A viruses of identical or different serotypes usually cocirculate in some regions during outbreaks and that coinfection of an individual with two or even three serotypes of HEVs is common. This gives different genotypes the opportunity to undergo recombination, and recombination between different serotypes would be favored when several strains are circulating in the same geographical area simultaneously (30). One way to envision the selective pressure for recombination is that an enterovirus could be thought of as a capsid sequence in search of noncapsid sequences with the highest fitness to provide a selective advantage (28). Thus, the cocirculating serotypes in the outbreak, which possess different capsid sequences, underwent recombination, and strains with common noncapsid sequences were more competitive. This resulted in different enteroviruses with similar noncapsid genome regions. Given that the analyzed epidemic CVA2 and CVA4 strains derived from recombination, the donor of noncapsid sequences of the genome could have been either a CVA2 or a CVA4 strain, and the CVA5SD and CVA10SD strains have the same pattern. However, because few HEV-A genome sequences are available, we did not find the progenitors of the noncapsid sequences. To investigate the putative parents of the recombinant viruses, more genome sequences of modern HEV-A viruses are needed.
As discussed by Oberste et al. (29), the lack of temporal and geographical heterogeneity in HEV-A relative to that of HEV-B and the small number of HEV-A strains analyzed had led to the conclusion that recombination events are fewer in HEV-A viruses than in HEV-B viruses. Our study analyzed genome sequences of clinical isolates from a wider geographical area and covering a longer period of time (about 60 years), and we found evidence for frequent recombination among different cocirculating HEV-A serotypes. This recombination appeared to be as frequent as that for other HEV species (8, 12, 49). Cocirculation and recombination of various HEV-A strains within certain regions and human populations during outbreaks may result in unexpected enterovirus diversity and could result in the emergence of a virus that causes a new disease manifestation. Therefore, more genome sequences of modern enterovirus strains should be determined to foster a better understanding of the evolution of these circulating viruses.
The 3′ partial VP1-coding sequences are widely used for enterovirus taxonomy, identification of new enterovirus types, and molecular epidemiology of enterovirus disease outbreaks. In the present study, these regions of the four 2009 viruses were aligned with all available GenBank sequences and phylogenetic trees were constructed on the basis of this common region. Phylogenetic analyses revealed that CVA2 strains consisted of five genotypes and that the A2SD09 strain was most closely related to a CVA2 strain isolated from Mongolia in 2003, with 90.8% nucleotide sequence identity, implying that A2SD09 was possibly imported from Mongolia. CVA4 strains have been detected in mainland China since 1998. The A4SZ09 strain, together with other isolates in China, had 89.6 to 97.9% nucleotide sequence identity and formed a genotype cluster III, implying that this virus has been circulating for 12 years in China. The phylogenetic tree showed that the Chinese isolates belong to an independent genotype; however, few CVA5 sequences are available from GenBank, which limits the scope of these analyses. Finally, it was reported that both CVA6 and CVA10 are equally common causes of HFMD as CVA16 and EV71 in Singapore, cocirculation of CVA6 and CVA10 caused the 2008 HFMD outbreak in Finland, and both viruses were new variants (1, 2). The A10SD09 Chinese isolate belonged to a different genotype, which contained strains mainly from Asia. Non-EV71 or CVA16, including CVA2, CVA4, CVA5, and CVA10, were rarely detected in China during the HFMD outbreak, though sporadic cases caused by several different coxsackieviruses have been reported (1, 42). Although the major HFMD pathogens in China are currently EV71 and CVA16, it is possible that other cocirculating enteroviruses might become important HFMD pathogens, considering that the HFMD outbreak in Finland was caused by cocirculating CVA6 and CVA10. Therefore, continued surveillance of HEV circulation in China should not focus only on EV71 but should be more comprehensive and include other HEV serotypes.
Supplementary Material
ACKNOWLEDGMENTS
The work was supported by the National Basic Research Program (grant no. 2011CB504902) from the Ministry of Science and Technology of China National Science and Technology Key Projects on Major Infectious Diseases such as HIV/AIDS, Viral Hepatitis Prevention and Treatment (2009ZX10004-102), and an intramural grant from the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (2009IPB112).
We thank the Municipal Hospital in Linyi City, Shandong Province, and Donghu Hospital, in Shenzhen, Guangdong Province, China, for providing clinical samples.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Ang L. W., et al. 2009. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001-2007. Ann. Acad. Med. Singapore 38:106–112 [PubMed] [Google Scholar]
- 2. Blomqvist S., et al. 2010. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J. Clin. Virol. 48:49–54 [DOI] [PubMed] [Google Scholar]
- 3. Brown B., Oberste M. S., Maher K., Pallansch M. A. 2003. Complete genomic sequencing shows that polioviruses and members of human enterovirus species C are closely related in the noncapsid coding region. J. Virol. 77:8973–8984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caro V., Guillot S., Delpeyroux F., Crainic R. 2001. Molecular strategy for ‘serotyping' of human enteroviruses. J. Gen. Virol. 82:79–91 [DOI] [PubMed] [Google Scholar]
- 5. Casas I., et al. 2001. Molecular characterization of human enteroviruses in clinical samples: comparison between VP2, VP1, and RNA polymerase regions using RT nested PCR assays and direct sequencing of products. J. Med. Virol. 65:138–148 [PubMed] [Google Scholar]
- 6. Chang W. L. W., Kirchoff V., Pari G. S., Barry P. A. 2002. Replication of rhesus cytomegalovirus in life-expanded rhesus fibroblasts expressing human telomerase. J. Virol. Methods 104:135–146 [DOI] [PubMed] [Google Scholar]
- 7. Chen K.-T., Chang H.-L., Wang S.-T., Cheng Y.-T., Yang J.-Y. 2007. Epidemiologic features of hand-foot-mouth disease and herpangina caused by enterovirus 71 in Taiwan, 1998-2005. Pediatrics 120:e244–e252 [DOI] [PubMed] [Google Scholar]
- 8. Chen X., et al. 2010. Analysis of recombination and natural selection in human enterovirus 71. Virology 398:251–261 [DOI] [PubMed] [Google Scholar]
- 9. Guindon S., Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 10. Hirst G. K. 1962. Genetic recombination with Newcastle disease virus, polioviruses, and influenza. Cold Spring Harbor Symp. Quant. Biol. 27:303–309 [DOI] [PubMed] [Google Scholar]
- 11. Holm S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6:65–70 [Google Scholar]
- 12. Huang S.-C., et al. 2008. Appearance of intratypic recombination of enterovirus 71 in Taiwan from 2002 to 2005. Virus Res. 131:250–259 [DOI] [PubMed] [Google Scholar]
- 13. Hyypiä T., Hovi T., Knowles N. J., Stanway G. 1997. Classification of enteroviruses based on molecular and biological properties. J. Gen. Virol. 78(Pt 1):1–11 [DOI] [PubMed] [Google Scholar]
- 14. Jegouic S., et al. 2009. Recombination between polioviruses and circulating coxsackie A viruses: role in the emergence of pathogenic vaccine-derived polioviruses. PLoS Pathog. 5:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kosakovsky Pond S. L., Posada D., Gravenor M. B., Woelk C. H., Frost S. D. W. 2006. Automated phylogenetic detection of recombination using a genetic algorithm. Mol. Biol. Evol. 23:1891–1901 [DOI] [PubMed] [Google Scholar]
- 16. Kyriakopoulou Z., et al. 2010. Full-genome sequence analysis of a multirecombinant echovirus 3 strain isolated from sewage in Greece. J. Clin. Microbiol. 48:1513–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ledinko N. 1963. Genetic recombination with poliovirus type 1. Virology 20:107–119 [DOI] [PubMed] [Google Scholar]
- 18. Legrand-Abravanel F., et al. 2007. New natural intergenotypic (2/5) recombinant of hepatitis C virus. J. Virol. 81:4357–4362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li L., et al. 2005. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. J. Clin. Microbiol. 43:3835–3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lindberg A. M., Andersson P., Savolainen C., Mulders M. N., Hovi T. 2003. Evolution of the genome of human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J. Gen. Virol. 84:1223–1235 [DOI] [PubMed] [Google Scholar]
- 21. Lole K. S., et al. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melnick J. L. 1996. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 655-712. In Fields B. N., Knipe D. M., Howley P. M. (ed.) Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- 23. Nix W. A., Oberste M. S., Pallansch M. A. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 44:2698–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Notredame C., Higgins D. G., Heringa J. 2000. T-coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302:205–217 [DOI] [PubMed] [Google Scholar]
- 25. Oberste M. S., et al. 1999. Typing of human enteroviruses by partial sequencing of VP1. J. Clin. Microbiol. 37:1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oberste M. S., Maher K., Kilpatrick D. R., Pallansch M. A. 1999. Molecular evolution of human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73:1941–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oberste M. S., et al. 2005. Enteroviruses 76, 89, 90 and 91 represent a novel group within the species human enterovirus A. J. Gen. Virol. 86:445–451 [DOI] [PubMed] [Google Scholar]
- 28. Oberste M. S., Maher K., Pallansch M. A. 2004. Evidence for frequent recombination within species human enterovirus B based on complete genomic sequences of all thirty-seven serotypes. J. Virol. 78:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oberste M. S., Peñaranda S., Maher K., Pallansch M. A. 2004. Complete genome sequences of all members of the species human enterovirus A. J. Gen. Virol. 85:1597–1607 [DOI] [PubMed] [Google Scholar]
- 30. Oprisan G., et al. 2002. Natural genetic recombination between co-circulating heterotypic enteroviruses. J. Gen. Virol. 83:2193–2200 [DOI] [PubMed] [Google Scholar]
- 31. Osterback R., et al. 2009. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg. Infect. Dis. 15:1485–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Posada D. 2002. Evaluation of methods for detecting recombination from DNA sequences: empirical data. Mol. Biol. Evol. 19:708–717 [DOI] [PubMed] [Google Scholar]
- 33. Posada D. 2008. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25:1253–1256 [DOI] [PubMed] [Google Scholar]
- 34. Posada D., Crandall K. A. 2001. Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc. Natl. Acad. Sci. U. S. A. 98:13757–13762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pöyry T., et al. 1996. Genetic and phylogenetic clustering of enteroviruses. J. Gen. Virol. 77(Pt 8):1699–1717 [DOI] [PubMed] [Google Scholar]
- 36. Rakoto-Andrianarivelo M., et al. 2007. Co-circulation and evolution of polioviruses and species C enteroviruses in a district of Madagascar. PLoS Pathog. 3:e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rico-Hesse R., Pallansch M. A., Nottay B. K., Kew O. M. 1987. Geographic distribution of wild poliovirus type 1 genotypes. Virology 160:311–322 [DOI] [PubMed] [Google Scholar]
- 38. Sedaghatinia A., Atan R. B., Arifin K. T., Murad M. A. B. A. 2009. Comparison and evaluation of multiple sequence alignment tools in bioinformatics. Int. J. Comput. Sci. Netw. Secur. 9:51–56 [Google Scholar]
- 39. Simmonds P., Welch J. 2006. Frequency and dynamics of recombination within different species of human enteroviruses. J. Virol. 80:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tao Z.-X., et al. 2009. Genotype distribution of enterovirus A species isolated in Shandong Province, China. Bing Du Xue Bao (Chinese J. Virol.) 25:410–414 (In Chinese) [PubMed] [Google Scholar]
- 41. Tebruegge M., Curtis N. 2009. Enterovirus infections in neonates. Semin. Fetal Neonatal Med. 14:222–227 [DOI] [PubMed] [Google Scholar]
- 42. Tu P. V., et al. 2007. Epidemiologic and virologic investigation of hand, foot, and mouth disease, southern Vietnam, 2005. Emerg. Infect. Dis. 13:1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Waterhouse A. M., Procter J. B., Martin D. M. A., Clamp M., Barton G. J. 2009. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. WHO 1992. WHO manual for the virological investigation of polio. WHO, Geneva, Switzerland [Google Scholar]
- 45. Witsø E., et al. 2006. High prevalence of human enterovirus A infections in natural circulation of human enteroviruses. J. Clin. Microbiol. 44:4095–4100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu Y., et al. 2010. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int. J. Infect. Dis. 14:e1076–e1081 [DOI] [PubMed] [Google Scholar]
- 47. Yamashita T., Ito M., Taniguchi A., Sakae K. 2005. Prevalence of coxsackievirus A5, A6, and A10 in patients with herpangina in Aichi Prefecture, 2005. Jpn. J. Infect. Dis. 58:390–391 [PubMed] [Google Scholar]
- 48. Yang F., et al. 2009. Enterovirus 71 outbreak in the People's Republic of China in 2008. J. Clin. Microbiol. 47:2351–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoke-Fun C., AbuBakar S. 2006. Phylogenetic evidence for inter-typic recombination in the emergence of human enterovirus 71 subgenotypes. BMC Microbiol. 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yozwiak N. L., et al. Human enterovirus 109: a novel interspecies recombinant enterovirus isolated from a case of acute pediatric respiratory illness in Nicaragua. J. Virol. 84:9047–9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang Y. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virol. J. 7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao Y. N., et al. 2006. FDJS03 isolates causing an outbreak of aseptic meningitis in china that evolved from a distinct echovirus 30 lineage imported from countries of the Commonwealth of Independent States. J. Clin. Microbiol. 44:4142–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu Z. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008. Virol. J. 7:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.