Abstract
Retrovirus transmission via direct cell-cell contact is more efficient than diffusion through the extracellular milieu. This is believed to be due to the ability of viruses to efficiently coordinate several steps of the retroviral life cycle at cell-cell contact sites (D. C. Johnson et al., J. Virol. 76:1–8, 2002; D. M. Phillips, AIDS 8:719–731, 1994; Q. Sattenau, Nat. Rev. Microbiol. 6:815–826, 2008). Using the murine leukemia virus (MLV) as a model retrovirus, we have previously shown that interaction between viral envelope (Env) and receptor directs viral assembly to cell-cell contact sites to promote efficient viral spreading (J. Jin et al., PLoS Biol. 7:e1000163, 2009). In addressing the underlying mechanism, we observed that Env cytoplasmic tail directs this contact-induced polarized assembly. We present here the viral determinants in the Env cytoplasmic tail and Gag that are important in this process. A tyrosine residue within the cytoplasmic tail of Env was identified, which directs polarized assembly. MLV matrix-mediated membrane targeting is required for Gag recruitment to sites of cell-cell contact. Our results suggest that MLV polarized assembly is mediated by a direct or indirect interaction between both domains, thereby coupling Gag recruitment and virus assembly to Env accumulation at the cell-cell interface. In contrast, HIV Gag that assembles outside of cell-cell interfaces can subsequently be drawn into contact zones mediated by MLV Env and receptor, a finding that is consistent with the previously observed lateral movement of HIV into the virological synapse (W. Hubner et al., Science 323:1743–1747, 2009; D. Rudnicka et al., J. Virol. 83:6234–6246, 2009). As such, we observed two distinct modes of virus cell-to-cell transmission that involve either polarized or nonpolarized assembly, but both result in virus transmission.
INTRODUCTION
Viruses exploit and manipulate cell-cell contacts for efficient spreading (12, 18, 32, 37, 43). In the case of the human immunodeficiency virus (HIV) and the human T cell lymphoma virus these cell-cell contacts share features with immunological synapses and have been designated infectious or virological synapses (13, 14, 19, 29, 41). Using the murine leukemia virus (MLV) as a model system, we demonstrated that the interaction between the viral Env glycoprotein (Env), expressed on the infected cell and the viral receptor, expressed on the uninfected target cell, leads to the establishment of cell-cell contacts (32, 45). Importantly, following the establishment of cell-cell adhesion, assembly was polarized toward the cell-cell contact (16). These data suggested that the infected cell can “sense” the uninfected cell and direct the viral components needed to assemble viruses toward the uninfected cell. Sensing of the target cell turned out to be mediated by the accumulation of Env and receptor at the cell-cell interface and depending on the presence of a cytoplasmic tail (C-tail) of Env, MLV Gag was recruited toward the site of cell-cell contact. Here, we identify a tyrosine residue within the C-tail of Env and matrix within Gag as the viral determinants responsible for this process.
MATERIALS AND METHODS
Plasmids and reagents.
Mutant MLV envelope expression vectors were generated by site-directed mutagenesis based on the Friend MLV envelope expression vector previously described (16, 46). MLV Gag-GFP expression vector was made by fusing enhanced green fluorescent protein (eGFP) to C-terminal of Gag and deleting a large fragment of Pol in full-length Friend57 MLV expression vector pLRB303 (23, 35). Individual domains of MLV Gag-GFP were deleted or replaced by a standard overlapping PCR approach. MLV Gag-YFP expression vector was previously described (46). Chimeric MLV Gag with MA swapped with HIV MA was kindly provided by Marc Johnson, University of Missouri. HIV Gag-GFP expression vector and chimeric HIV Gag with MA swapped with MLV MA were described previously (17). HEK293 and XC cells were previously described (16).
Visualizing virus cell-to-cell transmission.
HEK293 cells were transfected with indicated plasmids by FuGENE 6 and cocultured 4 h posttransfection with XC cells expressing mCherry or cyan fluorescent protein (CFP)-tagged mCAT-1 as previously described (16). The formation of MLV synapses was reproducibly observed 2 to 6 postinitiation of cocultures for MLV generated by the tripartite system (MLV GagPol, Gag-YFP, MLV LTR, and MLV Env) or 4 to 10 h postinitiation of cocultures for MLV generated using proviral pLRB303-derived constructs. Imaging of fixed samples, as well as live-cell imaging, was performed using spinning disc confocal microscopy as described previously (16). The extent of polarization of assembly to the MLV synapse in fixed images was quantified using Volocity (Perkin-Elmer) as described in Fig. S1 in the supplemental material. Briefly, a polarization index was determined based on the ratio of Gag fluorescence intensity in the cell-cell interface (as identified by the accumulation of mCAT-1) compared to that outside of the contact zone. Any value >2.5 indicated polarization.
VLP budding assay.
HEK293 cells were transfected with the indicated MLV or HIV Gag constructs. At 24 h after transfection, VLPs were pelleted by centrifugation of 1 ml of 0.45-μm-pore-size-filtered culture supernatants at 14,000 rpm for 2 h over a 15% sucrose cushion. Cells are lysed using radioimmunoprecipitation assay buffer. VLP and cell lysates were separated on polyacrylamide gels and transferred to polyvinylidene difluoride membranes, followed by Western blotting using anti-p30 or anti-GFP (Invitrogen, California) primary antibody and peroxidase-conjugated secondary antibodies.
RESULTS
A visual assay to measure polarized MLV assembly.
To identify the viral determinants within the C-tail of MLV Env needed for polarized assembly, we had to address the high fusogenicity of Env. MLV Env lacking the R-peptide (Env ΔR), the fragment that is cleaved off by the viral protease during maturation, is hyperfusogenic, and cell-cell contact leads to cell-cell fusion (30, 38, 40). Thus, our approach did not permit us to use the spreading of infectivity for MLV carrying mutant Env as an experimental readout. We decided to use a visual assay instead whereby we suppressed the fusogenicity of Env by deleting histidine 8 in Env without affecting receptor binding (3, 49). The complete panel of Env mutants that were generated in the ΔH8 background and tested in the present study are shown in Fig. 1.
Fig. 1.
MLV Env mutants tested. The C-tail of wt MLV Env is displayed on the top. Env ΔR that fails to polarize MLV assembly lacks the C-terminal 16 amino acids. Serial truncations were constructed, followed by the indicated point mutations. All mutants were made in the context of ΔH8 to suppress their membrane fusion activity.
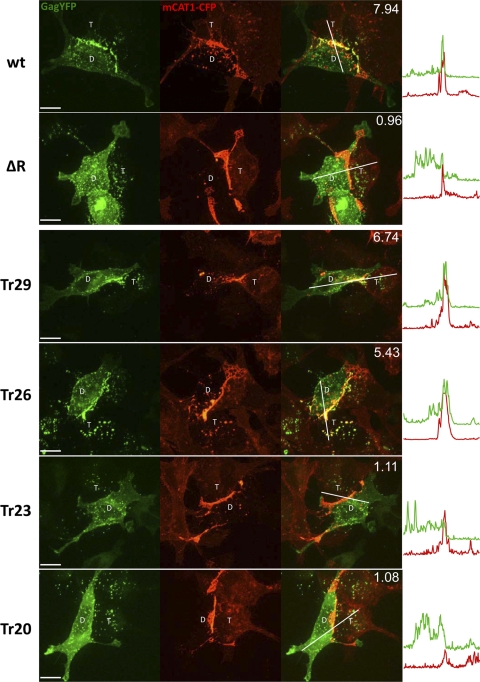
We first reproduced our previous results, which implicated the C-tail of Env (R-peptide) in the polarized assembly of MLV (Fig. 2) (16). HEK293 cells were transfected with plasmids encoding wild-type (wt) or mutant Env ΔR and MLV Gag-YFP. At 4 h posttransfection, producer cells were cocultured for 6 h with XC cells expressing mCAT1-CFP. Cells were fixed and imaged using a spinning disc confocal microscope (Fig. 2). Virological synapses between Env-expressing cells and mCAT1-CFP-expressing XC cells were identified due to the strong accumulation of mCAT1-CFP in the cell-cell interface and the exchange of material between donor and target cell. The MLV synapse is endocytically active, which leads to the internalization of mCAT1-CFP into the donor cell (16, 45). When wt Env was expressed in virus-producing cells, MLV Gag-YFP puncta appeared in the cell-cell interface and colocalized with accumulated mCAT1-CFP. In contrast, when the virus producing cells expressed Env ΔR, polarized assembly was not observed. Particles that were already transmitted into the target cell could be detected in both cases since nonpolarized assembly neither favor nor exclude assembly at sites of cell-cell contact (16). Fluorescence intensity profiles for Gag-YFP and mCAT1-CFP confirmed the extent of Gag polarization to the Env/receptor interface (Fig. 2). We quantified the extent of polarization by determining a polarization index defined as the ratio of Gag fluorescence intensity within the contact zone (marked by the accumulation of mCAT-1) compared to that outside of the contact zone (see Fig. S1A, B, and E in the supplemental material). A value >2.5 indicated polarization. The average polarization index for 10 synapses formed by wt Env was 7.94 (Fig. 2; see also Fig. S1E in the supplemental material). In contrast, the polarization index for Env ΔR was 0.96, which is close to the theoretical value of 1 for random assembly.
Fig. 2.
Visualizing MLV assembly in the presence of cell-cell contacts mediated by wild-type or serial truncated Env. Cells expressing wild-type (wt) or indicated mutant Env and MLV Gag-YFP (green) were cocultured with XC cells expressing mCAT1-CFP (red). Cells were fixed after 6 h of coculture. MLV synapses between donor (D) and target (T) cell were visualized using spinning disc confocal microscopy and images of the resulting 4.5-μm Z-stack were merged into a single extended focus view. The accumulation of Env/receptor complexes (red) at the cell-cell interface and distribution of Gag-YFP (green) was quantified by using intensity profiles along the indicated lines. Moreover, the polarization index as determined in Fig. S1 in the supplemental material is displayed in the upper right corner of the merged images. Scale bar, 10 μm.
Polarized assembly involves a tyrosine residue within the R peptide of MLV Env.
We generated successive truncations from the C terminus to determine at what point the polarizing features of Env were lost (Fig. 1 and 2). Interestingly, truncation at residue 23 (Tr23) but not at residue 26 (Tr26) of the C-tail disrupted polarized assembly (Fig. 1 and 2). The polarization index for Tr23 dropped to ∼1, and the fluorescence intensity profiles illustrated the striking mismatch between receptor accumulation in the cell-cell interface and random assembly at the plasma membrane. The residues 23 to 26 of the C-tail constitute a typical YxxØ motif (Ø = hydrophobic amino acid) known to interact with the μ subunit of AP1-4 complexes that regulate specific endosomal or secretory trafficking pathways (5). When these four residues were individually substituted with alanine, only mutation of the tyrosine 23 (Y23A) failed to support polarized MLV assembly (Fig. 3). Because mutation of the hydrophobic residue of the YxxØ motif that is critical for interaction with μ subunit (L26A) still supported the polarization of assembly (Fig. 3), it is unlikely that endocytic factors recognizing YxxØ motifs play a role in this process. Notably, these mutations did not affect the surface expression of MLV Env (see Fig. S2 in the supplemental material) despite the finding that this tyrosine motif can redirect surface molecules such as CD25 to endocytic compartments (4). However, in the context of the trimeric MLV Env glycoprotein, this endocytic motif is not functional. Tyrosines within YxxL motifs may be phosphorylated to induce downstream signaling (22, 47). To test whether phosphorylation of Tyr23 is required for recruitment of Gag molecules, we also substituted it with phenylalanine. Env Y23F did not affect polarized assembly, indicating that phosphorylation of this tyrosine is not required (Fig. 3).
Fig. 3.
A tyrosine residue within Env cytoplasmic tail is required for polarized assembly. An experiment as described in Fig. 2 was performed for point mutations within the YxxL motif of MLV Env. Scale bar, 10 μm.
MA-dependent membrane targeting is required for Gag recruitment to cell-cell contact sites.
Next, we wanted to understand how retroviral Gag is trafficked to sites of cell-cell contact. Retroviral assembly is driven by the Gag polyprotein that is composed of several functional domains (31). MLV matrix (MA) targets Gag to the plasma membrane by myristoylation at an N-terminal glycine. p12 contains the late domain motif PPPY needed for virus release. Capsid (CA) and nucleocapsid (NC) mediate oligomerization of Gag polyprotein. NC also mediates viral genome packaging into virions. To closely mimic the full-length virus that efficiently coordinates the packing of Env and genome into particles formed by Gag, we deleted individual domains of Gag in the context of the full-length virus (Fig. 4 and 5). Consistent with the results generated by coexpression of Env, Gag, and viral genome (16), Env expressed from the full-length viral construct can efficiently form viral synapses and polarize Gag assembly to the sites of cell-cell contact (Fig. 4; see Video S1 in the supplemental material). Myristoylation-mediated membrane binding was required for MLV Gag recruitment to cell-cell contact sites since the G2A mutant remained entirely cytoplasmic without enrichment at cell-cell contacts (Fig. 4). Interestingly, replacing the entire MA with a constitutive membrane targeting domain from Lck kinase (LckΔMA) prevented Gag recruitment to sites of cell-cell contact (Fig. 4). Polarized assembly was restored when MA was reinserted behind the N-terminal Lck myristoylation/palmitoylation motif (LckGag) (Fig. 4), indicating that MA is required for Gag recruitment to cell-cell contact sites. Likewise, when MA was deleted and MLV Gag targeted to the plasma membrane using the PH domain of PLCδ that binds PIP2 (11, 21, 36), MLV Gag failed to be recruited to sites of cell-cell contact (Fig. 4). In contrast, disrupting the budding ability of Gag by p12 deletion or mutation of PPPY late domain did not prevent assembled MLV Gag from accumulating at cell-cell contact sites (Fig. 5), indicating that the accumulation of MLV Gag at cell-cell contact sites is a process independent of virus release. This is consistent with our previous work in which we used live cell imaging coupled with single particle tracking to show that de novo assembly is polarized toward cell-cell contact sites (16). Deletion of CA and NC did not affect the ability of monomeric MLV Gag to accumulate at cell-cell contact sites, indicating that assembly is not required to target MLV Gag to contact sites (Fig. 5). Replacing NC with the trimeric leucine zipper domain from yeast transcription factor GCN4 rendered Gag assembly independent of genomic RNA (1). This mutant assembled preferentially at cell-cell contact sites (Fig. 5), indicating that genomic RNA cotrafficking and packaging is not required for polarized assembly. These results clearly demonstrate that MLV MA-dependent membrane targeting before Gag assembly directs polarized assembly to sites of cell-cell contact.
Fig. 4.
MLV Gag lacking matrix is not recruited to contact sites. (A) MLV Gag mutants tested. The scheme displays tested wild-type (wt) and mutant MLV Gag variants. The mutant G2A is impaired in N-terminal myristoylation. The entire MA domain or the first 10 amino acids were replaced with the membrane-targeting signal of Lck or the PH domain of PLCδ (LckΔMA, PHΔMA, and LckGag). Although only Gag is displayed, all mutations and deletions were made as Gag-GFP fusions within the context of the full-length Friend57 MLV that also expresses Env but lacks Pol. (B) An experiment as in Fig. 2 was performed in which HEK293 cells expressing wild-type or mutant MLV Gag-GFP (green) depicted in panel A were cocultured for 8 h with XC mCAT1-mCherry (red) cells, and the extent of Gag polarization was quantified. For the complete time-lapse video for wt MLV, see Video S1 in the supplemental material. Scale bar, 10 μm.
Fig. 5.
Assembly and budding of MLV Gag is not required for Gag recruitment to Env-mediated cell-cell contact sites. (A) MLV Gag mutants tested. The scheme displays tested mutant MLV Gag variants. CA and NC represent for capsid and nucleocapsid. In the last mutant, NC was replaced with the trimerizing leucine zipper of the yeast transcription factor GCN4 (1). As in Fig. 4, all mutations and deletions were made as Gag-GFP fusions within the context of the full-length Friend57 MLV that also expresses Env but lacks Pol. (B) The ability of these Gag-GFP mutants (green) to be directed to sites of cell-cell contact (red) was tested and quantified as described in Fig. 4. Scale bar, 10 μm.
MLV MA-dependent polarized assembly in MLV/HIV chimera.
In contrast to HIV Gag that efficiently assembles in the absence of MA (39), MLV Gag lacking MA is impaired in assembly and release (see Fig. S3A in the supplemental material). The inability of MLV lacking MA to assemble could potentially affect our visual readout in the above described experiment. We therefore decided to repeat all experiments using chimera between HIV and MLV Gag (Fig. 6 A) that all efficiently assemble and release virus-like particles in the absence of coculture (see Fig. S3B in the supplemental material). To this end, we coexpressed wild-type and MA-swapped chimeric MLV Gag-YFP and HIV Gag-GFP with MLV Env and cocultured these cells with mCAT1-CFP or mCherry target cells (Fig. 6). Under these conditions, HIV Gag can efficiently incorporate MLV Env into its viral envelope (20). In contrast to wt MLV Gag, HIV Gag assembly was not polarized to cell-cell interfaces generated by MLV Env and mCAT1 interactions (Fig. 6; see also Videos S2 and S3 in the supplemental material). Assembly of MLV Gag carrying the HIV MA protein (MLV-hMA) was similarly nonpolarized (Fig. 6). In contrast, HIV Gag carrying MLV MA (HIV mMA) polarized to MLV Env/mCAT1 cell interfaces (Fig. 6). These data confirm the critical role of MLV matrix in the targeting of Gag to MLV synapses in the context of assembly competent Gag chimera.
Fig. 6.
MLV matrix confers polarized assembly to HIV. (A) Wild-type and MA-swapped chimeric MLV and HIV Gag tested. (B) An experiment as in Fig. 4 and 5 was performed in which HEK293 cells expressing the wild-type MLV or HIV or their chimera (green) depicted in panel A, together with MLV Env, and cocultured with mCAT1-CFP- or mCherry-expressing target cells (red). Cells were fixed 4 or 8 h postinitiation of coculture for HIV Gag and MLV Gag, respectively. Scale bar, 10 μm.
Signaling via Env lays upstream of polarized assembly.
Work by our laboratory had previously shown that the establishment of cell-cell contact precedes polarized assembly, and this depends on Env C-tail (16). As such, we wanted to confirm that the MLV MA-dependent recruitment of Gag into MLV cell-cell interfaces similarly depends on the Env C-tail. Indeed, HIV Gag carrying MLV MA domain and MLV LckGag failed to be recruited to cell-cell interfaces established by MLV Env ΔR (Fig. 7).
Fig. 7.
Signaling via Env lies upstream of polarized assembly. (A) An experiment as described in Fig. 4 was performed. Env expression was disrupted by insertion of stop codon in the Env open reading frame of the LckGag construct used in Fig. 4. HEK293 cells coexpressing this LckGag with wild-type MLV Env or Env ΔR were cocultured with mCAT1-mCherry-expressing target cells (red). (B) An experiment as described in Fig. 6 was performed in which HEK293 cells expressing HIV Gag-GFP (green), together with wild-type MLV Env or Env ΔR, were cocultured with mCAT1-mCherry-expressing target cells (red). Scale bar, 10 μm.
HIV Gag is eventually recruited to MLV synapses after assembly outside of synapses.
We occasionally observed the accumulation of HIV particles carrying MLV Env in the cell-cell interface, particularly late after initiating coculture. We therefore performed time-lapse microscopy to gain further insight into this unexpected observation. Surprisingly, these experiments revealed that while HIV Gag assembled outside of the contact zone, viral assemblons were subsequently drawn into the cell-cell interface and transmitted to the target cell (Fig. 8). As such, these experiments document that virus surfacing (44) can result in the accumulation of viral particles on the surface of virus producing cells as previously discussed (15, 16).
Fig. 8.
HIV Gag carrying MLV Env can be drawn toward MLV synapses following the completion of assembly. (A) Selected frames from Video S3 in the supplemental material of an experiment as performed in Fig. 6 confirm the previously described polarized assembly of MLV at the cell-cell interface of the MLV Env/receptor synapse (16). (B) Selected frames from Video S2 in the supplemental material reveal that HIV particles carrying MLV Env that assembled outside of the contact zone were subsequently drawn into the MLV Env synapse. The 00:24:59 time point corresponds to the image displayed in Fig. 6B. Scale bar, 8 μm.
DISCUSSION
To address the mechanism of polarized MLV assembly toward Env/receptor-induced synapse, we applied a visual assay that could overcome two limitations of the classic approach by measuring spreading of viral infectivity. First, as already discussed, the C-tail of Env modulates the fusogenicity of Env causing cell-cell fusion. The visual approach allowed us to separate the ability of various Env mutants in directing viral assembly to cell-cell contact sites from their ability to mediate cell-cell fusion. Second, it also allowed us to study polarization of assembly and budding-incompetent Gag variants. Using this visual assay, we identified a tyrosine residue within the C-tail of Env and MA in Gag as the viral determinants required for polarized assembly. Recruitment of Gag was dependent on MA and the C-tail of Env. Myristoylation at the N terminus of MA that mediates membrane targeting was also required. In contrast, Gag lacking CA and NC were still efficiently recruited to contact sites. As such, targeting of Gag to sites of cell-cell contacts likely occurs at the cytoplasmic leaflet of the plasma membrane. Local concentration of Gag likely allows the nucleation of assembly explaining how assembly is polarized to contact sites. The absence of a role for NC, the main genome binding domain of Gag, likely excludes a possible role of viral genome in the sorting of Gag.
Various previous reports point to a role of tyrosine motifs in the cell-to-cell spread and in the coupling of Env and Gag trafficking toward the basolateral side in polarized epithelial cells (8–10, 25, 26). In vivo studies indicate that tyrosine mutations affect MLV latency and pathogenesis (8). In the case of MLV, the involvement of a tyrosine motif has been assumed to point to a role in endocytic trafficking despite the fact that overall surface levels did not change, as also observed by us (8, 25). Indeed, our detailed analysis did not confirm a role for the classic YxxØ motif in polarized assembly in cell-cell contact condition. We also tested the possible involvement of tyrosine phosphorylation by substituting Y with F and found that this Env mutant behaved like the wild type. As such, our analysis identifies a tyrosine residue within Env C-tail that is required for polarized assembly. Our characterization of the viral determinants for polarized assembly will assist the isolation of cellular factors that directly interact with the tyrosine motif and/or are required for a C-tail and matrix-dependent recruitment of Gag to the MLV synapse.
The molecular mechanism by which Gag is recruited to Env/receptor complexes at the cell-cell interface remains unknown. The best evidence for an interaction between the C-tail of Env and MA has been presented for HIV (7, 27, 33, 34, 48). Coupling between the trafficking of Env and Gag has also been observed for MLV (42). The inability of myristoylation defective MLV Gag to polarize indicates that if a direct interaction between the C-tail of Env and MA plays a role, it would depend on prior membrane association. The establishment of an Env/receptor-dependent synapse precedes the recruitment of Gag (16). At this point, we favor a model by which the C-tail of Env first modulates the cell-cell interface to generate an adhesive milieu that is required for polarized assembly. This view is supported by the correlation between the residues identified in our study as being responsible for polarity and residues that affect the cell-cell fusion activity of MLV Env (2; J. Jin and W. Mothes, unpublished data). The generation of a stable and long-lived cell-cell interface that prevents cell-cell fusion likely precedes Gag recruitment. The molecular mechanism of MA targeting to cell-cell interface may involve a subsequent direct or indirect interaction between the C-tail and MA. The hypothesized dual function of the C-tail of Env in the modulation of adhesive interfaces and the recruitment of MA would explain why the determinants identified here are distinct from the requirements for Env incorporation into virions (28; Marc Johnson, unpublished data). It is also worth mentioning that the adhesion-induced polarity described here is distinct from the polarity observed in primary migrating T cells, in which HIV Gag is polarized to the uropod by an Env-independent mechanism (24).
By comparing Gag assembly of two different retroviruses, HIV and MLV, we documented two potential modes of virus cell-to-cell transmission. After establishment of MLV Env-receptor adhesion interface, MLV Gag can be recruited to the contact sites prior to assembly. This process depends on MLV MA-mediated membrane targeting and/or MA-Env C-tail interaction. Presumably, this recruitment is along the inner leaflet of the plasma membrane. In contrast, HIV Gag assembles along the cell surface outside of the synapse and is then recruited to MLV synapse by outer leaflet flow. These two modes of adhesion-induced transmission can both contribute to efficient virus cell-to-cell transmission. The here observed behavior of HIV Gag in the context of an MLV Env/receptor synapse is consistent with the observation of lateral movement of HIV to the virological synapse in T cells (13, 41). The ability of HIV to utilize surface movement may also explain independence from the cytoplasmic tail of the envelope, although a dependence has also been reported (6, 10). Further work will be required to understand how the virological synapse can generate flow along the membrane to promote virus cell-to-cell transmission.
Supplementary Material
ACKNOWLEDGMENTS
We thank Marc Johnson for reagents and James Munro, Xaver Sewald, and Luis Agosto for critical reading of the manuscript.
This study is supported by the NIH R01 CA098727 to W.M. and a fellowship from amfAR, the Foundation for AIDS Research, to J.J.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 18 May 2011.
REFERENCES
- 1. Accola M. A., Strack B., Gottlinger H. G. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 74:5395–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aguilar H. C., Anderson W. F., Cannon P. M. 2003. Cytoplasmic tail of Moloney murine leukemia virus envelope protein influences the conformation of the extracellular domain: implications for mechanism of action of the R peptide. J. Virol. 77:1281–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bae Y., Kingsman S. M., Kingsman A. J. 1997. Functional dissection of the Moloney murine leukemia virus envelope protein gp70. J. Virol. 71:2092–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blot V., et al. 2006. The conserved dileucine- and tyrosine-based motifs in MLV and MPMV envelope glycoproteins are both important to regulate a common Env intracellular trafficking. Retrovirology 3:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonifacino J. S., Traub L. M. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–447 [DOI] [PubMed] [Google Scholar]
- 6. Chen P., Hubner W., Spinelli M. A., Chen B. K. 2007. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J. Virol. 81:12582–12595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosson P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783–5788 [PMC free article] [PubMed] [Google Scholar]
- 8. Danis C., et al. 2004. The tyrosine-based YXXO targeting motif of murine leukemia virus envelope glycoprotein affects pathogenesis. Virology 324:173–183 [DOI] [PubMed] [Google Scholar]
- 9. Delamarre L., et al. 1999. The Y-S-L-I tyrosine-based motif in the cytoplasmic domain of the human T-cell leukemia virus type 1 envelope is essential for cell-to-cell transmission. J. Virol. 73:9659–9663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Emerson V., Haller C., Pfeiffer T., Fackler O. T., Bosch V. 2010. Role of the C-terminal domain of the HIV-1 glycoprotein in cell-to-cell viral transmission between T lymphocytes. Retrovirology 7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamard-Peron E., et al. 2010. Targeting of murine leukemia virus gag to the plasma membrane is mediated by PI(4,5)P2/PS and a polybasic region in the matrix. J. Virol. 84:503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hope T. J. 2007. Bridging efficient viral infection. Nat. Cell Biol. 9:243–244 [DOI] [PubMed] [Google Scholar]
- 13. Hubner W., et al. 2009. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323:1743–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Igakura T., et al. 2003. Spread of HTLV-1 between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713–1716 [DOI] [PubMed] [Google Scholar]
- 15. Jin J., Sherer N., Mothes W. 2010. Surface transmission or polarized egress? Lessons learned from HTLV cell-to-cell transmission. Viruses 2:601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin J., Sherer N. M., Heidecker G., Derse D., Mothes W. 2009. Assembly of the murine leukemia virus is directed toward sites of cell-cell contact. PLoS Biol. 7:e1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin J., Sturgeon T., Weisz O. A., Mothes W., Montelaro R. C. 2009. HIV-1 matrix-dependent membrane targeting is regulated by Gag mRNA trafficking. PLoS One 4:e6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson D. C., Huber M. T. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jolly C., Sattentau Q. J. 2004. Retroviral spread by induction of virological synapses. Traffic 5:643–650 [DOI] [PubMed] [Google Scholar]
- 20. Jorgenson R. L., Vogt V. M., Johnson M. C. 2009. Foreign glycoproteins can be actively recruited to virus assembly sites during pseudotyping. J. Virol. 83:4060–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jouvenet N., et al. 2006. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 4:e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kane L. P., Lin J., Weiss A. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242–249 [DOI] [PubMed] [Google Scholar]
- 23. Kayman S. C., Park H., Saxon M., Pinter A. 1999. The hypervariable domain of the murine leukemia virus surface protein tolerates large insertions and deletions, enabling development of a retroviral particle display system. J. Virol. 73:1802–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Llewellyn G. N., Hogue I. B., Grover J. R., Ono A. 2010. Nucleocapsid promotes localization of HIV-1 gag to uropods that participate in virological synapses between T cells. PLoS Pathog. 6:e1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lodge R., et al. 1997. Two distinct oncornaviruses harbor an intracytoplasmic tyrosine-based basolateral targeting signal in their viral envelope glycoprotein. J. Virol. 71:5696–5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lodge R., Lalonde J. P., Lemay G., Cohen E. A. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lopez-Verges S., et al. 2006. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc. Natl. Acad. Sci. U. S. A. 103:14947–14952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lucas T. M., Lyddon T. D., Grosse S. A., Johnson M. C. 2010. Two distinct mechanisms regulate recruitment of murine leukemia virus envelope protein to retroviral assembly sites. Virology 405:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDonald D., et al. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295–1297 [DOI] [PubMed] [Google Scholar]
- 30. Melikyan G. B., Markosyan R. M., Brener S. A., Rozenberg Y., Cohen F. S. 2000. Role of the cytoplasmic tail of ecotropic Moloney murine leukemia virus Env protein in fusion pore formation. J. Virol. 74:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morita E., Sundquist W. I. 2004. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20:395–425 [DOI] [PubMed] [Google Scholar]
- 32. Mothes W., Sherer N. M., Jin J., Zhong P. 2010. Virus cell-to-cell transmission. J. Virol. 84:8360–8368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murakami T., Freed E. O. 2000. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J. Virol. 74:3548–3554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murakami T., Freed E. O. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. U. S. A. 97:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliff A. I., et al. 1980. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J. Virol. 33:475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ono A., Ablan S. D., Lockett S. J., Nagashima K., Freed E. O. 2004. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. U. S. A. 101:14889–14894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Phillips D. M. 1994. The role of cell-to-cell transmission in HIV infection. AIDS 8:719–731 [DOI] [PubMed] [Google Scholar]
- 38. Ragheb J. A., Anderson W. F. 1994. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J. Virol. 68:3220–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reil H., Bukovsky A. A., Gelderblom H. R., Göttlinger H. G. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rein A., Mirro J., Haynes J. G., Ernst S. M., Nagashima K. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rudnicka D., et al. 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 83:6234–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sandrin V., Muriaux D., Darlix J. L., Cosset F. L. 2004. Intracellular trafficking of Gag and Env proteins and their interactions modulate pseudotyping of retroviruses. J. Virol. 78:7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sattentau Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6:815–826 [DOI] [PubMed] [Google Scholar]
- 44. Sherer N. M., Jin J., Mothes W. 2010. Directional spread of surface-associated retroviruses regulated by differential virus-cell interactions. J. Virol. 84:3248–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sherer N. M., et al. 2007. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat. Cell Biol. 9:310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sherer N. M., et al. 2003. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic 4:785–801 [DOI] [PubMed] [Google Scholar]
- 47. Weiss A., Koretzky G., Schatzman R. C., Kadlecek T. 1991. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc. Natl. Acad. Sci. U. S. A. 88:5484–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu X., Yuan X., Matsuda Z., Lee T. H., Essex M. 1992. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J. Virol. 66:4966–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zavorotinskaya T., Qian Z., Franks J., Albritton L. M. 2004. A point mutation in the binding subunit of a retroviral envelope protein arrests virus entry at hemifusion. J. Virol. 78:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.