Abstract
Twenty of 1,279 nontyphoid Salmonella strains isolated from food animals and humans produced CTX-M-type extended-spectrum β-lactamase. All expressed CTX-M-15, except two which coexpressed CTX-M-14 and TEM-1. Insertion sequence ISEcp1 was identified upstream of blaCTX-M genes. The blaCTX-M-15 and blaCTX-M-14 genes were disseminated by large conjugative IncFIIs and IncI1-Iγ plasmids, respectively.
TEXT
Extended-spectrum cephalosporin (ESC) and fluoroquinolone are the drugs of choice for invasive Salmonella infections (14). Unfortunately, resistance to both drug classes has emerged among the Salmonella species worldwide, causing a serious problem in both human and veterinary medicine (2, 4). Similarly, reduced susceptibility to fluoroquinolones in Salmonella has been associated with clinical treatment failure, causing significant therapeutic problems in the clinical setting (1). During the past couple of decades, CTX-M-type extended-spectrum β-lactamases (ESBLs) or cefotaximases have been increasingly reported in many countries of the world (23). Currently, the CTX-M β-lactamase family consists of at least 92 different CTX-M β-lactamases that are clustered into five groups based on their amino acid identities, including CTX-M-1, -2, -8, -9, and -25 groups. ESBL production in Salmonella was first identified in 1988 (13). Since then, the number of studies reporting ESBL-mediated resistance in nontyphoid Salmonella (NTS) has been increasing (2, 4, 23), including from the Republic of Korea (20, 32), which has become a cause of concern. Thus, in the present study the phenotypic and genotypic characteristics of cefotaxime-resistant NTS strains isolated from food animals and humans in South Korea were investigated.
A total of 1,279 NTS isolates were obtained from food animals (n = 692) and human patients between January 1995 and December 2009. Animal isolates of NTS were recovered from various samples of food animals collected from all the nine provinces of South Korea and were isolated from pigs (n = 455), pork (n = 5), poultry (n = 54), chicken meat (n = 171), and cattle (n = 7). The human NTS strains were received from the Gwangju Research Institute of Public Health and Environment, South Korea. Identification and serotyping of Salmonella isolates were done as described previously (22). Overall, 20 NTS strains were resistant to cefotaxime. Except one isolate of Salmonella enterica serovar Essen, all of them belonged to serotype Enteritidis. Screening of these isolates by double disk synergy test (9) demonstrated production of ESBL, which was subsequently confirmed by the Epsilometer test (Etest) (AB Biodisk, Sweden). The geographical, temporal, and serotype distributions and origins of these 20 isolates are shown in Table 1. The MICs for selected antimicrobial agents were determined using the Etest strips according to Clinical and Laboratory Standards Institute (CLSI) guidelines (9). All of them were resistant to ampicillin (MIC, >256 mg/liter) and cefotaxime (MIC, >16 mg/liter). The presence of clavulanic acid reduced the MICs of cefotaxime for all the isolates by ≥128-fold (Table 2).
Table 1.
Phenotypic and molecular characteristics of nontyphoid Salmonella strains isolated from food animals and humans carrying blaCTX-M genesa
| Strain | Serotype | Origin | Farm | Province | Isolation date (yr.mo.day) | blaCTX-M gene | Other bla gene | Self-transferb | Phage type | PFGE profile |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| XbaI | AvrII | ||||||||||
| 09-V06-15 | S. Enteritidis | Chicken meat | C1 | Jeonbuk | 2009.01.28 | CTX-M-15 | − | Yes | PT21 | X4 | A3 |
| 09-V06-16 | S. Enteritidis | Chicken meat | C1 | Jeonbuk | 2009.01.28 | CTX-M-15 | − | Yes | PT21 | X5 | A1 |
| 09-V06-20 | S. Enteritidis | Diseased chicken | C2 | Jeonbuk | 2009.03.12 | CTX-M-15 | − | Yes | RDNC | X4 | A3 |
| 09-V06-21 | S. Enteritidis | Diseased chicken | C2 | Jeonbuk | 2009.03.12 | CTX-M-15 | − | Yes | PT21 | X4 | A3 |
| 09-V06-22 | S. Enteritidis | Diseased chicken | C2 | Jeonbuk | 2009.03.12 | CTX-M-15 | − | Yes | RDNC | X3 | A2 |
| 09-V06-23 | S. Enteritidis | Diseased chicken | C2 | Jeonbuk | 2009.03.12 | CTX-M-15 | − | Yes | RDNC | X4 | A1 |
| 09-V06-25 | S. Essen | Diseased chicken | C3 | Jeonbuk | 2009.03.27 | CTX-M-15 | − | No | ND | ND | ND |
| 09-V06-28 | S. Enteritidis | Diseased chicken | C4 | Jeonbuk | 2009.04.22 | CTX-M-15 | − | Yes | RDNC | X2 | A1 |
| 09-V05-54 | S. Enteritidis | Chicken meat | C6 | Chungnam | 2009.04.13 | CTX-M-15 | − | No | PT21 | X6 | A4 |
| 09-V05-56 | S. Enteritidis | Chicken feces | C6 | Chungnam | 2009.04.13 | CTX-M-15 | − | No | PT21 | X6 | A4 |
| 09-V05-57 | S. Enteritidis | Chicken feces | C6 | Chungnam | 2009.04.13 | CTX-M-15 | − | No | PT1 | X6 | A4 |
| 09-V05-58 | S. Enteritidis | Chicken feces | C7 | Chungnam | 2009.05.18 | CTX-M-15 | − | Yes | PT21 | X1 | A3 |
| 09-V05-59 | S. Enteritidis | Chicken feces | C6 | Chungnam | 2009.04.13 | CTX-M-15 | − | No | PT1 | X6 | A4 |
| 09-G-022 | S. Enteritidis | Human stool | Gwangju | 2009.06.01 | CTX-M-15 | − | No | PT1 | X8 | A6 | |
| 09-G-081 | S. Enteritidis | Human stool | Gwangju | 2009.10.05 | CTX-M-15 | − | No | PT21 | X6 | A5 | |
| 09-G-004 | S. Enteritidis | Human stool | Gwangju | 2009.02.02 | CTX-M-15 | − | Yes | PT1 | X4 | A3 | |
| 09-G-097 | S. Enteritidis | Human stool | Gwangju | 2009.11.23 | CTX-M-15 | − | Yes | PT21 | X4 | A3 | |
| 09-G-063 | S. Enteritidis | Human stool | Gwangju | 2009.08.17 | CTX-M-15 | − | Yes | PT21 | X4 | A3 | |
| 09-G-069 | S. Enteritidis | Human stool | Gwangju | 2009.09.07 | CTX-M-14 | TEM-1 | Yes | PT6a | X7 | A4 | |
| 09-G-076 | S. Enteritidis | Human stool | Gwangju | 2009.09.14 | CTX-M-14 | TEM-1 | Yes | PT6a | X7 | A6 | |
Abbreviations: PFGE, pulsed-field gel electrophoresis; RDNC, reacted but did not conform to any recognized phage types; ND, phage or PFGE typing not done; −, negative.
Self-transfer of plasmid carrying blaCTX-M gene in conjugation experiments.
Table 2.
Antimicrobial susceptibility and point mutation in DNA topoisomerase of nontyphoid Salmonella strains isolated from food animals and humans carrying blaCTX-M genea
| Strain | DDT | Etest MIC (mg/liter)b |
Point mutation within QRDR of: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP | CTX | CTX + CA | CAZ | CAZ + CA | FEP | ATM | FOX | NAL | CIP | gyrA | parC | ||
| 09-V06-15 | + | >256 | >16 | 0.064 | <0.5 | 0.125 | 16 | 24 | 1.5 | 256 | 0.125 | Asp87→Asn | Wild type |
| 09-V06-16 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 16 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-V06-20 | + | >256 | >16 | 0.094 | <0.5 | 0.25 | 24 | 24 | 2 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-V06-21 | + | >256 | >16 | 0.064 | <0.5 | 0.19 | 16 | 32 | 1.5 | 256 | 0.125 | Asp87→Asn | Wild type |
| 09-V06-22 | + | >256 | >16 | 0.094 | <0.5 | 0.25 | 16 | 24 | 2 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-V06-23 | + | >256 | >16 | 0.125 | <0.5 | 0.25 | 24 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-V06-25 | + | >256 | >16 | 0.125 | <0.5 | 0.25 | 16 | 24 | 2 | 512 | 0.25 | Asp87→Tyr | Wild type |
| 09-V06-28 | + | >256 | >16 | 0.064 | 24 | 0.125 | 12 | 16 | 1.5 | 128 | 0.125 | Asp87→Asn | Wild type |
| 09-V05-54 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 12 | 24 | 1.5 | 256 | 0.125 | Asp87→Asn | Wild type |
| 09-V05-56 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 16 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-V05-57 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 12 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-V05-58 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 16 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-V05-59 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 16 | 24 | 1.5 | 512 | 0.125 | Asp87→Asn | Wild type |
| 09-G-022 | + | >256 | >16 | 0.064 | <0.5 | 0.19 | 16 | 24 | 1.5 | 256 | 0.125 | Asp87→Asn | Wild type |
| 09-G-081 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 16 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-G-004 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 16 | 24 | 1.5 | 256 | 0.125 | Asp87→Asn | Wild type |
| 09-G-097 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 16 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-G-063 | + | >256 | >16 | 0.094 | <0.5 | 0.19 | 12 | 24 | 1.5 | 512 | 0.25 | Asp87→Asn | Wild type |
| 09-G-069 | + | >256 | >16 | 0.094 | 1.5 | 0.19 | 6 | 3 | 1.5 | 256 | 0.125 | Asp87→Gly | Wild type |
| 09-G-076 | + | >256 | >16 | 0.094 | 1.5 | 0.19 | 6 | 3 | 1.5 | 256 | 0.125 | Asp87→Gly | Wild type |
Abbreviations: DDT, double disk diffusion test; Etest, Epsilometer test; QRDR, quinolone resistance-determining region; AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime; ATM, aztreonam; FEP, cefepime; FOX, cefoxitin; CA, clavulanic acid; NAL, nalidixic acid; CIP, ciprofloxacin.
MICs of ciprofloxacin and nalidixic acid were determined by agar dilution method following the CLSI guidelines.
The PCR amplification and sequencing of entire blaTEM and blaSHV genes were done as described previously (26). The presence of the blaCTX-M gene was screened for using CTX-M universal primer sets as described previously (3). All 20 cefotaxime-resistant NTS strains carried blaCTX-M genes. These were confirmed by a second PCR using group-specific primers for CTX-M-1, CTX-M-2, CTX-M-8, and CTX-M-9 groups as described previously (5). Finally, combinations of CTX-M group-specific and ISECP1-U1 primers (27) were employed to amplify and sequence the complete blaCTX-M genes. Among them, 18 strains harbored the blaCTX-M-15 gene only whereas two remaining strains isolated from human patients coharbored blaCTX-M-14 and blaTEM-1 genes. Since blaCTX-M-15 was first identified in Enterobacteriaceae in 2003 in South Korea (18), blaCTX-M genes have been increasingly reported in Enterobacteriaceae either singly or in combination with other resistance determinants (21, 29). However, until recently, reports on CTX-M-type β-lactamase in Salmonella were limited to human isolates, particularly those causing salmonellosis in pediatric patients (19, 32). Thus, to our knowledge, cefotaximase has never been reported in Salmonella strains of animal origin from South Korea.
Additionally, all the 20 NTS strains exhibited high-level resistance to nalidixic acid (MICs, 128 to 512 mg/liter) and reduced susceptibility to ciprofloxacin (MICs, 0.125 to 0.25 mg/liter). Screening of plasmid-mediated quinolone resistance determinants using the primer sets and conditions as described previously (16, 17, 24) revealed that none of them carried the qnr, aac(6′)-Ib-cr, or qepA gene. Thus, PCR amplification and sequencing of the quinolone resistance-determining regions (QRDRs) of gyrA and parC genes were carried out as described previously (12). It was found that all of them contained a single mutation within the QRDR of gyrA at codon 87 (Table 2). Although fluoroquinolones are important alternative antimicrobials for treatment of invasive salmonellosis in adults, there are increasing reports of treatment failures for Salmonella infections caused by strains with decreased susceptibility to fluoroquinolones (1). Thus, it is worrisome to find the reduced susceptibility to fluoroquinolones among the ESBL-producing NTS strains, which would further exacerbate the complexity of the problem.
Conjugation experiments performed as described previously (28) demonstrated the transfer of the ESC resistance phenotype from 13 blaCTX-M-positive S. enterica serovar Enteritidis isolates to the sodium azide-resistant Escherichia coli J53 recipients. PCR analysis showed the presence of respective blaCTX-M genes in all the transconjugants. However, the blaTEM-1 gene did not transfer and was not amplified in the corresponding transconjugants. In addition to cephalosporin resistance, resistance to non-β-lactams also cotransferred along with the blaCTX-M-15 gene (Table 3). In contrast, no additional non-β-lactam resistance cotransferred along with the blaCTX-M-14 gene from the isolates that expressed them. These findings strongly suggest the association between blaCTX-M-15 and multidrug resistance (MDR), in contrast to blaCTX-M-14.
Table 3.
Characteristics of E. coli J53 transconjugants carrying blaCTX-M genes described in this studya
| Transconjugant | Donor strain | bla gene transferred | Approximate plasmid size (kb) | Replicon type by PCR | ISEcp1 upstream of blaCTX-M | IS903 downstream of blaCTX-M | Resistance transferred |
|---|---|---|---|---|---|---|---|
| p9V0615-1J | 09-V06-15 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9V0616-1J | 09-V06-16 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9V0620-1J | 09-V06-20 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9V0621-1J | 09-V06-21 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9V0622-1J | 09-V06-22 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9V0623-1J | 09-V06-23 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9V0628-1J | 09-V06-28 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9V0558-1J | 09-V05-58 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9G004-1J | 09-G-004 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9G097-1J | 09-G-097 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9G063-1J | 09-G-063 | CTX-M-15 | 95 | FIIs | + | − | AMP CEP XNL CTX ATM GEN STR TET SUL |
| p9G069-1J | 09-G-069 | CTX-M-14 | 95 | I1-Iγ | + | + | AMP CEP XNL CTX |
| p9G076-1J | 09-G-076 | CTX-M-14 | 95 | I1-Iγ | + | + | AMP CEP XNL CTX |
Abbreviations: AMP, ampicillin; CEP, cephalothin; XNL, ceftiofur; CTX, cefotaxime; ATM, aztreonam; GEN, gentamicin; STR, streptomycin; TET, tetracycline; SUL, sulfamethoxazole; ISEcp1, insertion sequence ISEcp1; IS903, insertion sequence IS903; +, positive; −, negative.
The plasmid DNA preparation using the QuickGene plasmid kit S II plasmid isolation system (Fujifilm Corporation, Japan) from the transconjugants revealed a large conjugative plasmid of approximately 95 kb, whereas the donor strains demonstrated at least one large plasmid of ∼95 kb common to all strains. A PCR-based Inc/replicon typing was done using plasmid DNA from both donors and transconjugants, as described previously (7). The results of replicon typing of plasmids from the transconjugants showed that the IncFIIs plasmid was involved in the dissemination of the blaCTX-M-15 gene in association with multidrug resistance (MDR) in both animal and human populations, whereas the IncI1-Iγ plasmid was involved in the dissemination of blaCTX-M-14 genes with no connection to MDR phenotype, suggesting a possible relationship among specific replicon type, CTX-M type, and MDR. These results are in agreement with those of a previous study in which the association of blaCTX-M-15 and blaCTX-M-14 genes with plasmids of IncFII and IncI1 replicon types, respectively, has been reported in two different clinical S. Enteritidis isolates in addition to a number of E. coli isolates (15). In fact, the dissemination of blaCTX-M-15 genes by IncFII plasmids or of blaCTX-M-14 genes by IncI1 in the members of the Enterobacteriaceae has been well documented in several reports (6).
PCR amplification and sequencing of the regions surrounding blaCTX-M genes using various CTX-M and insertion sequence primer sets as described previously (10, 27) identified insertion sequence ISEcp1 upstream of all the blaCTX-M genes in both the wild NTS strains and their transconjugants. Furthermore, insertion sequence IS903 was detected downstream of the blaCTX-M-14 gene in the two human NTS strains and their transconjugants. The identification of the blaCTX-M gene in Salmonella in association with insertion sequence ISEcp1 or IS903 is a serious concern because these insertion sequences play an important role in the capture, expression, and continuous spread of these genes to other susceptible bacteria in the environment (25).
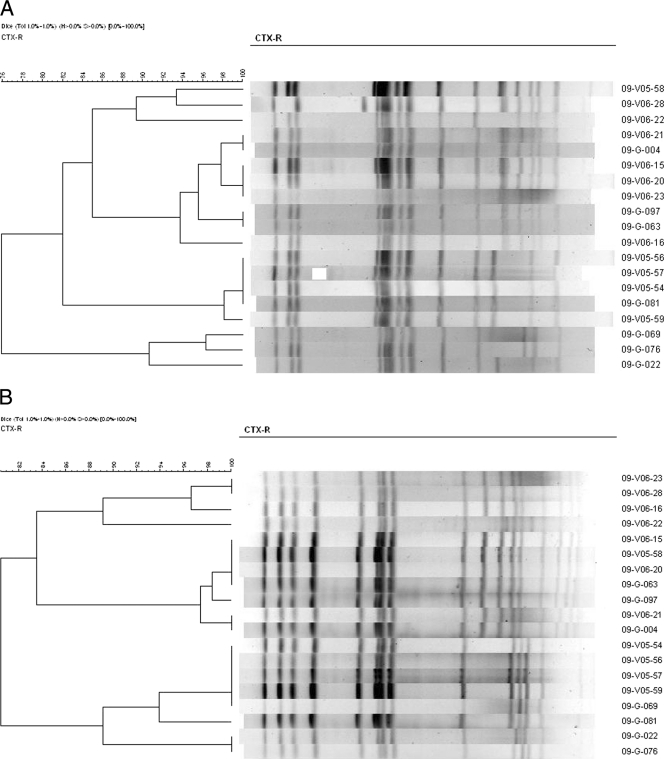
Among the 20 animal and human NTS strains carrying blaCTX-M genes, phage typing of 19 S. enterica serovar Enteritidis isolates as described previously (31) identified three different phage types, and four of them did not react with any of the standard typing phages used (Table 1). In order to compare the genetic relatedness of NTS strains isolated from food animals and humans, molecular typing of all the 19 blaCTX-M-positive S. Enteritidis isolates was done by pulsed-field gel electrophoresis (PFGE) using XbaI (Takara Bio Inc., Japan)- or AvrII (New England BioLabs)-digested genomic DNA according to the Centers for Disease Control and Prevention pulseNet standardized procedure as described previously (11). The XbaI-PFGE showed eight (X1 to X8) and the AvrII-PFGE revealed six (A1 to A6) arbitrary profiles among the 19 isolates. Identical PFGE profiles were found for strains from both animal and human sources by either XbaI- or AvrII-PFGE (Fig. 1).
Fig. 1.
Dendrogram generated by Bionumerics software showing the cluster analysis of XbaI- (A) and AvrII-PFGE (B) patterns of cefotaxime-resistant Salmonella serovar Enteritidis strains isolated from animals and humans. A cutoff similarity value of 95% was used to define clones. Similarity analysis was performed by using the Dice coefficient, and clustering was done by the unweighted-pair group method with arithmetic averages (UPGMA).
For further discrimination, XbaI- and AvrII-PFGE profiles were combined to produce 11 different arbitrary combination profiles. The most predominant XbaI-AvrII combined profile was X4A3, followed by X6A4. Finally, we combined the results of phage typing and molecular typing to produce arbitrary phage-genotype profiles. The combination of phage type and XbaI-AvrII genotype showed that certain common identical profiles were present among NTS strains from food animals as well as humans, suggesting a clonal relationship between blaCTX-M-15-positive S. Enteritidis isolates of animal and human sources, although the human isolates did not seem to have any epidemiological link with the animal isolates. Our result is in agreement with a previous study done in South Korea which showed a clonal relationship between human and broiler chicken NTS strains and suggested the transmission of specific clones of NTS from livestock to humans (8). A similar study carried out in Taiwan also reported the transmission of certain common clones of NTS between human and animal sources and reported that these remained in circulation between humans and animals as epidemic strains for years (30). Furthermore, clonal relationships between the food animal and human isolates of NTS producing CTX-M-type ESBL or plasmid-mediated AmpC β-lactamase and the transmission of these organisms to humans through the food chain have been reported from Europe (4) and North America (33), respectively.
In conclusion, our results suggest that a combination of clonal and horizontal transmission is spreading blaCTX-M genes among NTS strains in South Korea. To the best of our knowledge, this represents the first report of a blaCTX-M gene in S. enterica serotype Essen and the first report of CTX-M-14 and CTX-M-15 β-lactamases among NTS strains of animal origin with decreased susceptibility to ciprofloxacin in South Korea. The emergence of cefotaximase-producing NTS strains with reduced susceptibility to fluoroquinolones among food animals and humans is of great public health concern and should be carefully monitored.
Acknowledgments
This work was supported by a grant from the National Veterinary Research and Quarantine Service, Ministry of Food, Agriculture, Forestry, and Fisheries, Republic of Korea.
Footnotes
Published ahead of print on 25 May 2011.
REFERENCES
- 1. Aarestrup F. M., Wiuff C., Mølbak K., Threlfall E. J. 2003. Is it time to change fluoroquinolone breakpoints for Salmonella spp.? Antimicrob. Agents Chemother. 47:827–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arlet G., et al. 2006. Salmonella resistant to extended-spectrum cephalosporins: prevalence and epidemiology. Microbes Infect. 8:1945–1954 [DOI] [PubMed] [Google Scholar]
- 3. Batchelor M., et al. 2005. blaCTX-M genes in clinical Salmonella isolates recovered from humans in England and Wales from 1992 to 2003. Antimicrob. Agents Chemother. 49:1319–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bertrand S., et al. 2006. Clonal emergence of extended-spectrum β-lactamase (CTX-M-2)-producing Salmonella enterica serovar Virchow isolates with reduced susceptibilities to ciprofloxacin among poultry and humans in Belgium and France (2000 to 2003). J. Clin. Microbiol. 44:2897–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Branger C., et al. 2005. Genetic background of Escherichia coli and extended-spectrum β-lactamase type. Emerg. Infect. Dis. 11:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 53:2227–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carattoli A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 8. Cheong H. J., et al. 2007. Characteristics of non-typhoidal Salmonella isolates from human and broiler-chickens in southwestern Seoul, Korea. J. Korean Med. Sci. 22:773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. M100-S20-U. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Eckert C., Gautier V., Arlet G. 2006. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J. Antimicrob. Chemother. 57:14–23 [DOI] [PubMed] [Google Scholar]
- 11. Gautom R. K. 1997. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J. Clin. Microbiol. 35:2977–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giraud E., Brisabois A., Martel J. L., Chaslus-Dancla E. 1999. Comparative studies of mutations in animal isolates and experimental in vitro- and in vivo-selected mutants of Salmonella spp. suggest a counterselection of highly fluoroquinolone-resistant strains in the field. Antimicrob. Agents Chemother. 43:2131–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hammami A., et al. 1991. Nosocomial outbreak of acute gastroenteritis in a neonatal intensive care unit in Tunisia caused by multiply drug resistant Salmonella wien producing SHV-2 beta-lactamase. Eur. J. Clin. Microbiol. Infect. Dis. 10:641–646 [DOI] [PubMed] [Google Scholar]
- 14. Hohmann E. L. 2001. Nontyphoidal salmonellosis. Clin. Infect. Dis. 32:263–269 [DOI] [PubMed] [Google Scholar]
- 15. Hopkins K. L., et al. 2006. Replicon typing of plasmids carrying CTX-M or CMY β-lactamases circulating among Salmonella and Escherichia coli isolates. Antimicrob. Agents Chemother. 50:3203–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacoby G. A., Gacharna N., Black T. A., Miller G. H., Hooper D. C. 2009. Temporal appearance of plasmid-mediated quinolone resistance genes. Antimicrob. Agents Chemother. 53:1665–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim H. B., et al. 2009. Prevalence of plasmid-mediated quinolone resistance determinants over a nine-year period. Antimicrob. Agents Chemother. 53:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim J., Lim Y.-M., Jeong Y.-S., Seol S.-Y. 2005. Occurrence of CTX-M-3, CTX-M-15, CTX-M-14, and CTX-M-9 extended-spectrum β-lactamases in Enterobacteriaceae clinical isolates in Korea. Antimicrob. Agents Chemother. 49:1572–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee K. H., et al. 2009. Case report of pediatric gastroenteritis due to CTX-M-15 extended-spectrum β-lactamase-producing Salmonella enterica serotype Enteritidis. Korean J. Lab. Med. 29:446–464 [DOI] [PubMed] [Google Scholar]
- 20. Lee K., Yong D., Yum J. H., Kim H. H., Chong Y. 2003. Diversity of TEM-52 extended-spectrum beta-lactamase-producing non-typhoidal Salmonella isolates in Korea. J. Antimicrob. Chemother. 52:493–496 [DOI] [PubMed] [Google Scholar]
- 21. Lim S. K., Lee H. S., Nam H. M., Jung S. C., Bae Y. C. 2009. CTX-M-type beta-lactamase in Escherichia coli isolated from sick animals in Korea. Microb. Drug Resist. 15:139–142 [DOI] [PubMed] [Google Scholar]
- 22. Lim S. K., et al. 2009. Antimicrobial resistance and phage types of Salmonella isolates from healthy and diarrheic pigs in Korea. Foodborne Pathog. Dis. 6:981–987 [DOI] [PubMed] [Google Scholar]
- 23. Miriagou V., Tassios P. T., Legakis N. J., Tzouvelekis L. S. 2004. Expanded-spectrum cephalosporin resistance in non-typhoid Salmonella. Int. J. Antimicrob. Agents 23:547–555 [DOI] [PubMed] [Google Scholar]
- 24. Park C. H., Robicsek A., Jacoby G. A., Sahm D., Hooper D. C. 2006. Prevalence in the United States of aac(6′)Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 50:3953–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poirel L., Decousser J.-W., Nordmann P. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 47:2938–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rayamajhi N., et al. 2008. Characterization of TEM-, SHV- and AmpC-type beta-lactamases from cephalosporin-resistant Enterobacteriaceae isolated from swine. Int. J. Food Microbiol. 124:183–187 [DOI] [PubMed] [Google Scholar]
- 27. Saladin M., et al. 2002. Diversity of CTX-M beta-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 209:161–168 [DOI] [PubMed] [Google Scholar]
- 28. Tamang M. D., et al. 2007. Emergence of multidrug-resistant Salmonella enterica serovar Typhi associated with a class 1 integron carrying the dfrA7 gene cassette in Nepal. Int. J. Antimicrob. Agents 30:330–335 [DOI] [PubMed] [Google Scholar]
- 29. Tamang M. D., et al. 2008. Plasmid-mediated quinolone resistance determinants qnrA, qnrB, and qnrS among clinical isolates of Enterobacteriaceae in a Korean hospital. Antimicrob. Agents Chemother. 52:4159–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsen H.-Y., Lin J.-S., Hsih H.-Y. 2002. Pulse field electrophoresis for animal Salmonella enterica serovar Typhimurium isolates in Taiwan. Vet. Microbiol. 87:73–80 [DOI] [PubMed] [Google Scholar]
- 31. Ward L. D., De Sa J. D. H., Rowe B. 1987. A phage typing scheme for Salmonella enteritidis. Epidemiol. Infect. 99:291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yong D., et al. 2005. Nosocomial outbreak of pediatric gastroenteritis caused by CTX-M-14-type extended-spectrum beta-lactamase-producing strains of Salmonella enterica serovar London. J. Clin. Microbiol. 43:3519–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao S., et al. 2003. Characterization of Salmonella enterica serotype Newport isolated from humans and food animals. J. Clin. Microbiol. 41:5366–5371 [DOI] [PMC free article] [PubMed] [Google Scholar]