Abstract
Transient lymphopenia is a hallmark of measles virus (MV)-induced immunosuppression. To address to what extent replenishment of the peripheral lymphocyte compartment from bone marrow (BM) progenitor/stem cells might be affected, we analyzed the interaction of wild-type MV with hematopoietic stem and progenitor cells (HS/PCs) and stroma cells in vitro. Infection of human CD34+ HS/PCs or stroma cells with wild-type MV is highly inefficient yet noncytolytic. It occurs independently of CD150 in stroma cells but also in HS/PCs, where infection is established in CD34+ CD150− and CD34+ CD150+ (in humans representing HS/PC oligopotent precursors) subsets. Stroma cells and HS/PCs can mutually transmit MV and may thereby create a possible niche for continuous viral exchange in the BM. Infected lymphocytes homing to this compartment may serve as sources for HS/PC or stroma cell infection, as reflected by highly efficient transmission of MV from both populations in cocultures with MV-infected B or T cells. Though MV exposure does not detectably affect the viability, expansion, and colony-forming activity of either CD150+ or CD150− HS/PCs in vitro, it efficiently interferes with short- but not long-term hematopoietic reconstitution in NOD/SCID mice. Altogether, these findings support the hypothesis that MV accession of the BM compartment by infected lymphocytes may contribute to peripheral blood mononuclear cell lymphopenia at the level of BM suppression.
INTRODUCTION
Generalized immunosuppression induced by measles virus (MV) is the major cause of the continued high morbidity/mortality rate for acute measles infections (13, 32). In vitro systems and animal models, including experimentally infected macaques, have been widely used to explain the major hallmarks of MV-induced immunosuppression which are defined mainly by quantitative and qualitative alterations of the peripheral lympho/monocytic compartment (7, 41). Suppression of cellular immunity has been partially attributed to inadequate or aberrant activation and maturation of professional antigen-presenting cells, especially dendritic cells (DCs), by MV (14, 34, 41, 48). In addition to affecting polarization of T cell responses, MV DC infection was suggested to be important in MV uptake and dissemination to secondary lymphatic tissues and then viral transmission to T cells and their subsequent loss (8, 10, 11). Infection-mediated loss of lymphocytes in secondary lymphoid tissues may largely account for the substantial panleukopenia seen during acute measles, which, in addition to lymphocytes, also affects monocytes and neutrophils (19, 25, 38). Independently of infection, surface interaction of MV proteins with lympho/monocytic cells has been found to promote functional paralysis or apoptosis of these cells (21, 40).
The tropism of wild-type MV for cells of the hematopoietic lineage clearly segregates with the expression profile of its entry receptor CD150 (6, 8, 10), which is confined mainly to activated T and B cells, macrophages, and activated DCs (12, 45, 46). In contrast to wild-type MV, laboratory-adapted strains can also use CD46 as an entry receptor, which is expressed on all nucleated human cells (18, 49). The structural basis for MV receptor usage has recently been established (16, 17, 35, 36).
The Ig superfamily member CD150 (also referred to as SLAMF1) is the type species of the SLAM (signaling lymphocytic activation molecule) family (SLAMF), which consists of 9 proteins that are expressed on most hematopoietic cells and are activated to signal on homo- or heterotypic interactions (9). Within this family, CD150 seems to be unique in acting both as an MV uptake receptor and a general microbial sensor (2). SLAMF receptors are also involved in early phases of lineage commitment during hematopoiesis, and differential expression of CD150, CD48 (SLAMF2), and CD244 (SLAMF4) marks subsets of murine hematopoietic stem and progenitor cells (HS/PCs). This led to the term “SLAM code,” whose applicability to human CD34+ HS/PCs is as yet controversial (24, 26, 30, 43, 50).
In contrast to the involvement of classical lymphoid tissue such as lymph nodes, spleen, and thymus in MV-induced immunosuppression, little is known about the role of MV-dependent regulation of leukocyte homeostasis or activation at the level of the bone marrow (BM). As revealed by the presence of infected lymphocytes, MV accesses this compartment in infected macaques (8), and high levels of BM output correlated with fast recovery from MV-induced lymphopenia and immunosuppression, indicating that short-term repopulation from the BM is an important parameter for their resolution (29, 38). In vitro, MV interference with HS/PC viability and colony formation was not apparent on direct exposure but rather was mediated by undefined components released from infected stroma cells in long-term initiating cultures (30). In that study, a dual-tropic MV was used, and thus it remained unclear how CD150-restricted wild-type MVs would act on these cell types.
To evaluate the potential BM suppression by wild-type MV, we first reanalyzed the applicability of the SLAM code for human CD34+ HS/PCs enriched from umbilical cord blood (UCB) or peripheral blood of myeloid leukemia (ML) patients and their susceptibility to MV infection. Confirming a recent study (26), a minor subpopulation of CD34+ HS/PCs expressing CD150 was detectable, which represent progenitor rather than stem cells. Wild-type MV infection of CD34+ HS/PCs was inefficient, with little evidence of cell destruction. It occurred independently of CD150, as did that of stroma cells, which also followed a noncytolytic course, and both cell types mutually transmitted MV in cocultures. Importantly, exposure of HS/PCs to MV, while not detectably affecting HS/PC viability, stimulated expansion and colony formation in vitro and interfered with short-term repopulation of the leukocyte compartment in the BM upon engraftment of NOD/SCID IL-2Rγc−/− mice. Suppression of short-term repopulation occurred independently of loss of HS/PC homing efficiency, indicating that signals provided on viral transfer or contact with infected HS/PCs (or, in humans, infected stroma cells and HS/PCs) may be central to MV BM suppression.
MATERIALS AND METHODS
Viruses and cell culture.
MV wild-type strain WTF and the MV recombinants IC-323-GFP (wild type) (based on the wild-type strain IC-323, kindly provided by Y. Yanagi, Fukuoka, Japan) and ED-GFP (attenuated) (kindly provided by P. Duprex, Belfast, Northern Ireland) were grown on human lymphoblastoid BJAB cells in RPMI 1640–10% fetal calf serum (FCS) (wild-type MV) or on Vero cells in minimal essential medium (MEM)-5% FCS (ED-GFP) and were titrated on marmoset lymphoblastoid B95a cells (kept in RPMI 1640-5% FCS). Green fluorescent protein (GFP)-tagged MVs were generally used to determine infection levels and the untagged MV wild-type strain WTF for experiments involving detection of cellular surface markers or fluorescent dyes. A sham-infected BJAB cell culture, harvested identically to virus-infected cultures, was used as a mock preparation at concentrations corresponding to those used for viruses. If not stated otherwise, viruses were used at a multiplicity of infection (MOI) of 5 in Iscove's modified Dulbecco's medium (IMDM) for 1 h with subsequent replenishment with maintenance medium or direct plating onto cytokine-enriched methylcellulose.
Isolation and culture of human CD34 +cells.
Umbilical cord blood cells or peripheral blood leukapheresis preparations from mobilized patients with myeloid leukemia, which were used as sources for CD34+ cells, were obtained from the maternity ward of the University Hospital or the Missionary Hospital or from the Department for Transfusion Medicine, University of Würzburg, respectively, and analyzed anonymously. All experiments involving human material were conducted according to the principles expressed in the Declaration of Helsinki and received ethics approval from the Ethical Committee of the Medical Faculty of the University of Würzburg. Mononuclear cells were isolated by Ficoll-Paque density centrifugation followed by enrichment of CD34+ cells by immunomagnetic sorting (Miltenyi Biotech, Bergisch Gladbach, Germany) to purities ranging between 75 and 99%. When indicated, CD150+ cells were enriched by flow cytometric sorting from CD34+ cultures by using a CD150-specific fluorescein isothiocyanate (FITC)-conjugated antibody (A12; AbD Serotec, Oxford, United Kingdom).
MAbs and fluorescent dyes.
Mouse anti-human monoclonal antibodies (MAbs) used for phenotyping were CD150 (A12) and CD244 (2B4.69) (both from AbD Serotec, Oxford, United Kingdom), CD34 (AC136) and CD133 (AC133) (both from Miltenyi Biotech, Bergisch Gladbach, Germany), and CD38 (HIT2), CD45 (2D1 and HI30), CD46 (E4.3), CD34 (581), CD48 (TÜ145), CD14 (MφP9), and CD19 (HIB19) (all from BD Biosciences, Heidelberg, Germany). MAbs used as isotype controls were IgG2a (S43.10; Miltenyi, Bergisch Gladbach, Germany) and IgG1 (X40 and MOPC-21; BD Biosciences, Heidelberg, Germany). The Abs were labeled with either FITC, Alexa 488, phycoerythrin (PE), or PE-Cy5. 7-Aminoactinomycin D (7AAD) (BD Biosciences, Heidelberg, Germany) was used for viability staining. HS/PCs were stained with 0.83 μM carboxyfluorescein succinimidyl ester (CFSE) for 5 min for proliferation assay.
Flow cytometry and cell sorting.
Surface marker expression was analyzed on a FACSCalibur, and CD34+ CD150+ and CD34+ CD150− cell populations were sorted on a FACSDiva (both from BD Immunocytometry Systems). The purity of CD34+ cultures was determined by gating sequentially on CD45+ cells, on 7AAD-negative living cells, and then on the CD34+-population. Purity was calculated as the ratio of CD34+ cells to living CD45+ cells. SLAMF member expression and infection rate were analyzed by first setting a lymphocyte gate on forward scatter and side scatter and then gating on 7AAD-negative cells before analyzing marker expression or GFP expression by the virus on CD34-PE+ cells. For analysis of 13- and 1-week repopulation, at least 200,000 events were acquired, and for homing experiments, at least 500,000 events were acquired. Gates were set as described for analysis of CD150 expression. For sorting of CD34+ CD150+ and CD34+ CD150− populations, cells were not treated with 7AAD to avoid cytotoxic effects in subsequent culture experiments.
Colony formation assay (CFA).
CD34+ cells were exposed to MV or a mock preparation for 1 h at 37°C, washed twice with phosphate-buffered saline (PBS), and resuspended in IMDM–10% FCS at a concentration of 4,000 cells/ml. A 125-μl portion of this suspension was mixed with 1,125 μl methylcellulose medium (MethoCult H4434 Classic; Stemcell Technologies, Grenoble, France) containing recombinant human stem cell factor, recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF), recombinant human interleukin-3 (IL-3), and recombinant human erythropoietin prior to seeding each 1 ml in 35-mm petri dishes (Greiner, Frickenhausen, Germany) and subsequent culture at 37°C and 5% CO2. If not stated otherwise, colonies were counted after 14 days.
Stroma cells, coculture, and transmission experiments.
BM obtained from iliac crest was passed through a 70-μm cell strainer (BD Biosciences, Heidelberg, Germany). Single-cell suspensions were diluted 1:2 with PBS and subjected to Ficoll-Paque density centrifugation, and mononuclear cells were obtained after seeding in DMEM–20% FCS (while nonadherent cells were removed). The engraftment and purity of stroma cell cultures were determined morphologically by microscopy. Stroma cells were exposed to MV at an MOI of 2 or mock exposed prior to culture in DMEM–20% FCS supplemented with 5% 2-mercaptoethanol. For coculture experiments, stroma cells were kept in the medium of the cocultured cell type. Either CD34+ cells were exposed to MV and then cultured in basal bone marrow medium (BBMM) (IMDM containing stem cell factor [SCF] [50 ng/ml], IL-3 [10 ng/ml], IL-6 [50 U/ml], thrombopoietin [TPO] [10 ng/ml], and flt3 ligand [10 ng/ml] [all from PeproTech, Hamburg, Germany] and 100 μM 2-mercaptoethanol and supplemented with 30% FCS and 1% bovine serum albumin [BSA]) on uninfected stroma cells or CD34+ cells were cultured in BBMM on stroma cells previously exposed to MV at an MOI of 2. For transmission experiments, BJAB cells or primary human T cells (preactivated with phorbol myristate acetate [PMA]-ionomycin over night) were infected with IC323-GFP at the indicated MOI for 1 h, extensively washed and resuspended in RPMI 1640–10% FCS, and added to stroma cells or HS/PCs (105 lymphocytes per well of a 6-well plate of confluent stroma cells or per well of a 96-well plate containing 105 HS/PCs). Lymphocyte-stroma cell cocultures were analyzed over time by fluorescence microscopy and lymphocyte/HS/PC cultures by flow cytometry at 48 h after onset of the coculture.
Transplantation.
CD34+ UCB cells (3 × 104 to 2 × 105 for repopulation assays and 3 × 105 to 5 × 105 for homing assays) were exposed to MV (MOI of 5) or a corresponding amount of mock preparation, mixed with 1 × 105 murine spleen cells, and transfused through the tail vein into sublethally irradiated (2.5 grays, single dose) 8- to 12-week-old NOD/SCID IL-2Rγc−/− mice (colony name, NOD.Cγ-Prkdcscid Il2rgtm1Wjl/SzJ). At 12 or 13 weeks (the standard interval used for determining long-term engraftment by HS cells [HSCs]), 1 week (later than 10 days following transfer, hematopoietic PCs [HPCs] undergo apoptosis), or 40 h (prior to expansion of transplanted HS/PCs) posttransplantation, mice were sacrificed and single-cell suspensions from BM (both tibiae and femurs) and spleen were prepared and processed for flow cytometry. On average, rates of recovery of hCD45+ cells were 70 to 90% after 13 weeks, 1 to 11% after 1 week, and <0.5% after 40 h.
RESULTS
Human CD150+ HS/PCs are OPPs rather than HSCs.
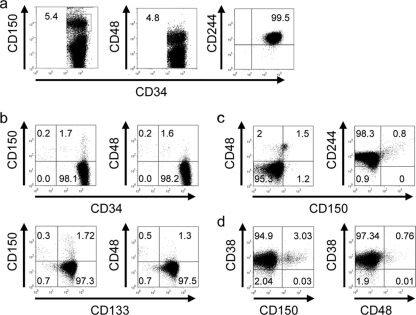
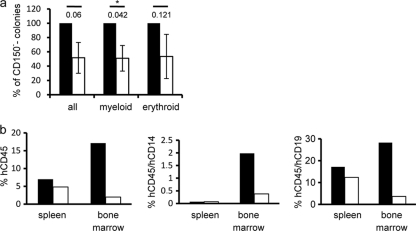
SLAMF members are differentially expressed on subpopulations of murine HS/PCs (“SLAM code”), and hence HSCs are CD150+ CD244− CD48−, multipotent progenitors (MPPs) are CD150− CD244+ CD48−, and oligopotent precursors (OPPs) are CD150− CD244+ CD48+ (24). To analyze whether this also applies to human CD34+ HS/PCs, these were isolated by immunomagnetic sorting using α-CD34-coated beads (the purity ranged between 75 and 99%) from umbilical cord blood (UCB) or peripheral blood of mobilized myeloid leukemia (ML) patients. In line with recent studies (43), CD244 was ubiquitously expressed on human CD34+ HS/PCs, while CD48 was detected on a minor subpopulation in all samples without source-specific differences being noted (exemplified in Fig. 1a, middle and right panels, for a UCB sample; Table 1). A CD34+ subpopulation expressing CD150 was consistently detected (mean frequency, 5.53%) (Fig. 1a, left panel, and Table 1), which also costained for CD133 (Fig. 1b). Ubiquitous expression of CD244 indicated that the murine SLAM code (where CD150 marks CD48− CD244− HSCs) does not apply to human HS/PCs, and we thus codetected CD150 with CD244 but also with CD48 (Fig. 1c). Similar to the case for murine HS/PCs, where CD48 is expressed on OPPs (24), both UCB and ML CD34+ CD48+ and CD34+ CD150+ populations coexpressed CD38 (exemplified in Fig. 1d). Altogether, our analysis revealed that CD150, the MV wild-type receptor, is expressed by a CD34+ CD133+ CD38+ HS/PC subset, which represents OPPs. To corroborate this hypothesis in functional terms, we comparatively analyzed the ability of sorted CD34+ UCB cells enriched for (CD34+ CD150+; purity, >95%) or depleted of CD150 (CD34+ CD150−) to form colonies in vitro in cytokine-supplemented methylcellulose cultures (colony formation assay [CFA]) or to reconstitute the lymphomyeloid compartment at 12 weeks posttransplantation into irradiated NOD/SCID IL-2Rγc−/− mice. As shown in Fig. 2a, CD34+ CD150+ UCB cells developed into colonies in vitro less efficiently than the CD150-depleted population regardless of whether all colonies, myeloid colonies (white colonies, for which the difference reached statistical significance), or erythroid (red) colonies were counted. Similarly, CD150+ UCB cells were less efficient in multilineage engraftment than CD150− UCB cells as revealed by detection of total hCD45+ donor cells or the myeloid (detected by CD14 staining) and lymphoid (representatively detected by CD19 staining) subpopulations thereof in spleen and BM (Fig. 2b). The higher CFA results and repopulating efficiency of the CD150− fraction most likely reflect the presence of HSCs in CD34+ UCB fractions.
Fig. 1.
The murine SLAM code does not apply to human CD34+ HS/PCs. CD34+ fractions of UCB and leukemia-mobilized peripheral blood lymphocytes were analyzed for surface expression of CD150, CD48, or CD244 (a), for CD150 and CD48 alone or in combination with CD133 (b), for CD150 together with CD48 or CD244 (c), or for CD38 in combination with CD150 or CD48 (d) by flow cytometry. Representative stainings of a total of 72 samples analyzed are shown, with the percentages of doubly positive (a and b [upper panels]) or triply positive cells (b [bottom panels], c, and d).
Table 1.
Levels of expression of SLAMF members on the surface of UBC (n = 54) or ML (n = 18) CD34+ hematopoietic cells
| SLAMF member (n) | Expression level (%) |
||
|---|---|---|---|
| Median | Range | Mean | |
| CD150 (72) | 3.3 | 0.5–25.8 | 5.53 |
| CD48 (25) | 1.43 | 0.64–4.5 | 2.42 |
| CD244 (16) | 90 | 75.6–99 | 89.15 |
Fig. 2.
CD34+ CD150+ UCB fractions are not lineage committed. (a) CD34+ UCB fractions enriched for (CD150+) (white bars) or depleted of (CD150−) (black bars) CD150+ cells were analyzed for their ability to promote colony formation (white [myeloid] or red [erythroid]) in methylcellulose in vitro after 2 weeks. Total colony numbers ranged between 68 and 147. Values shown are means from three independent experiments done in duplicates, with standard errors of the means (SEMs) and P values (determined by paired t test) indicated. The asterisks denote significant differences. (b) CD34+ CD150+ (white bars) or CD34+ CD150− (black bars) UCB cells were transplanted into NOD/SCID IL-2Rγc−/− mice (n = 3 each), and the frequencies of total hCD45+ donor cells (left panel) and of myeloid (hCD45+ CD14+ cells, middle panel) or lymphoid (hCD45+ CD19+ cells, right panel) donor cells in spleen and bone marrow were determined at 12 weeks posttransplantation by flow cytometry. Results of one out of two independent experiments are shown.
Wild-type MV infects HS/PCs independent of CD150.
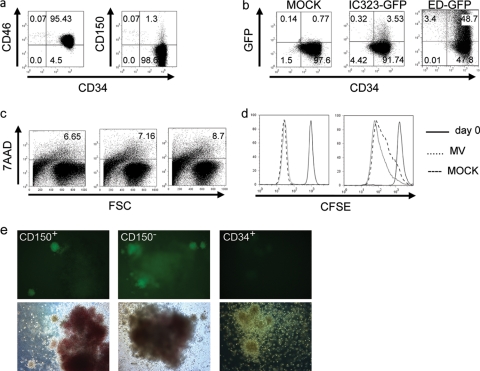
In contrast to CD150, CD46, the receptor for attenuated MV strains, is expressed by most if not all HS/PCs (Fig. 1; exemplified for UCB cells in Fig. 3a). On infection with recombinant attenuated (ED-GFP) or wild-type (IC323-GFP) MV, regardless of the input dose, HS/PCs released very limited amounts of infectious virus into the supernatant within 48 h, which could not be reliably titrated. Indicating that HS/PCs are well able to support MV replication, ED-GFP, which can use CD46 for entry, efficiently infected these cells within 48 h (average infection rate, 61.65%) (Fig. 3b, third panel). In contrast, wild-type MV infection (IC323-GFP) was very limited (using an MOI of as high as 5, the average infection rate was 4.02%) (Fig. 3b, second panel). Exposure to wild-type or attenuated MV (the latter efficiently replicates in these cells) did not detectably affect HS/PC viability (as detected by 7AAD staining) and expansion in response to SCF–IL-3–IL-6–TPO–flt3 ligand (as revealed by CFSE dilution) within 2 and 6 days compared to mock-treated controls (Fig. 3c [exemplified for 2 days postexposure] and d [2 days {left panel} and 6 days {right panel}, representatively shown for exposure to an untagged MV wild-type strain, WTF]).
Fig. 3.
CD34+ UCB cells support MV replication to a differential extent. (a) CD46 (left panel) or CD150 (right panel) was codetected with CD34 on UCB cells (representative examples for CD150 [n = 72]; Table 1) and for CD46 [n = 9]; average expression level, 98.39% ± 1.56%) are shown). An FITC-conjugated monoclonal mouse IgG1 antibody was used as an isotype control (not shown). (b) The percentage of GFP-expressing CD34+ UCB cells was determined at 48 h after exposure to a mock preparation (left panel) or wild-type IC323-GFP or attenuated ED-GFP MV (each used at an MOI of 5). Results from one out of four independent experiments are shown; the frequency of CD34+ CD150+ cells was 6.7% in this particular culture. (c) HS/PC cultures were stained for 7AAD at 2 days following exposure to IC323-GFP (middle panel) or ED-GFP (right panel) or the corresponding amounts of a mock preparation (left panel). (d) CFSE was detected immediately after labeling (both panels, solid line) or 2 (left panel) or 6 (right panel) days following exposure of HS/PCs to mock preparation (dashed line) or an untagged MV wild-type strain, WTF (dotted line). (e) CD34+ CD150+ cultures (left panels), CD34+ CD150− cultures (middle panels) or, total CD34+ HS/PCs (right panels) were seeded immediately after exposure to IC323-GFP (left and middle panels) or a mock preparation (right panels) into methylcellulose containing differentiating cytokines, and results were recorded by fluorescence (upper panels) and bright-field microscopy (bottom panels) after 14 days. Magnification, ×100. For all experiments, results of one representative out of at least three independent experiments are shown.
In IC323-GFP-exposed cultures, the frequency of GFP-expressing (GFP+) cells approximated that of CD150+ cells (which was 6.7% in the example culture). CD150-enriched or -depleted CD34+ UCB cells were exposed to IC323-GFP to evaluate the importance of CD150 in wild-type MV infection. Because the numbers of CD150+ cells were too low for flow cytometry analyses, infected CD150+ or CD150− cultures were plated into cytokine-enriched methylcellulose and GFP expression was detected after 14 days. Indicating that IC323-GFP entry was CD150 independent, CD150+ and CD150− HS/PC fractions gave rise to small satellite colonies expressing MV-encoded GFP with equal frequency (Fig. 3e). Satellite colonies were found in association with both red and white colonies and appeared white yet did not allow for lineage distinction. Large colonies exhibited autofluorescence (Fig. 3e) yet consistently excluded the strong GFP signal. These findings indicate that wild-type MV infects a subpopulation of HS/PCs, but independently of CD150, and MV exposure of HS/PCs does not detectably interfere with cell viability and expansion in vitro.
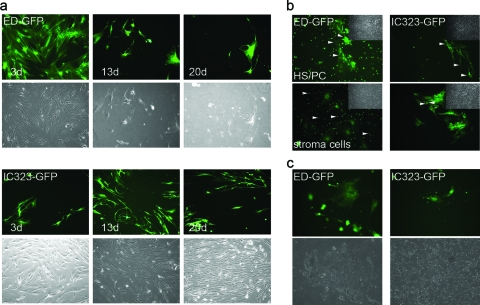
Wild-type MV establishes a noncytolytic infection in stroma cells and is mutually transferred between stroma cells and HS/PCs.
Disintegration of HS/PCs cocultured with MV-exposed stroma cells has been described (30), though modes of MV entry into stroma cells and mechanisms of HS/PC loss were not clarified. We thus reinvestigated whether HS/PCs could acquire MV from infected stroma cells and if this, rather than direct infection (see above), would enhance HS/PC infection and/or loss. ED-GFP-exposed stroma cell layers disintegrated within a few days due to cytolytic infection, which was not accompanied by efficient virus production (Fig. 4a, upper panels) (average virus release, 2.3 × 102 PFU/ml). As expected, these cells expressed CD46, and thus antibodies directed against this molecule interfered with infection and giant cell formation (not shown). In contrast, CD150 was not detected, and hence infection with IC323-GFP was insensitive to CD150-specific antibodies (not shown). Confined to a limited amount of cells, wild-type MV caused a noncytolytic infection in stroma cells. Cell fusions were hardly detectable, and virus production was very low (average, 3.7 × 102 PFU/ml), yet the infection slowly spread within the culture (Fig. 4a, bottom panels). To analyze potential MV transmission, we used HS/PC-stroma cell cocultures where either HS/PCs or stroma cells were initially infected. In line with our previous findings (Fig. 3b and 4a), ED-GFP efficiently infected, and was transmitted by, either cell population (Fig. 4b, left panels). Moreover, cell fusions were readily detectable in the stroma cell layer, while stroma cell-HS/PC fusions were not obvious (Fig. 4b, left bottom panel). Interestingly, wild-type-MV-infected stroma cells efficiently transferred virus to cocultured HS/PCs, and in turn these, when initially infected, caused stroma cell infection which appeared to be even more pronounced than with direct infection of these cells (Fig. 4b, right panels).
Fig. 4.
Differential infection of stroma and HS/PCs by MV strains and mutual transmission. (a) Infection in stroma cell cultures exposed to ED-GFP (upper panel set) or IC323-GFP (bottom panel set) (each at an MOI of 2) was recorded after the indicated time intervals. Differential interference contrast (DIC) images of the cultures are provided (each bottom row) to monitor the level of monolayer destruction over time. (b) Transmission of ED-GFP (left panels) or IC323-GFP (right panels) from infected HS/PCs to uninfected stroma cell cultures (upper panels, with arrows depicting GFP+ stroma cells) or to HS/PCs from infected stroma cell cultures (bottom panels, with arrows depicting GFP+ HS/PCs) was recorded at 5 days following coculture. DIC images in insets are provided to reveal the integrity of the respective stroma cell layers. (c) Stroma cells were exposed to ED-GFP (left panels) or IC323-GFP (right panels), and loss of these cells and cocultured HS/PCs was monitored after 12 days. DIC images (bottom panels) were included to microscopically monitor integrity of the stroma cell monolayer. Magnification, ×100.
As seen on infection of HS/PC or stroma cell cultures only (Fig. 3c and 4a), exposure to wild-type MV also did not interfere with cell integrity to a detectable extent in cocultures of these cells within 12 days (Fig. 4c, right panels). In contrast, rapid destruction and loss of stroma cells and HS/PCs (due to infection or loss of stroma cell support) were observed on ED-GFP exposure (Fig. 4c, left panels). Thus, MV can be mutually transferred between HS/PCs and stroma cells, yet infection and transmission of wild-type MV are nondestructive over prolonged periods for both HS/PCs and stroma cells.
Stroma cells and CD34+ HS/PCs can acquire MV from infected lymphocytes.
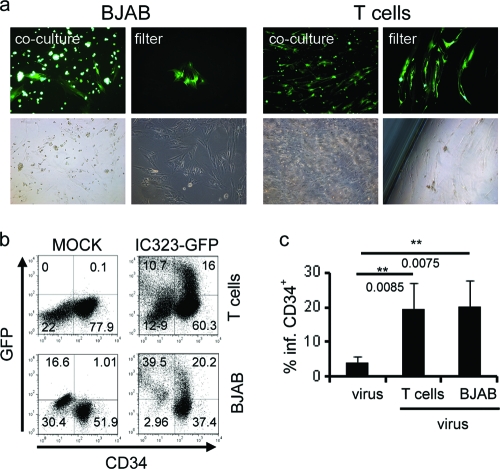
In vivo, MV might get access to the stem cell niche in the BM via infected lymphocytes homing to this compartment, and in line with this hypothesis, activated GFP-expressing lymphocytes were detected in the BM of IC323-GFP-infected macaques (8). To assess potential transmission from this source to stroma cells and HS/PCs, we monitored GFP acquisition of HS/PCs or stroma cells in cocultures with B lymphoblastoid BJAB or primary preactivated T cells which were infected with IC323-GFP 1 h prior to initiation of the cocultures. GFP-expressing stroma cells were readily detectable after 24 h, and GFP expression spread through the culture over time (Fig. 5a, first and third columns). When infected donor cells were separated from stroma cells by a 4-μm transwell filter, MV transfer was strongly delayed yet not abrogated, and GFP+ stroma cells were detected after 4 to 7 days (Fig. 5a, second and fourth columns). During this period, leakage of GFP+ lymphocytes to the lower compartment through the filter was not observed. GFP-expressing HS/PCs were detected after 48 h of coculture with infected BJAB or preactivated T cells by flow cytometry. Acquisition of virus by HS/PCs on coculture with infected T or BJAB cells reached on average 19.39% and 20.23%, respectively (exemplified in Fig. 5b) and thus significantly exceeded that seen on direct infection using cell-free virus (Fig. 5c and 3b, middle panel). These data imply that MV might access BM stroma cells and HS/PCs upon interaction with infected lymphocytes homing to this compartment.
Fig. 5.
Transmission of wild-type MV to stroma cells and HS/PCs by infected lymphocytes. (a) BJAB cells (left panels) or preactivated T cells (right panels) were infected with IC323-eGFP (MOI of 0.5) and cocultured with stroma cells in the absence (first and third columns) or presence (second and fourth columns) of a 4-μm transwell filter. Representative pictures from 5 (direct contact) or 3 (transwell filter) independent experiments recorded after 7 (first and third columns) and 20 (second and fourth columns) days are shown. Magnification, ×100. (b) Preactivated T cells (upper row) or BJAB cells (bottom row) infected with IC323-eGFP (MOI of 0.1) were cocultured with HS/PCs for 48 h prior to flow cytometry analysis. Results from one out of four (T cells) or five (BJAB cells) independent experiments are shown. (c) Average rates of infection of HS/PCs at 48 h postinfection with cell-free virus or after onset of coculture with infected preactivated T or BJAB cells.
MV affects short-term BM immune reconstitution in vivo.
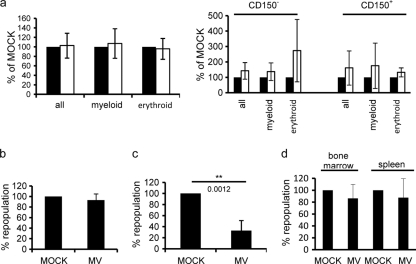
To address the impact of MV on HS/PC differentiation, CD150-enriched (CD150+) or -depleted (CD150−) subfractions were exposed to high doses of wild-type MV (or a corresponding amount of a mock preparation), and formation of total, myeloid, or erythroid colonies was recorded within 14 days following plating. Since in this experiment infection of colonies was not addressed, an unmodified rather than a recombinant wild-type MV (WTF) was used. No MV-related differences were detected in the CFA regardless of whether total HS/PCs (Fig. 6a, left panel), CD150+ and CD150− fractions thereof, or colony formation over time (on daily monitoring starting 5 days after seeding) was evaluated (Fig. 5a, right panels, and not shown), indicating that wild-type MV does not interfere with HS/PC differentiation in vitro. To assess the impact of MV on immune reconstitution by HS/PCs, cells were transplanted after MV exposure (MOI of 5) into NOD/SCID IL-2Rγc−/− mice, and the frequency of hCD45+ BM cells was determined. For these experiments, again the WTF rather than the IC323-GFP strain was used because the frequency of hCD45+ cells (and CD14+ or CD19+ subpopulations thereof) was recorded. As for the in vitro assays, no virus-related differences were recorded for total hCD45+ cells (or CD14+ or CD19+ subpopulations thereof [not shown]) after 13 weeks in these animals, which is the standard time interval to determine long-term BM reconstitution due to expansion from HSCs, which is initiated only later than 14 days following transfer (Fig. 6b, left panel) (3, 47). For short-term reconstitution, CD34+ CD38+ HPCs rather than CD34+ CD38− HSCs expand and subsequently undergo apoptosis within 2 weeks following transfer (3, 47), and thus this is routinely determined after 1 week. Strikingly, the engraftment efficiency (reflecting short-term repopulation) of MV-exposed HS/PCs was reduced by about 67%, indicating that MV infection caused an early effect on immune reconstitution, the extent of which does not correlate with the frequency of infected cells (which was on average 4.02% in vitro) (Fig. 6c and 3b). Impairment of early repopulation did not relate to detectable interference with HS/PC homing, because the frequency of viable, MV-exposed hCD45+ cells in BM and spleen after transfer corresponded to that of mock-treated cells when analyzed at 40 h following transplantation (prior to initiation of HPC expansion) (Fig. 6d). Thus, MV exposure impairs early immune reconstitution by HSPCs in vivo.
Fig. 6.
MV does not affect the overall colony formation efficiency of CD34+ HS/PCs in vitro yet interferes with early rather than long-term repopulation by these cells. (a) CD34+ UCB cells left unselected (left panel) or enriched for (CD150+, middle panel) or depleted of (CD150−, right panel) CD150+ cells and exposed to wild-type MV (IC323 [left panel] and WTF [right panel], each at an MOI of 5) (black bars) or to a mock preparation (white bars) were plated into methylcellulose and formation of total, myeloid, and erythroid colonies was determined after 14 days. Values shown represent means and standard deviations from 12 (left panel) or 3 (right panel) experiments. (b to d) Frequencies of hCD45-expressing cells were determined at 13 weeks (n = 3 for each mock or MV preparation) (b), 1 week (n = 5 for each mock or MV preparation) (c), or 40 h (n = 3 for each mock or MV preparation) (d) following transfer of CD34+ UCB cells exposed to MV (MOI of 5) or mock preparation in BM (b to d) and spleens (d) of NOD/SCID IL-2Rγc−/− mice. Values shown represent the means and standard deviations from at least three independent experiments. P values were determined using the t test.
DISCUSSION
BM suppression might be important in the understanding of MV-induced immunosuppression; however, there is as yet only one study that has addressed the interaction of MV with HS/PCs in detail (30). Because that study employed a dual-tropic MV strain for infection of HS/PCs and stroma cells, it was difficult to evaluate the results obtained with regard to receptor usage, especially that of wild-type MV, which is restricted to CD150 as an entry receptor (16, 17). We aimed to readdress this particular interaction. CD150 marks the murine HSC compartment, while other SLAMF members distinguish more restricted multi- or oligopotent subpopulations of HS/PCs (24). Using a statistically relevant number of UCB and ML samples, we were able to confirm that the SLAM code does not apply to human CD34+ HS/PCs (26, 43) (Fig. 1). In line with earlier findings (26, 30), we consistently detected CD150 on an HS/PC subpopulation, albeit with donor- but not ML- or UCB-dependent frequencies (Fig. 1; Table 1). It is unclear why CD150 was not detected on CD34+ HS/PCs in another study (43), which included the same antibodies for CD150 detection as our study. Based on coexpression of CD38, human CD150+ HS/PCs represent an OPP rather than, as in mice, an HSC compartment (Fig. 1) (24). In line with this hypothesis and recent findings (26), the ability of the CD150-enriched CD34+ UCB cells to promote colony formation in vitro and immune reconstitution in vivo is inferior to that of the CD150-depleted fraction, which should contain the HSCs (Fig. 2).
The susceptibility of CD34+ HS/PCs to MV infection has been analyzed earlier, when infection of a very limited subpopulation numerically roughly corresponding to the frequency of CD150+ cells was described (30). In that study, a dual-tropic MV able to use CD150 and CD46 was used, and HS/PC infection proved to be independent of CD150 (30). Wild-type MV restricted to CD150 usage obviously also enters independently of CD150 into HS/PCs as revealed by the appearance of GFP-expressing colonies in both the CD150+ and the CD150− fractions, the latter of which is unlikely to reflect the presence of CD150+ cells having escaped selection (Fig. 3e). This is because in this case the frequency of GFP+ satellite colonies should differ substantially between CD150+ and CD150− cultures, which has not been observed (Fig. 3e). Similar to our observations made for HS/PCs, infection of stroma cell cultures (which as expected expressed CD46 both on the RNA and protein levels [not shown]) with attenuated MV was CD46 dependent and followed a cytolytic course, while infection with wild-type MV was CD150 independent (CD150 was also not expressed in these cultures) and noncytolytic (Fig. 4a). This clear distinction is contrary to observations made in the earlier study, where MV exposure caused destruction of the stroma cell layer which would be compatible with CD46-dependent entry into these cells (30). Because disintegration of the stroma cells causes loss of the local environment for HS/PC development, abrogation of HS/PC viability in long-term cultures as described in this study is not surprising and may not require production of additional damaging mediators (30). Stroma cells, in common with other nonhematopoietic cells (1, 27, 28) but most interestingly also HS/PCs, acquired wild-type MV CD150 and CD46 independently (Fig. 3 and 4), yet the scarcity and, as intrinsic to primary cell cultures, heterogeneity of the cellular material and the focus of the present study did not allow for further analysis of the molecules involved.
Mutual exchange of MV between HS/PCs and stroma cells can occur, and the negligible amount of virus released suggests preferential cell-associated transmission, although fusion cells involving homologous or heterologous cell types were virtually undetectable in cocultures involving wild-type-MV-infected cells (Fig. 4b). Importantly, HS/PC and stroma cell infection can be acquired from infected lymphocytes (Fig. 5), the homing of which to the BM was confirmed in experimental MV infection (8). In this study, CD20+ and CD3+ GFP+ cells were detected throughout the observation period of 3 to 12 days after IC323-GFP infection, and the activated phenotype of these cells is in line with BM homing profiles of circulating lymphocytes (20). This is unlikely to occur with attenuated strains, which preferentially use CD46 in vitro but not in vivo (10), spread very inefficiently to lymphatic tissues, and thus are not likely to enter into the BM to cause stroma cell disintegration (10, 37, 39). Spread of IC323-GFP to HS/PCs or stroma cells, however, has not yet been found to occur in infected macaques (8).
Targeting of CD34+ CD38− HS/PCs for latent HIV infection, which on reactivation caused cell loss, was recently indicated as a means to exploit these cells as long-term reservoirs but also as a mechanism in HIV BM suppression (5). Though wild-type MV established a noncytolytic, carrier-like infection in both stroma cells and HS/PCs in vitro (Fig. 4a), it is unlikely that these cells serve as long terms reservoirs or persistence as revealed by reverse transcription-PCR (RT-PCR)-based cohort studies (31, 44).
In line with previous findings (30), wild-type MV exposure did not detectably affect the ability of HS/PCs to promote colony formation in vitro regardless of CD150 expression (Fig. 6a). Furthermore, long-term immune reconstitution by these cells when transferred into NOD/SCID IL-2Rγc−/− mice was also unaffected (Fig. 6b). In contrast, short-term BM repopulation was markedly affected by MV (Fig. 6c) but was not entirely ablated. Residual short-term reconstitution activity of MV-exposed HS/PCs might rely on a subpopulation of cells having escaped or, more likely, being insensitive to the MV exposure inhibitory signal, which is conceivable given the heterogeneity of the transplant (Fig. 1). Whether these would possibly be targeted in the BM (due to exposure to MV produced by stroma cells) could not be analyzed because murine stroma cells do not support MV replication. These observations seem to indicate that MV targets CD34+ CD38+ OPPs, which are known to home to this compartment, where they expand for short-term immune reconstitution and subsequently undergo apoptosis (22, 23, 51). Human CD34+ CD38+ HS/PCs thus cannot durably engraft NOD/SCID IL-2Rγc−/− mice, while sustained reconstitution after stem cell transplantation relies on CD34+ CD38− HSCs, which, however, expand only later than 2 weeks after transfer (3, 47). We cannot, however, exclude that CD34+ CD38− cells are targeted by MV as well, because these start to expand from the BM only 2 weeks after transfer and the MV inhibitory activity, if transient, could have resolved once the CD38− population had entered the expansion phase.
At present, the mechanistic basis of MV-mediated BM suppression at the level of HS/PCs in vivo is still unknown. It most likely does not relate to interaction with CD150 or MV infection, because both are confined to a minor subpopulation of these cells (Fig. 3). In vitro, however, MV exposure does not result in significant cell loss or lack of expansion (Fig. 3c and d), and thus, though not directly evaluated in the present study, MV contact-dependent abrogation of phosphatidylinositol-3-kinase/Akt kinase (PI3K) activation, as shown in lymphocytes, may not occur (40, 41). Further arguing for the integrity of this pathway in MV-exposed HS/PCs, their lineage commitment in vitro and the homing efficiency of these cells in vivo are retained (Fig. 6d) (4, 15).
The BM is accessed by wild-type MV via infected lymphocytes (8), and our in vitro study implies that MV might be transferred to HS/PCs and/or stroma cells which mutually exchange virus without detectable cell loss. Whether this also occurs in vivo can be evaluated only in infected macaques, where both cell populations could acquire MV. Evidently, however, early repopulation from the BM compartment is affected, and this is likely to target not MV-specific immune responses that have been initiated by then from differentiated lymphocyte precursors but mobilization and differentiation of HS/PCs, e.g., on reduction of BM cellularity or Toll-like receptor (TLR)-dependent or -independent danger signals (33, 42), thereby contributing to immunosuppression.
ACKNOWLEDGMENTS
We thank Juergen Schneider-Schaulies, Elita Avota, Karen Bieback, Horst Hummel, and Hermann Einsele for helpful discussion, Charlene Boertlein, Carolin Goetz, and Belinda Aul for technical help, and Paul Duprex and Yusuke Yanagi for providing recombinant viruses.
We thank the Interdisciplinary Center of Clinical Research, Medical Faculty of the University of Wuerzburg, and the Deutsche Forschungsgemeinschaft for financial support of the study.
Footnotes
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Andres O., Obojes K., Kim K. S., ter Meulen V., Schneider-Schaulies J. 2003. CD46- and CD150-independent endothelial cell infection with wild-type measles viruses. J. Gen. Virol. 84:1189–1197 [DOI] [PubMed] [Google Scholar]
- 2. Berger S. B., et al. 2010. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nature Immunology. 11:920–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatia M., Wang J. C., Kapp U., Bonnet D., Dick J. E. 1997. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 94:5320–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buitenhuis M., et al. 2008. Protein kinase B (c-akt) regulates hematopoietic lineage choice decisions during myelopoiesis. Blood 111:112–121 [DOI] [PubMed] [Google Scholar]
- 5. Carter C. C., et al. 2010. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat. Med. 16:446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Condack C., Grivel J. C., Devaux P., Margolis L., Cattaneo R. 2007. Measles virus vaccine attenuation: suboptimal infection of lymphatic tissue and tropism alteration. J. Infect. Dis. 196:541–549 [DOI] [PubMed] [Google Scholar]
- 7. de Swart R. L. 2009. Measles studies in the macaque model. Curr. Top. Microbiol. Immunol. 330:55–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Swart R. L., et al. 2007. Predominant infection of CD150+ lymphocytes and dendritic cells during measles virus infection of macaques. PLoS Pathog. 3:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Detre C., Keszei M., Romero X., Tsokos G. C., Terhorst C. 2010. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin. Immunopathol. 32:157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Vries R. D., et al. 2010. In vivo tropism of attenuated and pathogenic measles virus expressing green fluorescent protein in macaques. J. Virol. 84:4714–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Witte L., et al. 2008. DC-SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T-lymphocytes. PLoS Pathog. 4:e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engel P., Eck M. J., Terhorst C. 2003. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat. Rev. Immunol. 3:813–821 [DOI] [PubMed] [Google Scholar]
- 13. Griffin D. E. 2010. Measles virus-induced suppression of immune responses. Immunol. Rev. 236:176–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hahm B. 2009. Hostile communication of measles virus with host innate immunity and dendritic cells. Curr. Top. Microbiol. Immunol. 330:271–287 [DOI] [PubMed] [Google Scholar]
- 15. Haneline L. S., et al. 2006. Genetic reduction of class IA PI-3 kinase activity alters fetal hematopoiesis and competitive repopulating ability of hematopoietic stem cells in vivo. Blood 107:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hashiguchi T., Maenaka K., Yanagi Y. 2008. X-ray crystallographic analysis of measles virus hemagglutinin. Uirusu 58:1–10 [DOI] [PubMed] [Google Scholar]
- 17. Hashiguchi T., et al. 2011. Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat. Struct. Mol. Biol. 18:135–141 [DOI] [PubMed] [Google Scholar]
- 18. Hawkins E. D., Oliaro J. 2010. CD46 signaling in T cells: linking pathogens with polarity. FEBS Lett. 584:4838–4844 [DOI] [PubMed] [Google Scholar]
- 19. Hoffman S. J., Polack F. P., Hauer D. A., Griffin D. E. 2003. Measles virus infection of rhesus macaques affects neutrophil expression of IL-12 and IL-10. Viral Immunol. 16:369–379 [DOI] [PubMed] [Google Scholar]
- 20. Howie D., et al. 2005. The SLAM family receptor Ly108 controls T cell and neutrophil functions. J. Immunol. 174:5931–5935 [DOI] [PubMed] [Google Scholar]
- 21. Kerdiles Y. M., Sellin C. I., Druelle J., Horvat B. 2006. Immunosuppression caused by measles virus: role of viral proteins. Rev. Med. Virol. 16:49–63 [DOI] [PubMed] [Google Scholar]
- 22. Kerre T. C., et al. 2001. Both CD34+38+ and CD34+38− cells home specifically to the bone marrow of NOD/LtSZ scid/scid mice but show different kinetics in expansion. J. Immunol. 167:3692–3698 [DOI] [PubMed] [Google Scholar]
- 23. Kerre T. C. C., et al. 2002. Adapted NOD/SCID model supports development of phenotypically and functionally mature T cells from human umbilical cord blood CD34(+) cells. Blood 99:1620–1626 [DOI] [PubMed] [Google Scholar]
- 24. Kiel M. J., Yilmaz O. H., Iwashita T., Terhorst C., Morrison S. J. 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121 [DOI] [PubMed] [Google Scholar]
- 25. Kim Y. J., et al. 2002. Quantities of receptor molecules for colony stimulating factors on leukocytes in measles. Yonsei Med. J. 43:43–47 [DOI] [PubMed] [Google Scholar]
- 26. Larochelle A., et al. 2011. Human and rhesus macaque hematopoietic stem cells cannot be purified based only upon SLAM family markers. Blood 117:1550–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leonard V. H., et al. 2008. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 118:2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ludlow M., et al. 2010. Wild-type measles virus infection of primary epithelial cells occurs via the basolateral surface without syncytium formation or release of infectious virus. J. Gen. Virol. 91:971–979 [DOI] [PubMed] [Google Scholar]
- 29. Mackall C. L., Hakim F. T., Gress R. E. 1997. Restoration of T-cell homeostasis after T-cell depletion. Semin. Immunol. 9:339–346 [DOI] [PubMed] [Google Scholar]
- 30. Manchester M., Smith K. A., Eto D. S., Perkin H. B., Torbett B. E. 2002. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J. Virol. 76:6636–6642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matthews B. G., et al. 2008. Failure to detect measles virus ribonucleic acid in bone cells from patients with Paget's disease. J. Clin. Endocrinol Metab. 93:1398–1401 [DOI] [PubMed] [Google Scholar]
- 32. Moss W. J., Griffin D. E. 2006. Global measles elimination. Nat. Rev. Microbiol. 4:900–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nagai Y., et al. 2006. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 24:801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naniche D. 2009. Human immunology of measles virus infection. Curr. Top. Microbiol. Immunol. 330:151–171 [DOI] [PubMed] [Google Scholar]
- 35. Navaratnarajah C. K., et al. 2011. The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat. Struct. Mol. Biol. 18:128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navaratnarajah C. K., et al. 2008. Dynamic interaction of the measles virus hemagglutinin with its receptor signaling lymphocytic activation molecule (SLAM, CD150). J. Biol. Chem. 283:11763–11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohgimoto S., et al. 2001. The haemagglutinin protein is an important determinant of measles virus tropism for dendritic cells in vitro. J. Gen. Virol. 82:1835–1844 [DOI] [PubMed] [Google Scholar]
- 38. Okada H., et al. 2001. Comparative analysis of host responses related to immunosuppression between measles patients and vaccine recipients with live attenuated measles vaccines. Arch. Virol. 146:859–874 [DOI] [PubMed] [Google Scholar]
- 39. Pfeuffer J., Puschel K., Meulen V., Schneider-Schaulies J., Niewiesk S. 2003. Extent of measles virus spread and immune suppression differentiates between wild-type and vaccine strains in the cotton rat model (Sigmodon hispidus). J. Virol. 77:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schneider-Schaulies S., Dittmer U. 2006. Silencing T cells or T-cell silencing: concepts in virus-induced immunosuppression. J. Gen. Virol. 87:1423–1438 [DOI] [PubMed] [Google Scholar]
- 41. Schneider-Schaulies S., Schneider-Schaulies J. 2009. Measles virus-induced immunosuppression. Curr. Top. Microbiol. Immunol. 330:243–269 [DOI] [PubMed] [Google Scholar]
- 42. Scumpia P. O., et al. 2010. Bacterial infection induces hematopoietic stem and progenitor cell expansion in the absence of TLR signaling. J. Immunol. 184:2247–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sintes J., Romero X., Marin P., Terhorst C., Engel P. 2008. Differential expression of CD150 (SLAM) family receptors by human hematopoietic stem and progenitor cells. Exp. Hematol. 36:1199–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sonoda S., Kitahara M., Nakayama T. 2002. Detection of measles virus genome in bone-marrow aspirates from adults. J. Gen. Virol. 83:2485–2488 [DOI] [PubMed] [Google Scholar]
- 45. Veillette A. 2004. SLAM family receptors regulate immunity with and without SAP-related adaptors. J. Exp. Med. 199:1175–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Veillette A., Latour S. 2003. The SLAM family of immune-cell receptors. Curr. Opin. Immunol. 15:277–285 [DOI] [PubMed] [Google Scholar]
- 47. Verstegen M. M., et al. 1998. Transplantation of human umbilical cord blood cells in macrophage-depleted SCID mice: evidence for accessory cell involvement in expansion of immature CD34+CD38− cells. Blood 91:1966–1976 [PubMed] [Google Scholar]
- 48. Ward B. J., Griffin D. E. 1993. Changes in cytokine production after measles virus vaccination: predominant production of IL-4 suggests induction of a Th2 response. Clin. Immunol. Immunopathol. 67:171–177 [DOI] [PubMed] [Google Scholar]
- 49. Yanagi Y., Takeda M., Ohno S. 2006. Measles virus: cellular receptors, tropism and pathogenesis. J. Gen. Virol. 87:2767–2779 [DOI] [PubMed] [Google Scholar]
- 50. Yilmaz O. H., Kiel M. J., Morrison S. J. 2006. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood 107:924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zijlmans J. M., et al. 1998. The early phase of engraftment after murine blood cell transplantation is mediated by hematopoietic stem cells. Proc. Natl. Acad. Sci. U. S. A. 95:725–729 [DOI] [PMC free article] [PubMed] [Google Scholar]