Abstract
Drug resistance in tuberculosis (TB) is a matter of grave concern for TB control programs, as there is currently no cure for some extensively drug-resistant (XDR) strains. There is concern that this resistance could transmit, stressing the need for additional control measures, rapid diagnostic methods, and newer drugs for treatment. We developed an in-house assay that can rapidly detect resistance to drugs involved in the definition of XDR-TB directly from smear-positive specimens. Two hundred fifteen phenotypically XDR-TB isolates and 50 pansusceptible isolates were analyzed using a reverse line blot hybridization (RLBH) assay. The assay was also successfully applied to 73 smear-positive clinical specimens. The RLBH assay exhibited good sensitivity for the detection of resistance to isoniazid (99%), rifampin (99%), fluoroquinolones (95.3%), and second-line aminoglycosides (94.8%). The results from application of this assay on direct smear-positive clinical specimens revealed 93% concordance with the phenotypic drug susceptibility test (DST) results for the above-mentioned drugs. The time to accurate DST results was significantly reduced from weeks to 3 days. This molecular assay is a highly accurate tool for screening for XDR-TB, which achieves a substantial reduction in diagnostic delays.
INTRODUCTION
Estimates of the incidence of Mycobacterium tuberculosis infections continue to increase globally, and in 1993 the WHO declared tuberculosis (TB) a global public health emergency, being the only disease so far to warrant that designation. In many countries where the incidence of TB is high, the major driving force of the epidemic is transmission. The foremost reason for this is that there are prolonged delays from the onset of the TB disease in individuals to the time of actual diagnosis. During this period, an active TB case may infect numerous other people, thereby contributing to the increase of the epidemic (20). The emergence of multidrug-resistant (MDR) tuberculosis, defined as resistance to both rifampin (RIF) and isoniazid (INH), is a compounding factor for the control of the disease. Patients harboring MDR M. tuberculosis strains need alternative treatment regimens involving second-line drugs that are more expensive, toxic, and less effective. Moreover, extensively drug-resistant (XDR) tuberculosis (resistance to INH, RIF, fluoroquinolones [FQs] and any 1 second-line injectable, i.e., kanamycin [KAN], amikacin [AM], or capreomycin [CM]) constitutes an emerging threat for the control of the disease over the period (4). Accurate diagnosis and initiation of early treatment are essential to reduce transmission and improve the outcome for patients with tuberculosis. Timely identification of drug resistance is especially important to avoid subjecting patients with resistance to inadequate first-line treatment for months while awaiting drug susceptibility testing (DST) results. This exposure to inadequate therapy results in amplified drug resistance (21).
Drug resistance in M. tuberculosis is attributed to random mutations in the Mycobacterium genome. Mutations in the specific codons can therefore be exploited to rapidly detect drug resistance (8, 9, 12, 19, 22). Resistance to first-line drugs (INH, RIF) is attributed to mutations in inhA and katG for INH and rpoB for RIF (18, 19, 22, 23). Resistance to fluoroquinolones and second-line aminoglycosides is most frequently associated with mutations in gyrA and gyrB and in rrs, respectively. Studies have demonstrated that by targeting mutations in codons 90, 91, and 94 in gyrA, approximately 70 to 90% of all fluoroquinolone resistance can be accurately predicted (2, 5, 17). Similarly, mutations in the rrs gene, particularly the hot-spot region from nucleotides 1401 to 1484, can accurately predict resistance to second-line aminoglycosides (10, 16). The reverse line blot hybridization (RLBH) assay (11, 24) is a probe-based method where multiple oligonucleotide probes carrying the mutant sequence and wild-type sequence are immobilized on nitrocellulose strips and hybridized with biotin-labeled PCR products. This technique had been successfully applied for the identification of mutations related to resistance to isoniazid, rifampin, and streptomycin at our center. The assay was optimized to additionally detect mutations conferring FQ and second-line aminoglycoside (KAN, AM, CM) resistance under the same hybridization conditions, such that a single blot could be used to detect resistance to the antitubercular drugs included in the XDR-TB definition. This enabled us to reduce the time to accurate DST results.
MATERIALS AND METHODS
Setting.
The study was carried at the mycobacteriology laboratory at the P. D. Hinduja National Hospital (PDHNH) and Medical Research Centre, a tertiary care center in central Mumbai, India, with a referral bias toward complicated and nonresponding cases. The study was approved by the Institutional Review Board (IRB). The laboratory receives about 10,000 samples for culture annually, with a culture positivity rate of 40%. Being private, tertiary laboratory susceptibility testing is done only on request, wherein of the 3,609 requests for DST from January 2008 to December 2009, 65% of isolates are MDR M. tuberculosis, and of these, 9.1% are XDR M. tuberculosis.
Bacterial isolates.
Two hundred fifteen consecutive XDR-TB isolates tested by the mycobacterial growth indicator tube (MGIT 960) TB system were collected over a period of 24 months (January 2008 to December 2009). Fifty consecutive pansusceptible isolates tested by the MGIT 960 system were also tested using the RLBH assay. All the collected strains were stored at −70°C. DNA extraction from mycobacterial culture isolates was carried out by the cetyltrimethylammonium bromide-sodium chloride (CTAB-NaCl) method (15).
Clinical specimens.
Seventy-three consecutive acid-fast bacillus (AFB) smear-positive clinical specimens (1+ and above) collected over a period of 5 weeks were also tested by the RLBH method. Of the 73 specimens, 31 specimens were 1+ on acid-fast bacillus smear, 15 were 2+, and 27 were 3+ on AFB smear. Smear-positive specimens were subjected to decontamination by the N-acetyl-l-cysteine-sodium citrate-NaOH (6) method, and the sediment was used for DNA extraction, which was carried out using a Qiagen DNA extraction kit.
Amplification of all targets in multiplex PCR.
Table 1 lists the PCR primer sequences, wherein the primers for inhA, katG, and rpoB are the same as those described previously (14). Novel primers were designed for amplifying gyrA, gyrB, and rrs and are also listed in Table 1. The cycling conditions for the touchdown multiplex assay for the isolates and direct clinical specimens are provided in the supplemental material.
Table 1.
Primer sequences for inhA, katG, rpoB, gyrA, gyrB, and rrs
| Target region | Primer | Sequence |
|---|---|---|
| inhA | TB 92 M | 5′-CCT CGC TGC CCA GAA AGG GA TCC-3′ |
| TB 93 M | 5′-biotin-CCG GGT TTC CTC CGG T-3′ | |
| katG | KGF | 5′-biotin-AGA GCT CGT ATG CCG GA-3′ |
| KGR | 5′-GCG AAT GAC CTT GCG CAG ATC-3′ | |
| rpoB | Rpo F | 5′-TCA AGG AGA AGC GCT ACG ACC TGG-3′ |
| Rpo R | 5′-biotin-GGG TCT CGA TCG GGC ACA T-3′ | |
| gyrA | gyA F | 5′-CGA ACC GGT TGA CAT CGA GCA GGA G-3′ |
| gyA R | 5′-biotin-CAG CAT CTC CAT CGC CAA CG-3′ | |
| gyrB | gyrB F | 5′-GAA GCC AAC CCC ACC GAC GCG A-3′ |
| gyrB R | 5′-biotin-CTG CGC TGC CAC TTG AGT TTG-3′ | |
| rrs | rrs F | 5′-CGT TCC CTT GTG GCC TGT GTG CAG-3′ |
| rrs R | 5′-biotin-GTT GGG GCG TTT TCG TGG TGC-3′ |
Designing specific sequence probes for fluoroquinolones and second-line aminoglycosides.
The initial 150 XDR-TB isolates were amplified as single targets and sequenced (1). On the basis of the mutations, specific amino acid-labeled oligonucleotide probes for fluoroquinolones and second-line aminoglycosides were designed so that they could be processed under the same hybridization and washing conditions used for the MDR blot and were covalently linked to a nylon membrane in parallel lines. Six mutant probes and 2 wild-type probes were designed for gyrA, and 1 wild-type and 1 mutant probe were designed for gyrB. For aminoglycosides, the rrs region was targeted with 2 wild-type probes and 3 mutant probes correlating with high-level resistance to KAN, AM, and CM (Table 2). The uniqueness and cross-reactivity of all the newly designed and modified probes were analyzed with a BLAST search (http://www.ncbi.nlm.nih.gov).
Table 2.
Probe sequences for fluoroquinolone and aminoglycosides
| Gene and probe no. | Probe | Description | Sequencea |
|---|---|---|---|
| gyrA | |||
| 1 | Codon 90 | Wild type | 5′-ACC ACC CGC ACG GCG ACG CGT C-3′ |
| 2 | Codon 90 | Mutant type | 5′-ACCACC CGC ACG GCG ACG TGT C-3′ |
| 3 | Codon 91 | Mutant type | 5′-ACC CGC ACG GCG ACG CGC C-3′ |
| 4 | Codon 94 | Wild type | 5′-CG ATC TAC GAC AGC CTG GTG-3′ |
| 5 | Codon 94 | Mutant type I | 5′-CG ATC TAC GCC AGC CTG GTG-3′ |
| 6 | Codon 94 | Mutant type II | 5′-CG ATC TAC GGC AGC CTG GTG-3′ |
| 7 | Codon 94 | Mutant type III | 5′-CGA TCT ACT ACA CCC TGG TG-3′ |
| 8 | Codon 94 | Mutant type IV | 5′-CGA TCT ACA ACA CCC TGG TG-3′ |
| 9 | Codon 95 | Polymorphism | 5′-CGA TCT ACG ACA CCC TGG TG-3′ |
| gyrB | |||
| 10 | 510 | Wild type | 5′-GTG CTA AAG AAC ACC GAA G-3′ |
| 11 | 510 | Mutant type | 5′-CGA CCG GGT GCT AAA GAG CA-3′ |
| rrs | |||
| 12 | 1401 | Wild type | 5′-ACC GCC CGT CAC GTC ATG AA-3′ |
| 13 | 1401 | Mutant type | 5′-ACC GCC CGT CGC GTC ATG AA-3′ |
| 14 | 1402 | Mutant type | 5′-ACC GCC CGT CAA GTC ATG AA-3′ |
| 15 | 1484 | Wild type | 5′-CGG CGA TTG GGA CGA AGT C-3′ |
| 16 | 1484 | Mutant type | 5′-CGG CGA TTG GGA CTA AGT C-3′ |
The boldface with an underscore indicates the change in the nucleotide sequence.
Hybridization with amplified products.
Heat-denatured PCR products were applied on the membrane in the miniblotter and hybridized at 53°C for 60 min. The membrane was washed twice with gentle shaking in 250 ml 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])-0.5% SDS for 10 min at 60°C. The membrane was subsequently incubated at 42°C with 1:4,000-diluted streptavidin-peroxidase conjugate in 2× SSPE-0.5% SDS for 60 min, washed twice with 250 ml 2× SSPE-0.5% SDS at 42°C for 10 min, rinsed twice with 2× SSPE at room temperature (RT) for 5 min, and subjected to luminescent detection of hybrids with an enhanced chemiluminescence (ECL) detection system, followed by exposure to ECL Hyperfilm (Amersham Biosciences). The presence of a clearly visible black signal was considered a positive hybridization reaction. The results were easy to interpret (Fig. 1). For reuse, the membranes were stripped in 1% SDS solution at 80°C (twice for 40 min) and rinsed in 20 mM EDTA, pH 8.0, at RT. The membrane can be stripped and reused up to 7 times without compromising the results.
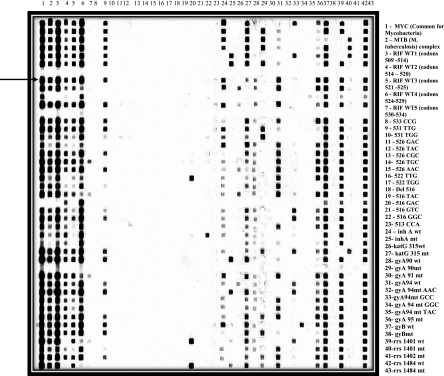
Fig. 1.
RLBH performed for rpoB, katG, inhA, gyrA, gyrB, and rrs. All the different probes are blotted vertically, and patient samples (PCR products) are applied horizontally. The probe in lane 1 is a common probe for all mycobacteria, and then there is a probe for the M. tuberculosis complex. Then there are 5 probes for the wild-type rpoB gene to check for rifampin-susceptible cases and 16 mutant probes for detection of rifampin resistance. Similarly, we targeted the inhA wild type and mutant with a probe for the promoter region, followed by probes for the wild type and mutant at codon 315 of the katG gene. This is further followed by probes for the wild type and mutant for gyrA mutations at codons 90, 91, 94, and 95. Similarly, we also targeted the gyrB codon 510 region with wild-type and mutant probes. For the second-line aminoglycosides, the rrs region at codons 1401, 1402, and 1484 were used. To illustrate an example, the patient sample marked by the arrow has mycobacterial infection due to M. tuberculosis complex and shows binding with probes 3 to 6, which are for the rpoB wild-type codon 509 to 529. It does not show binding with probe 7 but shows binding with probe 9, indicating RIF resistance due to a position 531 TTG mutation. Further, the same sample shows binding with probe 24, which is for the inhA promoter wild type, and shows binding with probe 27, which is the probe for a katG mutation at codon 315, thereby indicating INH resistance due to a katG mutation. The same sample shows binding with the 28th, 31st, 36th, 37th, 39th, and 42nd probes, which correspond to the gyrA codon 90 wild type, gyrA codon 94 wild type, gyrA codon 95 polymorphism, gyrB wild type, rrs codon 1401 wild type, and rrs codon 1484 wild type, respectively, thus giving a result for that particular sample indicating resistance to INH and RIF and susceptibility to FQs and KAN, AM, and CM. WT and wt, wild type; mt, mutant.
Reproducibility of the RLBH assay was validated by testing 215 clinical XDR M. tuberculosis isolates (of these, 150 were also sequenced and results were compared with those of sequencing [1] as well as the RLBH assay), 50 susceptible isolates, and 73 smear-positive clinical specimens (+1 and above). The results for the clinical specimens were compared with phenotypic susceptibilities determined on MGIT.
Statistical analysis.
A 2-by-2 table was used to calculate the sensitivity and specificity of the RLBH assay in comparison with the phenotypic DST, i.e., MGIT. A P value (significance) of <0.05 is deemed statistically significant. To compare two tests, e.g., RLBH and MGIT, kappa was used as a measure of agreement with the 95% confidence interval (CI).
RESULTS
Probe designing.
The initial 150 XDR M. tuberculosis isolates and 50 pansusceptible isolates were sent for sequencing to screen for mutations in gyrA, gyrB, and rrs, and on the basis of the mutations seen, the respective wild-type and mutant probes were designed for the fluoroquinolones and second-line aminoglycosides and used for standardizing the RLBH assay conditions.
Evaluation of RLBH assay.
The initial 150 sequenced isolates were then used as known controls for the respective mutations. The latter 65 of the total 215 XDR M. tuberculosis isolates were screened only by the RLBH assay.
Performance of the assay. (i) Rifampin resistance.
Of the total 215 XDR M. tuberculosis and 50 pansusceptible isolates screened by the RLBH assay, 1 isolate showed RIF resistance by MGIT (phenotypic assay) but did not show any mutation by the RLBH assay. The diagnostic sensitivity of the assay to detect RIF resistance was calculated to be 99%, with the specificity being 100%, as none of the 50 pansusceptible isolates showed any mutation with RLBH. The kappa value was 0.99 (95% CI), indicating good agreement between RLBH and phenotypic susceptibility.
(ii) Isoniazid resistance.
Of the total 215 XDR M. tuberculosis and 50 pansusceptible isolates, 1 isolate showed INH resistance by MGIT but did not bind with any mutant probes on the RLBH membrane. The diagnostic sensitivity of the assay to detect INH resistance was calculated to be 99%, with the specificity being 100%, as none of the 50 pansusceptible isolates showed any mutation with RLBH. The kappa value was 0.99 (95% CI), indicating good agreement between RLBH and phenotypic susceptibility.
(iii) Fluoroquinolone resistance.
Of the total 215 XDR M. tuberculosis and 50 pansusceptible isolates, 10 isolates phenotypically resistant to fluoroquinolones did not reveal any mutation by the RLBH assay. These samples were sent for sequencing to check for the mutations in gyrA and gyrB. Sequencing did not show any mutations, indicating mutations outside the targeted region or other mechanisms of resistance, i.e., efflux mechanism. The diagnostic sensitivity of the RLBH assay to detect fluoroquinolone resistance was calculated to be 95.34%, with the specificity being 100%, as none of the 50 pansusceptible isolates showed any mutation with RLBH. The kappa value was 0.89 (95% CI), indicating good agreement between RLBH and phenotypic susceptibility.
(iv) Second-line aminoglycosides (kanamycin and amikacin).
Of the 215 XDR-TB and 50 pansusceptible isolates, 11 isolates were phenotypically resistant to KAN and AM but did not reveal any mutation by the RLBH assay. These samples were sent for sequencing of the rrs region to check for the mutations. Sequencing did not show any mutations, indicating mutations outside the targeted region or other mechanisms of resistance. The diagnostic sensitivity for the detection of resistance to second-line aminoglycosides by the RLBH assay was 94.8%, with the specificity being 100%. The kappa value was 0.87 (95% CI), indicating good agreement between RLBH and phenotypic susceptibility.
The various mutations seen in the 215 XDR-TB isolates are listed in Table 3. Of all the XDR-TB isolates, 213/215 (99%) showed the katG S315T substitution correlating with isoniazid resistance. A total of 199/215 (92%) showed the S531L substitution in the rpoB gene. The mutations in gyrA were seen on the 90th, 91st, and 94th codons. The frequency of the D94G substitution was seen in 101/215 (46%) of XDR M. tuberculosis isolates. The other substitutions seen on the 94th codon were D94A and D94N, seen in 31/215 (14%) and 27/215 (12.5%) isolates, respectively. A total of 106/215 XDR M. tuberculosis isolates showed the A1401G mutation in the rrs region and were phenotypically resistant to KAN and AMK but susceptible to CM. A further 56/215 XDR-TB isolates with the same mutation showed phenotypic resistance to KAN, AMK, and CM. The G1484T mutation in rrs resulted in resistance to all 3 second-line injectables by the phenotypic method.
Table 3.
Mutations seen in XDR M. tuberculosis-infected patients
| Drug | Gene(s) | Mutation(s) | Amino acid change | No. of isolates |
|---|---|---|---|---|
| Isoniazid | katG | G315C | S315T | 213 |
| Isoniazid | inhA + katG | C-15T | 44 | |
| Rifampin | rpoB | C531T | S531L | 199 |
| Rifampin | rpoB | A526G | H526Y | 7 |
| Rifampin | rpoB | G516T + A526G | D516T | 2 |
| Rifampin | rpoB | A516T + A526G | D516E | 2 |
| Rifampin | rpoB | A516T | D516E | 2 |
| Rifampin | rpoB | G516T | D516T | 1 |
| Fluoroquinolones | ||||
| Ofloxacin | gyrA | C90T + C95G | A90V | 23 |
| gyrA | C90T | A90V | 21 | |
| Ofloxacin and moxifloxacin | gyrA | T91C | S91P | 2 |
| Ofloxacin and moxifloxacin | gyrA | A94G + C95G | D94G | 83 |
| Ofloxacin and moxifloxacin | gyrA | A94G | D94G | 18 |
| Ofloxacin and moxifloxacin | gyrA | A94C + C95G | D94A | 19 |
| Ofloxacin and moxifloxacin | gyrA | A94C | D94A | 12 |
| Ofloxacin and moxifloxacin | gyrA | G94A + C95G | D94N | 19 |
| Ofloxacin and moxifloxacin | gyrA | G94A | D94N | 8 |
| Aminoglycosides | ||||
| Kanamycin and amikacin | rrs | A1401G | 106 | |
| Kanamycin, amikacin, and capreomycin | rrs | A1401G | 56 | |
| Kanamycin, amikacin, and capreomycin | rrs | G1484T | 42 |
Applicability on direct smear-positive clinical specimens.
The results obtained on isolates were promising, but the assay was time-consuming; thus, the assay was tested on direct clinical specimens. Seventy-three specimens were tested. Of these, 8 proven by culture to be mycobacteria other than M. tuberculosis (MOTT) showed binding only with the mycobacterial complex probe and no binding with the Mycobacterium tuberculosis probe on the membrane, indicating atypical infection, which was in concordance with the results of the phenotypic MGIT method. There were 7 samples that showed inhibition with RLBH. The percent agreement for susceptibility testing between the phenotypic test and RLBH assay was calculated to be 93% for all the targeted drugs. With the application of the assay on direct clinical specimens, the turnaround time to results was reduced to 3 days (assay takes about 4 to 5 h, but times for DNA extraction and PCR amplification total the time to results as 3 days), compared to 2 to 4 weeks in the case of the phenotypic liquid culture system.
The RLBH membrane was used 7 times without compromising the signal strength, but the strength of the signal was reduced from the 8th use, thereby causing difficulty with accurate reading of the results.
Time to DST.
On average, the time to culture positivity for a smear 3+ positive is within 7 days (13), and thereafter, another 10 to 14 days is needed for DST results. The total time to DST for a smear 3+-positive specimen would therefore be 17 to 21 days. This is increased with smear 2+-positive specimens, wherein time to identification is 8 to 10 days, and thereafter, another 10 to 14 days is needed for DST, with the total time being 18 to 24 days. Similarly, for smear 1+-positive specimens, the time to identification is 11 days, and thereafter, 10 to 14 days is needed for DST, with the total time being 21 to 25 days. In comparison, the RLBH assay takes only 3 days from a direct smear 1+-positive specimen (including the DNA extraction, PCR amplification, and assay procedure).
DISCUSSION
The present study is a retrospective study that shows the greater rapidity of the RLBH assay using a probe-based technique over the phenotypic methods, with sensitivities for detection of resistance to INH, RIF, FQs, and second-line aminoglycosides being 99%, 99%, 95.34%, and 94.8%, respectively. These performance characteristics are indicative of equivalence with conventional DST. In most developing countries, DST is usually performed on solid medium, such as Lowenstein-Jensen, wherein results are available after 8 to 18 weeks, during which time many patients with MDR-TB or XDR-TB may have died, transmitted their disease, or both. Conventional DST for second-line drug testing is far less standardized than DST for first-line drugs. The critical concentrations are not as clear-cut, often because the MIC values are close to the assumed critical concentrations. The RLBH assay detects the presence of M. tuberculosis isolates with patterns of resistance to INH, RIF, FQ, KAN, AM, and CM within a short span of 3 days directly from a smear-positive clinical specimen by detection of point mutations present in rpoB, katG, inhA, rrs, gyrA, and gyrB. This could help clinicians in initiating appropriate therapy and implement infection control measures to curb the spread of resistance. Compared with PCR-based sequencing and DNA microarray techniques, RLBH can be performed without expensive devices. Though mutations to the first-line drugs are well studied, among the Indian XDR M. tuberculosis isolates in a study conducted in Delhi (7), the key mutations observed were S531L in rpoB and S315T in katG, which are similar to our findings. A previous study from this center (14) done on MDR isolates revealed an array of point mutations from positions 511 to 531 in rpoB, which seems to have been selected out to be Ser531Leu, in the case of XDR M. tuberculosis isolates. Among rifampin-resistant isolates, our findings are in concordance with those of other studies (1) that are dominated by the Ser531Leu mutation among the XDR M. tuberculosis isolates. There are limited mutation studies for the second-line drugs. FQ resistance is correlated with mutations in the quinolone resistance-determining regions (QRDRs), predominantly in gyrA and in gyrB, and a 90% accuracy of prediction of FQ resistance was achieved when mutations are detected at the 90th and 94th codons in this study, which are similar to the findings for Delhi (7) XDR M. tuberculosis isolates. Studies from outside of India also show mutations in the QRDR of gyrA, similar to the mutations observed among our XDR M. tuberculosis isolates (1). The resistance to second-line injectables can be predicted by the nucleotide changes in the 3′ part of the 16S rRNA (rrs) gene. With this assay, 89% of the second-line aminoglycoside resistance could be detected. The key mutations were seen at A1401G and G1484T, similar to the results from characterization of XDR M. tuberculosis isolates from Delhi (7). This study was dominated by the A1401G and G1484T mutations in rrs, whereas other studies (1) have reported several other point mutations in the rrs gene.
Commercial assays MTBDRplus and MTBDRsl (Hain Lifesciences) (8) are strip based and use two strips per patient, which are priced at approximately $49 (separate strips are needed for the drugs involved in the definition of MDR and FQs, plus injectables), excluding the equipment cost. In contrast, RLBH uses a single membrane to identify mutations for XDR-TB, and once it is prepared, the membrane can be stripped and reused at least 7 times without affecting the signal strength. Forty samples along with a positive control and a negative control can be tested simultaneously, and this reduces the cost of the assay, making it suitable for application in routine clinical laboratories in developing countries. Though the GeneXpert system (3), also evaluated at our center, is the new state-of-the-art technology, it can detect resistance only to rifampin, and the cost of the test at a reduced price for India is $17, excluding the cost of the instrument, whereas our assay targets all the drugs involved in the XDR-TB definition. Thus, the present assay is an adequate tool to rapidly detect these point mutations that confer resistance to INH, RIF, FQ, KAN, AM, and CM. Besides being cost-effective, this assay enables initiation of early treatment, which could curb the spread of resistant strains, thus helping in control of tuberculosis.
The assay can detect only those mutations that are covered by the wild-type or mutant probe. The inability to detect novel mutations at locations other than the probe region or outside the amplified region would be a shortcoming, but the system is an open system, wherein newer probes can be added. Though molecular assays have a short turnaround times and are the state of the art, they cannot completely replace the conventional methods, as there are multiple genes involved in resistance and the molecular assays target only known mutations.
Conclusion.
In this study, we have attempted to characterize the genetic mechanism of resistance in the XDR isolates, wherein we saw good concordance with the phenotypic resistance in the cases of isoniazid, rifampin, fluoroquinolones, and second-line aminoglycosides. As XDR M. tuberculosis is emerging as a highly threatening pathogen in a pervasive association with the AIDS epidemic, rapid tests to promptly identify resistance to first- and second-line antitubercular agents are urgently required. It has been observed that point mutations in rpoB, inhA, katG, gyrA, gyrB, and rrs can accurately predict resistance to these drugs. The RLBH assay is proposed to be an adequate tool for direct analysis of XDR M. tuberculosis from clinical specimens, thus fulfilling many attributes of an ideal TB diagnostic test, as proposed by the WHO for developing countries.
Supplementary Material
Acknowledgements
We sincerely thank the National Health Education Society, P. D. Hinduja National Hospital and Medical Research Centre, Mumbai, India, for funding this study.
We thank the entire staff of the Mycobacteriology Department at P. D. Hinduja National Hospital and Medical Research Centre and Ranjit. Mankeshwar for his esteemed guidance in the statistical analysis.
There are no conflicts of interest for the study reported here.
Footnotes
Supplemental material for this article may be found at http://jcm.asm.org/.
Published ahead of print on 25 May 2011.
REFERENCES
- 1. Ajbani K., Rodrigues C., Shenai S., Mehta A. 2011. Mutation detection and accurate diagnosis of extensively drug resistant tuberculosis: report from a tertiary care center in India. J. Clin. Microbiol. 49:1588–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alangaden G., Manavathu E., Vakulenko S., Zvonok N., Lerner S. 1995. Characterization of fluoroquinolone-resistant mutant strains of Mycobacterium tuberculosis selected in the laboratory and isolated from patients. Antimicrob. Agents Chemother. 39:1700–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boehme C. C., et al. 2010. Rapid molecular detection of tuberculosis and rifampicin resistance. N. Engl. J. Med. 363:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi N., et al. 2010. Multidrug resistant and extensively drug resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843 [DOI] [PubMed] [Google Scholar]
- 5. Ginsburg A., Grosset J., Bishai W. 2003. Fluoroquinolones, tuberculosis, and resistance. Lancet Infect. Dis. 3:432–442 [DOI] [PubMed] [Google Scholar]
- 6. Kent P. T., Kubica G. P. 1985. Public health mycobacteriology: a guide for the level III laboratory, p. 64-68. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Atlanta, GA [Google Scholar]
- 7. Khanna A., et al. 2010. Emergence of extensively drug-resistant Mycobacterium tuberculosis clinical isolates from the Delhi Region in India. Antimicrob. Agents Chemother. 54:4789–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kiet V. S., et al. 2010. Evaluation of the MTBDRsl test for detection of second-line drug resistance in Mycobacterium tuberculosis. J. Clin. Microbiol. 48:2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martin-Casabona N., Mimo D. X., Gonzalez T., Rossello J., Arcalis L. 1997. Rapid method for testing susceptibility of Mycobacterium tuberculosis by using DNA probes. J. Clin. Microbiol. 35:2521–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maus C., Plikaytis B., Shinnick T. 2005. Molecular analysis of cross-resistance to capreomycin, kanamycin, amikacin, and viomycin in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 49:3192–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mokrousov I., et al. 2004. Multicentre evaluation of reverse line blot assay for detection of drug resistance in Mycobacterium tuberculosis clinical isolates. J. Microbiol. Methods 57:323–335 [DOI] [PubMed] [Google Scholar]
- 12. Musser J. M. 1995. Antimycobacterial agent resistance in mycobacteria: molecular genetic insights. Clin. Microbiol. Rev. 8:496–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodrigues C., et al. 2009. Evaluation of the BACTEC MGIT 960 system for the recovery and identification of Mycobacterium tuberculosis complex in a high throughput tertiary care centre. Int J. Med. Microbiol. 27:217–221 [DOI] [PubMed] [Google Scholar]
- 14. Shenai S., Rodrigues C., Mehta A. 2009. Rapid speciation of 15 clinically relevant mycobacteria with simultaneous detection of resistance to rifampin, isoniazid, and streptomycin in Mycobacterium tuberculosis. Int. J. Infect. Dis. 13:46–58 [DOI] [PubMed] [Google Scholar]
- 15. Somerville W., Thibert L., Schwartzman K., Behr M. 2005. Extraction of Mycobacterium tuberculosis DNA: a question of containment. J. Clin. Microbiol. 43:2996–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki, et al. 1998. Detection of kanamycin-resistant Mycobacterium tuberculosis by identifying mutations in the 16S rRNA gene. J. Clin. Microbiol. 36:1220–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takiff H., et al. 1994. Cloning and nucleotide Sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Telenti A., et al. 1997. Genotypic assessment of isoniazid and rifampin resistance in Mycobacterium tuberculosis: a blind study at reference laboratory level. J. Clin. Microbiol. 35:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torres M. J., Criado A., Palomares J. C., Aznar J. 2000. Use of real-time PCR and fluorimetry for rapid detection of rifampin and isoniazid resistance-associated mutations in Mycobacterium tuberculosis. J. Clin. Microbiol. 38:3194–3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uys P., Warren R., Helden P. 2007. A threshold value for the time delay to TB diagnosis. PLoS One 2:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yagui M., et al. 2006. Timely diagnosis of MDR-TB under program conditions: is rapid drug susceptibility testing sufficient? Int. J. Tuberc. Lung Dis. 10:838–843 [PMC free article] [PubMed] [Google Scholar]
- 22. Yam W. C., et al. 2004. Direct detection of rifampin resistant Mycobacterium tuberculosis in respiratory specimens by PCR-DNA sequencing. J. Clin. Microbiol. 42:4438–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang M., et al. 2005. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. J. Clin. Microbiol. 43:5477–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y., Coyne M. Y., Will S. G., Levenson C. H., Kawasaki E. S. 2000. Single base mutational analysis of cancer and genetic diseases using membrane bound modified oligonucleotides. Nucleic Acids Res. 19:3929–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.