Abstract
Propionibacterium acnes is increasingly recognized as an important agent of prosthetic joint infection (PJI). However, the optimum culture conditions for recovery of this organism from PJI specimens have not been determined. By applying a prolonged 28-day culture incubation to all periprosthetic specimens received for bacterial culture from 198 revision arthroplasty procedures, we retrospectively determined that a 13-day culture incubation period is necessary for the recovery of P. acnes from patients with PJI. Incubation beyond this period was associated with increasing recovery of nondiagnostic isolates: 21.7% of P. acnes isolates believed to be clinically unimportant were recovered after 13 days of incubation. Importantly, a diagnosis of P. acnes PJI would have been missed in 29.4% of patients had extended culture incubation been applied only to anaerobic culture media. Although specimens from P. acnes PJIs were more commonly associated with the presence of ≥2 culture media positive for growth, acute inflammation (≥5 neutrophils/high-power field) was observed in only 40% of patients with PJIs that had more than one specimen submitted for bacterial culture. These results support the need for a minimum culture incubation period of 13 days to be applied to both aerobic and anaerobic culture media for all periprosthetic specimens. Optimal recovery of infecting organisms from PJI specimens will be an important component in generating a universal definition for PJI due to indolent agents of infection, such as P. acnes.
INTRODUCTION
Prosthetic joint replacement, or arthroplasty, successfully alleviates joint pain and dysfunction in hundreds of thousands of individuals annually. Despite this success, failure of the new joint can occur for a variety of reasons, such as aseptic loosening or fracture of the prosthesis (5). However, the most serious cause of prosthetic joint failure is infection. Patients with infected prosthetic joints typically require debridement, replacement of the prosthesis, as well as an extended course of antimicrobial therapy (17).
Prosthetic joint infections (PJIs) are classified temporally (32), with infections diagnosed within 3 months of arthroplasty being defined as “early.” “Delayed” PJIs, in contrast, are diagnosed thereafter through the first 24 months after surgery, with infections diagnosed beyond this period being defined as “late” PJIs. The temporal nature of this classification is believed to result from the innate virulence capabilities of the etiological agents of infection (28). Thus, early PJIs are typically caused by bacteria classically thought to be more virulent, such as Staphylococcus aureus. Delayed PJIs, in contrast, are caused by bacteria generally considered to be of lesser virulence, for example, coagulase-negative staphylococci. Not surprisingly, presentation of infection generally differs between early and delayed PJIs. Patients with early PJIs present acutely with classic signs of infection (e.g., fever, erythema, and leukocytosis), whereas patients with delayed PJIs often present with joint pain as the sole symptom (5). Because laboratory markers of infection are generally unremarkable in the latter group of patients (e.g., C-reactive protein, white blood cell [WBC] count, erythrocyte sedimentation rate) (20), obtaining a diagnosis of infection can often be challenging. Frequently, delayed PJIs can therefore be diagnosed only by using bacterial cultures of periprosthetic tissue and fluid specimens. Obtaining specimens for bacterial culture is also essential in cases of suspected PJI because identification of the etiological agent of infection is crucial in the selection of optimal antimicrobial therapy (16, 17).
Propionibacterium acnes is recognized as an agent of infection of prosthetic devices, such as ventriculoperitoneal shunts and prosthetic heart valves (2, 5, 14, 18). The importance of this Gram-positive, non-spore-forming, anaerobic bacterium as an agent of PJI is increasingly appreciated (6, 13, 15, 24). It has been hypothesized that because P. acnes is commonly associated with the sebaceous follicles of the axilla (19), this bacterium is more frequently associated with PJIs of the shoulder than of the hips or knees (13). However, because P. acnes is also a common contaminant of bacterial cultures, the significance of recovery of this organism from periprosthetic specimens is not always clear. Unfortunately, the interpretation of the significance of culture results is confounded by inconsistency among the culture methods employed in previous studies. In particular, a variety of culture incubation periods have been employed in studies described in the literature.
Typically, the majority of bacterial cultures of tissues and fluids obtained from sterile sites are incubated for 5 days or less (26). However, a number of studies have demonstrated the importance of extending culture incubation beyond this period for specimens where PJI is a consideration (13, 22). Extended culture incubation appears to be necessary to maximize the recovery of P. acnes from infected prosthetic joints due to the known prolonged doubling time of this organism (10). When described, extended culture incubation has generally been limited to a 14-day incubation of anaerobic culture media. It was therefore previously unknown whether recovery of P. acnes from PJI specimens would be further enhanced by additional culture incubation or whether such an extension would lead solely to increased recovery of nondiagnostic isolates. Furthermore, because many strains of P. acnes are aerotolerant (25), it is possible that extended culture incubation of both aerobic and anaerobic culture media would improve the recovery of P. acnes from PJI specimens.
Previous studies have not determined the specific culture requirements for the diagnosis of PJI due to P. acnes. Thus, the aims of this study were 2-fold: first, to retrospectively investigate the utility of extended culture incubation of both aerobic and anaerobic culture media for the recovery of P. acnes from PJI specimens and, second, to compare culture characteristics among P. acnes-positive specimens from PJIs compared to those among specimens where recovery of this bacterium was considered nondiagnostic. Any differences noted could have future utility in the generation of a universal definition for the diagnosis of PJIs. The use of optimized culture incubation conditions by clinical microbiology laboratories for the recovery of P. acnes from PJI specimens will be crucial in the process of generating such a definition in the future.
MATERIALS AND METHODS
Study population.
Records from the University of Washington clinical microbiology laboratory for July 2006 to January 2009 were reviewed to identify patients with at least one periprosthetic tissue and/or fluid specimen positive for bacterial growth that was subject to the specific culture methods detailed in the following section. Administration of prophylactic antibiotics was withheld until specimens for bacterial culture had been obtained. The Investigational Review Board (IRB) of the University of Washington approved this study (IRB approval no. 38891).
Culture methods.
All tissue and fluid specimens were processed within 1 h of surgery in a class 2 laminar-flow biological safety cabinet. Tissue specimens were homogenized in 3 ml of sterile saline using a Seward Stomacher 80, and the sterility of each vial of saline was confirmed at the time of use by bacterial culture. Sterile disposable Pasteur pipettes were used to inoculate fluid and homogenized tissue specimens onto the following microbiological media: blood agar (Trypticase soy agar with 5% sheep blood), chocolate agar, brucella agar (with blood, hemin, and vitamin K), and brain heart infusion broth (all from Remel, Lenexa, KS). All agar media were inoculated for single-colony isolation using sterile disposable loops. MycoSeal sealant (Hardy Diagnostics, Santa Maria, CA) was applied to the exterior of all agar plates to prevent desiccation during culture incubation. All media, with the exception of the brucella agar, were incubated at 37°C with 5% CO2 for 28 days. Brucella agar plates were incubated anaerobically at 37°C for 28 days. Media were visually examined daily for growth and were opened only if growth was noted. All specimens were also inoculated onto MacConkey agar with lactose (Remel); however, these results were excluded from the analysis due to the inability of P. acnes to grow on this culture medium.
Identification of P. acnes.
All bacteria isolated were subject to further identification. Specifically, a full species-level identification and antimicrobial susceptibility testing were performed per colony morphology type isolated. Gram stain, catalase, and indole test spot results were used to refer an identification of P. acnes among morphologically identical colonies isolated from additional media or from additional specimens from the same patient event. Species-level identification of bacterial isolates was achieved by 16S rRNA gene sequencing. Genomic DNA was extracted using a NucliSENS easyMag kit (bioMérieux, Marcy l'Etoile, France), and PCR amplification and DNA sequencing of the 16S rRNA genes were performed as described previously (30). Analysis with the BLAST program was used to search the NCBI database for each sequence obtained.
Antimicrobial susceptibility testing was performed by Etest (AB Biodisk, bioMérieux), according to previously established guidelines (3), using a panel of antibiotics that are routinely tested against anaerobic bacteria isolated in our laboratory. The overall rates of susceptibility observed for all P. acnes isolates tested during the course of this study were as follows: metronidazole, 0%; clindamycin, 95.3%; cefotetan, 98.4%, imipenem, 100%; piperacillin-tazobactam, 100%; ampicillin-sulbactam, 100%.
Microbiological definition of infection.
The following previously published criteria for infection were applied (15): (i) ≥2 cultures positive for growth with a morphologically identical organism(s) or (ii) 1 culture positive for bacterial growth with ≥5 neutrophils/high-power field observed on histologic analysis. Recovery of P. acnes from an event that did not meet either of the previous criteria was classified as a nondiagnostic event. Patient events where only one periprosthetic specimen was submitted for bacterial culture were included only if an accompanying histologic examination was also performed.
Data and statistical analyses.
The following data were obtained for each patient event with at least one periprosthetic specimen positive for bacterial growth: (i) number of specimens submitted for culture and (ii) number of specimens positive for bacterial growth. The following data were also obtained for each specimen positive for growth with P. acnes: (i) direct Gram stain results, (ii) time to culture positivity, (iii) number and type of culture media positive for growth, and (iv) results of any histologic examination performed. All data obtained were compared among P. acnes-infected and nondiagnostic groups. The Wilcoxon rank sum test was used to test for differences by infection status in the following measures: number of samples obtained, time to culture positivity among positive specimens, and number of media (out of four) positive for growth. For each of the last two tests, a single positive specimen was randomly selected from all culture-positive specimens for that patient event.
RESULTS
Specimens from 173 patients undergoing revision arthroplasty (198 surgeries) were submitted to the University of Washington Clinical Microbiology Laboratory for bacterial culture during the period from July 2006 to January 2009. In all, 557 tissue and fluid specimens were received for bacterial culture, corresponding to approximately 2.8 specimens submitted per surgery. Retrospective analysis of laboratory records revealed that 82 patients (87 surgeries; hereafter referred to as “events”) had at least one intraoperative specimen positive for bacterial growth. The majority of specimens from these culture-positive events were from prosthetic shoulders (83%). Of these, 42 events met the criteria for infection, corresponding to an overall infection rate of 21% (42/198). We restricted our consideration to these 87 events in which bacterial growth was detected.
As shown in Table 1, P. acnes was the organism most commonly isolated from infected patients. In all, 23 P. acnes-infected events (corresponding to 20 patients) were noted. Of these, four were polymicrobial infections and were excluded from further analysis. Thus, 19 P. acnes-infected events were included in this study. Consistent with previously published studies, P. acnes was also the organism most commonly isolated from nondiagnostic events (Table 1). Twenty-six events had a single periprosthetic specimen positive for growth with P. acnes. Of these, 3 events had only a single specimen submitted for culture in the absence of an accompanying histologic examination and were excluded from further analysis. Of the 23 remaining nondiagnostic P. acnes events, 10 were polymicrobial. However, because any additional organisms recovered were also isolated only from a single specimen, these events were included in the analysis so that a comparable number of patient events could be analyzed.
Table 1.
Organisms isolated from culture-positive patient events
| Organism | No. of events in which organisms were recovered |
|
|---|---|---|
| Infected events (n = 42) | Nondiagnostic eventsb (n = 45) | |
| Propionibacterium acnes | 23 | 26 |
| Coagulase-negative staphylococci | 7 | 17 |
| Staphylococcus aureusa | 6 | |
| Candida albicans | 2 | |
| Finegoldia magna | 2 | |
| Pseudomonas putida | 1 | |
| Mycobacterium marinum | 1 | |
| Enterococcus faecalisa | 1 | |
| Otherc | 16 | |
One instance of an S. aureus and E. faecalis polymicrobial infection.
Sixteen patient events had more than one organism recovered.
Includes Bacillus, Micrococcus, Paenibacillus, Kocuria, Dermacoccus, Corynebacterium, and Micromonospora species.
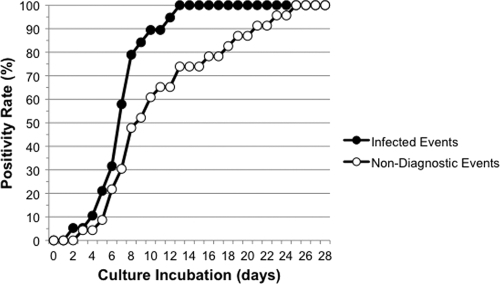
Incubation beyond 13 days is associated with increasing recovery of nondiagnostic P. acnes isolates.
The utility of culture incubation extending beyond 14 days for the recovery of P. acnes from patients with PJI had not previously been determined. As shown in Fig. 1, all P. acnes-infected events were positive for growth by 13 days. Furthermore, incubation beyond this period was associated with increasing recovery of P. acnes that were nondiagnostic: 21.7% of nondiagnostic P. acnes events became positive for growth after 13 days. These data suggested that P. acnes-infected events might be associated with a reduced time to positivity compared with nondiagnostic events. Although a trend toward a reduced time to positivity was indeed observed for P. acnes-infected events (7.3 ± 2.6 days versus 10.7 ± 5.6 days), a Wilcoxon test based on a randomly sampled culture from each event did not detect a difference (P = 0.34). Despite being necessary for the recovery of P. acnes from PJI specimens, it is possible that a 13-day culture incubation period might not be sufficient. Of note, two patient events would not have met the criteria for infection had a 13-day culture incubation period been employed, suggesting that incubation beyond this period might be required.
Fig. 1.
Time to culture positivity for P. acnes-positive patient events. For infected patient events with ≥2 culture-positive specimens (n = 17), only data from the first specimen positive for growth were included. The median number and range of specimens submitted for bacterial culture were similar among infected patient events (median = 4; range = 2 to 8) and nondiagnostic events (median = 4; range = 1 to 7). No difference in the number of samples submitted was detected by Wilcoxon test (P = 0.33).
Optimum recovery of P. acnes from PJI specimens requires extended incubation of both aerobic and anaerobic culture media.
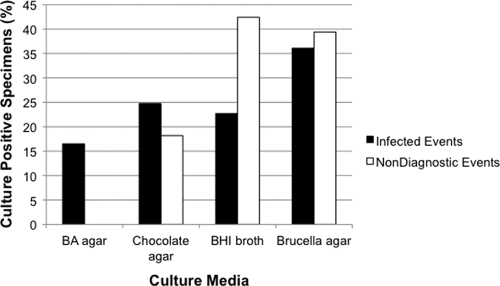
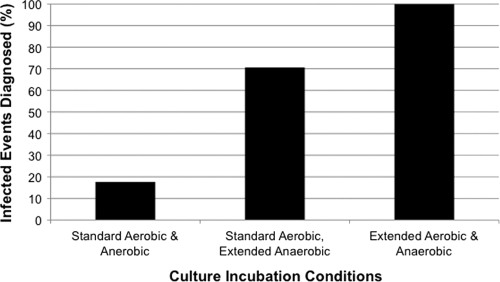
Because strains of P. acnes can be aerotolerant (25), we hypothesized that application of extended incubation to both aerobic and anaerobic culture media would maximize the recovery of P. acnes from PJI specimens. Although no individual culture medium was superior for the detection of P. acnes PJI, recovery of P. acnes from sheep blood agar was exclusively associated with the presence of PJI (Fig. 2). However, all specimens with P. acnes recovered from sheep blood agar also had P. acnes recovered from additional culture media. Importantly, a diagnosis of P. acnes PJI would have been missed in 29.4% of patients had extended culture incubation only been employed for the anaerobic culture media (Fig. 3). These data clearly demonstrate the importance of applying an extended culture incubation period to both aerobic and anaerobic culture media for optimum recovery of P. acnes.
Fig. 2.
Frequency of component culture media positive for growth with P. acnes. Recovery of P. acnes from blood agar was exclusively associated with the presence of infection (n = 16 specimens). However, all specimens positive for growth of P. acnes on blood agar were also positive for growth on at least one additional component medium. BA, blood agar; BHI, brain heart infusion.
Fig. 3.
Effect of culture incubation conditions on the diagnosis of PJI due to P. acnes.
Histologic analyses are frequently negative in patients with P. acnes PJI.
There were 27 P. acnes-positive patient events that had ≥2 specimens submitted for bacterial culture where histologic analyses were performed. Of the patient events with ≥2 specimens positive for growth with P. acnes, only 40% were found to have histologic evidence of active inflammation, i.e., ≥5 neutrophils/high-power field (Table 2). Similarly, P. acnes-infected events were only 1.5 times more likely to have a specimen with ≥2+ WBCs than specimens from nondiagnostic P. acnes events when the results of semiquantitative Gram stain WBC counts were examined (data not shown). These data further reiterate the frequently indolent nature of P. acnes PJI.
Table 2.
Association of P. acnes-positive specimens with histologic findings
| Histologic analysis result | No. of events |
||
|---|---|---|---|
| ≥2 culture-positive specimens | 1 culture-positive specimen | Total | |
| Positive | 6 | 0 | 6 |
| Negative | 9 | 12 | 21 |
| All | 15 | 12 | 27 |
Specimens from P. acnes-infected events are more likely to have multiple culture media positive for growth.
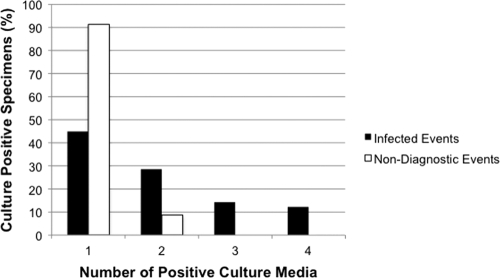
The reporting of positive bacterial cultures to clinicians typically includes a semiquantitative assessment of bacterial growth. However, information on the number of component media positive for bacterial growth is only rarely provided. In particular, such information is generally reserved for instances where an organism is isolated from broth only, i.e., where it is implied that the recovered organism is most likely a contaminant. Although we did not observe any difference in the semiquantitative enumeration of P. acnes recovered from solid media (data not shown), we hypothesized that P. acnes-infected events would be more commonly associated with growth in multiple media than nondiagnostic P. acnes events.
To address this, the number of culture media positive for growth with P. acnes was compared between infected and nondiagnostic patient events. As shown in Fig. 4, specimens from P. acnes-infected events were 6.3 times more likely to have ≥2 media positive for growth than nondiagnostic P. acnes events (P = 0.002). This difference could not be accounted for solely by the 28-day culture incubation period used in this study: had a 14-day culture incubation period been employed, P. acnes-infected events were 9 times more likely to have ≥2 media positive for growth with P. acnes. Importantly, the recovery of P. acnes from 3 or more culture media was exclusively associated with infection. Thus, while recovery of P. acnes from only a single culture medium does not exclude the possibility of clinical infection, recovery of this organism from multiple culture media is more commonly associated with the presence of infection.
Fig. 4.
Number of culture media positive for growth with P. acnes. Fifty-five percent of culture-positive specimens from infected patient events had ≥2 media positive for growth with P. acnes, compared with 8.7% of culture-positive specimens from nondiagnostic events.
DISCUSSION
The diagnosis of prosthetic joint infections with low-virulence organisms, such as P. acnes, is challenging because of the absence of classical clinical evidence of infection, as well as the challenges associated with culturing these organisms. We have analyzed the culture results for a large series of patients having upper extremity revision surgery for painful or loose prosthetic arthroplasty. Using a 28-day culture incubation period for both aerobic and anaerobic culture media, we retrospectively compared the characteristics of culture-positive specimens between P. acnes PJI and nondiagnostic events. In this regard, we have shown that (i) incubation beyond 13 days is associated with increasing recovery of nondiagnostic isolates, (ii) both aerobic and anaerobic culture media should be incubated for a minimum of 13 days to maximize recovery of P. acnes from PJI specimens, (iii) negative histologic findings were frequently observed in P. acnes-infected events, and (iv) PJI specimens were 6.3 times more likely to have ≥2 culture media positive for growth with P. acnes than specimens from nondiagnostic events.
Prevalence of infection subsequent to shoulder arthroplasty has previously been estimated to range from 0.4 to 15.4% (4, 23). At 21%, the infection rate observed in this study is higher than these published estimates, although it is lower than that in a recently published study (22). While it is possible that this higher infection rate could reflect increased recovery of contaminants that met the criteria for infection due to the extended culture incubation period employed, i.e., false positives, we do not believe this to be the case for the following reasons: first, published infection rates are based on a collection of studies where various culture incubation periods, frequently of 1 week or less in duration, were employed. Thus, the prevalence of infection subsequent to shoulder arthroplasty in many previous reports may have been underestimated. Second, a high prevalence of infection is not unexpected given the status of our institution as a reference center for revision arthroplasty. Had a 1-week culture incubation period been employed in this study, the infection rate would have been 15.6%, a figure in line with previously published rates. These data therefore demonstrate the importance of utilizing optimized culture incubation conditions to maximize recovery of P. acnes from PJI specimens.
The optimum culture incubation period for the recovery of P. acnes from PJI specimens has not been determined previously. Consistent with previous findings, we retrospectively determined that a 13-day culture incubation is necessary for the recovery of P. acnes from PJI specimens, although incubation beyond this period was associated with increasing recovery of P. acnes isolates that were nondiagnostic. Furthermore, we have demonstrated that a diagnosis of PJI would have been missed in 29.4% of infected patient events had extended culture incubation only been applied to anaerobic culture media. Although it is possible that additional culture incubation may be required, determination of the significance of recovery of P. acnes from specimens after 13 days of incubation will ultimately require large outcome studies with many patients. In addition, even if such data were available, incubation beyond 13 days would be impractical for most laboratories due to increased labor costs and the costs associated with increased recovery of nondiagnostic isolates.
One limitation of this study, as well as of others, is the absence of an accepted universal definition for the diagnosis of PJI. This is particularly problematic in cases of PJI due to more indolent organisms, such as P. acnes, where overt clinical and laboratory signs of infection are often absent. A variety of criteria for the diagnosis of PJI can therefore be found in the literature, ranging from a single culture-positive periprosthetic specimen in the absence of histologic findings (11) to the requirement for a minimum of three culture-positive specimens (1). One of the criteria used in this study was the requirement for morphologically indistinguishable organisms to be recovered from two or more specimens. This criterion was previously shown to correlate well with the presence of inflammation on histologic analysis, although this association was observed for a collective of different bacterial species and not for P. acnes specifically (15). Using optimized culture incubation conditions, we observed that only 40% of patient events with ≥2 specimens positive for growth with P. acnes had evidence of acute inflammation, consistent with previously published findings (31). Previous studies suggest that the presence of other findings on histology may also be indicative of P. acnes infection, e.g., chronic inflammation and macrophages (29, 31). Interestingly, all patients diagnosed preoperatively with P. acnes PJI in a previous study (i.e., patients with classic signs of infection) had evidence of acute inflammation, while neutrophils were observed in only 50% of those patients diagnosed postsurgically with P. acnes PJI (6). Our data provide additional support for the assertion that acute inflammation is often absent in patients with delayed PJI due to P. acnes.
Like many previously published studies, this study considers a single culture-positive specimen in the absence of histologic findings to be nondiagnostic and most likely represents contamination. In spite of this, a number of instances have been described in the literature where this assumption has been inaccurate (8, 12). For example, Kelly and colleagues noted a patient with only one of four periprosthetic specimens positive for growth of P. acnes who subsequently developed recurrent P. acnes PJI (12). While recovery of P. acnes from a single periprosthetic specimen most likely represents contamination from either the patient's skin, the skin of the medical staff, or laboratory processing, it could also represent low-level colonization of the prosthetic joint. Such colonization may in turn lead to the development of subsequent infection. While this scenario may not represent clinically significant infection at the time of specimen harvesting, outcomes data are required to determine the clinical significance of such findings, as well as the most appropriate and clinically efficacious action to be taken by clinicians. These data also suggest that the generation of a universal definition for the diagnosis of PJI caused by more indolent organisms may need to also incorporate criteria for the diagnosis of joint colonization. Such definitions, as well as the criteria contained therein, will ultimately require a large study set with detailed outcome data, a task that is beyond the scope of this analysis.
Because the definition of infection used in this study was solely microbiological, it was not possible to determine the sensitivity of culture. However, the sensitivity of periprosthetic culture for the diagnosis of PJI in previous studies has often been determined using the presence of overt signs of infection as a comparator, e.g., presence of a sinus tract communicating with the prosthesis and the presence of acute inflammation. As mentioned previously, such a comparison may not be entirely appropriate for PJI due to more indolent agents, such as P. acnes, which can present either acutely or indolently. Recent data have demonstrated that the sensitivity of culture for the diagnosis of PJI can be further improved by subjecting the removed explant to sonication, followed by culture of the sonicate fluid (27). With respect to the diagnosis of shoulder PJI, explant sonication was shown to be 12% more sensitive than traditional periprosthetic culture (66.7% versus 54.5%) (21). Similar findings have also been observed for the diagnosis of PJI of additional joints such as prosthetic knees and elbows (27). The improved sensitivity observed is believed to result from the propensity of agents of PJI, including P. acnes, to form biofilms on the surface of the prosthesis (7, 9). However, in the case of shoulder PJI, the incubation period employed differed between periprosthetic tissue (5 to 7 days) and sonicate cultures (14 days; anaerobic culture media). It is therefore unknown to what extent the sensitivity of sonicate culture truly surpasses that of periprosthetic culture for the recovery of P. acnes from patients with PJI. Future studies will be required to determine whether the sensitivity of periprosthetic culture using extended incubation of both aerobic and anaerobic culture media is equivalent to that of sonicate cultures. Nonetheless, one would predict that the sensitivity of sonicate culture will be further improved by employing extended culture incubation of both aerobic and anaerobic culture media for this culture type.
A further aim of this study was to determine whether other parameters that are easily obtained within the microbiology laboratory, but generally not reported to clinicians, would correlate with the presence of infection. To our knowledge, this is the first study to specifically examine the culture characteristics of P. acnes-positive specimens, other than culture time to positivity. Although the latter characteristic did not appear to be useful in the differentiation of infected events from nondiagnostic events, the data observed raise important questions for future research. For example, it is unknown whether recovery of P. acnes from a single specimen is more likely to represent colonization than contamination if the organism is recovered earlier in the culture incubation process. Furthermore, it is also unknown whether differences in patient presentation (i.e., acute versus indolent infection) may correlate with culture time to positivity. However, it is reasonable to hypothesize that acute infections could be more likely to become culture positive earlier than indolent ones due to the possibility of increased numbers of microorganisms in the acutely, overtly infected prosthetic joint. Finally, it is unknown whether culture time to positivity could play a role in predicting patient outcome. Future outcome studies will be required to address these questions.
In this study, we also observed that specimens from P. acnes-infected events were more commonly associated with the presence of ≥2 media positive for growth. In particular, recovery of P. acnes from sheep blood agar was exclusively associated with the presence of infection. Because not all strains of P. acnes are aerotolerant, it is possible that aerotolerance may be associated with increased virulence. However, we do not believe this to be the explanation for our observation. Rather, we believe that this finding most likely represents a higher organism burden in patients with PJI than in those without, as P. acnes was also recovered from the anaerobic culture media for each of these patient events. In addition, aerotolerance was observed for P. acnes strains isolated from both infected and nondiagnostic events when this characteristic was examined (data not shown). Because no specific anaerobic device was used for the transport of periprosthetic specimens to the laboratory in this study, it is also possible that there could have been a selection bias in favor of the recovery of aerotolerant P. acnes strains. However, we do not believe this to be the case, as the majority of tissue specimens received were over 1 cm3 in size, a size known not to require strict anaerobic transport conditions (26). Furthermore, all specimens were processed within 1 h of collection. Clinical outcomes studies will ultimately be required to determine whether patient events with a single periprosthetic specimen positive for P. acnes where 2 culture media are positive for growth are more likely to represent a clinically significant or insignificant finding.
Other laboratory methods for pathogen detection, most notably, direct 16S rRNA gene PCR, were previously demonstrated to be of limited utility in the diagnosis of PJI due to P. acnes. Thus, bacterial cultures of periprosthetic and sonicate specimens currently remain the mainstay for identification of etiological agents of infection. As a result, it is critically important that clinical microbiology laboratories utilize optimized culture conditions for the recovery of P. acnes from prosthetic joint specimens. While the implementation of 13-day culture incubation for both aerobic and anaerobic culture media is clearly associated with increased expense to the laboratory, the improvement in recovery of P. acnes is clear. Laboratories should also consider implementation of an extended culture incubation period where P. acnes is a common agent of infection e.g., cerebrospinal fluid specimens obtained through shunt devices, heart valve specimens, etc. Finally, the recovery of P. acnes from multiple culture media where noted should also be reported to clinicians. The use of optimized culture conditions by laboratories, coupled with the reporting of additional available microbiology data, will likely facilitate the future generation of a universal definition of prosthetic joint infection and colonization.
Footnotes
Published ahead of print on 4 May 2011.
REFERENCES
- 1. Atkins B. L., et al. 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J. Clin. Microbiol. 36:2932–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brook I. 2002. Meningitis and shunt infection caused by anaerobic bacteria in children. Pediatr. Neurol. 26:99–105 [DOI] [PubMed] [Google Scholar]
- 3. Clinical Laboratory Standards Institute 2009. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard, seventh ed. Document M11-A7, vol. 27, no. 2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 4. Cofield R. H, Edgerton B. C. 1990. Total shoulder arthroplasty: complications and revision surgery. Instr. Course Lect. 39:449–462 [PubMed] [Google Scholar]
- 5. Del Pozo J. L, Patel R. 2009. Clinical practice. Infection associated with prosthetic joints. N. Engl. J. Med. 361:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodson C. C., et al. 2010. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J. Shoulder Elbow Surg. 19:303–307 [DOI] [PubMed] [Google Scholar]
- 7. Donlan R. M. 2005. New approaches for the characterization of prosthetic joint biofilms. Clin. Orthop. Relat. Res. 437:12–19 [DOI] [PubMed] [Google Scholar]
- 8. Dramis A., et al. 2009. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int. Orthop. 33:829–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gristina A. G. 1987. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237:1588–1595 [DOI] [PubMed] [Google Scholar]
- 10. Hall G. S., et al. 1994. Growth curve for Propionibacterium acnes. Curr. Eye Res. 13:465–466 [DOI] [PubMed] [Google Scholar]
- 11. Kanafani Z. A., et al. 2009. Postoperative joint infections due to Propionibacterium species: a case-control study. Clin. Infect. Dis. 49:1083–1085 [DOI] [PubMed] [Google Scholar]
- 12. Kelly J. D., II, Hobgood E. R. 2009. Positive culture rate in revision shoulder arthroplasty. Clin. Orthop. Relat. Res. 467:2343–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy P. Y., et al. 2008. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin. Infect. Dis. 46:1884–1886 [DOI] [PubMed] [Google Scholar]
- 14. List R. J, Sheikh N, Theologou T, Mitchell I. M, Mathew T. 2009. Propionibacterium acnes endocarditis of a prosthetic aortic valve. Clin. Cardiol. 32:E46–E47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lutz M. F., et al. 2005. Arthroplastic and osteosynthetic infections due to Propionibacterium acnes: a retrospective study of 52 cases, 1995-2002. Eur. J. Clin. Microbiol. Infect. Dis. 24:739–744 [DOI] [PubMed] [Google Scholar]
- 16. Matthews P. C, Berendt A. R, McNally M. A, Byren I. 2009. Diagnosis and management of prosthetic joint infection. BMJ 338:b1773. [DOI] [PubMed] [Google Scholar]
- 17. Moran E., et al. 2007. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J. Infect. 55:1–7 [DOI] [PubMed] [Google Scholar]
- 18. Moreira A. L, Haslett P. A, Symmans W. F. 2000. Propionibacterium acnes as the cause of endocarditis in a liver transplant recipient. Clin. Infect. Dis. 30:224–226 [DOI] [PubMed] [Google Scholar]
- 19. Patel A, Calfee R. P, Plante M, Fischer S. A, Green A. 2009. Propionibacterium acnes colonization of the human shoulder. J. Shoulder Elbow Surg. 18:897–902 [DOI] [PubMed] [Google Scholar]
- 20. Piper K. E., et al. 2010. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One 5:e9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piper K. E., et al. 2009. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J. Clin. Microbiol. 47:1878–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schafer P., et al. 2008. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin. Infect. Dis. 47:1403–1409 [DOI] [PubMed] [Google Scholar]
- 23. Sperling J. W, Kozak T. K, Hanssen A. D, Cofield R. H. 2001. Infection after shoulder arthroplasty. Clin. Orthop. Relat. Res. 382:206–216 [DOI] [PubMed] [Google Scholar]
- 24. Sulkowski M. S, Abolnik I. Z, Morris E. I, Granger D. L. 1994. Infectious arthritis due to Propionibacterium acnes in a prosthetic joint. Clin. Infect. Dis. 19:224–225 [DOI] [PubMed] [Google Scholar]
- 25. Tally F. P, Goldin B. R, Jacobus N. V, Gorbach S. L. 1977. Superoxide dismutase in anaerobic bacteria of clinical significance. Infect. Immun. 16:20–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomson R. B. 2007. Specimen collection, transport, and processing: bacteriology, p. 291–333.In Murray P. R., Baron E. J., Jorgensen J. H., Landry M.L., Pfaller M.A. Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC. [Google Scholar]
- 27. Trampuz A., et al. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 357:654–663 [DOI] [PubMed] [Google Scholar]
- 28. Trampuz A, Widmer A. F. 2006. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 19:349–356 [DOI] [PubMed] [Google Scholar]
- 29. Tunney M. M., et al. 1999. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J. Clin. Microbiol. 37:3281–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yager J. E., et al. 2010. Ureaplasma urealyticum continuous ambulatory peritoneal dialysis-associated peritonitis diagnosed by 16S rRNA gene PCR. J. Clin. Microbiol. 48:4310–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeller V., et al. 2007. Propionibacterium acnes: an agent of prosthetic joint infection and colonization. J. Infect. 55:119–124 [DOI] [PubMed] [Google Scholar]
- 32. Zimmerli W, Trampuz A, Ochsner P. E. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654 [DOI] [PubMed] [Google Scholar]