Abstract
We report incidental isolation of an OXA-48-producing Escherichia coli strain in urine of a 62-year-old woman recently returning from a 2-month vacation in Morocco. Commercially available extended-spectrum beta-lactamase (ESBL)-targeting medium failed to detect it in the patient's stools, although a locally developed and easy-to-implement method using ertapenem-supplemented brain heart infusion (BHI) broths could.
CASE REPORT
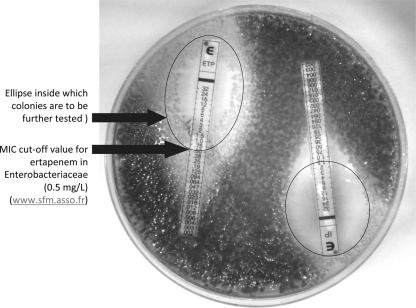
A 62-year-old woman was admitted to a medical ward of the Bichat-Claude Bernard University Teaching Hospital on 21 September 2010 for exacerbation of asthma. The patient was overweight and had type 2 diabetes, requiring insulin therapy. She had previously been hospitalized in our hospital 3 days in February for minor abdominal surgery and was not known to carry multidrug-resistant bacteria. As for all patients hospitalized for uncontrolled diabetes in this ward, urine analysis was performed at admission (21 September). The patient's urine contained 104 leukocytes/ml, and Escherichia coli (104 cells/ml) was isolated after culture on UriSelect 4 medium (Bio-Rad). This isolate was designated BOU-1. Because the patient showed no symptoms of cystitis, she was not treated with antibiotics. As tested by the disk-diffusion method and the MICs by Etest strips (bioMérieux) according to recommendations of the French Society for Microbiology (www.sfm.asso.fr), BOU-1 was resistant to co-amoxiclav (MIC > 256 mg/liter), piperacillin (MIC > 256 mg/liter), tazocillin (MIC > 256 mg/liter), and ertapenem (MIC, 1.5 mg/liter) and susceptible to cefotaxime (MIC, 1 mg/liter), ceftazidime (MIC, 0.25 mg/liter), cefepime (MIC, 0.25 mg/liter), aztreonam (MIC, 0.094 mg/liter), imipenem (MIC, 0.75 mg/liter), meropenem (MIC, 0.38 mg/liter), and doripenem (MIC, 0.19 mg/liter). No synergy between clavulanate and any extended-spectrum cephalosporin was observed. In order to elucidate its resistance phenotype, we extensively tested BOU-1 for beta-lactamase genes. Only blaOXA-48 (for oxacillinase) yielded positive amplification with specific primers (5). Subsequent sequencing confirmed the identification of blaOXA-48. The blaOXA-48-carrying plasmid could be transferred to a rifampin-resistant J53 E. coli strain after mating in brain heart infusion (BHI) broth. An interview with the patient revealed that she had spent a 2-month family vacation in Morocco in May and June 2010, but she did not have any direct contact with a Moroccan health care structure or health care personnel. No history of carbapenem usage was reported. The patient was placed on contact precautions on 28 September 2010 (day 8) and discharged home on 29 September 2010 (day 9). A list of 31 contact patients was established, of whom 12 were screened. All 13 patients (including the index patient) were negative for extended-spectrum beta-lactamase (ESBL) or OXA-48 intestinal carriage using the chromID ESBL medium (bioMérieux). Indeed, in vitro subculture of strain BOU-1 on chromID ESBL medium was negative. A new enrichment procedure was implemented to isolate putative OXA-48-producing E. coli from further rectal swabs, including overnight culture (18 to 24 h at 37°C without stirring) in 10 ml BHI broth supplemented with 0.5 mg/liter ertapenem before plating on Drigalski agar medium with ertapenem and imipenem Etest strips (bioMérieux) and being cultured overnight (18 to 24 h at 37°C). Etest strips were chosen instead of disks because of their better tolerance to inoculum effect (2). Enterobacteriaceae growing inside the inhibition ellipses of the MIC breakpoints were further tested for blaOXA-48. The patient was readmitted to our hospital on 24 November 2010 for scheduled surgery. A rectal swab culture using the method described above yielded colonies in the ertapenem and imipenem Etest strip ellipses that were identified as the same OXA-48-producing E. coli strain (Fig. 1). Direct plating of the swab on chromID ESBL medium was again negative. Urine culture was also negative. The patient was placed on isolation precaution throughout hospitalization, and no secondary case of colonization or infection was detected despite extensive screening of contacts.
Fig. 1.
Drigalski agar medium with ertapenem (ETP) and imipenem (IP) Etest strips (bioMérieux) plated with a dry cotton swab inserted into an overnight stool culture in a 0.5-mg/liter ertapenem-supplied BHI broth.
This case raises further concerns about the spread of OXA-48 resistance. If not for a routine urine culture, strain BOU-1 would not have been recovered and no control measures would have been implemented to prevent subsequent nosocomial transmission. Indeed, control of the spread of carbapenemase-carrying Enterobacteriaceae is important, because antibiotic options are limited to treat patients infected by such bacteria. The French High Committee for Public Health has recently recommended that patients hospitalized in French hospitals after hospitalization abroad should be screened for multiresistant bacteria, including carbapenemase producers (http://www.hcsp.fr/docspdf/avisrapports/hcspr20100518_bmrimportees.pdf). Although our patient had traveled in Morocco, where OXA-48 had already been reported (1), she did not have contacts with hospitals. Thus, no screening was performed. In addition, recommended screening procedures include plating on commercially available ESBL media that are likely to miss OXA-48-producing strains, such as BOU-1 and some OXA-48-producing strains with similar antibiotic susceptibility profiles (3–6). OXA-48-producing Enterobacteriaceae can thus be a “black hole” in carbapenemase detection in patients with a history of travel in areas where OXA-48-producing strains are endemic, using currently recommended methods. The two-step procedure described here is a simple means to fill this breach. It is, however, time- and resource-consuming and can be jeopardized if ertapenem-resistant Gram-negative bacilli, such as nonfermenters, are also present in the feces. We therefore suggest it should be used only in screening patients such as those who are returning from travel in foreign countries or are contacts of a documented case. This stresses the urgent need of molecular methods to detect colonization by all types of ESBL and carbapenemase producers in fecal specimens.
Acknowledgments
This work was supported by the Centre National de Référence Associé “Résistance dans les Flores Commensales,” Laboratoire de Bactériologie, Hôpital Bichat Claude Bernard, AP-HP, Paris, France.
Footnotes
Published ahead of print on 11 May 2011.
REFERENCES
- 1. Benouda A., Touzani O., Khairallah M. T., Araj G. F., Matar G. M. 2010. First detection of oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Morocco. Ann. Trop. Med. Parasitol. 104:327–330 [DOI] [PubMed] [Google Scholar]
- 2. Bouza E., et al. 2007. Direct E-test (AB Biodisk) of respiratory samples improves antimicrobial use in ventilator-associated pneumonia. Clin. Infect. Dis. 44:382–387 [DOI] [PubMed] [Google Scholar]
- 3. Carrer A., Fortineau N., Nordmann P. 2010. Use of ChromID extended-spectrum beta-lactamase medium for detecting carbapenemase-producing Enterobacteriaceae. J. Clin. Microbiol. 48:1913–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuzon G., et al. 2008. Plasmid-encoded carbapenem-hydrolyzing beta-lactamase OXA-48 in an imipenem-susceptible Klebsiella pneumoniae strain from Belgium. Antimicrob. Agents Chemother. 52:3463–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poirel L., Heritier C., Tolun V., Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woodford N., et al. 2010. Comparison of BD Phoenix, Vitek 2, and MicroScan automated systems for detection and inference of mechanisms responsible for carbapenem resistance in Enterobacteriaceae. J. Clin. Microbiol. 48:2999–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]