Abstract
A brain abscess caused by Encephalitozoon cuniculi genotype I together with Streptococcus intermedius occurred in a patient without major immunocompromise and with diabetes. The distinguishing clinical signs were hemiparesis and epilepsy. The microsporidium was observed in the abscess aspirate, and its specific DNA was also detected in stool and urine. The patient was successfully treated with albendazole and mebendazole.
CASE REPORT
A 56-year-old male patient with a history of myocardial infarction years ago, recently diagnosed with type II diabetes mellitus treated with metformin and hypertension treated with antihypertensives, was transferred from a county referral hospital with a 5-day history of illness that after two episodes of loss of conscience with generalized seizures left him with residual hemiparesis on the left side and severe cognitive impairment, along with a temperature of 39.5°C. By occupation, he is a groundskeeper in city parks. Prior to admission he spent most of his work time mowing lawns.
The initial cranial CT scan did not detect any pathology except for some fluid in his paranasal sinuses—right-sided frontal, ethmoid, and maxillary sinusitis. Lumbar puncture revealed a mild elevation in protein (99 mg/dl) and leukocytes (14 cells/μl, 63% segmented neutrophils and 37% lymphocytes) (corresponding to the initial stage of aseptic meningitis). General markers of systemic inflammation (leukocytes, C-reactive protein [CRP], and procalcitonin in serum) did not suggest bacterial infection. The preliminary diagnosis was viral encephalitis, most likely tick-borne, complicated by sinusitis.
The initial therapy included diazepam, phenytoin, omeprazole, dexamethasone, mannitol, tiapride, and empirical amoxicillin clavulanate due to the fluid present in the sinuses. Due to the progression of left-sided regional seizures to generalized seizures and to status epilepticus, he was intubated 5 days after admission and mechanically ventilated for 3 days. After extubation, his state of conscience improved to Glasgow coma scale 15, with no subsequent seizures, and the patient manifested only residual weakness of his left hand. His temperature normalized and antibiotics were discontinued after 10 days. CRP and procalcitonin never exceeded 35 mg/liter and 0.2 ng/ml, respectively.
The molecular examination of cerebrospinal fluid for viruses ruled out herpes simplex virus 1 (HSV1) and HSV2, varicella-zoster virus (VZV), enterovirus, cytomegalovirus (CMV), and Epstein-Barr virus (EBV), and serology was negative for tick-borne encephalitis and HIV-1/2. The CD4+ T-lymphocyte absolute count was normal (1.64 × 109/liter).
A cerebrospinal fluid (CSF) study on day 10 after admission showed a rather low leukocyte count, consistent with aseptic inflammation, and a cranial computed tomography (CT) scan on day 17 detected a developing subdural effusion on the right side, high in the fronto-temporal-parietal region. The effusion was 7 mm wide, with a density of 25 to 30 Hounsfield radiodensity units, suggestive of either suppuration or old hemorrhage. A neurosurgeon suggested conservative management and cefotaxime therapy. The patient was in good clinical condition, without fever and seizures; only minimal residual weakness in the left-side extremities persisted.
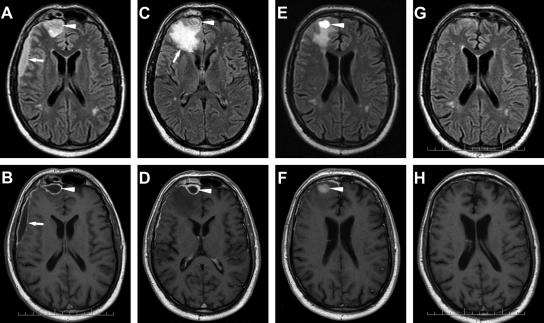
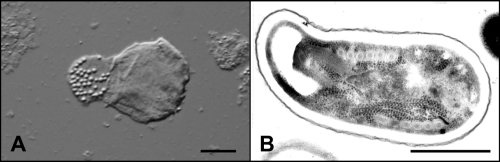
Ten days later, another follow-up CT scan showed the development of an abscess cavity in the subdural space of the fronto-temporo-parietal region. On the next day, a magnetic resonance imaging (MRI) scan (Fig. 1 A and B) in a well-feeling patient with almost intact neurologic findings showed spread of the subdural empyema into the interhemispheral space and the formation of a new abscess in the right frontal lobe adjacent to the previously affected frontal sinuses. A neurosurgeon performed needle evacuation of pus that in microscopic examination revealed masses of leukocytes with no bacteria. Culture, as well as PCR, yielded Streptococcus intermedius. The abscess aspirate has been sent to the Institute of Parasitology to exclude infection with amphizoic amoebae. Parasitological examination of the native wet mount, however, showed the presence of cells containing vacuoles with refractile particles (Fig. 2 A). The same sample was examined using transmission electron microscopy according to the standard protocol (6). This examination revealed spores of microsporidia with polar tube coils arranged in a single row (Fig. 2B), which is typical of the genus Encephalitozoon.
Fig. 1.
The course of development of the brain abscess. (A, B) Initial examination (IE). (A) Axial fluid attenuated inversion recovery (FLAIR) image demonstrates subdural collection of fluid frontolaterally on the right (arrow) and rounded right frontal cortical lesion dorsal of obliterated frontal sinus—frontal sinusitis (arrowhead). (B) Axial T1-weighted (T1W) contrast-enhanced MR image after gadolinium administration shows enhancement of the pyogenic membrane subdural empyema. (C, D) Checkup after the first surgery (20 days post-IE). (C) Axial FLAIR image after drainage of the lateral portion of empyema with persistent frontal part (arrowhead). Large regional swelling newly appears in the right frontal lobe dorsal of the empyema (arrow). (D) Axial T1W contrast-enhanced image shows only encapsulated fluid in the subdural space (arrowhead). The abscess in the brain tissue is not visible. (E, F) Checkup after the second surgery (38 days post-IE). (E) Axial FLAIR image after the MR-navigated neurosurgery intervention shows a postresection cyst with fluid containing blood remains (arrowhead). Decrease of swelling. (F) Axial T1W contrast-enhanced image shows only residual enhancement of the frontal meninges (arrowhead). (G, H) Final examination (83 days post-IE). (G) Minimal scarring changes in the right frontal lobe on FLAIR image. (H) Axial T1W contrast-enhanced image shows no contrast enhancement.
Fig. 2.
Parasitological findings from the brain abscess. (A) Native preparation of the abscess aspirate imaged by Nomarski differential interference contrast microscopy (bar = 10 μm). (B) Microsporidial spore in the abscess aspirate imaged by transmission electron microscopy (bar = 1 μm).
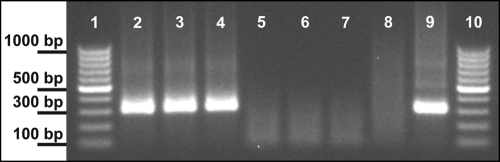
The abscess aspirate and stool and urine samples were used for standard molecular identification of the microsporidial species and genotype (5, 8). This revealed the presence of Encephalitozoon cuniculi genotype I in the abscess aspirate, as well as in stool and urine specimens, before treatment (Fig. 3).
Fig. 3.
Gel image of PCR products detected in the material from the patient. Lanes 1 and 10, molecular weight marker (100-bp ladder; Fermentas); lane 2, stool sample before treatment; lane 3, urinary sediment before treatment; lane 4, abscess aspirate before treatment; lane 5, stool sample after treatment; lane 6, urinary sediment sample after treatment; lane 7, cerebrospinal fluid during treatment; lane 8, negative control (Encephalitozoon-free sample); lane 9, Encephalitozoon intestinalis (positive control).
Due to the progressive MRI finding despite treatment with amoxicillin, therapy with intravenous chloramphenicol and albendazole was started; however, due to the expiration of albendazole's marketing authorization, 10 days later, albendazole was replaced by mebendazole, which is also effective (4). After 21 days of such therapy, a follow-up image-navigated needle aspiration from the residual abscess cavity was performed, which had negative bacterial and parasitological results (Fig. 1C and D). The imaging studies showed resorption of the subdural effusion; however, the swelling of the right frontal lobe decreased and completely disappeared 3 weeks later.
After 28 days, chloramphenicol was switched to oral amoxicillin and the patient was discharged to an outpatient setting. A follow-up cranial MRI scan showed regression of the abscess cavity into gliotic scar tissue (Fig. 1E and F). The parasitological examination of stool and urine was already negative.
The patient's condition kept improving; amoxicillin was discontinued after 6 weeks and mebendazole after 15 weeks. Three months after admission, a follow-up MRI showed almost normal findings (Fig. 1G and H). Six months after discontinuing the therapy, the patient claimed occasional headaches, without need to use any analgesics, and perhaps slightly deteriorated memory, which did not disturb him. Otherwise he felt well and resumed his normal work and lifestyle.
Encephalitozoon cuniculi is a common microsporidian that infects various mammals, such as rodents, carnivores, and primates, including humans. Although unapparent human infections seem to be frequent (8), the documented cases of clinical infections have been recorded in patients with severe immunodeficiency caused mostly by AIDS (6, 10, 11) or other damage to the immune system (7). Successful resolutions of such cases have been scarce (1).
At first, this case appeared quite typical of a severe form of endemic tick-borne encephalitis. With the development of subdural effusion, the differential diagnosis included hemorrhage after an unnoticed cranial trauma during seizures or developing abscess due to bacteria translocated from the frontal and ethmoid sinuses. With the follow-up imaging studies, the latter diagnosis turned out to be more likely.
After the unsuccessful presumptive antibiotic course, a neurosurgical intervention enabled the identification of etiological pathogens. Bacterial culture detected Streptococcus intermedius, a pathogen commonly seen under conditions like this. A problem was its resistance to the expanded-spectrum drug cephalosporin (MIC = 1 mg/liter), which surely contributed to the disease progression.
The detection of microsporidia warranted serious consideration of this parasite. Despite the normal immunological parameters, dexamethasone therapy in a diabetic patient could have facilitated the penetration of bacteria into the subdural space previously colonized with slow-growing microsporidia.
Genotyping microsporidium isolates is a prerequisite for characterizing their epidemiology (12). In human cases of symptomatic infections, E. cuniculi genotype III (“dog” strain) seems to be the most frequent (3, 9, 10); however, genotype I (“rabbit” or “rodent” strain) has been recorded as well (2). This genotype has also been detected in healthy humans (8).
In our patient, occupational exposure to dust from grass contaminated by rodents could be related to his acquiring the infection.
We are not able to determine to what extent the microsporidia participated in clinical symptoms, since concurrent bacterial infection was detected. However, we are inclined to believe that their participation was significant, because of their presence in pathological material and because of the symptom withdrawal after specific therapy.
A parasitological examination of sinusitis-induced brain abscess is not always performed, and the present study may prompt consideration of less-common pathogens participating in such illnesses.
Nucleotide sequence accession number.
The internal transcribed spacer (ITS) sequences obtained for the rRNA gene have been submitted to GenBank under accession numbers GU198946, GU198947, and GU198948.
Acknowledgments
This study was supported by the Grant Agency of the Czech Republic (grants 206/09/0927 and 505/11/1163) and Research Project Z60220518 of the Institute of Parasitology, ASCR.
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. De Groote M. A., et al. 1995. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J. Infect. Dis. 171:1375–1378 [DOI] [PubMed] [Google Scholar]
- 2. Deplazes P. A., Mathis A., Baumgartner R., Tanner I., Weber R. 1996. Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin. Infect. Dis. 22:557–559 [DOI] [PubMed] [Google Scholar]
- 3. Didier E. S., et al. 1996. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J. Clin. Microbiol. 34:2835–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Katiyar S. K., Edlind T. D. 1997. In vitro susceptibilities of the AIDS-associated microsporidian Encephalitozoon intestinalis to albendazole, its sulfoxide metabolite, and 12 additional benzimidazole derivatives. Antimicrob. Agents Chemother. 41:2729–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katzwinkel-Wladarsch S., Lieb M., Heise W., Löscher T., Rinder H. 1996. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1:373–378 [DOI] [PubMed] [Google Scholar]
- 6. Mertens R. B., et al. 1997. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod. Pathol. 10:68–77 [PubMed] [Google Scholar]
- 7. Orenstein J. M., et al. 2005. Fatal pulmonary microsporidiosis due to Encephalitozoon cuniculi following allogeneic bone marrow transplantation for acute myelogenous leukemia. Ultrastruct. Pathol. 29:269–276 [DOI] [PubMed] [Google Scholar]
- 8. Sak B., et al. 2011. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J. Clin. Microbiol. 49:1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snowden K., Logan K., Didier E. S. 1999. Encephalitozoon cuniculi strain III is a cause of encephalitozoonosis in both humans and dogs. J. Infect. Dis. 180:2086–2088 [DOI] [PubMed] [Google Scholar]
- 10. Tosoni A., et al. 2002. Disseminated microsporidiosis caused by Encephalitozoon cuniculi III (dog type) in an Italian AIDS patient: a retrospective study. Mod. Pathol. 15:577–583 [DOI] [PubMed] [Google Scholar]
- 11. Weber R., et al. 1997. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N. Engl. J. Med. 336:474–478 [DOI] [PubMed] [Google Scholar]
- 12. Xiao L., et al. 2001. Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences. J. Clin. Microbiol. 39:2248–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]